SUMMARY
Animals modulate the power output needed for different locomotor tasks by changing muscle forces and fascicle strain rates. To generate the necessary forces, appropriate motor units must be recruited. Faster motor units have faster activation–deactivation rates than slower motor units, and they contract at higher strain rates; therefore, recruitment of faster motor units may be advantageous for tasks that involve rapid movements or high rates of work. This study identified motor unit recruitment patterns in the gastrocnemii muscles of goats and examined whether faster motor units are recruited when locomotor speed is increased. The study also examined whether locomotor tasks that elicit faster (or slower) motor units are associated with increased (or decreased) in vivo tendon forces, force rise and relaxation rates, fascicle strains and/or strain rates. Electromyography (EMG), sonomicrometry and muscle-tendon force data were collected from the lateral and medial gastrocnemius muscles of goats during level walking, trotting and galloping and during inclined walking and trotting. EMG signals were analyzed using wavelet and principal component analyses to quantify changes in the EMG frequency spectra across the different locomotor conditions. Fascicle strain and strain rate were calculated from the sonomicrometric data, and force rise and relaxation rates were determined from the tendon force data. The results of this study showed that faster motor units were recruited as goats increased their locomotor speeds from level walking to galloping. Slow inclined walking elicited EMG intensities similar to those of fast level galloping but different EMG frequency spectra, indicating that recruitment of the different motor unit types depended, in part, on characteristics of the task. For the locomotor tasks and muscles analyzed here, recruitment patterns were generally associated with in vivo fascicle strain rates, EMG intensity and tendon force. Together, these data provide new evidence that changes in motor unit recruitment have an underlying mechanical basis, at least for certain locomotor tasks.
KEY WORDS: electromyography, gait, motor recruiment, activation
INTRODUCTION
Mammalian skeletal muscles are composed of multiple motor unit types, each of which has defined physiological and mechanical properties. The combination of innervating slow-twitch fibers (type I) and fast-twitch fibers (types IIA and IIB) (Burke et al., 1973) is thought to enable muscles to perform a wide range of locomotor tasks that require different forces and shortening velocities. For example, fast fibers have strain rates and activation–deactivation rates that are two to three times faster than those of slow fibers (He et al., 2000), making fast motor units potentially advantageous for tasks that involve rapid movements or high rates of mechanical work. However, the manner in which recruitment patterns change with the force- or strain-related demands of different locomotor tasks remains largely unknown. Many studies have made indirect assessments of muscle force, length and shortening velocity (e.g. Hodson-Tole and Wakeling, 2008b; Lichtwark et al., 2007; Gillis et al., 2001). However, few studies have measured muscle force and strain together directly during differing locomotor tasks, and even fewer have examined whether such measures are associated with the recruitment of faster or slower motor units (Hodson-Tole and Wakeling, 2007; Wakeling et al., 2006).
Recent in vivo studies have established that muscles modulate their work output to meet the changing mechanical demands associated with various locomotor tasks (Roberts et al., 1997, Daley and Biewener, 2003; Gabaldón et al., 2004; McGuigan et al., 2009). For example, when goats run uphill, extensor muscles at the knee and ankle increase their work output to raise the body's center of mass (Lee et al., 2008). Across a variety of species, in those muscles examined, muscle fascicle strain, strain rate and force have been shown to vary substantially during walking, hopping and running at different speeds or over different terrain (e.g. Roberts et al., 1997; Biewener, 1998; Biewener et al., 1998; Daley and Biewener, 2003; Gillis and Biewener, 2002; Fukunaga et al., 1997; Lichtwark and Wilson, 2006; Lichtwark et al., 2007; McGuigan et al., 2009). However, the manner in which animals modulate the mechanical output of their muscles by recruiting different motor unit types remains poorly understood.
When a task demands an increased level of muscle force, motor units are typically recruited in an orderly fashion from slowest to fastest, originally formulated as the size principle of motor unit recruitment by Henneman and others (Henneman and Olson, 1965; Henneman et al., 1965; Henneman et al., 1974) and others (Freund et al., 1975; Fedde et al., 1969). However, evidence has emerged across a range of species that motor units may be preferentially recruited (e.g. Gillespie et al., 1974; Grimby et al., 1981; Nardone et al., 1989; Sokoloff and Cope, 1996; Wakeling et al., 2006; Hodson-Tole and Wakeling, 2007; Hodson-Tole and Wakeling, 2008b; Hodson-Tole and Wakeling, 2010). For example, there is evidence that faster motor units may be recruited, independent of slower units, during rapid locomotor tasks. This evidence is based, in large part, on analyzing the time-varying frequency spectra of electromyographic (EMG) signals (see reviews by Hodson-Tole and Wakeling, 2009; Raez et al., 2006). Different motor unit types have muscle fibers with different electrical membrane properties (Luff and Atwood, 1972), and there is an intrinsic speed dependence of the EMG frequency such that faster fibers generate higher-frequency signals (Gerdle et al., 2000; Wakeling et al., 2002; Hodson-Tole and Wakeling, 2008b; Lee et al., 2011). For example, when human subjects cycle at increased cadences, EMG signals from the gastrocnemii shift to higher frequencies (Wakeling et al., 2006), consistent with the recruitment of faster motor units (Citterio and Agostoni, 1984).
The factors that trigger recruitment of the different motor unit types remain unclear. In tasks with fast shortening–lengthening cycles, such as a cat paw shake, recruitment of faster motor units – independent of slow units – may be advantageous (e.g. Smith et al., 1980) because faster units have faster activation–deactivation rates (Burke et al., 1973; Lee et al., 2011). In tasks where rapid force rise and relaxation rates are not required, or where sustained force at only low to moderate levels is needed, orderly motor unit recruitment according to the size principle is more likely to be sufficient. Interestingly, Hodson-Tole and Wakeling (Hodson-Tole and Wakeling, 2008b) reported that fascicle strain rates in rat hindlimb muscles were slower during inclined walking than during level walking and were associated with the recruitment of slower motor units, despite an increase in the total EMG intensity. Preferential recruitment of motor unit types was further supported when comparing the EMG frequency content during level running and incline walking where the EMG intensity was similar, but the EMG frequency content was significantly lower during incline walking. Such studies suggest that motor unit recruitment may be associated with task-dependent factors, but which factors and under what conditions remains largely unknown.
In this study, we examined the time-varying frequency spectra of EMG signals from the lateral and medial gastrocnemius (LG and MG) muscles of goats during level walking, trotting and galloping, and during inclined walking and trotting. We also explored whether changes in EMG frequency during the different locomotor tasks were associated with in vivo tendon forces, force rise and relaxation rates, fascicle strains and/or strain rates. In goats and other mammals, the LG and MG muscles are composed of both slow and fast fibers. Goats were selected for this study because they allowed EMG, fascicle length and tendon force to be measured directly under in vivo conditions. Based on a previous study in rats (Hodson-Tole and Wakeling, 2008b), we hypothesized that: (1) goats would recruit faster motor units at increased locomotor speeds, resulting in an increase in the high-frequency component of the EMG signal during galloping compared with walking; and (2) goats would recruit slower motor units at increased surface grades, resulting in an increase in the low-frequency component of the EMG signal during inclined versus level gait. We also hypothesized that: (3) EMG frequency content would be associated with in vivo tendon force as well as the force rise and relaxation rates; and (4) EMG frequency content would be associated with fascicle shortening strain rate. These associations are anticipated because increases in a muscle's force likely depend on the recruitment of faster motor units (in addition to slower units) and because increases in locomotor speed are likely linked to more rapid changes in muscle force and higher fascicle strain rates.
MATERIALS AND METHODS
Six African pygmy goats [Capra aegagrus hircus (L.); three males, three females; age: 21.0±15.5 months; body mass: 25.85±6.20 kg] were tested at Harvard University's Concord Field Station. EMG, fascicle strain and tendon force data were recorded in vivo during a variety of locomotor tasks, as detailed below. The experimental protocol was conducted over a 3-day period, which included surgical implantation of transducers, in vivo testing and in situ testing (Lee et al., 2011). The results of the in situ experiments were previously reported (Lee et al., 2011). All surgical and experimental procedures followed IACUC approval.
Surgical implantation of transducers
Initially, animals were sedated with a mixed injection of ketamine and xylazine (8 mg kg−1 body mass and 0.05 mg kg−1 body mass, respectively) into the jugular vein. The animals were then intubated and maintained on a closed-system anesthesia machine (Matrix, Orchard Park, NY, USA) at 0.5–1.0% isoflurane.
Offset twist-hook bipolar silver-wire electrodes (0.1 mm; California Fine Wire, Grover Beach, CA, USA), with tips bared of 0.5 mm of insulation and an offset of 2 mm, were implanted ~3 mm deep into proximal, mid-belly and distal regions of the LG and MG muscles (Fig. 1) (Lee et al., 2011).
Fig. 1.
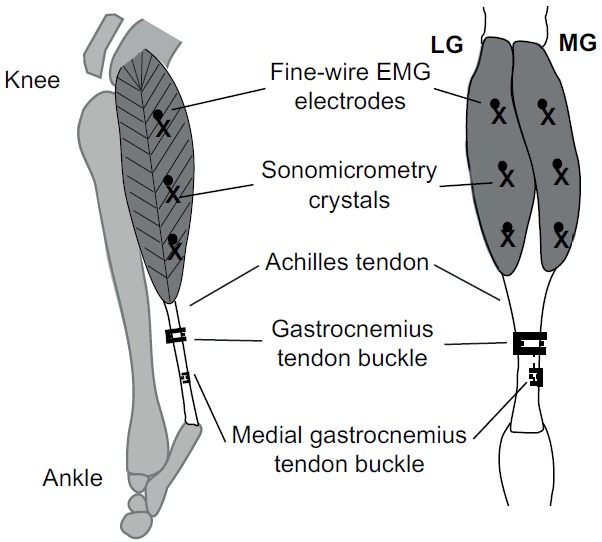
Lateral (left) and posterior (right) view of the goat lower hindlimb. The approximate locations of the electromyographic (EMG) electrodes and sonomicrometry crystals (proximal, mid-belly and distal) in the lateral and medial gastrocnemius (LG and MG) muscles, and the common gastrocnemius and MG tendon-buckle force transducers are shown (adapted from Lee et al., 2011).
Sonomicrometry crystals (2 mm; Sonometrics, London, ON, Canada) were implanted into the mid-belly region of the LG and MG muscles in either paired or triad configurations (Fig. 1) (Lee et al., 2011). For the paired configuration, the crystals were inserted parallel to the fascicles (proximo-superficial to distal-deep ~25 deg pennation angle), which allowed length changes of the fascicles to be estimated. For the triad configuration, an additional crystal was implanted directly below the superficial crystal to measure muscle depth, which also allowed changes in pennation angle to be estimated (these results will be reported elsewhere). The signal output was amplified (Triton 120.2; Triton Technology, San Diego, CA, USA) and monitored on an oscilloscope (2245A; Tektronix, Beaverton, OR, USA). The alignment of the crystals was optimized to maximize the signal-to-noise ratio.
A lateral incision proximal to the ankle joint was made to expose the underlying tendons. The common tendon sheath was then exposed to separate the gastrocnemius tendon from the superficial digital flexor tendon. Gas-sterilized E-shaped stainless-steel buckle transducers, equipped with a metal foil strain gauge (type FLA-1; Tokyo Sokki Kenkyujo. Tokyo, Japan) bonded to the central arm (Biewener and Baudinette, 1995), were attached to the Achilles tendon. On several goats, a longer 2 cm incision was made along the distal portion of the Achilles tendon and two separate tendon buckles were attached to the lateral and medial portions of the Achilles tendon, enabling separate recordings of LG and MG force (Fig. 1) (Lee et al., 2011).
Lead wires from all transducers were passed through a subcutaneous tunnel to a connector that was sutured to the skin proximal to the hip. Animals received post-operative analgesia (buprenorphine, 0.1 to 0.5 mg kg−1, subcutaneously) every 12 h during the following 40 h recovery and in vivo recording periods.
In vivo testing
Goats were allowed 20–24 h for recovery following surgery. The animals had been previously trained over a period of 2–3 weeks to walk, trot and gallop on a large, motorized treadmill (belt dimensions: 2.50 m long and 0.75 m wide) on the level and on a 15 deg incline. The animals' speeds ranged from 1.1 to 1.4 m s−1 (walking), 1.7 to 2.8 m s−1 (trotting) and 3.3 to 4.9 m s−1 (galloping). For each goat, the same set of speeds was kept for the level and incline conditions. Recordings were therefore made at typical walking and running speeds such that three trials of at least 15 to 20 strides each were collected for each condition. Goats were allowed to rest between trials to minimize fatigue and to ensure that they could complete the experiments. EMG signals were amplified (gain of 100–1000) and recorded with minimal filtering (band-pass 30–3000 Hz, notch at 60 Hz, P511 amplifier; Grass Technologies, West Warwick, RI, USA). Tendon buckle signals were connected to a bridge amplifier (Vishay 2120; Micro-Measurements, Raleigh, NC, USA). All signals were recorded at 5000 Hz using a 16-channel acquisition device (NI-6259; National Instruments, Austin, TX, USA).
Tendon buckle transducers were calibrated the following day, after the in situ experiments were completed and the animals were euthanized (sodium pentobarbital, 150 mg kg−1). For these calibrations, a cut was made across the distal aponeurosis of the LG and MG to free a portion of the Achilles tendon with the buckles intact. The cut end was clamped and frozen with liquid nitrogen. A series of cyclical loads were imposed using a force transducer (model 9203; Kistler, Amherst, MA, USA), and the relationship between buckle voltage and applied force was determined.
Analysis of force data
Tendon forces were filtered using a low-pass third-order Butterworth filter with a cut-off frequency of 115 Hz and notch filter at 60 Hz. The cut-off frequency was chosen to accommodate a complementary study, in which we tested muscle models against in situ experiments involving 40 Hz tetanic stimulations (Wakeling et al., 2012). Force rise and relaxation rates were calculated by taking the first derivative of tendon force with respect to time. Data from each condition were partitioned into 20 equal time windows, and the mean force and force rates were calculated for each time window (Fig. 2).
Fig. 2.
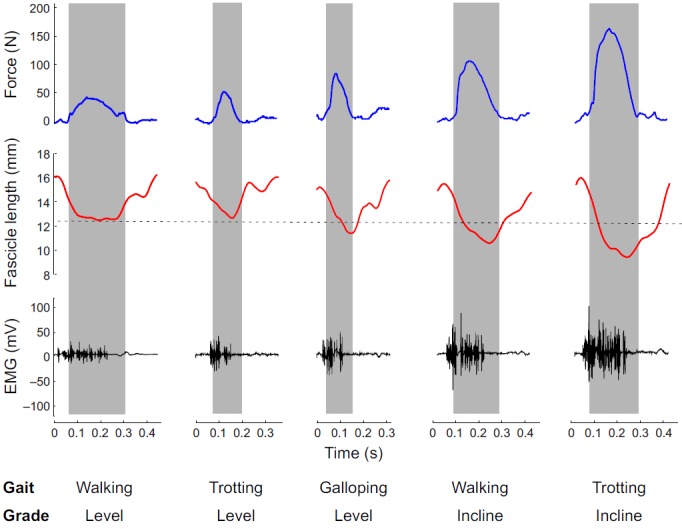
Representative LG force, fascicle length and EMG signals from fine-wire EMG of a goat during different locomotor tasks. Shaded bars indicate the stance phase. The dashed line indicates fascicle length during standing.
Stride times were determined from the tendon force profiles of each trial. The beginning of each stride was defined as 60 ms prior to when the force began to increase (Fig. 2). This ensured that our analysis captured the entire muscle activation profile.
Analysis of sonomicrometric data
Raw sonomicrometry signals were converted to a distance measurement between the crystals based on the speed of sound through skeletal muscles, adjusted by a +0.82 mm correction because of differences between the speed of sound through the epoxy coating of the crystals and the speed of sound through muscle (Daley and Biewener, 2003). Sonomicrometry signals were first filtered using a low-pass, second-order Butterworth filter with a cut-off frequency of 30 Hz. Filters with different parameters were tested, but the chosen parameters were most effective in smoothing out noise while still capturing time-varying features of the signal. A custom-written program was used to identify and remove ‘extraneous’ peaks, after which the signals were fit with a fourth-order polynomial using a 200-point (40 ms) size window. Extraneous peaks would occasionally occur in the signal when one or two values deviated greatly from the signal. Fascicle lengths were converted to strain by subtracting the resting length (distance measured between the crystals during quiet stance) and then dividing by the resting length (Gillis et al., 2001). Strain rate was calculated as the first derivative of strain with respect to time. Data from each condition were partitioned into 20 equal time windows, and the mean strain and strain rate were calculated for each time window (Fig. 2).
Analysis of EMG data
EMG signals from the LG and MG were analyzed using wavelet analysis, a time–frequency decomposition technique, similar to previously published methods (von Tscharner, 2000; Wakeling et al., 2002; Wakeling and Syme, 2002; Lee et al., 2011). A filter bank of 24 wavelets (0≤k≤23) was used to decompose the EMG signals into intensities as a function of time and frequency. To exclude low-frequency noise, the first four wavelet domains were excluded from further analysis (≤70 Hz) such that the data were analyzed for wavelets 3 to 23 (Hodson-Tole and Wakeling, 2007). Thus, the frequency band of 70–1857 Hz is presented in this analysis. This ensures that the signals of the slow motor units [central frequencies of ~150 Hz (Lee et al., 2011)] are included in the analysis and are consistent with the cut-off frequency used in other fine-wire EMG studies (Daley and Biewener, 2003; Gabaldón et al., 2004; Gillis and Biewener, 2001; Gillis and Biewener, 2002). As with the force data, EMG signals were partitioned into 20 equal time windows, and the mean intensity for each wavelet for each time window was calculated. The total intensity of the signal was calculated as the sum of the intensities determined using wavelets 3 to 23.
We used principal component analysis (PCA) to identify the major features of the intensity spectra (Wakeling, 2004; Hodson-Tole and Wakeling, 2008b; Lee et al., 2011). We randomly selected 30 strides per gait condition for statistical analysis. The spectra were compiled into a p × N matrix A, where p=20 wavelets and N=36,000 (6 goats × 2 muscles × 5 gait conditions × 30 strides × 20 partitioned time windows). The principal components (PCs) of the intensity spectra, defined in terms of eigenvector–eigenvalue pairs, were calculated from the covariance matrix B of the data matrix A without prior subtraction of the mean (Wakeling and Rozitis, 2004). This ensured that the whole signal, and not just its variance, was described. The weightings of each PC were given by the eigenvectors ξ of covariance matrix B, and the amount of the signal explained by each PC was determined from the eigenvalues. The PC loading scores were calculated from ξ′A, the product of the transpose of the weighting matrix and matrix A. We calculated the angle θ as the angle formed between the vector of the first and second PC (PCI–PCII) loading scores and the PCII loading score axis (Wakeling and Rozitis, 2004). θ describes the contribution of high- and low-frequency content in the signal: a small θ has a positive contribution of the PCII loading score and indicates relatively high-frequency content. Mean PCI and PCII loading scores were calculated for each of the 20 time windows of the stride, thus allowing changes in their contributions to be quantified throughout each stride for each gait condition.
Statistical analysis
A general linear model ANOVA was conducted to assess whether EMG intensity, tendon force, force rise and relaxation rates, fascicle strain and/or fascicle strain rate differed significantly between muscles, gait (walk, trot and gallop) and/or grade (incline and level). When significant differences were found, Tukey's post hoc tests were conducted to identify differences within each factor. A general linear model ANCOVA was conducted to identify significant associations between θ and intensity, between θ and the force rise and relaxation rates, and between θ and fascicle strain rate, with tendon force, force rate, fascicle strain, strain rate and EMG intensity as covariates. EMG intensity was included as a covariate so that associations between θ and strain rate could be detected that were independent from EMG intensity. Fascicle strain was also included as a covariate so that changes in strain would not confound the results (Wakeling et al., 2006), as greater strains have been found to be associated with decreased EMG frequency content (Doud and Walsh, 1995). When fascicle strain and θ were found to be significantly positively correlated, no further analysis was conducted. These analyses were carried out for time periods corresponding to concentric contraction (when the fascicle strain rate was negative), and separate analyses were conducted for the force-rise and force-relaxation periods (when the force rise and relaxation rates were positive and negative, respectively).
RESULTS
Changes in EMG intensity and frequency content in relation to muscle contractile patterns and locomotor tasks
During walking, trotting and galloping, the LG and MG consistently underwent a single shortening cycle during the stance phase, with typically one burst of muscle activity and one period of active force production per stride (e.g. Fig. 2). As locomotor speed increased from walking to trotting to galloping, the total EMG intensity and the tendon force increased (Figs 3, 4). The force rise and relaxation rates, as well as the fascicle shortening strains and strain rates, also tended to increase with speed (Figs 3, 4). All of these measures were generally even higher when animals walked and trotted uphill (Tables 1, 2, 3 and 4, Figs 3, 4).
Fig. 3.
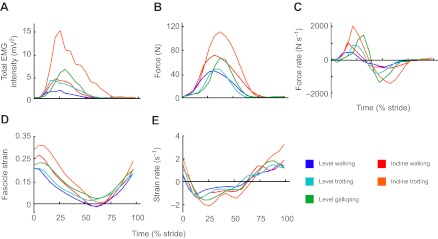
Mean values of LG (A) total EMG intensity, (B) force, (C) force rate, (D) fascicle strain and (E) strain rate for different locomotor tasks over a stride (level walking, trotting and galloping, incline walking and trotting).
Fig. 4.
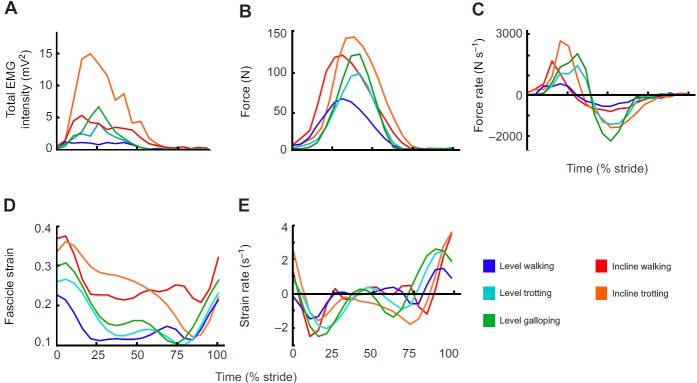
Mean values of MG (A) total EMG intensity, (B) force, (C) force rate, (D) fascicle strain and (E) strain rate for different locomotor tasks over a stride (level walking, trotting and galloping, incline walking and trotting).
Table 1.
Mechanical and excitation variables for the lateral gastrocnemius (LG) muscle, calculated during the force rise phase of concentric contractions during locomotion
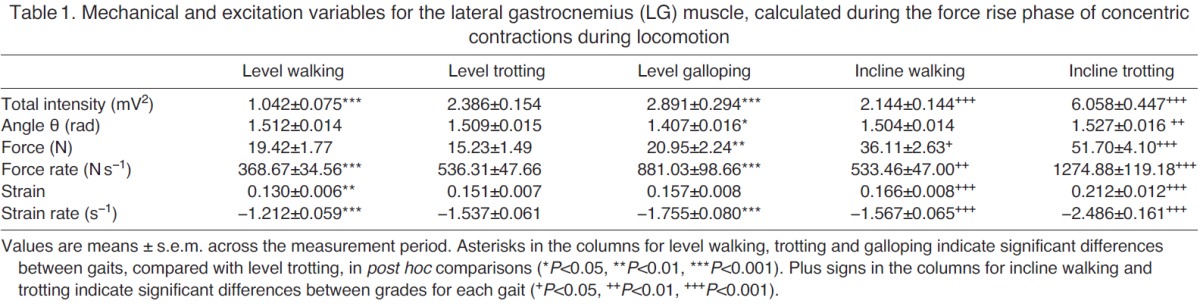
Table 2.
Mechanical and excitation variables for the LG muscle, calculated during the force relaxation phase of concentric contractions during locomotion
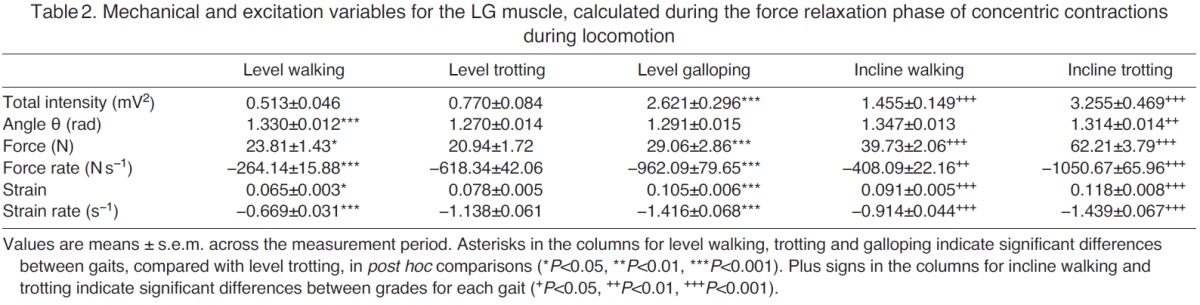
Table 3.
Mechanical and excitation variables for the medial gastrocnemius (MG) muscle, calculated during the force rise phase of concentric contractions during locomotion
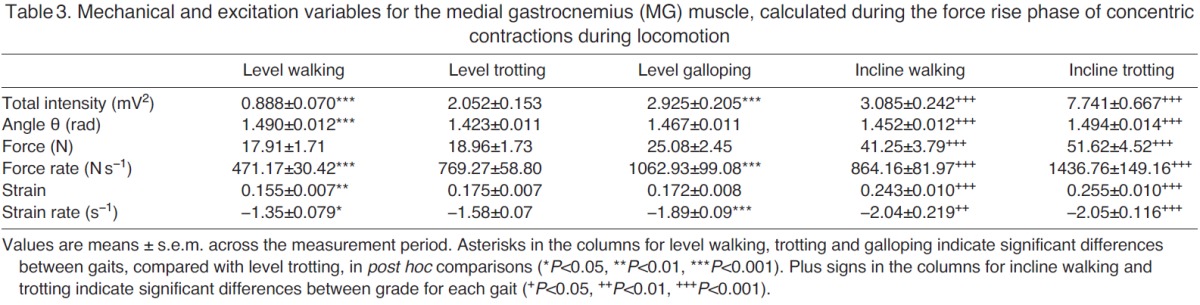
Table 4.
Mechanical and excitation variables for the MG muscle, calculated during the force relaxation phase of concentric contractions during locomotion
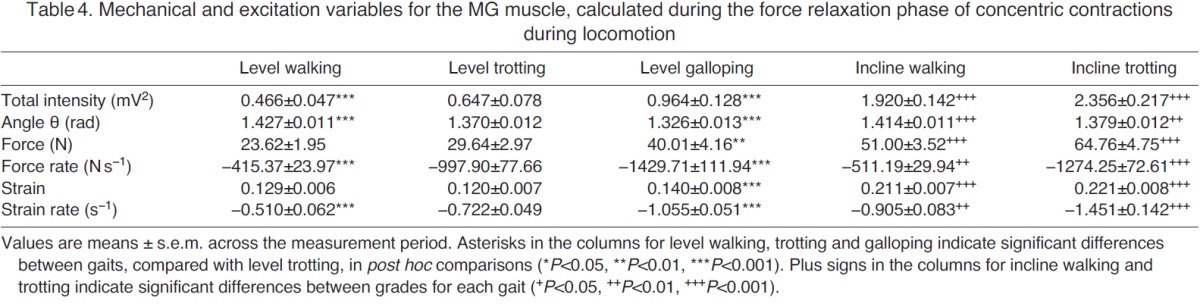
The frequency content of the EMG signals from LG and MG also changed with locomotor speed and grade (Fig. 5). From the PCA, the first two principal components explained ~76% of the EMG signal (Fig. 5). PCI had positive weightings for all frequencies and had a shape that was similar to the mean intensity spectrum (r2=0.98). The second PC had positive and negative weightings with a transition occurring at 387 Hz. In general, the angle θ tended to decrease as speed increased from level walking to trotting to galloping (Fig. 5), indicating an increase in the high-frequency component of the EMG signal with speed. By contrast, θ tended to increase as surface grade increased (Tables 1, 2, 3 and 4, Fig. 5), indicating an increase in the low-frequency component of the EMG signal with surface grade.
Fig. 5.
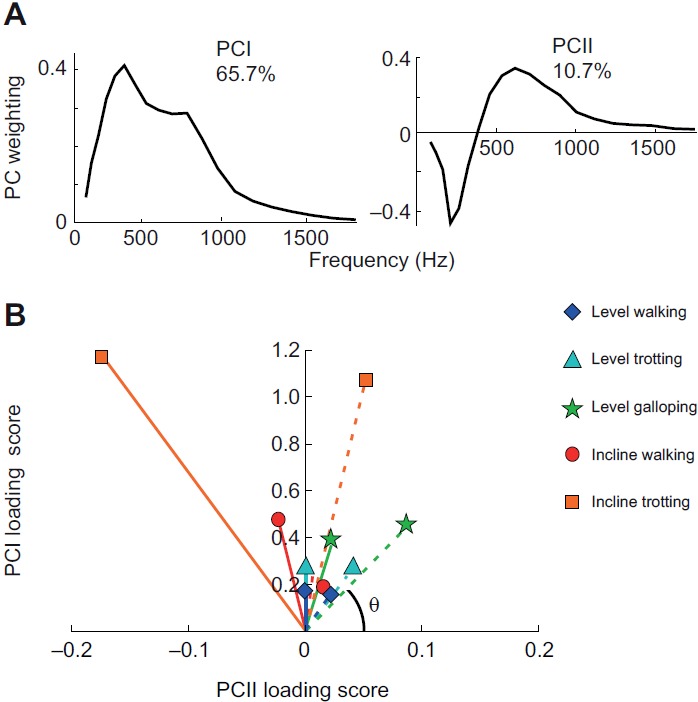
Principal component analysis (PCA) of the EMG signals. (A) The first two PCs defined from the EMG spectra of LG and MG explained ~76% of the signal; the percentage values shown reflect the percentage of the signal explained by each PC. (B) PCI and PCII loading scores for MG (solid) and LG (dashed) during the stance phase for different locomotor tasks (diamond, level walking; triangle, level trotting; star, level galloping; circle, incline walking; square, incline trotting). Angle θ describes the contribution of high- and low-frequency content in the EMG signal; smaller values of θ indicate higher-frequency content.
Associations between EMG frequency content and EMG intensity and force
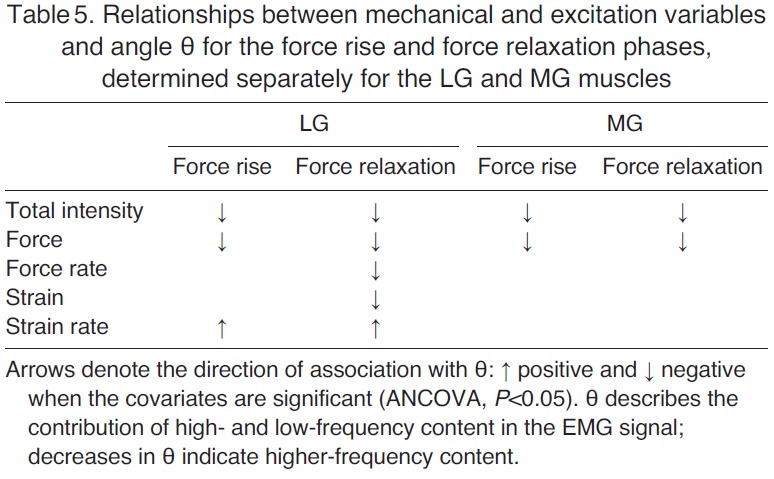
A significant negative association was detected between θ and EMG intensity (P<0.001), as well as between θ and force (P<0.001), for both the force rise and relaxation phases and both muscles (Table 5). Thus, the high-frequency component of the EMG signal increased as EMG intensity increased and force increased during locomotion.
Table 5.
Relationships between mechanical and excitation variables and angle θ for the force rise and force relaxation phases, determined separately for the LG and MG muscles
Association between EMG frequency content and force rate
A significant negative association was detected between θ and force rate during LG force relaxation (P=0.031; Table 5), but not during LG force rise, MG force rise or MG force relaxation. Thus, these data do not consistently support our hypothesis that EMG frequency content is associated with rates of force rise or force relaxation during locomotion.
Associations between EMG frequency content and fascicle strain and fascicle strain rate
A significant negative association was detected between θ and fascicle strain during LG force relaxation (P<0.001; Table 5), but not during LG force rise, MG force rise or MG force relaxation. This means that our interpretations of recruitment and factors associated with recruitment are generally not confounded by fascicle strain as changes in the EMG frequency content were not affected by strain, except during LG force relaxation.
For the LG, a significant positive association was detected between θ and fascicle shortening strain rate during both force-rise and force-relaxation phases (P<0.001; Table 5). This result indicates that higher-frequency signals are associated with faster shortening strain rates, and it supports our hypothesis that the frequency content of the EMG signal increases with faster shorting strain rates. For the MG, the association between θ and strain rate was not statistically significant (P=0.198 during force rise and P=0.256 during force relaxation).
DISCUSSION
Our study was designed to detect associations between EMG frequency content and intensity with tendon force, force rise and relaxation rates, fascicle strain and fascicle strain rate, all measured in vivo during different locomotor tasks. EMG frequency properties were characterized by the variable θ, which emerged from the PCA, with higher θ corresponding to lower-frequency content (and lower θ corresponding to higher-frequency content). In general, the results for goat LG and MG muscles revealed a positive association between θ and shortening strain rate and a negative association between θ and EMG intensity, demonstrating that faster motor units, associated with higher EMG frequency content, are recruited for locomotor tasks that involve increased fascicle strain rates and increased EMG intensity. This result was most clearly illustrated for level locomotion. During inclined locomotion, shifts in recruitment (changes in θ) were less clear and failed to correlate with fascicle strain rates. However, we did generally observe increases in EMG intensity, force, force rate, fascicle strain and fascicle strain rate as exercise demand increased from level walking to trotting to galloping, and from level walking and trotting to incline walking and trotting.
Changes in total EMG intensity, tendon force and rate, and fascicle strain and rate across different locomotor tasks
Several of our findings are consistent with previous in vivo experiments. For example, total EMG intensity increased with locomotor speed and surface grade (Tables 1, 2, 3 and 4, Figs 3, 4), which is consistent with previous studies of goats (McGuigan et al., 2009), rats (Gillis and Biewener, 2002), turkeys (Gabaldón et al., 2004) and horses (Wickler et al., 2005). The increase in EMG intensity was concurrent with increases in LG and MG force as goats increased speed from level walking to trotting to galloping, and when they moved from the level to the inclined surface (Tables 1, 2, 3 and 4, Figs 3, 4). These increases in tendon force are again consistent with data from goats reported by McGuigan et al. (McGuigan et al., 2009) and with data from guinea fowl reported by Daley and Biewener (Daley and Biewener, 2003). Our measures of increased fascicle shortening strain and strain rate during incline walking and trotting (Tables 1, 2, 3 and 4, Figs 3, 4) are also consistent with previous studies. For example, Roberts et al. (Roberts et al., 2007) reported increased shortening strain and strain rates when turkeys switched from level running to incline running. Using ultrasound to track human MG fascicles, Lichtwark and Wilson (Lichtwark and Wilson, 2006) also reported increased fascicle strain during walking and running as surface grade was increased. These previous studies have shown that the work output of individual muscles may be modulated by changes in force, fascicle shortening or both. The timing of muscle force relative to fascicle strain also contributes to changes in work output (Daley and Biewener, 2003; Gabaldón et al., 2004). Consequently, muscle activation and motor unit recruitment likely play important roles in modulating muscle work across different locomotor tasks (McGuigan et al., 2009). The present study extends previous research by demonstrating that the net work performed by muscles may also be modulated by recruiting different motor unit types.
Differences in EMG frequency spectra across locomotor tasks
Our study is also generally consistent with previous analyses of EMG frequency content during locomotor tasks. For example, our results support previous work showing that locomotor tasks associated with increasing grade elicit increasing θ values (Hodson-Tole and Wakeling, 2007; Hodson-Tole and Wakeling, 2008a), indicative of an increase in the low-frequency component of the EMG signal (Tables 1, 2, 3 and 4, Fig. 5). Our results also support previous work showing that locomotor tasks associated with increasing speed (walking to trotting to galloping) elicit decreasing θ values (Hodson-Tole and Wakeling, 2008a; Hodson-Tole and Wakeling, 2009), indicative of higher EMG frequency content at higher locomotor speeds (Tables 1, 2, 3 and 4, Fig. 5). Higher EMG frequencies are present when there is an increase in the activation level and proportion of faster fibers recruited. It is important to note, however, that higher EMG frequencies could result either from an orderly pattern of motor unit recruitment, from slow to fast fibers, or from preferential recruitment of faster fibers (Wakeling, 2004). Therefore, the observation of higher-frequency content alone is insufficient to detect differences in recruitment patterns between locomotor tasks. To make such a distinction, tasks must be studied that elicit similar levels of EMG intensity (corresponding to similar levels of activation), but differing EMG frequencies (indicating a shift in recruitment).
We initially sought to test for task-specific recruitment by comparing EMG intensity and frequency content for high-speed level locomotion (reduced θ, indicative of increased fast motor unit recruitment) with intensity and frequency content for slower-speed inclined locomotion (increased θ, indicative of increased slow motor unit recruitment). However, this comparison of tasks revealed that, in general, tasks with higher EMG frequency content also had greater EMG intensity. Thus, we were unable to directly resolve differences in recruitment patterns from these comparisons (Tables 1, 2, 3 and 4) because of the manner in which EMG intensity covaried with frequency. We therefore conducted an ANCOVA (Table 5, and discussed in the following two sections) so that the effect of intensity could be accounted for as a covariate, while still testing for associations between EMG frequency and tendon force, force rate, fascicle strain and fascicle strain rate. In some instances, the EMG intensities for level galloping were comparable to those measured during incline walking and/or trotting, and in these specific cases we observed distinct trends between θ and gait condition, consistent with increased recruitment of slow motor units during slower incline gait compared with increased recruitment of faster units during level galloping (Tables 1, 2, 3 and 4). However, these shifts in motor unit recruitment were not consistent with the measured LG and MG fascicle strain rates (Tables 1, 2, 3 and 4), complicating interpretation of these data. In goats, inclined walking and trotting generally resulted in greater fascicle strain rates than level walking and trotting because of the need for increased muscle shortening and work output when moving uphill. Nevertheless, differences in EMG frequency content were observed across some locomotor tasks when EMG intensity and fascicle strain rates were similar.
When evaluating EMG signals, it is important to consider fatigue, which can affect the frequency content. We designed our experiments to minimize the effects of fatigue. Specifically, our experiments involved five short recording intervals of ~20 s each for each locomotor task, with periods of rest between trials. We also varied the order of locomotor tasks among animals to minimize any bias that fatigue may have had. Therefore, we believe our results are not affected by fatigue.
One challenge in determining associations between EMG frequency content and strain rate relative to force rate is examining appropriate locomotor tasks. In this study, the incline locomotor tasks elicited an increase in θ (slower frequency content), but also a simultaneous increase in strain rates. An activity such as jumping is also not an ideal locomotor task because the total EMG intensity increases, in addition to strain rate and force. Thus, it is difficult to uncouple force, strain rate and EMG intensity during most locomotor tasks. Identifying a locomotor task that requires rapid, but small muscle forces would be ideal, and this challenge has been acknowledged in previous studies (Smith et al., 1980; Hodson-Tole and Wakeling, 2008b; Wakeling et al., 2006).
Differences in EMG frequency spectra across muscles
Although we observed similar shifts in EMG frequency for the LG and MG in the different gait conditions, we found that EMG recordings from the LG yielded smaller values of θ for all gait conditions (Fig. 5), indicating that the frequency content of LG signals was consistently higher than the frequency content of MG signals. This finding agrees with previously reported patterns of in situ motor unit recruitment from these muscles (Lee et al., 2011), and is likely caused by the higher proportion of fast fibers within the goat LG [which is supported by unpublished immunohistochemistry observations (J. A. Carr, M.B.M., S.S.M.L., J.M.W. and A.A.B., unpublished observations)]. This finding also likely explains why the LG generally had a greater total EMG intensity and faster fascicle strain rate than the MG during level galloping (Tables 1, 2, 3 and 4), when faster shortening is required. In addition, this finding and the supporting evidence that MG contains a higher proportion of slow fibers may explain why the MG generally had a greater total EMG intensity than the LG during incline walking and trotting (Tables 1, 2, 3 and 4), when recruitment of slower motor units was dominant. McGuigan et al. (McGuigan et al., 2009) also reported higher EMG intensity in the MG during incline walking and trotting, and higher intensity in the LG during level walking and trotting in goats.
Association between EMG frequency and force rise and relaxation rates
For single-twitch contractions, the time from an action potential to peak force (Burke et al., 1973), the total contraction time and the activation–deactivation rates (Lee et al., 2011) all differ between slow and fast motor units, and these properties affect the mechanical output of the muscle. Roberts and Gabaldón (Roberts and Gabaldón, 2008) examined the relaxation electromechanical delay of the turkey LG (from the end of EMG to the end of force) and found that this delay varied inversely with locomotor speed. Because the delay was not associated with fascicle shortening velocity, these investigators suggested that changes in motor unit recruitment might explain the observed association between the electromechanical relaxation delay and locomotor speed. In other words, preferential recruitment of faster motor units – accompanied by diminished recruitment of slower motor units – could explain why slower activation–deactivation rates and slower strain rates did not dominate at all locomotor speeds. If this is indeed the case, then a muscle's EMG frequency content is likely to be associated with its force rise and relaxation rates. To our knowledge, our study is the first to test this hypothesis. However, we found that our data from the goat LG and MG provide little supporting evidence. We detected a negative association between θ and force relaxation rate for the LG, suggesting that lower EMG frequency content was associated with faster relaxation rates for this muscle during the relaxation phase, in contrast to what we predicted. We also examined whether the faster force rise and relaxation rates may simply be a reflection of the increased force due to an increase in the number of motor units recruited, rather than a shift in the types motor units recruited. To do this, we conducted additional statistical analysis using force rise and relaxation rates calculated from the measured forces normalized to peak forces. However, this analysis also did not detect a significant association between the force rise and relaxation rates and θ.
Association between EMG frequency and strain rate
A major finding of our study was a shift from lower to higher EMG frequencies and a corresponding increase in the shortening strain rates of LG from slow to faster level locomotion. Using wavelets and PCA, and accounting for EMG intensity, we determined that this shift was due to a decrease in low-frequency components and an increase in high-frequency components of the EMG spectra. Also, using ANCOVA, we detected a significant positive association between fascicle strain rate and θ for the LG during both force rise and relaxation phases (Table 5). This indicates that as LG shortening rates increased for faster gaits (Fig. 3), the EMG signal contained greater high-frequency components.
Because we did not detect this same association for the MG during shortening, we conducted an additional analysis. Given that strain increased with surface grade, and the possibility of strain being a confounding factor [i.e. greater strains can be associated with decreased frequency content (Wakeling et al., 2006; Doud and Walsh, 1995)], we repeated the PCA and ANCOVA for only the level gait conditions. This yielded a positive association between θ and strain rate during MG force development, similar to the results obtained for LG, suggesting that recruitment of faster motor units may allow the faster strain rates needed to run faster on a level surface. These results are consistent with findings in the MG, soleus and plantaris muscles in rats (Hodson-Tole and Wakeling, 2008b) and in the MG muscles in humans (Wakeling et al., 2006), where increases in gait velocity (rats) and cycling frequency (humans) and increased strain rates were associated with shifts to higher frequencies in the EMG signal. One potential advantage of preferentially recruiting faster motor units during rapid movements is that maximum mechanical power and maximum mechanical efficiency occur at higher strain rates in faster motor units (He et al., 2000). A positive association between higher EMG frequencies and faster fascicle strain rates may therefore be driven by the necessity of generating high mechanical power at high efficiency. Preferential recruitment of faster motor units at faster gait speeds would also favor rates of muscle shortening that are better suited to the necessary increases in limb segment velocity.
Conclusions
Our results for the goat LG and MG support previous observations in other animals and in humans that recruitment of faster or slower motor units may depend, at least in part, on characteristics of the task. In particular, our analyses of EMG frequency content, in relation to in vivo measures of fascicle strain and tendon force, identified locomotor conditions for which recruitment of faster fibers was related to faster shortening strain rates. The shift to higher EMG frequencies was due to a decrease in low-frequency components and an increase in high-frequency components of the spectra, providing evidence that more fast motor units were recruited while fewer slow motor units were recruited. These results support hypotheses posed in previous studies, suggesting that motor units form task groups that are selectively recruited for rapid tasks (Hodson-Tole and Wakeling, 2008b; Wakeling, 2004; Wakeling et al., 2002; Loeb, 1985). The results also may have important and practical implications for muscle modeling, as new models that incorporate separate slow and fast contractile elements may yield better predictions of time-varying muscle force than existing, one-element models that do not consider differential recruitment (Wakeling et al., 2012). To our knowledge, this is only the third report that has identified significant associations between EMG frequency content and EMG intensity, strain rate and force on motor unit recruitment and investigated whether there is a mechanical basis for preferential recruitment of different motor unit types. Further work is needed to determine whether these associations are consistent across muscles and species and to identify other biomechanical factors that influence motor unit recruitment strategies.
ACKNOWLEDGEMENTS
We thank Pedro Ramirez for animal care and assistance during training, and Drs Jennifer Carr and Carlos Moreno for assistance during data collection.
FOOTNOTES
FUNDING
This work was supported by the National Institutes of Health [R01AR055648 to A.A.B. and J.M.W.]. Deposited in PMC for release after 12 months.
REFERENCES
- Biewener A. A. (1998). Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. 120B, 73-87 [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Baudinette R. (1995). In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (Macropus eugenii). J. Exp. Biol. 198, 1829-1841 [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Konieczynski D. D., Baudinette R. V. (1998). In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J. Exp. Biol. 201, 1681-1694 [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd (1973). Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 234, 723-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citterio G., Agostoni E. (1984). Selective activation of quadriceps muscle fibers according to bicycling rate. J. Appl. Physiol. 57, 371-379 [DOI] [PubMed] [Google Scholar]
- Daley M. A., Biewener A. A. (2003). Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941-2958 [DOI] [PubMed] [Google Scholar]
- Doud J. R., Walsh J. M. (1995). Muscle fatigue and muscle length interaction: effect on the EMG frequency components. Electromyogr. Clin. Neurophysiol. 35, 331-339 [PubMed] [Google Scholar]
- Fedde M. R., DeWet P. D., Kitchell R. L. (1969). Motor unit recruitment pattern and tonic activity in respiratory muscles of Gallus domesticus. J. Neurophysiol. 32, 995-1004 [DOI] [PubMed] [Google Scholar]
- Freund H. J., Büdingen H. J., Dietz V. (1975). Activity of single motor units from human forearm muscles during voluntary isometric contractions. J. Neurophysiol. 38, 933-946 [DOI] [PubMed] [Google Scholar]
- Fukunaga T., Ichinose Y., Ito M., Kawakami Y., Fukashiro S. (1997). Determination of fascicle length and pennation in a contracting human muscle in vivo. J. Appl. Physiol. 82, 354-358 [DOI] [PubMed] [Google Scholar]
- Gabaldón A. M., Nelson F. E., Roberts T. J. (2004). Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J. Exp. Biol. 207, 2277-2288 [DOI] [PubMed] [Google Scholar]
- Gerdle B., Karlsson S., Crenshaw A. G., Elert J., Fridén J. (2000). The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur. J. Appl. Physiol. 81, 2-10 [DOI] [PubMed] [Google Scholar]
- Gillespie C. A., Simpson D. R., Edgerton V. R. (1974). Motor unit recruitment as reflected by muscle fibre glycogen loss in a prosimian (bushbaby) after running and jumping. J. Neurol. Neurosurg. Psychiatry 37, 817-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A. (2001). Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J. Exp. Biol. 204, 2717-2731 [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A. (2002). Effects of surface grade on proximal hindlimb muscle strain and activation during rat locomotion. J. Appl. Physiol. 93, 1731-1743 [DOI] [PubMed] [Google Scholar]
- Grimby L., Hannerz J., Hedman B. (1981). The fatigue and voluntary discharge properties of single motor units in man. J. Physiol. 316, 545-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. H., Bottinelli R., Pellegrino M. A., Ferenczi M. A., Reggiani C. (2000). ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys. J. 79, 945-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E., Olson C. B. (1965). Relations between structure and function in the design of skeletal muscles. J. Neurophysiol. 28, 581-598 [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. (1965). Excitability and inhibitability of motoneurons of different sizes. J. Neurophysiol. 28, 599-620 [DOI] [PubMed] [Google Scholar]
- Henneman E., Clamann H. P., Gillies J. D., Skinner R. D. (1974). Rank order of motoneurons within a pool: law of combination. J. Neurophysiol. 37, 1338-1349 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E. F., Wakeling J. M. (2007). Variations in motor unit recruitment patterns occur within and between muscles in the running rat (Rattus norvegicus). J. Exp. Biol. 210, 2333-2345 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E. F., Wakeling J. M. (2008a). Motor unit recruitment patterns 1: responses to changes in locomotor velocity and incline. J. Exp. Biol. 211, 1882-1892 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E. F., Wakeling J. M. (2008b). Motor unit recruitment patterns 2: the influence of myoelectric intensity and muscle fascicle strain rate. J. Exp. Biol. 211, 1893-1902 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E. F., Wakeling J. M. (2009). Motor unit recruitment for dynamic tasks: current understanding and future directions. J. Comp. Physiol. B 179, 57-66 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E. F., Wakeling J. M. (2010). The influence of strain and activation on the locomotor function of rat ankle extensor muscles. J. Exp. Biol. 213, 318-330 [DOI] [PubMed] [Google Scholar]
- Lee D. V., McGuigan M. P., Yoo E. H., Biewener A. A. (2008). Compliance, acutation, and work characteristics of the goat foreleg and hindleg during level, uphill, and downhill running. J. Appl. Physiol. 104, 130-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S. M., Miara M. B., Arnold A. S., Biewener A. A., Wakeling J. M. (2011). EMG analysis tuned for determining the timing and level of activation in different motor units. J. Electromyogr. Kinesiol. 21, 557-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtwark G. A., Wilson A. M. (2006). Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Biol. 209, 4379-4388 [DOI] [PubMed] [Google Scholar]
- Lichtwark G. A., Bougoulias K., Wilson A. M. (2007). Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J. Biomech. 40, 157-164 [DOI] [PubMed] [Google Scholar]
- Loeb G. E. (1985). Motoneurone task groups: coping with kinematic heterogeneity. J. Exp. Biol. 115, 137-146 [DOI] [PubMed] [Google Scholar]
- Luff A. R., Atwood H. L. (1972). Membrane properties and contraction of single muscle fibers in the mouse. Am. J. Physiol. 222, 1435-1440 [DOI] [PubMed] [Google Scholar]
- McGuigan M. P., Yoo E., Lee D. V., Biewener A. A. (2009). Dynamics of goat distal hind limb muscle-tendon function in response to locomotor grade. J. Exp. Biol. 212, 2092-2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A., Romanò C., Schieppati M. (1989). Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J. Physiol. 409, 451-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raez M. B., Hussain M. S., Mohd-Yasin F. (2006). Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proced. Online 8, 11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. J., Gabaldón A. M. (2008). Interpreting muscle function from EMG: lessons learned from direct measurements of muscle force. Integr. Comp. Biol. 48, 312-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. J., Marsh R. L., Weyand P. G., Taylor C. R. (1997). Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113-1115 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Higginson B. K., Nelson F. E., Gabaldón A. M. (2007). Muscle strain is modulated more with running slope than speed in wild turkey knee and hip extensors. J. Exp. Biol. 210, 2510-2517 [DOI] [PubMed] [Google Scholar]
- Smith J. L., Betts B., Edgerton V. R., Zernicke R. F. (1980). Rapid ankle extension during paw shakes: selective recruitment of fast ankle extensors. J. Neurophysiol. 43, 612-620 [DOI] [PubMed] [Google Scholar]
- Sokoloff A. J., Cope T. C. (1996). Recruitment of triceps surae motor units in the decerebrate cat. II. Heterogeneity among soleus motor units. J. Neurophysiol. 75, 2005-2016 [DOI] [PubMed] [Google Scholar]
- von Tscharner V. (2000). Intensity analysis in time-frequency space of surface myoelectric signals by wavelets of specified resolution. J. Electromyogr. Kinesiol. 10, 433-445 [DOI] [PubMed] [Google Scholar]
- Wakeling J. M. (2004). Motor units are recruited in a task-dependent fashion during locomotion. J. Exp. Biol. 207, 3883-3890 [DOI] [PubMed] [Google Scholar]
- Wakeling J. M., Rozitis A. I. (2004). Spectral properties of myoelectric signals from different motor units distinguished during ramped contractions of the leg extensors. J. Exp. Biol. 207, 2519-2528 [DOI] [PubMed] [Google Scholar]
- Wakeling J. M., Syme D. A. (2002). Wave properties of action potential from fast and slow motor units. Muscle Nerve 26, 659-668 [DOI] [PubMed] [Google Scholar]
- Wakeling J. M., Kaya M., Temple G. K., Johnston I. A., Herzog W. (2002). Determining patterns of motor recruitment during locomotion. J. Exp. Biol. 205, 359-369 [DOI] [PubMed] [Google Scholar]
- Wakeling J. M., Uehli K., Rozitis A. I. (2006). Muscle fibre recruitment can respond to the mechanics of the muscle contraction. J. R. Soc. Interface 3, 533-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling J. M., Lee S. S. M., Arnold A. S., de Boef Miara M., Biewener A. A. (2012). A muscle's force depends on the recruitment patterns of its fibers. Ann. Biomed. Eng. 40, 1708-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickler S. J., Hoyt D. F., Biewener A. A., Cogger E. A., De La Pax K. L. (2005). In vivo muscle function vs speed. II. Muscle function trotting up an incline. J. Exp. Biol. 208, 1191-1200 [DOI] [PubMed] [Google Scholar]


