SUMMARY
Associative learning has been shown in a variety of insects, including the mosquitoes Culex quinquefasciatus and Anopheles gambiae. This study demonstrates associative learning for the first time in Aedes aegypti, an important vector of dengue, yellow fever and chikungunya viruses. This species prefers to rest on dark surfaces and is attracted to the odor of 1-octen-3-ol. After training in which a dark surface alone or a dark surface with odor was paired with electric shock, mosquitoes avoided the previously attractive area. The association was stronger when odor was included in training, was retained for at least 60 min but not for 24 h, and was equal for males and females. These results demonstrate the utility of a bulk-training paradigm for mosquitoes similar to that used with Drosophila melanogaster.
KEY WORDS: inhibitory avoidance, passive avoidance, conditioning
INTRODUCTION
It has long been known that insects are capable of associative learning, with most work concentrating on bees (Menzel, 1999; Giurfa, 2007; Giurfa and Sandoz, 2012) and flies (Davis, 2005; Keene and Waddell, 2007). While other insects have also been studied in depth, including cockroaches, moths, wasps, and solitary and social bees (Dukas, 2008), there have been few rigorous studies of learning in mosquitoes. This is surprising given their significant impact on human and animal health and aspects of their life cycle and mode of feeding that may be mediated by learning (Clements, 1992).
Aedes aegypti is a tropical mosquito that feeds preferentially on humans (Harrington et al., 2001) and is a vector for dengue, yellow fever and chikungunya viruses (Gubler, 1998; World Health Organization, 2002; Ligon, 2006). Nearly 40% of the world population may be exposed to dengue, with over 100 million infected and 22,000 fatalities annually (World Health Organization, 1997). There is no vaccine or treatment for dengue infection, so vector control is currently the only means of fighting the disease (Swaminathan and Khanna, 2009; Webster et al., 2009). However, the usual methods of control, reducing breeding sites and applying insecticides, have been of only limited success (World Health Organization, 1997; Gubler, 1998; Ooi et al., 2006). Understanding the role of learning in mosquito behavior could explain choice of breeding sites and hosts, which could in turn give rise to new control methods. Several researchers have suggested that learning could be involved in preference for nectar sources (Jhumur et al., 2006), host species (Hii et al., 1991; Mwandawiro et al., 2000) or even individuals of a host species (McCall and Kelly, 2002), choice of oviposition sites (Kaur et al., 2003), and home range (Charlwood et al., 1988; McCall et al., 2001).
The existing body of research into mosquito learning is small and contradictory, with some finding no evidence of learning in Ae. aegypti (Alonso et al., 2003) and others claiming learning in various species but with flawed methods (reviewed by Alonso and Schuck-Paim, 2006). Of the few studies with clear evidence of learning, two studies show associative learning with appetitive stimuli in Culex (Tomberlin et al., 2006; Sanford and Tomberlin, 2011) and one study shows associative learning with appetitive stimuli in Anopheles (Chilaka et al., 2012). Our current work examines associative learning with aversive stimuli in Ae. aegypti, using bulk training methods similar to those established with Drosophila melanogaster (Quinn et al., 1974).
There are many forms of associative learning. In most of them, an animal learns to associate a neutral stimulus (the conditional stimulus) with a positive or negative stimulus (the unconditional stimulus). After trials in which the two stimuli are paired, the subject responds to the conditional stimulus alone as if the unconditional stimulus were present. We used an inhibitory avoidance test to determine whether mosquitoes could learn to avoid innately attractive stimuli. Inhibitory avoidance learning is often used to test the effect of drugs and neuromodulators on learning in rodents (e.g. McIntyre et al., 2002). In one version of the task, a rat is placed in a brightly lit area (aversive) and permitted to enter a dark area (innately preferred). However, the floor of the dark area delivers a shock. Increased latency or reduced probability of entering the dark area is taken as evidence of learning. Inhibitory avoidance learning differs from classical conditioning in two important respects. First, the conditional stimulus must be attractive rather than neutral. Second, it is not possible to have a control in which conditional and unconditional stimuli are unpaired during training. Instead, control groups go through the training procedure without exposure to shock.
We tested two attractive stimuli, a color and an odor. Both sexes of Ae. aegypti prefer to rest on dark-colored surfaces (Gilbert and Gouck, 1957) and are attracted to the odor of 1-octen-3-ol (Takken and Kline, 1989; Grant and Dickens, 2011). These are the two conditional stimuli, while the aversive unconditional stimulus is an electric shock delivered through the dark surface. If associative learning occurs, the proportion of mosquitoes resting on the dark surface should decrease after training.
MATERIALS AND METHODS
Mosquitoes
For this study, Aedes aegypti L. came from a lab colony established from eggs collected in Tapachula, Mexico (14°54′N, 92°15′W) in 2006 and supplemented with field-collected eggs from the same region in 2008 and 2009. Mosquitoes were kept in an environmental chamber simulating natural conditions, with a 14 h:10 h light:dark cycle and 2 h of dawn and twilight, at 75±7% relative humidity and at 22–30°C fluctuating temperature. Eggs were vacuum-hatched in water to obtain simultaneous cohorts. Larvae were fed 1:1 lactalbumin and brewer's yeast. Male and female pupae were transferred to separate 2-liter containers with mesh lids and offered a 20% sucrose solution upon eclosion. No more than 45 pupae were placed in a container. Containers of adult mosquitoes were kept in the environmental chamber until the day of an experiment.
Experimental chamber
Experiments took place in a 46-cm long, 9.5-cm inner-diameter transparent Plexiglas(14°54′N, 92°15′W) in 2006 and supplemented with field-collected eggs from the same region in 2008 and 2009. Mosquitoes were kept in an environmental chamber simulating natural conditions, with a 14 h:10 h light:dark cycle and 2 h of dawn and twilight, at 75±7% relative humidity and at 22–30°C fluctuating temperature. Eggs were vacuum-hatched in water to obtain simultaneous cohorts. Larvae were fed 1:1 lactalbumin and brewer's yeast. Male and female pupae were transferred to separate 2-liter containers with mesh lids and offered a 20% sucrose solution upon eclosion. No more than 45 pupae were placed in a container. Containers of adult mosquitoes were kept in the environmental chamber until the day of an experiment.
Experimental chamber
Experiments took place in a 46-cm long, 9.5-cm inner-diameter transparent Plexiglas™ cylinder closed at both ends (Fig. 1). One end cap was white plastic, while the other end cap was a darker printed-circuit board (PCB) through which an electric shock could be applied. The PCB had interleaved hot and neutral contacts separated by 1 mm. The outside of the chamber was mostly covered with white paper; to facilitate counting of mosquitoes, 2 cm by the walls at each end and a strip at the top were left uncovered. Six 2.5 mm-diameter holes were drilled in the cylinder wall, 5 mm from the PCB end: two for delivery of odor, two for removal of odor, and two for pressure release. Four similar holes provided comparable air circulation at the other end of the cylinder. During experiments, a desk lamp illuminated the chamber, with intensity ranging from 700 lux at the end caps to 1270 lux in the center of the chamber (approximately the brightness of an overcast day).
Fig. 1.

Experimental chamber. (A) Mosquitoes were released in a 46 cm long, 9.5 cm inner-diameter cylinder. One end cap was a dark printed-circuit board (PCB) through which electric shock could be applied; the other end was white. To assess the preference of mosquitoes, we defined a dark area consisting of the PCB and adjacent 2 cm of cylinder, comprising 9% of the total interior surface area. (Darkness refers to surface color rather than illumination, which was similar throughout the chamber.) (B) For odor experiments, odor was pumped in through two holes at the dark end and simultaneously pumped out through two orthogonally placed holes.
We defined two areas of the chamber, a dark area consisting of the PCB and adjacent 2 cm of cylinder, and a light area consisting of the white end cap and the remaining 44 cm of cylinder (Fig. 1). The dark area was ~9% of the total interior surface of the chamber.
Stimuli
The unconditional stimulus was a 100 mA, 140 V AC shock applied through the PCB at the dark end of the experimental chamber for the 60 s of a training trial. This intensity caused most mosquitoes to leave the PCB without any evident harm. We tested two conditional stimuli: (1) the attractively dark-colored PCB wall and (2) the odor of 1-octen-3-ol (98%, Acros lot A0272468). The color was always present, while the odorant was delivered only during the 60 s of a trial. Since the dark PCB was always present, our two conditional stimuli were ‘dark surface color alone’ and ‘dark surface color with odor’. We henceforth refer to these as ‘color’ and ‘odor/color’, respectively.
To deliver odor to the dark area of the chamber, air was pumped through a 20 ml vial containing filter paper impregnated with 1.5 μl of odorant and then into the experimental chamber. The pump (Micro Air Pump, part 3A120INSN, 475 cm3 min−1) moved air into the chamber through two opposite ports near the PCB, while a vacuum pump (Metal Bellows model MB-41) simultaneously removed air through two orthogonal ports, and two additional holes in the chamber kept pressure equalized. To avoid odorant buildup, we cleaned the vial before each test. After each experiment, we cleaned the chamber with ethanol to remove any residues and left it to dry overnight.
Procedure
Training and testing procedures are shown schematically in Fig. 2. Before an experiment, a cohort of 35–40 mosquitoes of the same sex and age (2–10 days post-eclosion) was anesthetized by chilling for 30 s at 5°C and then transferred to the experimental chamber described above. They were given 5 min to acclimate to the chamber before experiments began.
Fig. 2.
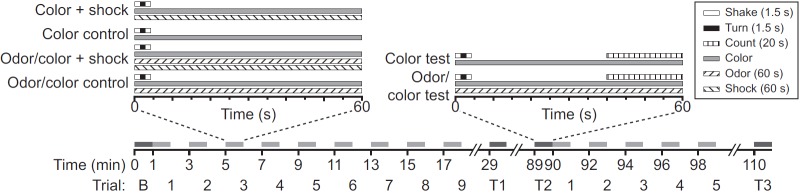
Procedure. The time line shows the pattern of test trials (dark bars) and training trials (light bars). On the Trial axis, B denotes the baseline preference test before training, T1 denotes a preference test 10 min after first training, T2 denotes a test 60 min after first training, and T3 denotes a test 10 min after a second training, while numbers represent training trials. The content of each trial or test depended on the type of experiment, as shown in the expanded time lines and described in the text. Color refers to trials or tests in which only the dark surface color was present; odor/color refers to trials or tests in which the odor was present along with the dark surface color.
We determined the dark-area preference of mosquitoes as follows. The chamber was gently shaken (to make mosquitoes fly), rotated (to avoid side bias), shaken again and then placed on a table. This took ~5 s, after which mosquitoes were given 35 s to settle. We then counted mosquitoes in the dark and light areas of the chamber and calculated the proportion resting in the dark area. We did this at the outset of each experiment, to establish a baseline, and at intervals after training to test learning and retention. In odor tests, the odor was present in the dark-surface area during this time.
There were four types of trials, each lasting 60 s: color + shock, color control, odor/color + shock, and odor/color control. Each training trial with shock was as follows. The chamber was shaken, turned and shaken as above. Electrical current to the PCB was turned on for 60 s. In odor trials, the odor was supplied for 60 s along with the current. Control trials were the same except that no current was supplied to the PCB. The interval between trials was 60 s, so a trial occurred every 120 s.
Each cohort was used in only one experiment with one type of training or control trial. An experiment consisted of an initial baseline test of dark-area preference, nine training or control trials, a 10 min rest period, a test of dark-area preference, a 60 min rest period, another test of dark-area preference, and five more training trials followed 10 min later by a final test of dark-area preference. This gave us four measures of dark-area preference: baseline, 10 min after first training, 60 min after first training, and 10 min after second training.
To test retention beyond 60 min, we ran a separate set of experiments (not shown in Fig. 2) with 12 cohorts trained with odor/color + shock and 12 odor/color control cohorts; all were female. Baseline preference was tested, followed by nine training or control trials, followed by a preference test after another 24 h in the chamber.
Because mosquitoes remained in the chamber between training and test trials, a further control was needed. It is possible that shocked mosquitoes emit a substance that repels other mosquitoes. To test this, we placed a cohort in the chamber, shook and rotated them, and counted the number in the dark area. Those mosquitoes then experienced nine trials with shock, shaking and rotation on the same schedule as the color + shock training trials. We then removed those mosquitoes, immediately replaced them with a naive cohort, shook and rotated the chamber, and counted the number in the dark area. If the shocked mosquitoes leave an alarm substance, then naïve mosquitoes should avoid the dark area.
Statistics
Each cohort of 35–40 mosquitoes was measured several times in the course of an experiment: once at the outset, twice after the initial training, and once after a second training. Each experimental treatment used 12 cohorts. To assess learning and retention, we tested several predetermined comparisons, each of them separately for males and females. Predetermined comparisons of baseline values were done with ANOVA. For predetermined comparisons between tests and baselines, between control and experimental groups, and between stimulus types, we used paired t-tests with Bonferroni corrections for multiple comparisons (four for any comparison with baseline, three for tests among post-baseline experimental and control values). All t-tests had 22 degrees of freedom, with 12 cohorts per sample. Finally, testing for differences between males and females was done with a multilevel model. Fixed effects in this model were sex (male or female), conditional stimulus (color or odor/color), experiment (shock or control), treatment (baseline, 10 min after first training, 60 min after first training, 10 min after second training), and all interactions. Cohort was set as a random effect (12 cohorts of each sex were run through each experiment). All tests were done with JMP software (SAS Institute, Inc., Cary, NC, USA). All P-values reported below are corrected for multiple comparisons as necessary.
RESULTS
Baseline measurements for both sexes confirmed that the dark area was attractive on its own, with or without the presence of the odor. The dark area was only 9% of the internal surface of the chamber, yet over 50% of mosquitoes were found in that area before training (Fig. 3). There were no statistically significant differences in baseline preference among cohorts used in the different experiments (ANOVA: female P=0.11, F3,22=2.62; male P=0.23, F3,22=1.48). For each sex, five replications of the control experiment for alarm substances showed no significant difference between the distribution of mosquitoes in a clean chamber and that of naive mosquitoes placed in a chamber after its prior occupants received nine electric shocks (ANOVA: female pre-shock 43±2% in dark area, naive 43±3% in dark area, F1,8=0.07, P=0.80; male pre-shock 46±2% in dark area, naive 51±4% in dark area, F1,8=0.90, P=0.37). Thus, shocked mosquitoes do not alter their surroundings in any way that deters other mosquitoes, so changes in mosquito distribution after training indicate learning rather than aversion to an alarm substance.
Fig. 3.
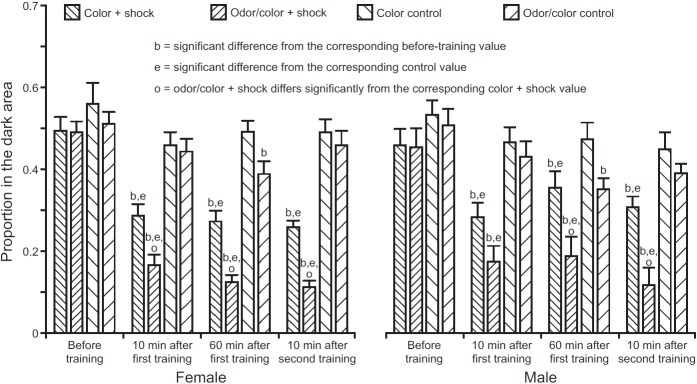
Results. Bars show the proportion of mosquitoes counted in the dark area of the chamber (mean ± s.e.m.); decrease from the initial value indicates aversive learning. Labels below bars correspond to tests described in Fig. 2. All experimental test values differed significantly from baseline; control values differed significantly from baseline in only two cases. All experimental values after the baseline test differed significantly from their corresponding controls. After training, values for odor/color + shock were significantly lower than for color + shock except in one case. There were no statistically significant differences between males and females. All t- and P-values are given in the text.
The inhibitory avoidance paradigm makes three straightforward predictions. (1) If mosquitoes learn to associate the conditional stimulus (color or odor/color) with the aversive stimulus (shock), then their preference for the dark or odor/dark area should decrease after training. (2) Control experiments, in which training trials lack a shock, should show no decline from the baseline preference for the dark area. (3) Preference of trained mosquitoes for the dark area should be less than that of control mosquitoes. Based on these predictions, we judged learning by the extent to which preference for the dark area decreased from baseline. We judged retention by the extent to which that preference remained depressed at intervals after training.
The first prediction was met, since the proportion of mosquitoes resting in the dark area 10 min after first training was less than baseline for both sexes and stimuli (P<0.0001 in all tests; female color, t=5.52; female odor/color, t=8.67; male color, t=4.65; male odor/color, t=7.45). The second prediction was met, since the proportion of control mosquitoes in the dark area did not differ significantly from the baseline after the first training for either sex or stimulus except in one case (female color P=0.03, t=2.69; female odor/color P=0.28, t=1.81; male color P=0.29, t=1.80; male odor/color P=0.16, t=2.07). The third prediction was also met, since the proportion of trained mosquitoes preferring the dark area after training was less than that of the control mosquitoes for both sexes and stimuli (female color P=0.0012, t=3.57; female odor/color P<0.0001, t=5.76; male color P=0.0006, t=3.77; male odor/color P<0.0001, t=5.31). Results after the second training period were comparable.
The association was retained for at least 60 min after training. Preference for the dark area remained below baseline for both sexes and stimuli (female color P<0.0001, t=5.89; female odor/color P<0.0001, t=5.52; male color P=0.025, t=2.75; male odor/color P<0.0001, t=7.07) and was less than that of control mosquitoes (female color P<0.0001, t=4.54; female odor/color P<0.0001, t=5.46; male color P=0.045, t=2.45; male odor/color P=0.003, t=3.33). However, dark-area preference of the control mosquitoes declined slightly and differed significantly from baseline in two cases (female color P=0.29, t=1.81; female odor/color P=0.005, t=3.29; male color P=0.44, t=1.61; male odor/color P=0.0002, t=4.18). In the 24-h retention test (all females, odor/color only), there was no difference between trained and control mosquitoes (P=1.0, t=0.031) and neither group differed significantly from its baseline (trained P=0.10, t=2.08; control P=0.25, t=1.60).
Association between the conditional stimulus and shock was stronger with the odor/color combination than with color alone. The dark-area preference was significantly lower after odor/color training than after color training in females (T1 P=0.04, t=2.51; T2 P=0.007, t=3.06; T3 P=0.008, t=3.02) and males except at 10 min after first training (T1 P=0.07, t=2.27; T2 P=0.002, t=3.44; T3 P=0.0003, t=3.94).
Although dark-area preference was sometimes lower for trained females than trained males, sex was never statistically significant either as a main effect, or in any interaction terms, or in any post-hoc comparisons in our mixed multilevel model.
DISCUSSION
In summary, our data show that Ae. aegypti learned to avoid previously attractive stimuli paired with a shock in a bulk-training paradigm. The association was retained for at least 60 min but not for 24 h, was stronger for color with odor than for color alone, and was equal for males and females. Reversal of preference after training has also been shown in honeybees, which learned aversion to attractant pheromones and floral odorants, but has been investigated in few other insects (Roussel et al., 2012).
Our findings are at odds with the only other study that has tested associative learning in Ae. aegypti (Alonso et al., 2003). That series of experiments paired aversive (shock or vibration) or positive (blood feeding or human breath) unconditional stimuli with neutral conditional stimuli (odors or visual patterns) and found no evidence of associative learning with any pairing. While their procedures differed from ours in many respects, including a relatively short training period, there are no obvious flaws in their design. Alonso et al. speculate that Ae. aegypti may not need to learn in nature but also acknowledge that their mosquitoes (from a colony founded in the 1950s) may show the effect of many generations of laboratory rearing (Alonso et al., 2003). In contrast, our mosquitoes came from a colony collected from the wild in 2006 and refreshed in 2009. We suspect that this accounts for the discrepant results. Recent work in D. melanogaster found a genetic polymorphism affecting learning (Mery et al., 2007) and metabolic costs to memory (Mery and Kawecki, 2005). This is probably also true of mosquitoes, making it likely that learning ability could be reduced over many generations of lab rearing, due to genetic drift and relaxed selection.
Three recent studies have successfully shown associative learning in other mosquito species. Two used appetitive stimuli with Culex quinquefasciatus (Tomberlin et al., 2006; Sanford and Tomberlin, 2011). In both cases, individual mosquitoes learned to associate previously neutral odors with food reward (sugar or blood). A bulk-training study showed that female Anopheles gambiae could associate visual or olfactory stimuli with desirable and undesirable feeders or normal and unpalatable blood (Chilaka et al., 2012). Learning was rapid, with mosquitoes reaching 100% accuracy on the fourth trial with visual stimuli and palatable versus unpalatable blood.
The initial bulk training studies in Drosophila (Quinn et al., 1974) were criticized for not directly measuring the learning of individuals, and the matter has been discussed at length (e.g. Holliday and Hirsch, 1986; McGuire, 1986; Tully, 1986). However, it is clear that group learning reflects individual learning even when individual learning is not directly measured. Indeed, if no individuals learn, there can be no change in the behavior of a trained group. Individual training (as in the Culex work) and bulk training (as in our work and the Anopheles work) have inevitable trade-offs. Individual training offers precise control over conditions such as age, appetitive state, and the timing of conditional and unconditional stimuli, and may reveal nuances of behavior and individual variation among subjects. However, it is time-consuming, requires careful handling of delicate insects and could be difficult to scale up for use with large numbers. Bulk training accepts some variability in the treatment experienced by each subject and uncertainty about variation among subjects in exchange for ease of implementation. There is no need to handle mosquitoes individually; response measurement is simple, requiring no judgments about the quality of a behavior; and large amounts of data can be rapidly collected. In our method, additionally, the appetitive state of the mosquitoes is not critical and the unconditional stimulus is salient to both sexes.
Bulk training was clearly effective in our tests. However, some aspects of the method may have reduced its sensitivity. (1) Not all mosquitoes in a cohort got the aversive stimulus in each trial. At the outset, about 50% were on the PCB and received a shock; as the number on the PCB declined, the number receiving the shock also declined. Thus, over nine training trials, mosquitoes experienced fewer than nine pairings of conditional and unconditional stimuli. (2) By necessity, the dark PCB was always present, so mosquitoes in color + shock and odor/color + shock experiments could land on it between trials without receiving a shock. These experiences amounted to extinction training, which may have reduced the association between color and shock. Although unlikely during training and short-term retention tests, extinction probably occurred in the 24-h retention test, since mosquitoes went through a circadian cycle and redistributed themselves. (3) If shaking is aversive, all mosquitoes got an aversive stimulus on each trial and may have associated it with whatever surface they were on. Since many mosquitoes started out on the dark surface, this may account for the small, generally non-significant, decline in dark-area preference over time in control experiments.
We found retention for 60 min but not 24 h after aversive training. This is in contrast to 24-h retention after appetitive conditioning in Culex (Sanford and Tomberlin, 2011) and 72-h retention after appetitive conditioning in Anopheles (Chilaka et al., 2012). There are four possible explanations. First, it has long been known that learning is retained longer after widely separated (spaced) than closely separated (massed) training trials. For example, honeybees trained with a 180-s intertrial interval showed significantly greater retention after 24–72 h than those trained with a 30-s intertrial interval (Menzel et al., 2001). Trial spacing may explain the 72-h retention in Anopheles [300-s interval (Chilaka et al., 2012)] but does not easily explain the difference between retention in Aedes (<24 h retention after 60-s interval; current study) and Culex [24-h retention after 30-s interval (Sanford and Tomberlin, 2011)]. The Culex and Aedes studies both used massed trials but differed in retention at 24 h. Second, appetitive and aversive learning may differ. One study of D. melanogaster larvae found that, with identical training paradigms, appetitive odor association long outlasted aversive odor association (Honjo and Furukubo-Tokunaga, 2009). This may also be true of mosquitoes, although we are cautious about drawing that conclusion from three different species and training paradigms. Third, learned aversion to an innately preferred stimulus may be weaker, and more readily lost, than an aversive association attached to an otherwise neutral stimulus. Finally, we cannot exclude the possibility that lack of retention in our test was due to de facto extinction training during the 24-h period, as described above.
Although the baseline attractiveness of the dark surface with odor did not differ from that of the dark surface alone, odor clearly affected learning. Mosquitoes learned to associate the dark surface with shock, but the association was stronger when odor was also present. Our experiments were not intended to address questions of blocking, overshadowing, configural versus elemental stimuli, or stimulus generalization that arise when conditional stimuli are used in combination (Pearce, 1987). Future work might address some of these issues by training with odor and color together and testing with color alone (to test overshadowing) or by training with odor in a chamber with dark PCBs at both ends (to test the strength of odor as a conditional stimulus on its own).
We found no sex difference in learning, nor have others who have looked for it in mosquitoes (Sanford and Tomberlin, 2011), fruit fly larvae (Neuser et al., 2005) or honeybees (Bitterman et al., 1983). However, one study found that female honeybees learn better than males in tasks specifically related to foraging behavior (Shafir et al., 2005). Given that female and male mosquitoes have very different feeding behaviors and risks to balance in foraging and reproduction, it is likely that sex-specific learning assays could be found for them as well.
Naturalistic studies of mosquito learning have been problematic (reviewed by Alonso and Schuck-Paim, 2006), but there are two that show clear evidence of learning. Mwandawiro et al. found that three species of Culex preferred hosts to which they had been previously exposed; they found no such preference in the species of Aedes that they tested (Mwandawiro et al., 2000). Similarly, female Culex raised as larvae in water containing skatole preferred as adults to oviposit in skatole-containing water over plain water or water containing p-cresol, despite the fact that skatole is normally repellant and p-cresol normally attractive (McCall and Eaton, 2001). Although these two studies showed learning and successfully excluded genetic predisposition (daughters did not show the same preference as their mothers), the type of learning underlying these behaviors was not established. Learned aversion to host odors may explain heterogeneous distribution of disease vectors among host individuals and may provide an avenue for targeted control methods (McCall and Kelly, 2002). Learned aversion to a host odor has been demonstrated in the laboratory with bloodsucking bugs (Vinauger et al., 2011) but has not been reported with mosquitoes.
Finding that Ae. aegypti is capable of associative learning is not surprising, given that it has been demonstrated in other insects of similar size and behavioral complexity. Indeed, the opposite finding, inability to learn, would require special explanation. A more practical question is whether mosquitoes use this ability in nature. Given their complex life history, there is likely to be an advantage to learning but this remains to be shown in free-ranging mosquito populations. Further lab studies could direct the design of well-controlled field experiments or naturalistic lab experiments by circumscribing the parameters within which mosquitoes learn. For example, lab work might show what stimuli are adequate as conditional or unconditional stimuli, reveal the minimum number of training trials required, or show the effects of age, sex, circadian state or appetitive state. Bulk training methods such as ours and the recent work on Anopheles (Chilaka et al., 2012) provide a framework for large-scale studies of mosquito learning.
ACKNOWLEDGEMENTS
We thank Sylvie Pitcher, Melissa Orteza, and other members of the Harrington laboratory for mosquito rearing and maintenance, Gary Oltz for assistance with construction of the test apparatus, Haim Bar for additional statistical consulting, and three anonymous reviewers for useful comments.
FOOTNOTES
FUNDING
This research was supported by the National Institutes of Health [5R01DC103-37 to R.R.H.] and a Hatch award [2010-11-184 to L.C.H.]. Deposited in PMC for release after 12 months.
REFERENCES
- Alonso W. J., Schuck-Paim C. (2006). The ‘ghosts’ that pester studies on learning in mosquitoes: guidelines to chase them off. Med. Vet. Entomol. 20, 157-165 [DOI] [PubMed] [Google Scholar]
- Alonso W. J., Wyatt T. D., Kelly D. W. (2003). Are vectors able to learn about their hosts? A case study with Aedes aegypti mosquitoes. Mem. Inst. Oswaldo Cruz 98, 665-672 [DOI] [PubMed] [Google Scholar]
- Bitterman M. E., Menzel R., Fietz A., Schäfer S. (1983). Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107-119 [PubMed] [Google Scholar]
- Charlwood J. D., Graves P. M., Marshall T. F. (1988). Evidence for a ‘memorized’ home range in Anopheles farauti females from Papua New Guinea. Med. Vet. Entomol. 2, 101-108 [DOI] [PubMed] [Google Scholar]
- Chilaka N., Perkins E., Tripet F. (2012). Visual and olfactory associative learning in the malaria vector Anopheles gambiae sensu stricto. Malar. J. 11, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A. (1992). The Biology of Mosquitoes: Development, Nutrition and Reproduction. London, UK: Chapman and Hall; [Google Scholar]
- Davis R. L. (2005). Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28, 275-302 [DOI] [PubMed] [Google Scholar]
- Dukas R. (2008). Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145-160 [DOI] [PubMed] [Google Scholar]
- Gilbert I., Gouck H. (1957). Influence of surface color on mosquito landing rates. J. Econ. Entomol. 50, 678-680 [Google Scholar]
- Giurfa M. (2007). Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 193, 801-824 [DOI] [PubMed] [Google Scholar]
- Giurfa M., Sandoz J. C. (2012). Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54-66 [DOI] [PubMed] [Google Scholar]
- Grant A. J., Dickens J. C. (2011). Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE 6, e21785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D. J. (1998). Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11, 480-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L. C., Edman J. D., Scott T. W. (2001). Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 38, 411-422 [DOI] [PubMed] [Google Scholar]
- Hii J. L., Chew M., Sang V. Y., Munstermann L. E., Tan S. G., Panyim S., Yasothornsrikul S. (1991). Population genetic analysis of host seeking and resting behaviors in the malaria vector, Anopheles balabacensis (Diptera: Culicidae). J. Med. Entomol. 28, 675-684 [DOI] [PubMed] [Google Scholar]
- Holliday M., Hirsch J. (1986). A comment on the evidence for learning in Diptera. Behav. Genet. 16, 439-447 [DOI] [PubMed] [Google Scholar]
- Honjo K., Furukubo-Tokunaga K. (2009). Distinctive neuronal networks and biochemical pathways for appetitive and aversive memory in Drosophila larvae. J. Neurosci. 29, 852-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhumur U. S., Dötterl S., Jürgens A. (2006). Naive and conditioned responses of Culex pipiens pipiens biotype molestus (Diptera: Culicidae) to flower odors. J. Med. Entomol. 43, 1164-1170 [PubMed] [Google Scholar]
- Kaur J. S., Lai Y. L., Giger A. D. (2003). Learning and memory in the mosquito Aedes aegypti shown by conditioning against oviposition deterrence. Med. Vet. Entomol. 17, 457-460 [DOI] [PubMed] [Google Scholar]
- Keene A. C., Waddell S. (2007). Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8, 341-354 [DOI] [PubMed] [Google Scholar]
- Ligon B. L. (2006). Reemergence of an unusual disease: the chikungunya epidemic. Semin. Pediatr. Infect. Dis. 17, 99-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall P. J., Eaton G. (2001). Olfactory memory in the mosquito Culex quinquefasciatus. Med. Vet. Entomol. 15, 197-203 [DOI] [PubMed] [Google Scholar]
- McCall P. J., Kelly D. W. (2002). Learning and memory in disease vectors. Trends Parasitol. 18, 429-433 [DOI] [PubMed] [Google Scholar]
- McCall P. J., Mosha F. W., Njunwa K. J., Sherlock K. (2001). Evidence for memorized site-fidelity in Anopheles arabiensis. Trans. R. Soc. Trop. Med. Hyg. 95, 587-590 [DOI] [PubMed] [Google Scholar]
- McGuire T. R. (1986). Further evidence for learning in Diptera: a reply to Holliday and Hirsch. Behav. Genet. 16, 457-473 [DOI] [PubMed] [Google Scholar]
- McIntyre C. K., Hatfield T., McGaugh J. L. (2002). Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur. J. Neurosci. 16, 1223-1226 [DOI] [PubMed] [Google Scholar]
- Menzel R. (1999). Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323-340 [Google Scholar]
- Menzel R., Manz G., Menzel R., Greggers U. (2001). Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn. Mem. 8, 198-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery F., Kawecki T. J. (2005). A cost of long-term memory in Drosophila. Science 308, 1148 [DOI] [PubMed] [Google Scholar]
- Mery F., Belay A. T., So A. K., Sokolowski M. B., Kawecki T. J. (2007). Natural polymorphism affecting learning and memory in Drosophila. Proc. Natl. Acad. Sci. USA 104, 13051-13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwandawiro C., Boots M., Tuno N., Suwonkerd W., Tsuda Y., Takagi M. (2000). Heterogeneity in the host preference of Japanese encephalitis vectors in Chiang Mai, northern Thailand. Trans. R. Soc. Trop. Med. Hyg. 94, 238-242 [DOI] [PubMed] [Google Scholar]
- Neuser K., Husse J., Stock P., Gerber B. (2005). Appetitive olfactory learning in Drosophila larvae: effects of repetition, reward strength, age, gender, assay type and memory span. Anim. Behav. 69, 891-898 [Google Scholar]
- Ooi E.-E., Goh K.-T., Gubler D. J. (2006). Dengue prevention and 35 years of vector control in Singapore. Emerg. Infect. Dis. 12, 887-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce J. M. (1987). A model for stimulus generalization in Pavlovian conditioning. Psychol. Rev. 94, 61-73 [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A., Benzer S. (1974). Conditioned behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 71, 708-712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel E., Padie S., Giurfa M. (2012). Aversive learning overcomes appetitive innate responding in honeybees. Anim. Cogn. 15, 135-141 [DOI] [PubMed] [Google Scholar]
- Sanford M. R., Tomberlin J. K. (2011). Conditioning individual mosquitoes to an odor: sex, source, and time. PLoS ONE 6, e24218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir S., Menda G., Smith B. H. (2005). Caste-specific differences in risk sensitivity in honeybees, Apis mellifera. Anim. Behav. 69, 859-868 [Google Scholar]
- Swaminathan S., Khanna N. (2009). Dengue: recent advances in biology and current status of translational research. Curr. Mol. Med. 9, 152-173 [DOI] [PubMed] [Google Scholar]
- Takken W., Kline D. L. (1989). Carbon dioxide and 1-octen-3-ol as mosquito attractants. J. Am. Mosq. Control Assoc. 5, 311-316 [PubMed] [Google Scholar]
- Tomberlin J. K., Rains G. C., Allan S. A., Sanford M. R., Lewis W. J. (2006). Associative learning of odor with food- or blood-meal by Culex quinquefasciatus Say (Diptera: Culicidae). Naturwissenschaften 93, 551-556 [DOI] [PubMed] [Google Scholar]
- Tully T. (1986). Measuring learning in individual flies is not necessary to study the effects of single-gene mutations in Drosophila: a reply to Holliday and Hirsch. Behav. Genet. 16, 449-455 [DOI] [PubMed] [Google Scholar]
- Vinauger C., Buratti L., Lazzari C. R. (2011). Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. II. Aversive learning. J. Exp. Biol. 214, 3039-3045 [DOI] [PubMed] [Google Scholar]
- Webster D. P., Farrar J., Rowland-Jones S. (2009). Progress towards a dengue vaccine. Lancet Infect. Dis. 9, 678-687 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1997). Vector Surveillance and Control Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva, CH: World Health Organization; [Google Scholar]
- World Health Organization (2002). Dengue and Dengue Haemorrhagic Fever. Geneva, CH: World Health Organization; [Google Scholar]


