Abstract
Purpose.
A fluorescent probe was used to identify mucin-depleted areas on the ocular surface and to test the hypothesis that tear lipocalin retrieves lipids from the eyes of normal and dry eye subjects.
Methods.
Fluorescein-labeled octadecyl ester, FODE, was characterized by mass spectrometry and absorbance spectrophotometry. The use of FODE to define mucin defects was studied with impression membranes under conditions that selectively deplete mucin. The kinetics of FODE removal from the ocular surface were analyzed by sampling tears from control and dry eye patients at various times. The tear protein–FODE complexes were isolated by gel filtration and ion exchange chromatographies, monitored with absorption and fluorescent spectroscopies, and analyzed by gel electrophoresis. Immunoprecipitation verified FODE complexed to tear lipocalin in tears.
Results.
FODE exhibits an isosbestic point at 473 nm, pKa of 7.5, and red shift relative to fluorescein. The low solubility of FODE in buffer is enhanced with 1% Tween 80 and ethanol. FODE adheres to the ocular surface of dry eye patients. FODE produces visible staining at the contact sites of membranes, which correlates with removal of mucin. Despite the fact that tear lipocalin is reduced in dry eye patients, FODE removal follows similar rapid exponential decay functions for all subjects. FODE is bound to tear lipocalin in tears.
Conclusions.
Tear lipocalin retrieves lipid rapidly from the human ocular surface in mild to moderate dry eye disease and controls. With improvements in solubility, FODE may have potential as a fluorescent probe to identify mucin-depleted areas.
A recently synthesized hydrophobic molecule is used for a novel application in dry eye disease to identify ocular surface mucin defects and to track lipid retrieval by tear lipocalin in human subjects with and without dry eye disease.
Introduction
Components of the tear film serve to moisten and protect the ocular surface. Transmembrane mucins extend long-chain hydrophilic carbohydrates from the outermost epithelium.1–6 Holly propounded that lipids could contaminate dry spots on the surface of the cornea to mitigate wettability.7,8 Long-chain lipids bound to mucin-depleted areas create a hydrophobic surface to exclude aqueous solutions from nourishing these cells. In dry eye disease, decreased mucin production has been associated with reduced expression of Notch transduction signaling molecules.9–11 Loss of corneal surface cells may also result in mucin deficits evident by an enhanced surface aqueous contact angle that reflects augmented hydrophobicity.12 These studies mesh well with mouse dry eye models13–15; human ocular surface disease models16; and histopathology of human dry eye disease showing that loss of mucins, particularly MUC16, occurs as a result of an exfoliative epitheliopathy.17 However, diagnostic tests in common clinical use such as Schirmer tests, tear breakup time, evaporation rates, and fluorescein staining do not directly delineate mucin loss on the ocular surface. Fluorescein, a hydrophilic agent, is not specific for dry eye and stains in many conditions, including normal eyes.18–20 Fluorescein rapidly penetrates normal corneal epithelium.21,22 A hydrophobic probe that more specifically reflects ocular surface loss of mucin would be useful. If such a probe could also be tracked, information regarding the interaction of surface lipids and lipid-binding proteins could be revealed. Dartt recently called for an in vivo demonstration of the lipid-scavenging function of the predominant lipid-binding protein in tears, tear lipocalin.23 Here we introduce the novel application of a fluorescent probe that binds mucin-depleted areas in the epithelium and demonstrates the scavenging of lipids by tear lipocalin in human subjects.
Materials and Methods
Chemicals
Fluorescein octadecyl ester (FODE), structure shown in Figure 1A, and 4′,6-diamidino-2-phenylindole, dilactate (DAPI), were purchased from Invitrogen (Carlsbad, CA). Bovine submaxillary mucin and Tween 80 were purchased from Sigma-Aldrich (St. Louis, MO). L(+)-arginine hydrochloride, tris(hydroxymethyl)aminomethane (Tris), sodium chloride, sodium borate, sodium citrate, and sodium phosphate were American Chemical Society (ACS) grade (Fisher Scientific, Pittsburgh, PA).
Figure 1. .
Mass spectrum (scan range m/z 100–600) of FODE after 6 months' storage in ethanol. Major peak at m/z 583.3 corresponds to the predicted mass of the MH− chemical structure (inset) of FODE (C38H48O5, molecular weight 584.78).
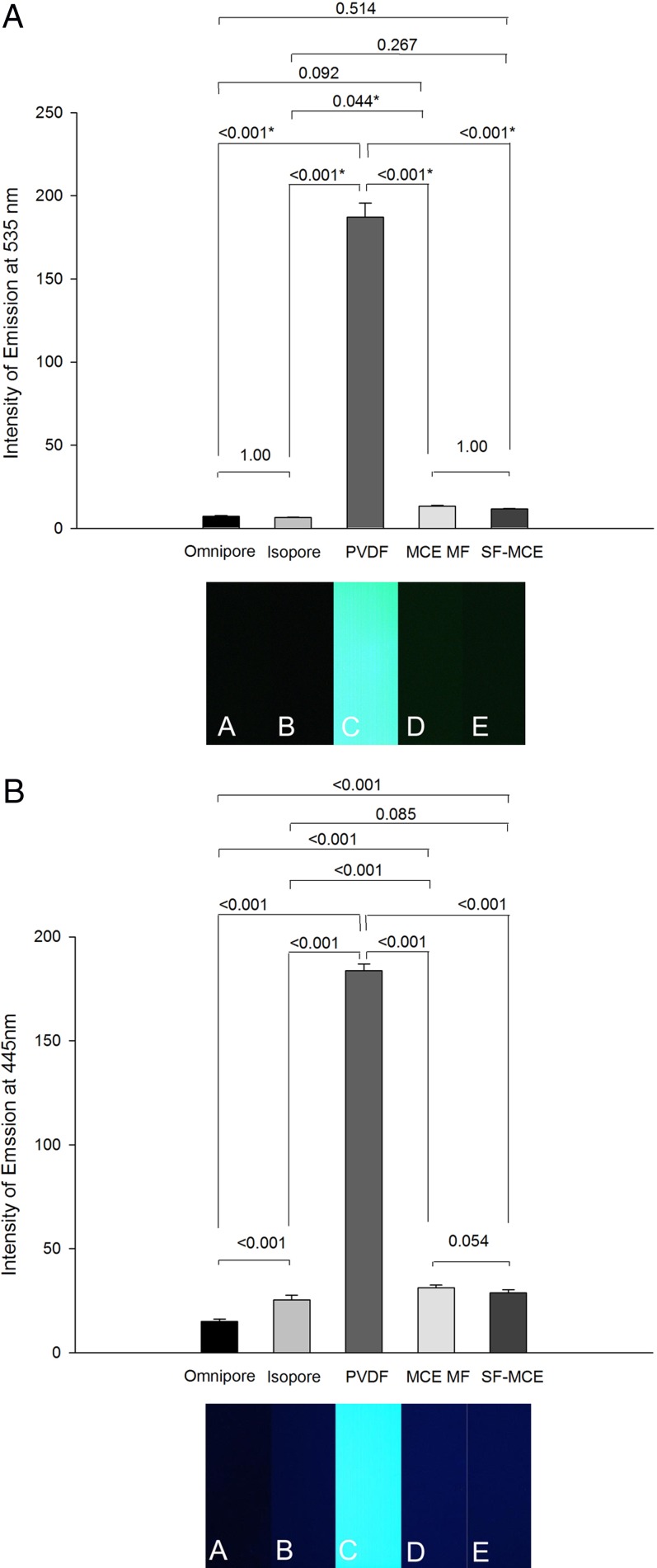
Figure 4. .
Intensity of autofluorescence of impression cytology membranes. Bar graphs expressed as mean (error bars ± SD) intensity of autofluorescence nm computed from six independent photographs in (A) at λem = 535 and in (B) at λem = 445 nm. *P < 0.05.
Mass Spectrometry of FODE
Mass spectrometry was performed with a PE Sciex/MDS API III+ biomolecular analyzer (PE Sciex/MDS, Framingham, MA) electrospray ionization (ESI) quadrupole in negative ion mode. Samples were dissolved in 5 mM ammonium acetate Φ = 0.495, acetonitrile Φ = 0.495, and methanol Φ = 0.01; ion spray voltage optimized at −3500 V and scanned over the range of 100 to 1000 Da. Background was subtracted from the signal intensity and expressed as percent relative intensity. To determine authentic signals, multiple samples were run, weeks apart.
Subject Enrollment
The research was performed in accordance with the tenets of the Declaration of Helsinki, and the procedures were approved by the Institutional Review Board of the University of California-Los Angeles. Written informed consent was obtained from each subject after explanation of the nature and possible consequences of the study. Six dry eye and six control subjects were enrolled. All prospective enrolled subjects completed a dry eye examination including an Ocular Surface Disease Index Questionnaire,24 slit-lamp examination, and Schirmer test with anesthesia. Dry eye severity was defined by the DEWS criteria.25 The six control subjects had no history of ocular surface disease, ocular surgery, inflammatory eye disease, or contact lens wear; and the subject tests listed above were within normal limits.
Absorbance Spectrophotometry
UV absorbance spectra were obtained on a spectrophotometer (Shimadzu UV-2401PC, Kyoto, Japan) in a cuvette of path length 1 cm. Baseline corrections were made for each individual buffer and subtracted from the sample absorbance. In each case at least two spectra were averaged.
The solubility of FODE was determined by saturating solutions of phosphate-buffered saline/1% ethanol with or without 1% Tween 80, followed by ultracentrifugation at 70,000 rpm, and dilution of the supernatant with ethanolic potassium hydroxide to pH 10.4 to measure absorbance using the published and verified molar extinction coefficient of 95,000 M−1 cm−1 at 504 nm.26
For pH titration studies, FODE/1% EtOH/1% Tween 80 was dissolved in various buffer solutions: sodium citrate 15 mM for pH 3.5, 4.4, 5.2, and 6.0; sodium phosphate 15 mM for pH 7.0 and 7.5; and sodium borate 15 mM for pH 8.0, 9.0, 9.5, 10, 11, and 12. The pKa and the slope of the pH titration curve were fit to the Hill1 equation (y = ymin + (ymax − ymin) * x ∧ n / (k ∧ n + x ∧ n); y = relative absorbance (%), x = pH value, n = Hill coefficient; k = dissociation constant)27 in Origin Pro 8.6 (MicroCal Software, Inc., Northampton, MA).
Tear Collection
The type of Schirmer strip for tear collection was selected on the basis of the least interfering absorbance at 280 nm from substances eluted in successive incubations in buffer over a total of 24 hours. Preweighed strips from three brands of Schirmer strips were tested: Tear Flo (Contacare Ophthalmics & Diagnostics, Gujarat, India), Schirmer Tear Test Strips (Alcon, Fort Worth, TX), and Schirmer Tear Test Strips (Clement Clarke, Harlow, Essex, UK). To identify absorbance due to contaminants eluted from Schirmer strips, absorbance spectra of elutions were measured. Each Schirmer strip was minced in a microcentrifuge tube and incubated in 400 μL PBS for 6 hours followed by centrifugation. The process was repeated with 200 μL PBS. The supernatants were conjoined (600 μL) and passed through a 0.22 μm filter (Fisher Scientific) to remove small filter particles. To calculate the absorbance at 280 nm from the contaminant in complex solutions, linear regression analysis (see Statistics below) was performed from graphs of absorbance at 280 vs. 310 nm (negligible absorbance by tears) from solutions at varied concentrations of the contaminant.
For instillation in subjects, FODE saturated in 100% anhydrous ethanol was diluted in phosphate buffer solution (PBS), pH 7.4, to a concentration of 0.74 μM so that the final ethanol concentration was less than 1%. FODE in 1% Tween 80, 1% ethanol PBS solution was prepared in a similar fashion.
Tear fluids were collected from all enrolled subjects with the Schirmer type II tear test.28 Proparacaine (Bausch & Lomb, Tampa, FL) was applied to the subject's conjunctival cul de sac. After 1 minute, 25 μL 1% EtOH/FODE in PBS (0.43 μg/mL) was instilled with a calibrated pipette. Tear Flo Schirmer strips (Contacare Ophthalmics & Diagnostics) were inserted in the inferior cul de sac for four successive 5-minute intervals. Each Schirmer strip was placed in a preweighed microcentrifuge tube and immediately reweighed and frozen at −20°C for analysis within 2 weeks. Each sample was analyzed by steady-state fluorometry for the presence of FODE (see below). The exponential regression analysis of fluorescence intensity was applicable for each subject using y = a*exp−bt where y = fluorescence intensity, a = fluorescence intensity at time = 0, b = fluorescence reduction constant, and t = time. The reduction in fluorescence over time was also expressed as the half-life (t1/2) of fluorescence (FODE) disappearance or t1/2 = ln2/ln(b).
Steady-State Fluorometry
Steady-state fluorescence intensities were measured on spectrofluorometers (either Cary Eclipse [Varian, Walnut Creek, CA] or Fluorologτ-3 [Jobin Yvon-SPEX, Edison, NJ]). Spectral parameters were λex = 490 nm and λem = 530 nm, 5 nm bandwidth, for excitation and emission bandwidths for the Cary Eclipse and 2 nm and 3 nm for the Fluorologτ-3.
Size-Exclusion and Ion Exchange Chromatographies
Tear samples were collected and pooled for two time intervals, a group at 5 minutes and a group at 10 to 20 minutes, keeping samples from dry eye and control subjects separate. The pooled tear samples were concentrated (Amicon Ultra-5, 5000 Da molecular weight cutoff; Millipore, Billerica, MA) to approximately 80 μL. Size-exclusion gel filtration chromatography was performed with two columns vertically stacked in a piggy-back configuration (TSK G3000SW gel columns; Toyo Soda Manufacturing, Tokyo, Japan) equilibrated in 0.05 M Tris HCl, 0.3 M NaCl, and 0.3 M arginine pH 8.5. Protein peaks were monitored continuously at 280 nm, and the peak areas were integrated (ÄKTA Purifier Versatile FPLC; GE Healthcare Biosciences, Pittsburgh, PA). Protein concentrations (c) for each of the major proteins were determined by individual molar extinction coefficients (ε) of tear lipocalin (13,760 M−1 cm−1),29 lysozyme (36,500 M−1 cm−1),30 and lactoferrin (107,640 M−1 cm−1),31,32 according to the Beer–Lambert equation: c = A/(ε λ) where A = absorbance (arbitrary units), λ = path length (cm). Spectrofluorometry (Cary Eclipse) and absorbance spectra (Shimadzu UV-2401PC) were performed on each fraction.
Gel filtration combined with anion exchange chromatography has been shown in many studies to result in high purity of tear lipocalin from tears.33–36 Therefore the gel filtration fractions containing fluorescence with putative tear lipocalin were subjected to anion exchange chromatography (strong anion spin column; Thermo Fisher Scientific, Waltham, MA). The pooled size-exclusion gel filtration fractions were first centrifugally concentrated with 10K membrane filters (Amicon) and the buffer was exchanged to 25 mM Tris, pH 8.4. The flow-through, wash, and eluent (1 M NaCl) fractions were separately collected and tested for fluorescence.
SDS Tricine Polyacrylamide Gel Electrophoresis (PAGE)
Protein fractions from the gel filtration chromatography were concentrated (Amicon 10K) and analyzed in gradient (4%–12%) polyacrylamide gels as previously described.37
Immunoprecipitation of Tear Lipocalin Complexed to FODE
To further establish that FODE in tears is bound to tear lipocalin, immunoprecipitation was performed. Rabbit antiserum to tear lipocalin had been prepared and tested previously.33 Rabbit anti-tear lipocalin and rabbit preimmune sera were bound to separate protein A cross-linked agar columns (Thermo Fisher Scientific). The manufacturer instructions were followed with kit reagents except that the additional washing step was added, with buffer containing 25 mM Tris, 50 mM NaCl, pH 8.4 (due to the low isoelectric point of tear lipocalin). Two microliters of tears containing FODE were combined and incubated overnight on the column at 4°C. The eluted fractions were tested by steady-state fluorometry.
Impression Cytology
After application of one drop of proparacaine 0.5% (Bausch & Lomb), 25 μL 0.74 μM FODE/1% EtOH/1% Tween 80 in PBS was applied to the ocular surface. After 3 minutes, the selected impression membrane was applied just below the central lower limbus for 8 seconds, removed, and air dried.
Fluorescence Microscopy
Impression cytology membranes were immediately examined by fluorescence microscopy (Axiovert 135M; Carl Zeiss, Oberkochen, Germany) with the following filters: for green fluorescence of FODE, λex = 483 ± 15 nm, λem = 535 ± 25 nm, and a dichroic filter (<506 nm cutoff) (Edmund Optics, Barrington, NJ); for DAPI, λex = 365 ± 20 nm, λem = 445 ± 25 nm, and dichroic filter (<395 nm cutoff, Carl Zeiss). Images were captured using the CoolSnap CCD camera (1.45 megapixels; Photometrics, Tucson, AZ) and Image Pro Express software (Media Cybernetics, Bethesda, MD) with constant exposure time.
Comparison of Impression Cytology Membranes
Polycarbonate (Isopore; Millipore), polyvinylidene fluoride (PVDF, Durapore; Millipore), polytetrafluoroethylene (PTFE, Omnipore, Biopore, Teflon; Millipore), mixed cellulose ester (MCE, MF filter; Millipore), and surfactant-free mixed cellulose ester (SF-MCE; Millipore) were compared for intensity of autofluorescence. Fluorescence intensity was quantitated from the grayscale in ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsbweb.nih.gov/ij/index.html).
To find the optimal membrane with the greatest periodic acid-Schiff (PAS) sensitivity for mucin, 2 μL bovine submaxillary mucin (1 mg/mL) (Sigma-Aldrich) was applied to each cytology membrane; membranes were then air dried and fixed in 10% formalin, immersed in 0.5% periodic acid for 10 minutes, rinsed with distilled water, immersed in Schiff base solution for 5 minutes, rinsed with warm distilled water, incubated in 0.5% sodium metabisulfite solution for 5 minutes, rinsed in water, and air dried. PAS intensity, ΔPAS, was calculated as the difference between staining and background intensities on a reverse grayscale (ImageJ). The membrane with the greatest ΔPAS was chosen to assess retrieval for mucin.
External Eye Photography
External eyes were photographed under a LED blue penlight (467 nm; LDP LLC, Carlstadt, NJ) source, excitation band-pass filter (315–540 nm, 80% transmittance; Beseler, Stroudsburg, PA), emission yellow gel filter 312 (460 nm cutoff <0.1% transmittance; 467 nm, 0.26% transmittance; 505 nm to 525 nm, >80% transmittance in the range of the fluorophore; Rosco Laboratories, Inc., Stamford, CT), and a 9× slit-lamp eyepiece (Leitz, Wetzlar, Germany). Manual camera settings for digital photography were ISO 400, 1/4,” F2.9, VR, no flash, fine 13 m (Coolpix P6000; Nikon, Tokyo, Japan).
Statistical Analysis
Absorbance spectra, fluorescence intensity, autofluorescence intensity, and ΔPAS intensity are presented as the mean ± standard deviation (SD). The Mann-Whitney U test was performed to make comparisons between dry eye and control groups at each time point of fluorescence decay. Differences among the absorbance of Schirmer strip extracts at 280 nm, intensity of the membrane autofluorescence at DAPI or green fluorescence settings, and ΔPAS staining on different membranes were evaluated by one-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparison test, as appropriate.
All of the statistical analyses were performed using STATA 8.2 software (StataCorp LP, College Station, TX). A P value less than 0.05 was considered statistically significant.
Regression analysis for linear and exponential functions was performed using SigmaPlot statistical analysis with either linear or two-parameter exponential regression method (Systat Software, Inc., San Jose, CA).
Results
Fluorescein Octadecyl Ester (FODE) Chemical Properties
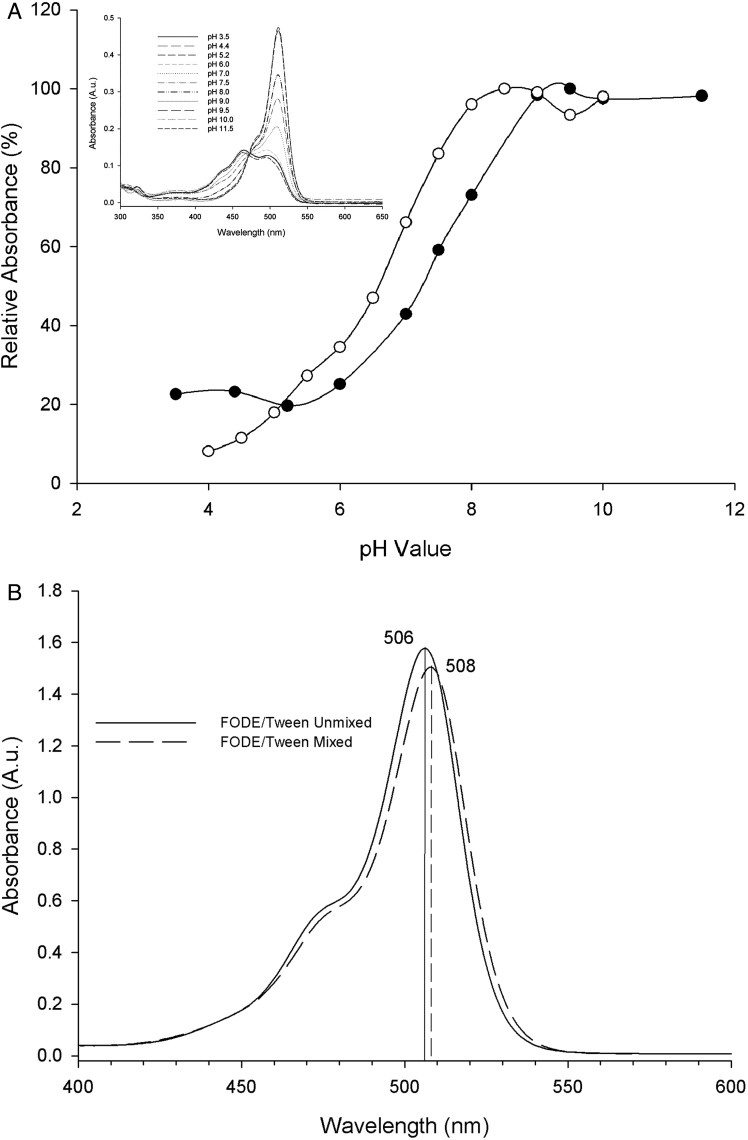
Mass spectrometry of FODE was unchanged after storage for over 15 months in ethanol at −20°C (Fig. 1) and showed a consistent monoisotopic peak at m/z = 583.3 on all runs consistent with the MH− ionized species. Smaller peaks accounted for less than 10% of the total ion intensity and were inconsistently present. Ion extraction chromatogram analysis showed that the ions from the smaller peaks were extant in the infusion buffer and therefore considered chemical noise. Figure 2 shows the effect of pH and Tween 80 on the spectral characteristics of FODE. With increasing pH there is a marked red shift in the absorbance peak, from 465 nm at pH 3 to pH 6 to 511 nm at pH 7 to pH 11.5 (Fig. 2A, inset). All spectra converge on a single isosbestic point at 473 nm (Fig. 2A, inset), indicative of two chemical forms with similar absorbance at 473 nm. The pH titration plots of absorbance at 511 nm for FODE and at 490 nm for sodium fluorescein, as adapted from a prior publication,38 are both sigmoidal (Fig. 2A). The pKa's of FODE and sodium fluorescein in aqueous buffer solution are 7.57 and 6.65, respectively. The solubility of FODE is 0.4 and 6.5 μM in PBS/1% ethanol and in PBS 1% ethanol/1% Tween 80, respectively.
Figure 2. .
(A) pH titration of absorbance for 0.74 μM FODE in 1% EtOH/1% Tween 80 (•) at 511 nm and fluorescein (○) at 490 nm of 0.005% fluorescein adapted with permission from Doughty MJ. pH dependent spectral properties of sodium fluorescein ophthalmic solutions revisited. Ophthalmic Physiol Opt. 2010;30:167–174. Copyright 2010 John Wiley and Sons. Inset: absorption spectra of 0.74 μM FODE/1% EtOH/1% Tween 80 in buffers from pH 3.5 to 12 show convergence at the isosbestic point at 473 nm. (B) Influence of Tween 80 on the spectra of FODE. A split cuvette separated FODE in buffer in one chamber and buffer with Tween in the second chamber (−) before mixing the two chambers and (– –) FODE/Tween 80 mixed. Peak absorbances shown in nm; 2 nm red shift of absorbance resulted from the mixture of FODE in Tween 80 solution.
To determine the effect of Tween 80 on the absorbance spectrum of FODE, a split cuvette was utilized. One chamber contained FODE in PBS pH 7.0, and the separate adjacent chamber held 1% Tween 80, PBS pH 7.0. Mixing the contents of the two chambers resulted in only 2 nm of red shift in the peak and 4.6% reduction in the peak height of absorbance (Fig. 2B).
Impression Cytology
Impression cytology membranes have different handling properties. Omnipore and Isopore are soft and prone to folding. MCE MF is rigid and fragile. SF-MCE is pliable and durable. PVDF is firm and resilient and has excellent clearing properties during processing that are superior for finding cells with histochemical staining.
To determine if FODE binds to cells of the ocular surface in dry eye, a PVDF membrane was placed on the ocular surface of a subject with dry eye after instillation of FODE. The photomicrographs of the membrane, corrected for autofluorescence, show fluorescence localized to some cells (Fig. 3). In other areas, fluorescence was unaccompanied by cells. However, the PVDF membrane was discovered to have background fluorescence; several impression cytology membranes were tested for autofluorescence. The results are shown in Figures 4A and 4B. The PVDF membrane shows the highest intensity of fluorescence for both DAPI and green fluorescence settings. At DAPI settings, Omnipore, Isopore, and SF-MCE membranes have the lowest autofluorescence. Omnipore has the lowest green autofluorescence.
Figure 3. .
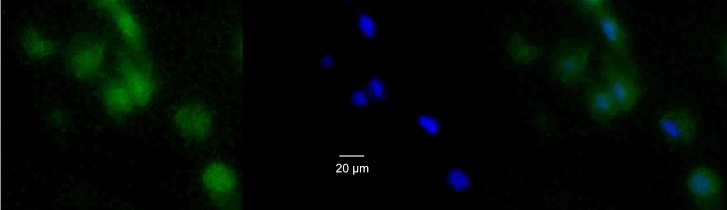
Photomicrograph of fluorescence image of impression cytology membrane taken from ocular surface 20 minutes after FODE instillation. (A) Green fluorescence of FODE: λex = 483 ± 15 nm, λem = 535 ± 25 nm, and a dichroic filter (<506 nm cutoff). (B) Same area after DAPI staining, λex = 365 ± 20 nm, λem = 445 ± 25 nm, and dichroic filter (<395 nm cutoff). (C) 3A and 3B merged. Original magnification ×200.
SF-MCE had relatively low autofluorescence and was chosen for assessing approximate duration of time for which residual FODE remained on the ocular surface. SF-MCE membranes were applied at time intervals of 1, 20, and 60 minutes after instillation of FODE. Membrane fluorescence is prominent at 1 minute (Fig. 5A) but has disappeared after 20 minutes (Figs. 5B, 5C).
Figure 5. .
Time-dependent pattern of FODE removal from ocular surface as marked by impressions taken from different areas of the same patient at the following times: (A) 1 minute after FODE instillation, (B) 20 minutes, (C) 60 minutes. Original magnification: 50×.
Tracking FODE Removal in Tears of Dry Eye and Control Subjects
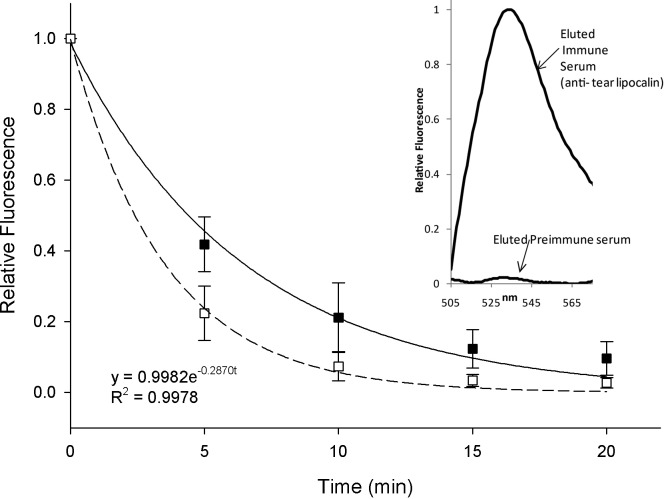
To determine if FODE was removed by components in tears and to better follow the pharmacokinetics of FODE, tears were collected with Schirmer strips from six dry eye and six control subjects after FODE instillation. The fluorescence intensities of the eluted Schirmer strips are shown at various time points (Fig. 6). The decay in fluorescence is rapid and fits an exponential curve. The exponential regression curves for dry eye and control subjects are also shown in Figure 6. No statistical difference is evident between dry eye and control subjects (P = 0.30). The mean half-life of decay was 5.00 ± 3.76 minutes for controls and 2.50 ± 1.56 minutes in the dry eye group (P = 0.15).
Figure 6. .
Fluorescence of tears versus time after FODE installation in dry eye (□) and control subjects (▪). Fluorescence intensity (FI) was measured at λex = 490 nm and λem = 511 nm. Individual exponential regression curve was calculated. FI at 0 minutes was set as 100%, and FI of subsequent time points were also represented as percentage of FI at 0 minutes. Mean intensities were not significantly different between control and dry eye subjects (P = 0.30). Inset: fluorescent intensity of eluted fractions from immunoprecipitation on columns of protein A cross-linked agar bound with either anti-tear lipocalin immune serum or preimmune serum as shown. Two microliters of combined tears and FODE were incubated on the columns and eluted at pH 2.
Verification that tear lipocalin was bound to FODE in tears was established by immunoprecipitation of tears containing FODE. Fluorescence (FODE) was found only in the eluted fractions from the protein A cross-linked column bound to tear lipocalin antisera. Negligible fluorescence was observed in the eluted fractions from the column bound to preimmune serum (Fig. 6, inset). The fluorescence from the preimmune column was present in the wash fractions.
Chromatography and SDS Tricine Polyacrylamide Gel Electrophoresis
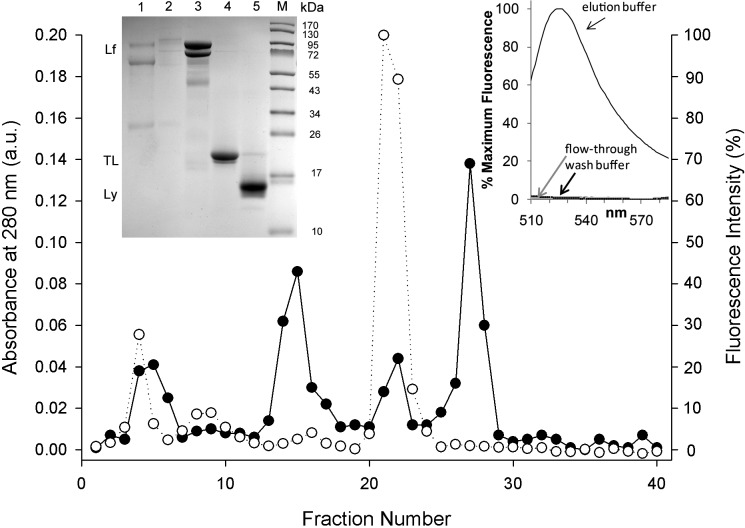
To further verify that FODE removed from the ocular surface is bound to a protein fraction, tears collected at time intervals were subjected to gel filtration chromatography. Figure 7 shows the elution profile of the tears pooled from dry eye subjects at 5 minutes for both absorbance and fluorescence of each fraction. The major protein-absorbing fractions at 280 nm were analyzed by tricine SDS-PAGE. The fractions containing tear lipocalin coincided with the major fluorescent peak (Fig. 7, inset, left, lane 4). A minor fluorescent peak in fraction 4 (Fig. 7) accounts for only ∼12.5% of total FODE and has a shorter elution time than tear lipocalin. Gel electrophoresis confirmed the lack of discernible tear lipocalin in these fractions. The logical candidate for binding FODE in these fractions seems to be a 30 kDa protein; this protein appears only in fractions 3 and 4 in gradient gels (Fig. 7, inset, left). One candidate protein for the minor peak is apolipoprotein D, a known tear glycoprotein (∼30 Kda) and member of the lipocalin family.39 The other proteins in fractions 3 and 4 are found to be more concentrated in later fractions where no FODE was detected.
Figure 7. .
Size-exclusion gel filtration of pooled tear samples from dry eye subjects collected at 5 minutes. (•) Absorbance at 280 nm was corrected by linear regression analysis of absorbance of 280 vs. 310 nm. (○) Fluorescence intensity (λem = 511 nm). Inset left: Coomasssie-stained SDS tricine (4%–12%) acrylamide gradient gel. Lane 1, fractions 4 to 6. Lane 2, fractions 8 to 10. Lane 3, fractions 14 to 16. Lane 4, fractions 21 to 23. Lane 5, fractions 26 to 28. Lane M, molecular weight markers, size shown at the right. Lactoferrin (Lf), lysozyme (Ly), and tear lipocalin (TL) are indicated at left. Inset right: fluorescence of anion exchange chromatography performed on fractions 21 to 23 of control subjects collected at 10 to 20 minutes. Fluorescence was seen only in the elution buffer (high salt) fractions. Gel filtration chromatography of control sample was nearly identical to that of dry eye.
Anion exchange spin chromatography of gel filtration fractions corresponding to tear lipocalin from control subjects showed no fluorescence in the flow-through or wash fractions. Fluorescence was found only in the elution buffer, which contained tear lipocalin (Fig. 7, inset, right).
Protein Concentrations in Dry Eye and Control Subjects
Tear protein concentration can be calculated from samples extracted from Schirmer strips, once cross-absorbing (280 nm) agents are corrected. Control spectra revealed that the extracts from Schirmer strips from all manufacturers tested contained a substance extractable in PBS that absorbs at 280 nm (Fig. 8). The absorbances at 280 nm, normalized per mass of Schirmer strip, were significantly different for some manufacturers. Tear Flo elution showed the lowest absorbance at 280 nm and 310 nm (P = 0.0005) (Fig. 8). The spectrum of 25 μL human tears in 475 μL PBS indicates that absorbance >310 nm is negligible (Fig. 8, top inset). Linear regression analysis of the absorbance plot of 280 vs. 310 nm shows the absorbance at 280 nm attributable to extracted contaminants from Schirmer strips (Fig. 8, bottom inset). Also the cross-absorbance of FODE at 280 nm was estimated. Because absorbance between 500 nm and 515 nm in the absorbance spectra of all gel filtration fractions (data not shown) was insignificant, and since the absorbance of FODE at 280 nm was 62% of that at 500 to 515 nm, the absorbance at 280 nm from FODE in the gel filtration samples was deemed negligible (Fig. 2B, inset).
Figure 8. .
Absorbance spectra of extracts from various Schirmer strips in buffer. Spectra are displayed as mean values (n = 6) for extracts from each strip. Inset top: absorbance spectrum of human tears shows negligible absorbance >310 nm. Inset bottom: linear regression analysis was performed from graphs of absorbance at 280 vs. 310 nm from extracts of the Tear Flo strip.
Peak integration of isolated protein fractions in the chromatogram of tears at 280 nm, in combination with the published molar extinction coefficients, permits the determination of the relative amounts of the major proteins in control and dry eye subjects (Table 1). The largest integrated peak area in both the dry eye and control group is lysozyme, followed in order by lactoferrin and tear lipocalin. However, compensating for the molar extinction coefficients, the protein with the greatest concentration in both dry eye and control tears is tear lipocalin, followed by lysozyme and lactoferrin.
Table 1. .
Comparison of Tear Proteins (μM) in Human Tears of Normal Subjects
|
Reference |
Methods of Measurement |
Lactoferrin |
Tear Lipocalin |
Lysozyme |
| 54 | Radial immunodiffusion and lysodeiticus assay | 17.95 | 131.84 | 146.80 |
| 51 | HPLC, A280, ELISA (recalculate using e) | 22.3 (23.83) | 96.15 (69.21) | 249.47 (184.86) |
| 52 | Bradford method, Lowry method, SDS-PAGE densitometry | 35.01 | 165.65 | 171.97 |
| 53 | HPLC, bicinchoninic acid technique | 88.27 | ||
| Current study | FPLC, A280, e | 10.7 | 56.29 | 45.21 |
FODE Staining at Membrane Impression Sites on the Ocular Surface
Fluorescence was evident at the site of impression of the Schirmer strips after FODE/1% EtOH/1% Tween 80 was applied (Fig. 9A). The tear film was also fluorescent, corroborating rapid retrieval by components in tears (Fig. 9B). Fluorescence on the ocular surfaces appeared to diminish quickly; exiguous staining was seen on the ocular surface after 5 minutes (Fig. 9B).
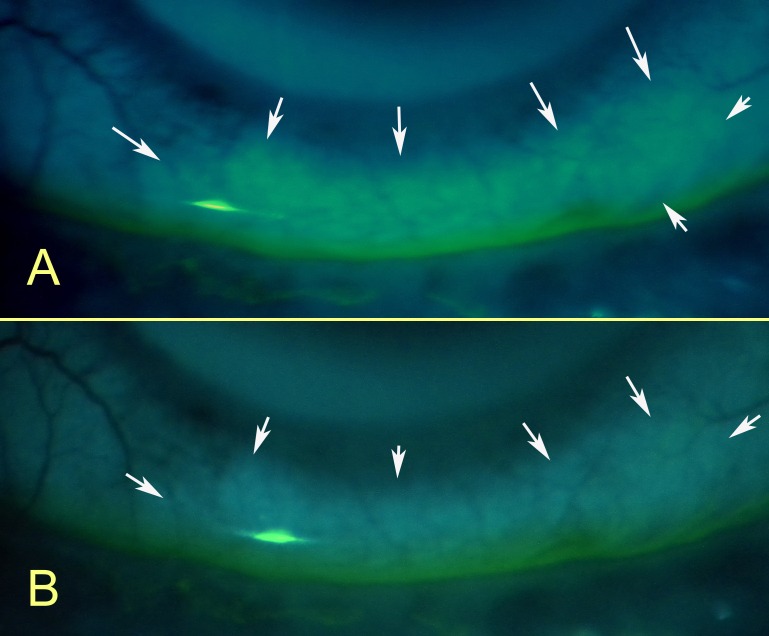
Figure 9. .
FODE staining of sites where Schirmer test II was performed (arrows). Photos taken after (A) 3 minutes and (B) 5 minutes. Photos were under a LED blue penlight (467 nm, LDP LLC) source, excitation band-pass filter (315–540 nm, 80% transmittance) (Beseler), emission yellow gel filter 312 (Rosco Laboratories) (cutoff <460 nm), and a 9× slit-lamp eyepiece (Leitz). Manual camera settings for photography were ISO 400, 1/4,” F2.9, VR, no flash, fine 13 m (Coolpix P6000; Nikon).
The possibility that staining was associated with removal of surface mucin was tested. Impression cytology membranes provide a flat surface for microscopy to distinguish cells from acellular mucins. Figure 10 shows that PAS staining intensity varied between background membrane and mucin spots according to the type of membrane and the amount of applied bovine submaxillary mucin. There were statistically significant differences in staining intensity between background and mucin spots, ΔPAS intensity, among most membranes. Omnipore membrane had the lowest ΔPAS intensity. SF-MCE membrane showed the greatest ΔPAS intensity and was chosen for ocular surface study (Fig. 10). SF-MCE membrane impression on the ocular surface left a residual area, which was susceptible to FODE staining (Fig. 11). The areas of PAS staining precisely matched the membrane impression area; no appreciable staining was seen in areas that did not touch the ocular surface (Fig. 12). FODE appeared to have been removed rapidly, and ocular surface and tears were bereft of fluorescence after 20 minutes (Fig. 11).
Figure 10. .
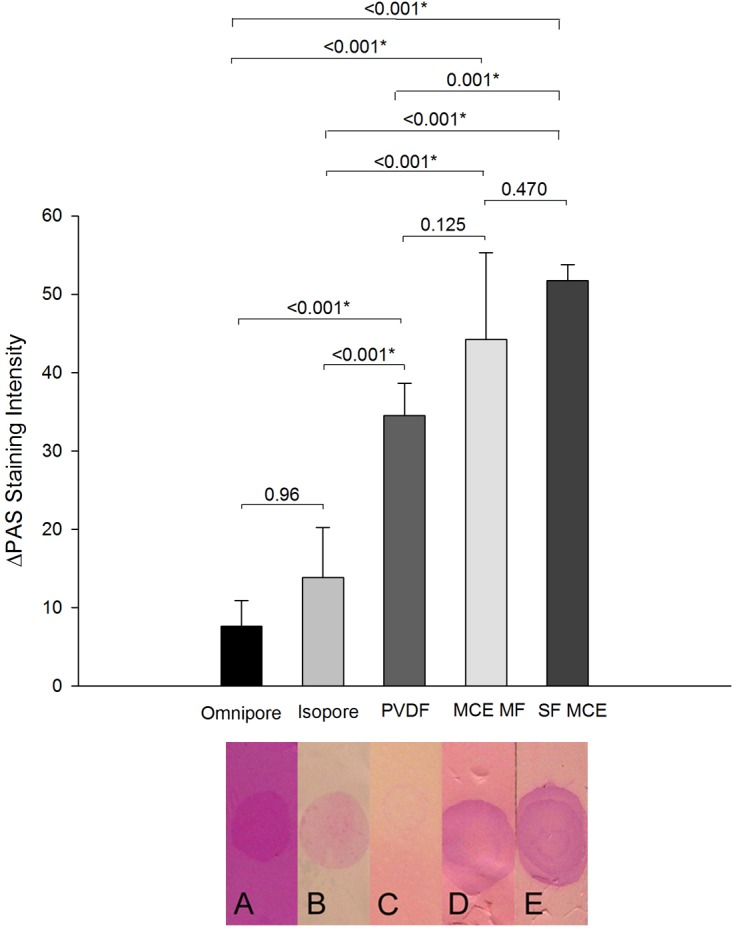
Specificity of periodic acid-Schiff (PAS) staining of bovine sialomucin in various types of impression cytology membranes. Bar graphs show the difference in PAS intensity of areas of the membrane without mucin compared to those with mucin. Intensity calculations were made from reverse grayscale program provided by ImageJ software (NIH). Measurements represent the mean for six individual experiments (±SD). *P < 0.05. Photographs of representative membranes from one experiment are shown below the graph.
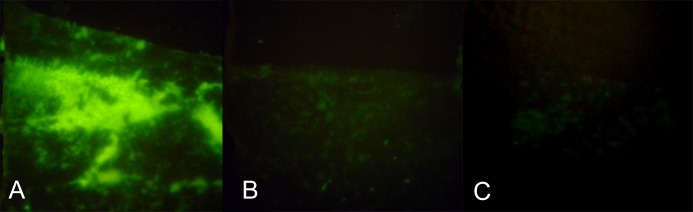
Figure 11. .
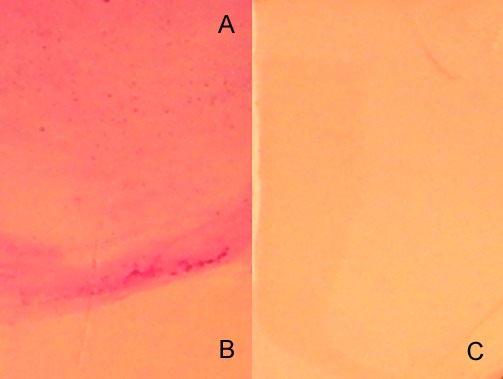
Periodic acid-Schiff staining of surfactant-free mixed cellulose ester membrane. (A) Image of an area that contacted the ocular surface. (B) Image of control region of the same membrane not in contact with ocular surface. (C) Membrane alone. Manual camera settings for photography were ISO 64, 1/284,” F2.9, VR, no flash, fine 13 m (Coolpix P6000).
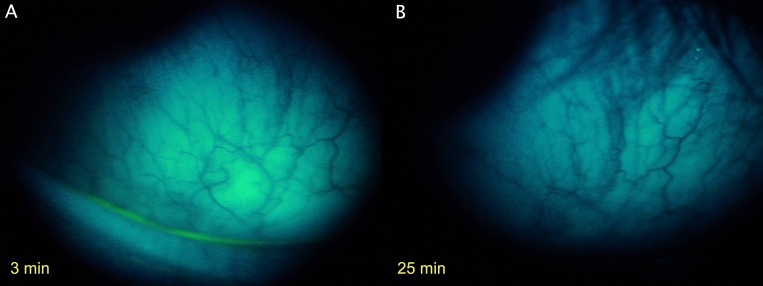
Figure 12. .
Clinical image of SF-MCE membrane impression site. (A) Three minutes after FODE instillation. (B) Same area 25 minutes after instillation. Images were under a LED blue penlight (467 nm, LDP LLC) source, excitation band-pass filter (315–540 nm, 80% transmittance) (Beseler), emission yellow gel filter 312 (Rosco Laboratories) (cutoff <460 nm), and a 9× slit-lamp eyepiece (Leitz). Manual camera settings for photography were ISO 400, 1/4,” F2.9, VR, no flash, fine 13 m (Coolpix P6000).
Discussion
Two major findings emerge from this study. First, tear lipocalin rapidly removes exogenous lipid from the ocular surface in vivo. Second, FODE has pharmacokinetic properties that differ from fluorescein and may aid in the identification of areas of mucin deficits on the ocular surface.
Removal of FODE from the Ocular Surface by Tear Lipocalin
A recent review called for exploring the function of tear lipocalin to retrieve lipids in vivo.23 The rapid removal of lipid from the ocular surface by tear lipocalin in patients is expected for a number of reasons. Tear lipocalin, as the major lipid-binding protein in tears, contains native ligands including fatty acids, fatty alcohols, phospholipids, glycolipid, and cholesterol.34,37 The binding is strong, with dissociation constants generally in the micromolar range.40 Tear lipocalin has been shown to bind to ferric stearate adherent to Teflon, to solubilize nitroxide-labeled lauric acid derivatives bound to polystyrene, to adsorb to meibomian lipid films, and to bind palmitic acid fluorescent derivatives in quartz cuvettes.41–43 Tear lipocalin retrieves lipids from both fixed and unfixed human corneas, with and without ocular surface disease, in vitro.17
The gel filtration, ion exchange, and immunoprecipitation chromatography experiments combined with gel electrophoresis show unambiguously that the predominant protein binding FODE in tears is tear lipocalin. The speed of removal of lipid from the human cornea in vivo is remarkably fast. As evident from the photographs, FODE disappears from the ocular surface and appears in the tear film in less than 1 minute. FODE reduction from tears occurs at approximately 10.0% to 20.0%/minute, a rate similar to that of turnover of tears measured by fluorophotometry.44,45 Unlike fluorescein, which showed no protein binding in the limited tear components studied,46 FODE appears exclusively protein bound in our chromatography studies. Taken together, these findings suggest that binding of tear lipocalin to the lipid is not the limiting factor of removal but probably reflects simple clearance of tear lipocalin. The removal of 16-(9-anthroyloxy)palmitic acid (fluorescein-labeled fatty acid; 16AP) from fresh corneal buttons in vitro was slower as measured by the time to retrieve half of the maximal fluorescence, or 35.9 vs. 5 minutes for FODE in vivo.17,42 A number of factors could account for the apparent accelerated retrieval in patients, including intact lid wiper action and a longer alkyl chain, which has a greater binding constant for tear lipocalin.34,40,47 Also consistent with an inverse relationship between rate of removal and alkyl chain length of the lipid, Mochizuki showed that a lipid bearing a C12 alkyl chain had a much slower turnover rate in human subjects than our data show for FODE, with only 50% removal at approximately 44 minutes.48
No statistical difference was noted between the removal of FODE from the ocular surfaces of normal and dry eye subjects. These data match the results obtained in vitro for corneal buttons from patients with bullous keratopathy.17 Bullous keratopathy models the desquamation of the superficial mucin-producing cells seen in dry eye disease.14,16,49,50 As in our previous work in vitro, the current data from human subjects clearly identify tear lipocalin as the predominant protein to remove lipids.17,41,42
Comparison of Tear Lipocalin in Dry Eye and Normal Subjects
The absence of differences between retrieval of lipids from dry eye and normal ocular surfaces both in vitro and in vivo suggests that the amount of tear lipocalin is more than ample to perform the task. Chromatographic isolation of the major tear proteins invites comparison of the amount of tear lipocalin in normal and dry eye subjects. A summary is shown in Table 1. Such a comparison is necessarily an approximation because the samples, methods, and patient populations are not identical. In one study, peak integration and similar SK 3000 column chromatography were employed, so detailed comparison is possible.51 Compared to the 5 peaks in our chromatogram (Fig. 7), 11 peaks, some of which have overlapping proteins, were seen in the previous study despite the use of 1 M NaCl to dampen column interactions. The fewer peaks in our chromatogram are probably due to the addition of arginine, an ion pairing agent. The integrated peak area for lipocalin is relatively greater and lysozyme slightly less in our chromatograms. Also affecting the calculated difference is the relatively low molar extinction coefficient used in our data for determining the concentration of tear lipocalin rather than less specific general protein assays. If one recalculates Fullard's data with recently acquired extinction coefficients,29 the values match more closely (Table 1). Similar convergence would be expected for studies that estimate proteins similarly.52,53 It is probable that additional volume of fluid instilled with FODE diluted the concentration of proteins in tears. Supporting this contention is the slightly decreased overall protein concentration measured from the 5-minute collection sample compared to the 10- to 15-minute sample. Schirmer contaminants are an unlikely source of error of individual protein concentrations since purification steps followed the collection that would eliminate small molecular weight contaminants.
In this study, tear lipocalin concentration was much lower in the mild to moderate dry eye versus control patients. Although the samples from multiple patients were pooled to represent an average of each group as a whole, formal statistical evaluation is obviated as sample size permitted only two chromatograms run in tandem with gel electrophoresis. The majority of prior studies have found a similar reduction in tear lipocalin in dry eye disease.52–59 Lysozyme and/or lactoferrin have also been found to be reduced in dry eye disease.54,55,58,60 These findings contrast with those of other studies in which no significant differences were seen in some or all of these proteins (Table 2).56,61
Table 2. .
Study Comparison of Concentration of Tear Proteins in Dry Eye Disease
|
Reference |
Method |
Dry Eye Disease |
Normal Subjects |
||||
|
Lactoferrin |
Tear Lipocalin |
Lysozyme |
Lactoferrin |
Tear Lipocalin |
Lysozyme |
||
| Current study | FPLC, A280, e | 0.20 | 0.68 | 0.62 | 0.19 | 1.00 | 0.80 |
| 53 | HPLC, bicinchoninic acid technique | 0.58 | 1.00 | ||||
| 71 | HPLC, A280 | 0.62 | 1.08 | 1.19 | 0.72 | 1.00 | 1.22 |
| 57 | DC Protein Assay Kit, Western blot | 0.45 | 1.00 | ||||
| 55 | iTRAQ, 2-dimensional Nano-LC-nano-ESI-MS/MS analysis | 0.23 | 0.76 | 1.71 | 0.29 | 1.00 | 2.00 |
| 56 | Western blot, ECL vs. DC Protein Assay | 0.25 | 0.77 | 0.29 | 1.00 | ||
Mechanism of Staining of FODE and Fluorescein
The data show that FODE has different mechanisms of interaction with the ocular surface than fluorescein, which may be useful diagnostically. The punctate staining of fluorescein is not specific to dry eye disease but occurs in numerous conditions, even from the use of contact lens solutions.22,62 In fact fluorescein can be found within corneal epithelial cells of normal subjects by fluorescent microscopy.46,63
The histologic basis of the punctate pattern of fluorescein staining in dry eyes has recently been correlated with hyperfluorescence of superficial epithelial cells in human specimens.22 The mechanisms by which the fluorescein enters the cells are uncertain. Diffusion is a plausible mechanism given the high concentration, approximately 3 mM, of fluorescein that is applied clinically and the polar nature of the fluorescein molecule. However, the distribution of hyperfluorescent cells in punctate spots seems incongruous with diffusion in some instances. For example, in some punctate spots, wing cells may stain in isolation, unassociated with staining of the overlying superficial cell layers.22 Hence, the pattern of fluorescein staining does not seem to necessarily reflect the biophysical characteristics of the surface. Because the hyperfluorescent cells generally appear intact rather than apoptotic, the punctate spot pattern seems to befit an upregulated active transport mechanism in the hyperfluorescent epithelium. Cellular influx of fluorescein has been demonstrated to occur through monocarboxylate transporters. The mechanism, a transmembrane proton-coupled exchange, has been documented in the epithelium of the jejunum and cornea.64–66 Monoanionic fluorescein cellular influx is accelerated at acidic pH.67 The monocarboxylate transport mechanism is particularly active under conditions of aerobic stress and speculatively may reflect local acidotic conditions in individual epithelial cells in dry eye.
The pH titration results indicate that FODE appears well suited to probe the biophysical nature of the ocular surface. The greater pKa of FODE (7.57), versus that of fluorescein (6.65), translates into fewer available monoanions at acidic pH and therefore is probably not as susceptible to the monocarboxlate transporter. Further, the hydrophobic nature of FODE may lead to insoluble complexes that could impede cell entry. Indeed, the surface deposition of FODE is evident in this study from removal by direct membrane contact. In most areas, the fluorophore was unaccompanied by cells on the membrane. The augmented deposition/staining of FODE on the ocular surface where mucin has been effectively removed by impression cytology membranes is provocative. The mechanism of cellular staining appears to be adherence to mucin-depleted areas, perhaps because binding of the hydrophobic alkyl chain of FODE is enhanced in the absence of the charged glycosylated moieties. Reduced surface mucins, including MUC16, have been reported in dry eye disease and bullous keratopathy as a reduction in expression and a result of an exfoliative epitheliopathy.16,68 However, cells captured in exfoliative cytology from postmenopausal women show increased protein levels perhaps suggesting compensatory production.69 The fact that FODE binds to corneas of normal subjects is consistent with the concept of ectoshedding of mucins.2 A comparison of the amount of FODE bound to normal versus dry eye subjects was not possible because of the extremely short transit time achieved from rapid removal by tear lipocalin. However, the enhanced binding of FODE to mucin-depleted areas may seem contradictory to work showing that soluble bovine mucins may be strongly absorbed to synthetic hydrophobic surfaces.70 Differences between the behavior of human and bovine surface mucin characteristics have been published.38 The binding properties of human ocular surface mucins require a more detailed study.
The mechanism of FODE removal is also different from that of fluorescein. While FODE is removed rapidly by tear lipocalin, instilled fluorescein is not protein bound in tear components.46
The use of FODE as a diagnostic agent to identify ocular surface areas bereft of mucin faces potential hurdles. The absence of a difference in FODE removal from normal subjects and mild to moderate dry eye subjects in these initial studies may reflect the lack of a clinically detectable difference in deposition in mild dry eye disease or simply indicate that retrieval by lipocalin is robust enough to overcome any differences. Improved solubility of FODE is probably necessary to achieve adequate clinically visible fluorescent staining under excitation with the cobalt blue filter commonly used on slit lamps. Solubility was enhanced by over an order of magnitude in 1% Tween 80, but the saturated concentration is still at least two orders of magnitude below that of fluorescein in clinical use. Fluorescein staining is likewise enhanced in areas where mucin has been removed by membrane impression.21 Therefore, in some cases the information reported may be similar to that from FODE, but the mechanism for fluorescein staining results in a lack of specificity. Fluorescein appears to involve intracellular migration, perhaps related to accelerated diffusion in the absence of a protective mucin coat.21,22 The clear demarcation of mucin-deprived surface areas by FODE at low concentration is alluring.
Acknowledgments
The authors thank Kym Faull, Alex Yoon, and the Pasarow Mass Spectrometry Laboratory for assistance.
Footnotes
Supported by NIH Grants EY11224 (BG), EY000331 (Core), and NIH S10-RR023718 A (Shared Instrumentation Grant) and the Edith and Lew Wasserman Professorship (BG).
Disclosure: P.-T. Yeh, None; R. Casey, None; B.J. Glasgow, None
References
- 1. Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004; 78: 379–388 [DOI] [PubMed] [Google Scholar]
- 2. Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003; 231: 1–49 [DOI] [PubMed] [Google Scholar]
- 3. Gipson IK, Inatomi T. Cellular origin of mucins of the ocular surface tear film. Adv Exp Med Biol. 1998; 438: 221–227 [DOI] [PubMed] [Google Scholar]
- 4. Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res. 2001; 73: 281–289 [DOI] [PubMed] [Google Scholar]
- 5. Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003; 44: 2487–2495 [DOI] [PubMed] [Google Scholar]
- 6. Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006; 47: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holly FJ. Physical chemistry of the normal and disordered tear film. Trans Ophthalmol Soc U K. 1985; 104 (pt 4): 374–380 [PubMed] [Google Scholar]
- 8. Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973; 15: 515–525 [DOI] [PubMed] [Google Scholar]
- 9. Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009; 50: 2666–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002; 43: 1004–1011 [PubMed] [Google Scholar]
- 11. Xiong L, Woodward AM, Argueso P. Notch signaling modulates MUC16 biosynthesis in an in vitro model of human corneal and conjunctival epithelial cell differentiation. Invest Ophthalmol Vis Sci. 2011; 52: 5641–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiffany JM. Measurement of wettability of the corneal epithelium. II. Contact angle method. Acta Ophthalmol (Copenh). 1990; 68: 182–187 [DOI] [PubMed] [Google Scholar]
- 13. De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006; 83: 526–535 [DOI] [PubMed] [Google Scholar]
- 14. Chen W, Zhang X, Zhang J, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci. 2008; 49: 1386–1391 [DOI] [PubMed] [Google Scholar]
- 15. Lin Z, Liu X, Zhou T, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011; 17: 257–264 [PMC free article] [PubMed] [Google Scholar]
- 16. Glasgow BJ, Gasymov OK, Casey RC. Exfoliative epitheliopathy of bullous keratopathy with breaches in the MUC16 glyocalyx. Invest Ophthalmol Vis Sci. 2009; 50: 4060–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glasgow BJ, Gasymov OK, Abduragimov AR, Engle JJ, Casey RC. Tear lipocalin captures exogenous lipid from abnormal corneal surfaces. Invest Ophthalmol Vis Sci. 2010; 51: 1981–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korb DR, Korb JM. Corneal staining prior to contact lens wearing. J Am Optom Assoc. 1970; 41: 228–232 [PubMed] [Google Scholar]
- 19. Norn MS. Micropunctate fluorescein vital staining of the cornea. Acta Ophthalmol (Copenh). 1970; 48: 108–118 [DOI] [PubMed] [Google Scholar]
- 20. Schwallie JD, McKenney CD, Long WD Jr, McNeil A. Corneal staining patterns in normal non-contact lens wearers. Optom Vis Sci. 1997; 74: 92–98 [DOI] [PubMed] [Google Scholar]
- 21. Thinda S, Sikh PK, Hopp LM, Glasgow BJ. Polycarbonate membrane impression cytology: evidence for fluorescein staining in normal and dry eye corneas. Br J Ophthalmol. 2010; 94: 406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mokhtarzadeh M, Casey R, Glasgow BJ. Fluorescein punctate staining traced to superficial corneal epithelial cells by impression cytology and confocal microscopy. Invest Ophthalmol Vis Sci. 2011; 52: 2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dartt DA. Tear lipocalin: structure and function. Ocul Surf. 2011; 9: 126–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003; 110: 1412–1419 [DOI] [PubMed] [Google Scholar]
- 25. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop ( 2007). Ocul Surf. 2007; 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 26. Wistrom CA, Jones GM, Tobias PS, Sklar LA. Fluorescence resonance energy transfer analysis of lipopolysaccharide in detergent micelles. Biophys J. 1996; 70: 988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goutelle S, Maurin M, Rougier F, et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol. 2008; 22: 633–648 [DOI] [PubMed] [Google Scholar]
- 28. Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966; 62: 47–60 [DOI] [PubMed] [Google Scholar]
- 29. Gasymov OK, Abduragimov AR, Glasgow BJ. The conserved disulfide bond of human tear lipocalin modulates conformation and lipid binding in a ligand selective manner. Biochim Biophys Acta. 2011; 1814: 671–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ito Y, Yoshikawa A, Hotani T, Fukuda S, Sugimura K, Imoto T. Amino acid sequences of lysozymes newly purified from invertebrates imply wide distribution of a novel class in the lysozyme family. Eur J Biochem. 1999; 259: 456–461 [DOI] [PubMed] [Google Scholar]
- 31. Franco I, Castillo E, Perez MD, Calvo M, Sanchez L. Effects of hydrostatic high pressure on the structure and antibacterial activity of recombinant human lactoferrin from transgenic rice. Biosci Biotechnol Biochem. 2012; 76: 53–59 [DOI] [PubMed] [Google Scholar]
- 32. Baker EN, Anderson BF, Baker HM, et al. Three-dimensional structure of lactoferrin. Implications for function, including comparisons with transferrin. Adv Exp Med Biol. 1998; 443: 1–14 [PubMed] [Google Scholar]
- 33. Glasgow BJ. Tissue expression of lipocalins in human lacrimal and von Ebner's glands: colocalization with lysozyme. Graefes Arch Clin Exp Ophthalmol. 1995; 233: 513–522 [DOI] [PubMed] [Google Scholar]
- 34. Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr Eye Res. 1995; 14: 363–372 [DOI] [PubMed] [Google Scholar]
- 35. Glasgow BJ, Abduragimov AR, Yusifov TN, et al. A conserved disulfide motif in human tear lipocalins influences ligand binding. Biochemistry. 1998; 37: 2215–2225 [DOI] [PubMed] [Google Scholar]
- 36. Selsted ME, Martinez RJ. Isolation and purification of bactericides from human tears. Exp Eye Res. 1982; 34: 305–318 [DOI] [PubMed] [Google Scholar]
- 37. Dean AW, Glasgow BJ. Mass spectrometric identification of phospholipids in human tears and tear lipocalin. Invest Ophthalmol Vis Sci. 2012; 53: 1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doughty MJ. pH dependent spectral properties of sodium fluorescein ophthalmic solutions revisited. Ophthalmic Physiol Opt. 2010; 30: 167–174 [DOI] [PubMed] [Google Scholar]
- 39. Rassart E, Bedirian A, Do Carmo S, et al. Apolipoprotein D. Biochim Biophys Acta. 2000; 1482: 185–198 [DOI] [PubMed] [Google Scholar]
- 40. Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim Biophys Acta. 1999; 1433: 307–320 [DOI] [PubMed] [Google Scholar]
- 41. Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999; 40: 3100–3107 [PubMed] [Google Scholar]
- 42. Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005; 46: 3589–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009; 50: 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nelson JD. Simultaneous evaluation of tear turnover and corneal epithelial permeability by fluorophotometry in normal subjects and patients with keratoconjunctivitis sicca (KCS). Trans Am Ophthalmol Soc. 1995; 93: 709–753 [PMC free article] [PubMed] [Google Scholar]
- 45. Sorbara L, Simpson T, Vaccari S, Jones L, Fonn D. Tear turnover rate is reduced in patients with symptomatic dry eye. Cont Lens Anterior Eye. 2004; 27: 15–20 [DOI] [PubMed] [Google Scholar]
- 46. Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992; 99: 605–617 [DOI] [PubMed] [Google Scholar]
- 47. Abduragimov AR, Gasymov OK, Yusifov TN, Glasgow BJ. Functional cavity dimensions of tear lipocalin. Curr Eye Res. 2000; 21: 824–832 [DOI] [PubMed] [Google Scholar]
- 48. Mochizuki H, Yamada M, Hatou S, Tsubota K. Turnover rate of tear-film lipid layer determined by fluorophotometry. Br J Ophthalmol. 2009; 93: 1535–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beardsley RM, De Paiva CS, Power DF, Pflugfelder SC. Desiccating stress decreases apical corneal epithelial cell size--modulation by the metalloproteinase inhibitor doxycycline. Cornea. 2008; 27: 935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006; 47: 2847–2856 [DOI] [PubMed] [Google Scholar]
- 51. Fullard RJ, Snyder C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest Ophthalmol Vis Sci. 1990; 31: 1119–1126 [PubMed] [Google Scholar]
- 52. Ng V, Cho P, To C. Tear proteins of normal young Hong Kong Chinese. Graefes Arch Clin Exp Ophthalmol. 2000; 238: 738–745 [DOI] [PubMed] [Google Scholar]
- 53. Yamada M, Mochizuki H, Kawai M, Tsubota K, Bryce TJ. Decreased tear lipocalin concentration in patients with meibomian gland dysfunction. Br J Ophthalmol. 2005; 89: 803–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Janssen PT, van Bijsterveld OP. Tear fluid proteins in Sjogren's syndrome. Scand J Rheumatol Suppl. 1986; 61: 224–227 [PubMed] [Google Scholar]
- 55. Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009; 8: 4889–4905 [DOI] [PubMed] [Google Scholar]
- 56. Srinivasan S, Joyce E, Boone A, Simpson T, Jones L, Senchyna M. Tear lipocalin and lysozyme concentrations in postmenopausal women. Ophthalmic Physiol Opt. 2010; 30: 257–266 [DOI] [PubMed] [Google Scholar]
- 57. Caffery B, Joyce E, Boone A, et al. Tear lipocalin and lysozyme in Sjogren and non-Sjogren dry eye. Optom Vis Sci. 2008; 85: 661–667 [DOI] [PubMed] [Google Scholar]
- 58. Srinivasan S, Thangavelu M, Zhang L, Green KB, Nichols KK. iTRAQ quantitative proteomics in the analysis of tears in dry eye patients. Invest Ophthalmol Vis Sci. 2012; 53: 5052–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Versura P, Nanni P, Bavelloni A, et al. Tear proteomics in evaporative dry eye disease. Eye (Lond). 2010; 24: 1396–1402 [DOI] [PubMed] [Google Scholar]
- 60. Ohashi Y, Ishida R, Kojima T, et al. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003; 136: 291–299 [DOI] [PubMed] [Google Scholar]
- 61. Grus FH, Sabuncuo P, Augustin AJ. Quantitative analysis of tear protein profile for soft contact lenses–a clinical study [in German]. Klin Monbl Augenheilkd. 2001; 218: 239–242 [DOI] [PubMed] [Google Scholar]
- 62. Lebow KA, Schachet JL. Evaluation of corneal staining and patient preference with use of three multi-purpose solutions and two brands of soft contact lenses. Eye Contact Lens. 2003; 29: 213–220 [DOI] [PubMed] [Google Scholar]
- 63. Ichijima H, Yokoi N, Nishizawa A, Kinoshita S. Fluorophotometric assessment of rabbit corneal epithelial barrier function after rigid contact lens wear. Cornea. 1999; 18: 87–91 [PubMed] [Google Scholar]
- 64. Kuwayama K, Miyauchi S, Tateoka R, Abe H, Kamo N. Fluorescein uptake by a monocarboxylic acid transporter in human intestinal Caco-2 cells. Biochem Pharmacol. 2002; 63: 81–88 [DOI] [PubMed] [Google Scholar]
- 65. Berginc K, Zakelj S, Levstik L, Ursic D, Kristl A. Fluorescein transport properties across artificial lipid membranes, Caco-2 cell monolayers and rat jejunum. Eur J Pharm Biopharm. 2007; 66: 281–285 [DOI] [PubMed] [Google Scholar]
- 66. Konishi Y, Hagiwara K, Shimizu M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci Biotechnol Biochem. 2002; 66: 2449–2457 [DOI] [PubMed] [Google Scholar]
- 67. Legen I, Zakelj S, Kristl A. Polarised transport of monocarboxylic acid type drugs across rat jejunum in vitro: the effect of mucolysis and ATP-depletion. Int J Pharm. 2003; 256: 161–166 [DOI] [PubMed] [Google Scholar]
- 68. Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010; 90: 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gipson IK, Spurr-Michaud SJ, Senchyna M, Ritter R III, Schaumberg D. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011; 30: 1346–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi L, Caldwell KD. Mucin adsorption to hydrophobic surfaces. J Colloid Interface Sci. 2000; 224: 372–381 [DOI] [PubMed] [Google Scholar]
- 71. Grus FH, Augustin AJ. High performance liquid chromatography analysis of tear protein patterns in diabetic and non-diabetic dry-eye patients. Eur J Ophthalmol. 2001; 11: 19–24 [DOI] [PubMed] [Google Scholar]