Abstract
Epithelial cell invasion through the extracellular matrix (ECM) is a crucial step in branching morphogenesis. The mechanisms by which the mammary epithelium integrates cues from the ECM with intracellular signaling in order to coordinate invasion through the stroma to make the mammary tree are poorly understood. Because the cell membrane-bound matrix metalloproteinase Mmp14 is known to play a key role in cancer cell invasion, we hypothesized that it could also be centrally involved in integrating signals for mammary epithelial cells (MECs) to navigate the collagen 1 (CL-1)-rich stroma of the mammary gland. Expression studies in nulliparous mice that carry a NLS-lacZ transgene downstream of the Mmp14 promoter revealed that Mmp14 is expressed in MECs at the tips of the branches. Using both mammary organoids and 3D organotypic cultures, we show that MMP activity is necessary for invasion through dense CL-1 (3 mg/ml) gels, but dispensable for MEC branching in sparse CL-1 (1 mg/ml) gels. Surprisingly, however, Mmp14 without its catalytic activity was still necessary for branching. Silencing Mmp14 prevented cell invasion through CL-1 and disrupted branching altogether; it also reduced integrin β1 (Itgb1) levels and attenuated MAPK signaling, disrupting Itgb1-dependent invasion/branching within CL-1 gels. FRET imaging revealed that Mmp14 associates directly with Itgb1. We identified a domain of Mmp14 that is required for modulating the levels of Itgb1, MEC signaling and the rate of invasion within CL-1. These results shed light on hitherto undescribed non-proteolytic activities of Mmp14 that are necessary for the Itgb1-dependent biochemical and mechanical signals that regulate branching in the mammary epithelium.
Keywords: Branching morphogenesis, Mammary epithelial invasion, Mmp14, Integrin-β1, Mouse
INTRODUCTION
The formation of branched organs involves coordinated invasion of epithelium into the surrounding stroma (Chuong, 1998; Fata et al., 2004; Lu and Werb, 2008; Yamada and Cukierman, 2007). In the mammary gland, both ductal branching and alveologenesis require integrin-mediated signaling, as well as the activity of matrix metalloproteinases (MMPs) (Fata et al., 2007; Simian et al., 2001; Sympson et al., 1994; Talhouk et al., 1991). These and other studies have demonstrated roles for secreted MMPs in the developing mammary gland (MG) (reviewed by Fata et al., 2004). Whereas the primary source of Mmp2 and Mmp3 is the mammary stroma, Mmp14 is expressed in both mammary stroma and epithelium of terminal end buds (TEBs) (Wiseman et al., 2003), suggesting that Mmp14 may be involved in epithelial invasion into the mammary fat pad. However, despite its position on the cell surface and its expression in TEBs, neither a role for Mmp14 in mammary branching morphogenesis nor its possible mechanism of action have been explored. Here, using a transgenic mouse model, we have found that Mmp14 is highly expressed at the invading edges of TEBs and that its expression peaks at the height of development of the mammary epithelial tree. We hypothesized that the localization of Mmp14 at the invading front of TEBs may indicate an important role for Mmp14 in the branching process. We postulated further that because of its location at the cell surface, Mmp14 may serve also as a bi-directional signal transducer between the invading cell and its surrounding ECM. Testing such a hypothesis in vivo would be complicated by multiple cell types, different ECM molecules and proteases present within the gland that change rapidly and continuously as development progresses. To overcome these obstacles, we used a combination of a transgenic mouse model, primary mammary organoids or a mammary cell line grown in three-dimensional (3D) collagen 1 (CL-1) gels (Simian et al., 2001).
Our studies reveal that Mmp14 proteolytic activity is required in dense – but not in sparse – CL-1 gels; surprisingly, however, non-catalytic activity of Mmp14 is still required for branching in sparse gels. Because it is known that Itgb1 is necessary for branching in vivo (Taddei et al., 2008), we explored the possibility of an association between Mmp14 and Itgb1. Using immunoprecipitation and FRET analysis, we show the physical interaction between the two molecules. This finding explains how Mmp14 can activate MAPK signaling, despite the absence of a kinase domain. Furthermore, we show that the extracellular domain with or without proteolytic activity as well as the transmembrane/cytoplasmic domain of Mmp14 are required for both branching in CL-1 gels and modulating the level of Itgb1 expression. In summary, our results demonstrate that Mmp14 is a central regulator of invasion and branching, and it mediates signals from the ECM via crosstalk and association with Itgb1.
MATERIALS AND METHODS
Cell culture and reagents
Functionally normal mouse mammary epithelial cells, EpH4 (Reichmann et al., 1989), were cultured in 1:1 Dulbecco’s Modified Eagle’s Medium: Ham’s F12 Nutrient Mixture (DMEM/F12), 2% fetal bovine serum, 5 μg/ml insulin and 50 μg/ml gentamycin (Sigma). The following inhibitors were used at the concentrations indicated: PD98059 (40 μM; Calbiochem, San Diego, USA); GM6001 (40 μM; Chemicon/EMD Millipore, Billerica, USA).
Whole-mount β-gal staining
Transgenic mice carrying the lacZ gene under the control of the Mmp14 promoter were used (Yana et al., 2007). Inguinal MGs were isolated from 12-week-old wild-type (+/+) and Mmp14 (+/–, lacZ) mice. Tissues were collected in ice-cold PBS and fixed for 15 minutes at room temperature in fix solution (2% formaldehyde, 0.2% glutaraldehyde, 0.02% Nonidet P-40 (NP-40) and 0.01% sodium deoxycholate in PBS). After fixation, tissues were rinsed several times in PBS and stained overnight at 37°C in the dark with stain solution (5 mM potassium ferricyanide and 5 mM potassium ferrocyanide in rinse buffer – 1 mg/ml β-Gal, 2 mM MgCl2, 0.02% NP-40 and 0.01% sodium deoxycholate in PBS). Dehydrated sections of MG tissue were stained with Hematoxylin and Eosin and inspected for β-gal staining.
Branching morphogenesis assays
Branching morphogenesis was induced using slight modifications of previously published protocols (Hirai et al., 1998; Simian et al., 2001). Cell clusters were prepared as follows: EpH4 cells suspended in growth medium containing DNase I were placed on top of agarose-coated wells and incubated at 100 rpm and 37°C on an orbital shaker overnight, yielding rounded and well-packed clusters. Single cells were removed by centrifugation and the clusters were then washed three times with DMEM/F12. Cell clusters and primary organoids were embedded in CL-1 gels. Briefly, acid-extracted type I collagen (Koken, Tokyo, Japan) was mixed gently on ice (eight volumes) with one volume of 10× DMEM/F12, then pH is adjusted to 7.4 with 0.1 N NaOH. Collagen solution was added into each well of a 48-well plate, which was then incubated at 37°C to allow gelation. EpH4 cell clusters or primary organoids were suspended in collagen at 3 mg/ml or 1 mg/ml, poured onto the basal collagen layer and placed at 37°C for gelation. After gelation of the collagen, growth medium containing 9 nM FGF2 was added to the wells. Branching morphogenesis was assessed using a Nikon Diaphot 300 microscope and clusters were scored as positive when displaying three or more branches of lengths that were at least half the diameter of the central cell cluster.
Preparation of lentivirus
To transduce FLAG-tagged human MMP14F and MMP14F-dCAT (deletion of Tyr112-Pro312) (Itoh et al., 1999; Mori et al., 2002), each cDNA was ligated into pLenti-EF1α-puro, generated in our laboratory. MMP14F-dEC (deletion of Tyr112-Cys516) was made by PCR, and sequence was confirmed by DNA sequencing. Lentivirus plasmids containing shRNA (Mission shRNA; Sigma, St Louis, USA) against mouse Mmp14 or Itgb1, or lentivirus plasmids containing Mmp14 or mutants were transfected into 293FT cells using FuGene6 (Roche, Basel, Switzerland). Transfected cells were cultured in DMEM containing 5% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Culture media were replaced after 24 hours with fresh media. Forty-eight hours later, recombinant lentivirus was concentrated from filtered culture media (0.45 μm filters) by ultracentrifugation at 100,000 g for 90 minutes (SW41Ti rotor; Beckman Coulter, Brea, USA). To transduce EpH4 cells, 1.0×105 cells were plated in each well of a six-well plate, infected with the lentivirus, treated with polybrene for 30 minutes and selected by adding 5 μg/ml puromycin to growth medium for 4 days. Lentivirus with scrambled sequence was used as a shRNA control. Target sequences of Mmp14 and Itgb1, and results of 3D CL-1 gel cultures are indicated in supplementary material Fig. S10. Template plasmids for Ypet and Cypet were purchased from Addgene (http://www.addgene.org) and mutated alanine 206 to lysine, as suggested for the monomeric form of these fluorophores (Shaner et al., 2005). Monomeric Ypet or Cypet were fused, respectively, with the C terminus of Itgb1 or Mmp14 by PCR. All the sequences were confirmed by sequencing.
Quantitative RT-PCR analysis
Total RNA was isolated using the QIAGEN RNeasy Mini kit (Valencia, USA). Total RNA (100 ng) was used to synthesize cDNA using SuperScript II First-Strand Synthesis System (Invitrogen, Carlsbad, USA). Mmp14 was amplified with 5′-GAGATCAAGGCCAATGTTCG and 5′-GTCCAGGGCTCGGCAGAATC primers or with 5′-CATCTTCTTGGTGGCTGTG and 5′-TGACCCTGACTTGCTTCC primers. Itgb1 was amplified with 5′-GGAGATGGGAAACTTGGTGG and 5′-CCCATTCACCCCATTCTTGC primers. As a control for total RNA, RT-PCR for 18S rRNA was performed with 5′-TCGGAACTGAGGCCATGATT and 5′-CCTCCGACTTTCGTTCTTGATT primers. Real-time PCR was performed using the LightCycler System and Fast Start DNA Master SYBR Green I (Roche) following manufacturer’s instructions.
Western blotting
Samples were lyzed using modified RIPA buffer [50 mM HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 10 mM sodium pyrophosphate containing 1.5 mM MgCl2, 1 mM EGTA, 1% sodium deoxycholate, 0.25 mM Na3VO4, 100 mM NaF and proteinase inhibitor cocktail (EMD Millipore, Billerica, USA)]. Protein concentration was determined using the BCA Protein Assay kit (Thermo Scientific, Waltham, USA), following the manufacturer’s instructions. Protein samples (10 μg) were mixed with Laemmeli sample buffer and heated at 95°C for 5 minutes. Samples were loaded into a pre-cast 4-20% tris-glycine polyacrylamide gel (Invitrogen) using the NOVEX system (Invitrogen). Resolved proteins were transferred to nitrocellulose membrane (Whatman, Maidstone, UK) followed by blocking in PBS, 0.05% Tween-20 with 5% w/v non-fat dry milk for 1 hour at room temperature. Membranes were incubated overnight at 4°C in 5% BSA, 0.1% Tween-20 in PBS containing antibodies that recognize either phosphorylated Erk1/2 or total Erk1/2 (Cell Signaling). Anti-Mmp14 (Abcam, Cambridge, USA), anti-Itgb1 (Santa Cruz Biotechnology, Santa Cruz, USA), anti-Itgb1 p788/789 (Abcam), anti-LAM A/C (Santa Cruz Biotechnology), anti-Actin (Abcam), anti-FLAG (Sigma) and rabbit IgG (Sigma) antibodies were used for immunoblotting. Primary antibodies were detected with HRP conjugated anti-IgG (Thermo) and the Pierce SuperSignal detection kit. Chemiluminescence signal was captured with a FluorChem 8900 analysis system (Alpha Innotech, San Leandro, USA).
Immunoprecipitation
Samples were lyzed at room temperature with 50 mM HEPES (pH 7.4), 150 mM NaCl, 1% Brij98 (Sigma), 1.5 mM MgCl2, phosphatase inhibitor cocktail (Sigma) and proteinase inhibitor cocktail (EMD Millipore). Lysate containing 5 mg protein was incubated with 10 μg of control rabbit-IgG or anti-Mmp14 for 16 hours at room temperature. Precipitation was performed with protein-G sepharose (GE Healthcare, Little Chalfont, UK). Ten percent of total precipitates were loaded to western blotting. For detecting Itgb1, anti-Itgb1 (Santa Cruz Biotechnology) was used.
Gel stiffness probed by AFM
Gels were prepared by adding 100 μl of collagen solutions (1 or 3 mg/ml) to the glass surface of a 35 mm culture dish with a 14 mm diameter bottom-glass coverslip (MatTek, Ashland, USA) and incubating the samples for 20 minutes at 37°C to allow gelation. Gel stiffness was characterized by measuring the Young’s elastic modulus (E) using an atomic force microscope (AFM) (Bioscope; Bruker AXS, Santa Barbara, USA) as previously described (Alcaraz et al., 2003; Alcaraz et al., 2011). Briefly, three force-indentation (F-δ) curves were acquired in at least nine gel locations for each independent experiment (n≥2). A contact elastic model was fitted to the loading part of each F-δ curve to obtain E.
RESULTS
Mmp14 expression peaks during puberty and is highly elevated at the invading front of mammary gland end buds
We measured the levels of Mmp14 expression in different stages of MG development in virgin, pregnant and lactating mice using quantitative RT-PCR. Mmp14 expression increased with development of the mammary epithelial tree during branching morphogenesis in virgin mice (Fig. 1A). However, Mmp14 expression plunged at the onset of pregnancy before mildly increasing during mid-pregnancy (coinciding with the stage of pregnancy that alveoli form) and subsiding again in late pregnancy and during lactation (Fig. 1A). β-Galactosidase staining of whole-mounted MGs isolated from 5-week-old mice carrying a lacZ reporter downstream of the endogenous Mmp14 promoter (Yana et al., 2007) revealed expression of Mmp14 in epithelial cells, especially at the tips of ducts and/or TEBs (Fig. 1Bi-iii). Analysis of tissue sections indicated that the Mmp14 promoter was also prominently active in myoepithelial cells (Fig. 1Biv), suggesting that Mmp14 functions at the interface of the invading epithelium and ECM.
Fig. 1.
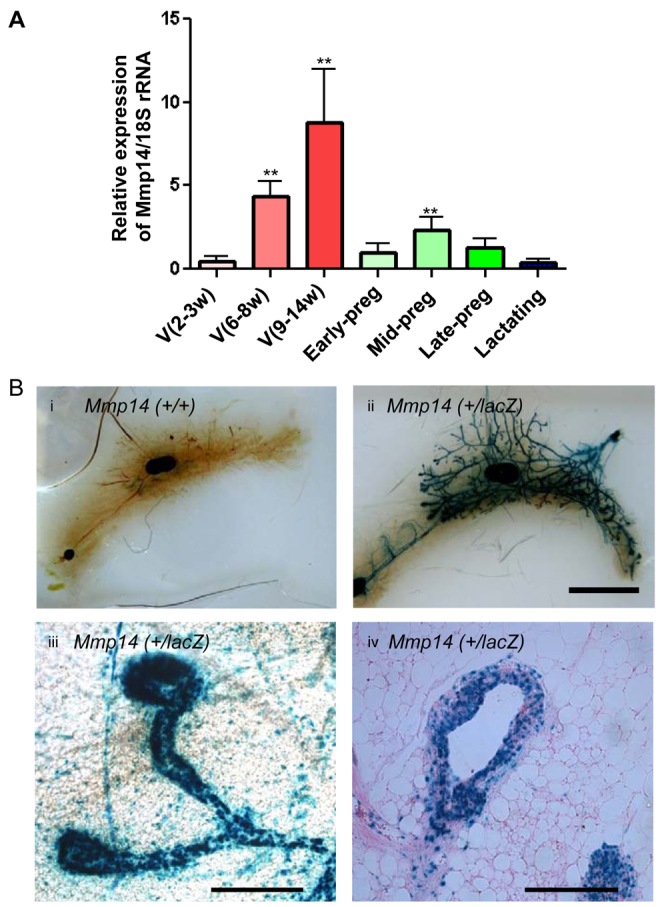
Mmp14 expression peaks during puberty, and is highly elevated at the invading front of mammary gland (MG) end buds. (A) Quantitative RT-PCR analysis of Mmp14 expression during development of the mouse MG from virgin (V; 2-3, 6-8 and 9-14 weeks after birth), early pregnancy (Early-preg; day 4), mid-pregnancy (Mid-preg; days 8-12), late pregnancy (Late-Preg; days 16-18) and lactating (days 1-10) mice, normalized to 18S rRNA. Data are mean±s.e.m. **P<0.01 when compared with V (2-3w). (B) Mmp14 promoter activity in MGs from Mmp14 (+/lacZ) mice. Images are of glands of 5-week-old mice. β-Gal staining of a whole-mount MG isolated from a virgin transgenic heterozygote mouse bearing the lacZ gene under the control of the endogenous Mmp14 promoter (Yana et al., 2007) indicates that Mmp14 promoter activity is high in mammary epithelial cells. (i) β-Gal-stained MG from Mmp14 (+/+) mouse as negative control. (ii) β-gal stained MG from Mmp14 (+/lacZ) mouse. Scale bar: 6.25 mm. (iii) β-Gal-stained mammary end buds in Mmp14 (+/lacZ) mouse showed intense promoter activity at the tip of the bud. (iv) β-Gal and Eosin stain of a MG tissue section from a 5-week-old Mmp14 (+/lacZ) transgenic mouse. Scale bars: 200 μm.
Mmp14 catalytic activity is required for invasion/branching only in dense- but not sparse-collagen gels
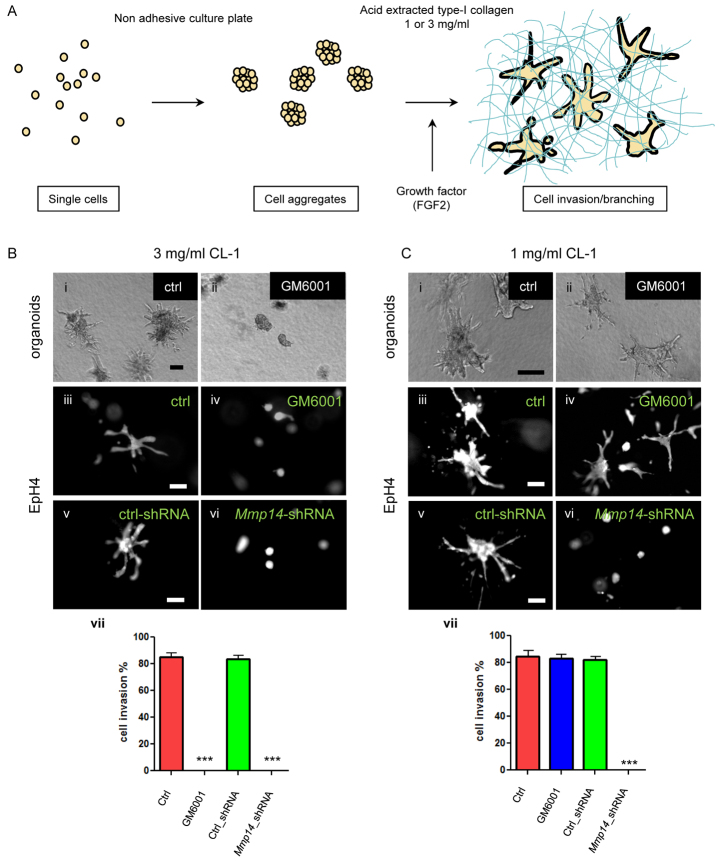
To dissect the role of Mmp14 in mammary invasion/branching, we used two culture models that simulate mammary epithelial branching: primary mammary organoids (Fata et al., 2007; Simian et al., 2001) and aggregated cellular clusters (Hirai et al., 1998) of a functionally normal mouse mammary epithelial cell line (EpH4) (Reichmann et al., 1989), in each case embedded within a CL-1 gel (Fig. 2A). The physiological relevance of this model is illustrated by the rich presence of CL-1 in the stroma surrounding epithelial ducts in the murine MG (supplementary material Fig. S1) (Williams and Daniel, 1983).
Fig. 2.
Mmp14 catalytic activity is required for invasion/branching in dense but not in sparse collagen gels. (A) A 3D organotypic culture model of mammary epithelial cell invasion/branching. Mammary organoids from Balb/c mice or clusters of EpH4 cells were induced to branch by addition of 9 nM FGF2 for 5 days in either 3 mg/ml or 1 mg/ml collagen 1 (CL-1). (B) CL-1 (3 mg/ml). (i,iii) Vehicle control (ctrl; DMSO), (ii,iv) MMP inhibitor GM6001 (40 μM) or pre-treatment with (v) control shRNA or (vi) Mmp14-shRNA. (i,ii) Bright-field image of primary organoids in 3 mg/ml CL-1 gel. (iii-vi) Live dye (Calcein AM) stained EpH4 cell aggregates in 3 mg/ml CL-1 gel. (vii) Invasion/branching of EpH4 cells was scored as positive when displaying three or more branches with lengths of at least half the diameter of the central cell cluster (as described in the Materials and methods). Percentages of cell invasion of control (DMSO treated; red bar) EpH4 cells versus EpH4 treated with GM6001 or infected with control (ctrl_shRNA; green bar) and shMmp14-containing lentivirus (Mmp14_shRNA). Two-hundred colonies were analyzed for each condition in three separate experiments. Data are mean±s.e.m. ***P<0.001 compared with control. (C) Invasion/branching of MECs in sparse CL-1 gels (1 mg/ml) in the presence of (i,iii) vehicle control (ctrl; DMSO), (ii,iv) MMP inhibitor GM6001 (40 μM) or pre-treated with (v) control- or (vi) Mmp14-shRNA. (i,ii) Bright-field image of primary organoids in CL-1 gel. (iii-vi) Live dye (Calcein AM) stained EpH4 cell aggregates in CL-1 gel. (vii) Percentages of cells invading control EpH4 cells (DMSO treated; red bar), EpH4 cells treated with GM6001 (blue bar), EpH4 cells infected with control shRNA (ctrl, green bar) or EpH4 cells infected with Mmp14 shRNA-containing lentivirus. Two-hundred colonies were analyzed for each condition over three separate experiments. Data are mean±s.e.m. ***P<0.001 when compared with control. Scale bars: 200 μm.
To mimic the CL-1-rich ECM found in the MG, we used two different concentrations of CL-1: 1 mg/ml (sparse) and 3 mg/ml (dense). The denser concentration is actually representative of the pre-malignant MG (Levental et al., 2009), but has been used commonly nonetheless by us and others to model branching of the mammary epithelium (Brinkmann et al., 1995; Hirai et al., 1998; Janda et al., 2002; Mori et al., 2009; Simian et al., 2001). Upon addition of fibroblast growth factor 2 (FGF2), both primary organoids and EpH4 cells invaded dense CL-1 gels (Fig. 2Bi,iii). Invasion was completely abrogated by addition of either the broad-spectrum MMP inhibitor GM6001 (Fig. 2Bii,iv) or tissue inhibitor of metalloproteinases (TIMP) 2 (Simian et al., 2001) (not shown) in these gels, which have an average pore size that is smaller than the size of an average cell (Alcaraz et al., 2011). Silencing Mmp14 with shRNA decreased Mmp14 expression by ∼90% (supplementary material Fig. S2) and also completely inhibited MEC invasion in CL-1 under these conditions (Fig. 2Bv-vii).
Using atomic force microscopy, we found that sparse 1 mg/ml CL-1 gels better approximate the mechanical microenvironment of the murine mammary gland (supplementary material Fig. S3) (Levental et al., 2009; Paszek et al., 2005). Under these conditions, primary organoids and MECs still invaded in response to FGF2 (Fig. 2Ci,iii), regardless of whether GM6001 (Fig. 2Cii,iv) or Timp2 (data not shown) were added or not. Unexpectedly, however, expression of Mmp14 itself was still required: shRNA knockdown of Mmp14 blocked MEC invasion completely (Fig. 2Cv-vii). These data suggest that Mmp14 has multiple activities involved in the regulation of invasion/branching morphogenesis in the MG, at least one of which is non-proteolytic in nature. Therefore, we concentrated on elucidating the mechanism by which the non-catalytic activity of Mmp14 is involved in branching morphogenesis in gels containing physiological concentrations of CL-1.
MAPK activity and cross-signaling between Mmp14, Erk and Itgb1 are involved in branching morphogenesis
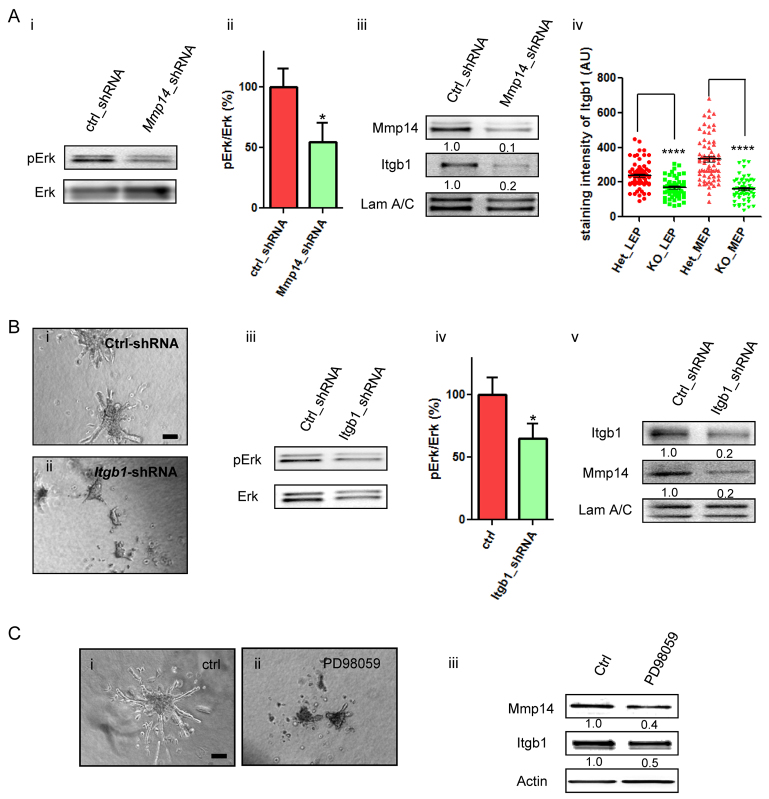
We have previously shown the necessity of MAPK signaling for alveologenesis using primary MECs within 3D laminin-rich ECMs (Fata et al., 2007). We asked whether or not the non-catalytic mechanism through which Mmp14 orchestrates branching at a physiological concentration of CL-1 in gels involves MAPK activity. Silencing Mmp14 with shRNA reduced MAPK activation by half (Fig. 3Ai,ii). However, MAPK activity did not change in response to addition of GM6001 (supplementary material Fig. S4), suggesting that MAPK activation does not depend on the proteolytic activity of MMPs and that a non-proteolytic function of Mmp14 is responsible for MAPK activity. However, our analysis of 20 amino acids sequence of Mmp14 cytoplasmic tail (Sato et al., 1994) did not reveal any homology to known kinase domains. How, then, might Mmp14 affect MAPK activity? One possibility is that Mmp14 regulates downstream signaling through a partner molecule with kinase activity. A search of molecules that were altered when Mmp14 was silenced by shRNA showed Itgb1 was dramatically reduced (Fig. 3Aiii). This was not the result of an off-target effect of Mmp14 shRNA: we observed this with multiple shRNA, and examination of MGs from Mmp14 knockout mice (supplementary material Fig. S5) also demonstrated a significant reduction in Itgb1 expression in both luminal and myoepithelial cells (Fig. 3Aiv). A small shRNA screen of collagen receptors with known kinase activity [Itgb1, discoidin domain receptors (Ddr1 and DDr2)] confirmed that silencing Itgb1 also blocked branching of EpH4 cells in 1mg/ml CL-1 gels (Fig. 3Bi,ii). Silencing Ddr1 or Ddr2 did not block branching under the same conditions (data not shown). Itgb1-silenced cells showed significantly reduced MAPK activation (Fig. 3Biii,iv). Significantly, silencing Itgb1 resulted in a dramatic decrease in Mmp14 levels (Fig. 3Bv). These results indicated that Mmp14 and Itgb1 modulate each other’s expression in addition to modulating MAPK activity. Preventing MAPK activation using a small molecule MEK inhibitor (PD95089) reduced invasion/branching (Fig. 3Ci,ii) and also reduced expression of Mmp14 and Itgb1 (Fig. 3Ciii), confirming the three-way connection. Src inhibition showed similar inhibition of the levels of Mmp14 and Itgb1 (data not shown), indicating the involvement of downstream intermediates. In summary, these data suggest that Mmp14, Itgb1 and MAPK activation are all connected reciprocally during branching morphogenesis, and, specifically, that Mmp14 cooperates in a proteolytically independent manner with Itgb1 to activate Erk and facilitate branching in sparse CL-1 gels.
Fig. 3.
MAPK activity and cross-signaling between Mmp14, Erk and Itgb1 are involved in branching morphogenesis. (A) Silencing Mmp14 reduces Erk activity and the level of Itgb1. (i) Immunoblots of phospho-Erk (pErk) in control and Mmp14-silenced EpH4 cells. (ii) Quantification of Erk activity in i. Data are mean±s.e.m. *P<0.05. n=3. (iii) Western blot of Mmp14 and Itgb1 in control and Mmp14-silenced EpH4 cells. LAM A/C was used as the loading control. (iv) MECs from Mmp14 knockout had reduced levels of Itgb1. Immunofluorescence intensity of Itgb1 measured in MG tissues from Mmp14 (+/–, HET) or Mmp14 (–/–, KO) mice (see also supplementary material Fig. S5). Analysis was performed on luminal epithelial cells (LEP) and myoepithelial cells (MEP). Measurement was performed with IMARIS software (Bitplane). At least 50 cells were analyzed per tissue section, n=3 tissue sections. ****P<0.0001. Horizontal lines indicate the mean. (B) Silencing Itgb1 reduced MEC branching, MAPK activity and the Mmp14 levels in sparse CL-1 gels. Branching of (i) control or (ii) Itgb1 shRNA-treated MECs in CL-1 gels of 1 mg/ml. (iii) Silencing Itgb1 reduced Erk phosphorylation. (iv) Quantification of the ratio between pErk and total Erk in Itgb1-shRNA-treated EpH4 cells in sparse CL-1. Data are mean±s.e.m. *P<0.05. (v) Silencing Itgb1 reduced the expression levels of Mmp14 (mean intensity values normalized to Lamin A/C (LAM A/C) calculated via band densitometry from n=3 immunoblots shown below each band). (C) MEK activity is required for cell invasion in CL-1. (i,ii) Control (ctrl; DMSO) and PD98059-treated EpH4 cells in CL-1 gels of 1 mg/ml. (iii) MEK inhibition reduced Mmp14 and Itgb1 levels, as determined by immunoblot (normalized mean intensity values calculated as above are given below each band). Scale bars: 200 μm.
Co-immunoprecipitation and FRET reveal a direct association between Mmp14 and Itgb1
To explore the nature of the Itgb1 and Mmp14 partnership that activates MAPK, we immunoprecipitated endogenous Mmp14 from 3D cultures of branching EpH4 cells and immunoblotted for Itgb1 in the precipitated fraction. Itgb1 was present in this fraction (Fig. 4A). Reverse immunoprecipitation was also performed to confirm this association (supplementary material Fig. S6). As a demonstration of co-precipitation alone is not sufficient to show direct interactions, we sought additional evidence for protein-protein association. We performed FRET analysis (Nguyen and Daugherty, 2005; Shaner et al., 2005) using monomeric (m) Cypet-tagged MMP14 and mYpet-tagged ITGB1. Excitation of mCypet-MMP14 elicited fluorescence of mYpet-Itgb1 in a substantial number of EpH4 cells, supporting a direct association [within ∼10-100 Å (Förster, 2012)] between these two molecules (Fig. 4B).
Fig. 4.
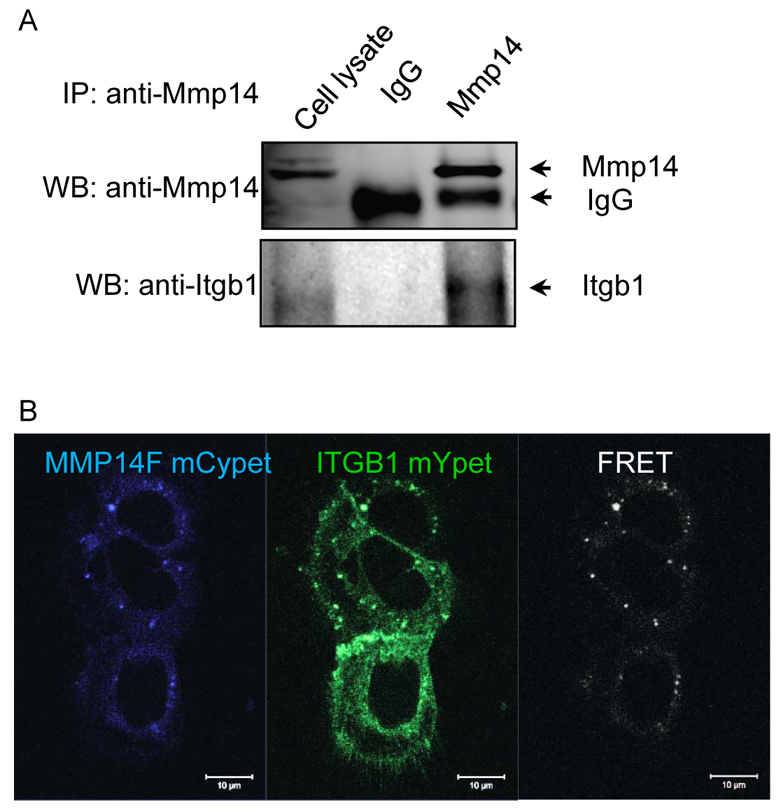
Co-immunoprecipitation and FRET reveal a direct association between Mmp14 and Itgb1. (A) Co-immunoprecipitation of endogenous Mmp14 and Itgb1. Protein complexes containing Mmp14 were immunoprecipitated from EpH4 cells cultured on a collagen 1-coated dish and probed for Mmp14 (top row) or Itgb1 (bottom row). (B) FRET analysis of monomeric Cypet-tagged MMP14F (FLAG tagged human MMP14) and monomeric Ypet-tagged ITGB1 exogenously expressed in EpH4 MECs. Ypet emission signal was detected as FRET signal when Cypet was excited. Scale bars: 10 μm.
Assigning functional activities to the non-catalytic domains of MMP14
To dissect the non-catalytic activities of Mmp14 for functional relevance, we engineered two constructs: catalytic domain-deleted mutant (MMP14F-dCAT; Fig. 5A) and catalytic/hemopexin domains-deleted mutant (MMP14-dCAT/dPEX; Fig. 5A). To prevent interference from endogenous Mmp14, we silenced the endogenous enzyme with shRNA, and then introduced the exogenous FLAG-tagged full-length Mmp14 (MMP14F-FL; Fig. 5A), MMP14F-dCAT, MMP14F-dCAT/dPEX and the vector control using lentivirus. Overexpression of MMP14F-FL restored the level of Itgb1 as expected (Fig. 3, Fig. 5B). However, whereas MMP14F-dCAT/dPEX also restored the Itgb1 level and its activity (Itgb1 pT788/789; Fig. 5B), MMP14F-dCAT did not [Fig. 5B; the ratios of Itgb1 level/loading control (LAM A/C) are shown below each lane]. To establish that the biochemical analysis is relevant to morphogenetic behavior and to observe the behavior of mutants more directly, we tagged all the constructs at the C terminus with mYpet, and confirmed that the mYpet tag did not interfere with the functional behavior (supplementary material Fig. S7). We tested parallel transduced-cultures in the branching assays, and found that MMP14F-FL and MMP14F-dCAT/dPEX restored the invasion in cells that were silenced for endogenous Mmp14, but not in cells expressing MMP14F-dCAT (Fig. 5C). To confirm the association between each mutant and Itgb1, FRET analyses were performed between mCypet-tagged ITGB1 and mYpet tagged MMP14-FL, -dCAT and -dCAT/dPEX (supplementary material Fig. S8). MMP14-FL and dCAT/dPEXs showed significantly higher FRET signals compared with MMP14-dCAT, further supporting the results shown in Fig. 5. These findings indicate that the transmembrane/cytoplasmic domain of MMP14 is required for regulation of Itgb1 levels and activity, as well as for the ability of the cell to invade and branch in CL-1, and that the catalytic activity and the hemopexin domain are not necessary for these functions. It is surprising that the construct, MMP14F-dCAT, does not allow either of the two functions described above, despite the fact that the transmembrane/cytoplasmic domain is still present. This finding raises the possibility that the hemopexin domain exerts inhibitory function on the transmembrane/cytoplasmic domain only if the catalytic domain is not present. The action of MMP14F-dCAT was confirmed further by re-expressing MMP14 catalytic inactive mutant in Mmp14-silenced EpH4 cells, and it restored branching/invasion in sparse gels (data not shown). It is likely that the protein folding and/or localization of MMP14 are altered without the catalytic domain. This mechanism is under investigation in our laboratory.
Fig. 5.
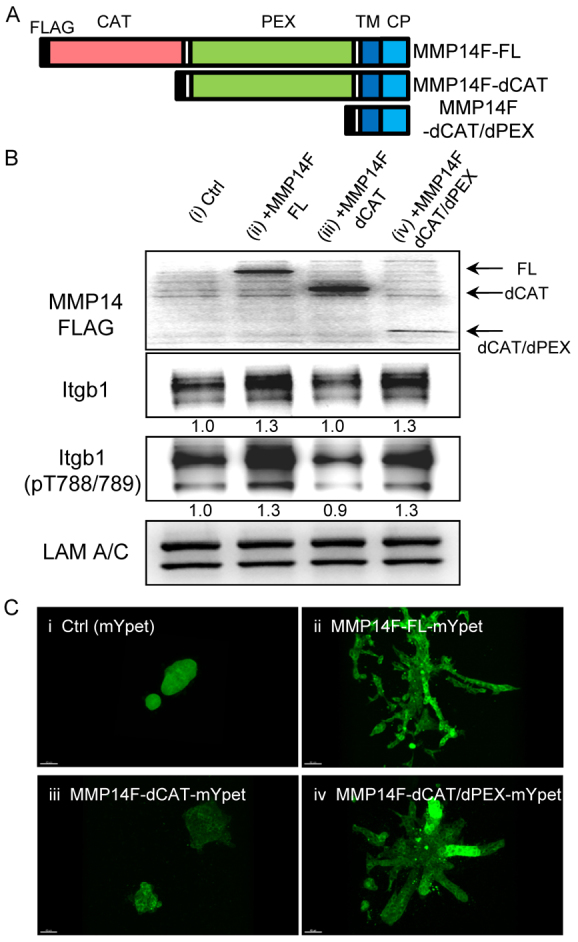
Assigning functional activity to the non-catalytic domains of MMP14. (A) FLAG-tagged full-length human MMP14 (MMP14F FL) catalytic domain-deleted mutant (MMP14F dCAT) and catalytic/hemopexin domain-deleted mutant (MMP14F dCAT/dPEX). (B) MMP14 overexpression rescued the level of Itgb1 in Mmp14-silenced cells. Expression of MMP14F-FL or other mutant proteins was performed on Mmp14-silenced EpH4 cells at the passage 3 after infection with Mmp14 shRNA containing lentivirus. Samples for cell lysate or branching were used at passage 3 or 4 from silencing Mmp14. Immunoblot analysis of Mmp14 (with an anti-FLAG antibody) and Itgb1 (total and phospho-T788/T789) are indicated. The level of total and phospho-T788/T789 were up-modulated when cells overexpressed MMP14F-FL or MMP14F-dCAT/dPEX. LAM A/C is shown as loading control. Numbers below blots indicate the ratio between Itgb1 (or phospho-T788/T789) and LAM A/C. (C) Full-length MMP14 or MMP14F dCAT/dPEX (i.e. only the transmembrane/cytoplasmic domain) mutant rescued invasion/branching in Mmp14-silenced EpH4 cells in sparse CL-1 gels. (i-iv) Mmp14-silenced EpH4 cells were infected with lentivirus containing (i) control lentivirus (mYpet), and mYpet tagged- (ii) MMP14F-FL, (iii) MMP14F-dCAT and (iv) MMP14F-dCAT/dPEX. Cells were cultured in sparse (1 mg/ml) CL-1 gel. Scale bars: 40 μm.
We have shown previously that mammary epithelial cells use the hemopexin domain of Mmp14 to sort cells to the branching/initiating front (Mori et al., 2009). We also have shown recently that the proteolytic activity of Mmp14 is needed to degrade dense collagen gels to generate a path for branching (Alcaraz et al., 2011). Here, we demonstrate that it is the transmembrane/cytoplasmic domain that is needed for signaling to MAPK via its interaction with Itgb1 (Fig. 6C). Thus, there is division of labor between the different domains of MMP14: depending on the context – the density of the ECM in this case – the domains perform different tasks to complete branching morphogenesis in the mammary gland.
Fig. 6.
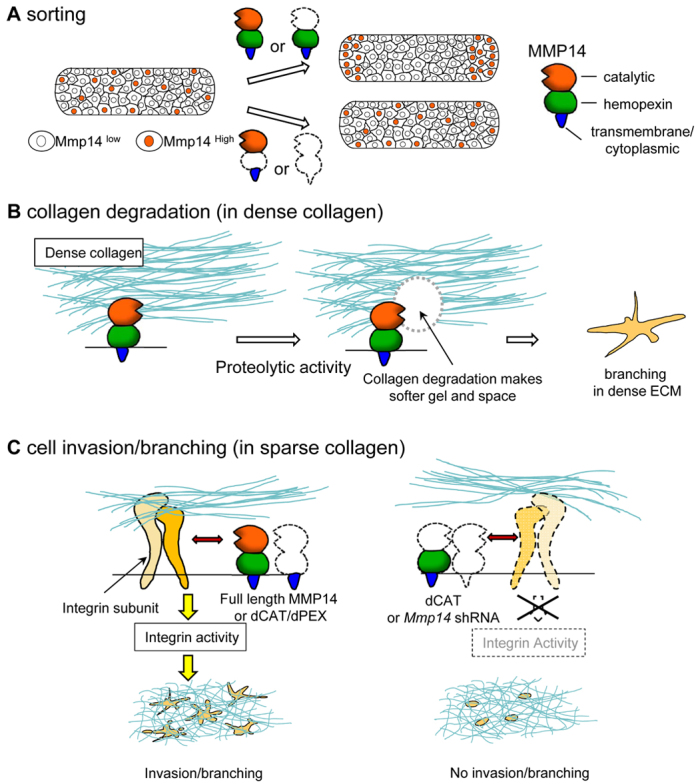
Different steps involving Mmp14 and Itgb1 during MEC invasion/branching in a collagen 1 microenvironment. (A) Non-proteolytic activity of MMP14 is involved in mammary epithelial cell sorting in a CL-1 microenvironment (Mori et al., 2009). The relationship between Mmp14 expression and MEC sorting. Whereas MECs expressing full-length Mmp14 (FL-Mmp14) or the catalytic domain-deleted mutant (dCAT) sort to the invasive front, the hemopexin domain-deleted mutant (dPEX) or MECs with silenced Mmp14 expression do not. (B) Proteolytic activity of Mmp14 is required for MECs to invade/branch in dense CL-1 (Alcaraz et al., 2011). MECs need to degrade collagen 1 to generate a path for invasion/branching in dense collagen. Mmp14 is at the hub of this proteolytic activity for collagen degradation. (C) The association between Mmp14 and Itgb1 during MEC invasion/branching in a sparse CL-1 microenvironment. Whereas MECs do not need MMP activity for invasion/branching in sparse CL-1 gels, Mmp14 itself is required. Specifically, Mmp14 association with Itgb1 is necessary for MEC invasion/branching. Expressing FL-MMP14 or dCAT/dPEX in Mmp14-silenced MECs results in restoration of Itgb1 levels and activity to facilitate branching. Expression of the catalytic domain-deleted mutant (dCAT) was unable to rescue branching and the activation of Itgb1 in sparse CL-1 gels when the catalytic domain is absent. These events (from A to C) suggest that cells use different functions and domains of Mmp14 in a context-dependent manner during branching in collagenous microenvironments.
DISCUSSION
During branching morphogenesis, epithelia form tubular/branching structures through well-controlled processes of cellular invasion and proliferation. MMPs are key metalloproteinases that degrade ECM, and are involved in both mammary branching morphogenesis (Fata et al., 2004; Lu and Werb, 2008; Simian et al., 2001; Sympson et al., 1994) and cancer cell invasion (Brinckerhoff and Matrisian, 2002). MMP14 is a membrane-tethered MMP (Sato et al., 1994) and is characterized as a collagenase (Alcaraz et al., 2011; Ohuchi et al., 1997). It is also a known activator of other MMPs (Knäuper et al., 1996; Sato et al., 1994) and a sheddase for surface molecules (Endo et al., 2003; Kajita et al., 2001). The enzyme plays a role in morphogenesis of ureteric buds (Meyer et al., 2004) where Mmp14KO mice show a substantial decrease in ureteric branching (Riggins et al., 2010); it is also instrumental in lung alveologenesis (Atkinson et al., 2005; Kheradmand et al., 2002). However, in these and other reports on the role of Mmp14 in morphogenesis, the emphasis has been essentially on its proteolytic/catalytic activity, and very little attention has been paid to the non-catalytic domains of this important molecule.
Mmp14 is expressed in normal mammary glands during ductal branching (Szabova et al., 2005; Wiseman et al., 2003). It is highly upregulated in β1,4-galactosyltransferase 1 knockout mice, which display enhanced mammary glands side-branching (Steffgen et al., 2002). Our data presented here also show that Mmp14 is highly expressed in the mammary glands of virgin mice during branching morphogenesis. However, whereas Mmp14 expression was reported mainly in the stroma of mammary glands of FVB mice (Szabova et al., 2005; Wiseman et al., 2003), our analysis using Mmp14 (+/lacZ, heterozygote, C57BL/6) revealed high promoter activity essentially in mammary epithelial cells, especially in the end buds (Fig. 1B). We have confirmed that the expression of Mmp14 tracks with the promoter activity in our study (data not shown). It is possible that the differences in Mmp14 localization in our study and those mentioned above are due to differences in the mouse background. However, in FVB backcrosses, we have also confirmed the expression of Mmp14 in the epithelia. Therefore, we believe that the epithelial Mmp14 plays a direct role in controlling growth and extension of epithelial branching. In fact, the mammary glands from backcrossed Mmp14 knockout (C57BL/6) mice showed reduced ductal elongation, branching intervals and branching points compared with the wild type (supplementary material Fig. S9).
The complex surges in growth factors and hormones during mammary gland development in vivo make it difficult to understand the molecular mechanisms by which Mmp14 contributes to specific stages of branching morphogenesis. We thus chose 3D culture models of collagen-1 (CL-1) gels using both mammary organoids or mammary epithelial cells (MECs) previously used by us and others (Brinkmann et al., 1995; Hirai et al., 1998; Provenzano et al., 2009; Simian et al., 2001). Here, we used different concentrations of native acid-extracted CL-1 to mimic the conditions similar to those found in vivo. We demonstrate that whereas mammary epithelial cells use the proteolytic activity of Mmp14 for invasion/branching in dense (3 mg/ml) CL-1, this activity is dispensable in sparse (1 mg/ml) CL-1 gels. However, intriguingly, we found that the Mmp14 molecule itself is still necessary for mammary epithelial cells to branch, indicating that Mmp14 possesses previously unknown non-proteolytic functions in development. Mammary epithelial ducts are surrounded by basement membrane components such as laminins/type-IV or type I collagen (supplementary material Fig. S1 and data not shown for type IV collagen). The proteolytic functions of Mmp14 may be direct or indirect (e.g. induction of Mmp2) and are undoubtedly used for degrading and remodeling these basement membrane components during branching morphogenesis.
To demonstrate the non-proteolytic function of Mmp14, a physiologically relevant assay was needed. In addition to using branching as an end point, we wanted to know the pathways by which non-catalytic domains would signal for branching. A number of intracellular signaling cascades are essential transducers of microenvironmental stimuli in MECs and are known to mediate morphogenesis. For example, Src–/– mice have defects in mammary ductal elongation (Kim et al., 2005), and in MEK-inhibited mammary epithelial organoids in 3D laminin-rich gels, there are defects in alveologenesis (Fata et al., 2007). We thus considered using MAPK activation as an additional end point in sparse CL-1 gels where proteolytic activity of Mmp14 was not necessary. However, our analysis of the Mmp14 short cytoplasmic domain indicated it did not contain any known kinase domain, suggesting that Mmp14 may have to couple to a partner with such activity. As we assayed invasion and branching in collagen gels, we suspected involvement of a collagen receptor. A selective silencing of different collagen receptors revealed that silencing Itgb1 exhibited the same phenotype as silencing Mmp14. We demonstrate that Mmp14 indeed associates directly with Itgb1 (Fig. 4), indicating a possible link between Mmp14 and Itgb1 in activation of MAPK signaling. Surprisingly, silencing Mmp14 affected the level of Itgb1 in MECs. This finding has a physiological counterpart in vivo: analysis of mammary gland tissue showed that the level of Itgb1 was dramatically lower in Mmp14 knockout mice than in the heterozygotes (Fig. 3Aiv; supplementary material Fig. S5). In a 3D model of human breast cancer cells (Petersen et al., 1992), we have shown previously that ITGB1 and MAPK modulate each other’s levels reciprocally, and are involved in regulating 3D architecture in laminin-rich gels (Wang et al., 1998). Our results here indicate that Mmp14 is involved in the reciprocal association between Itgb1 and the activation of MAP kinase in mammary epithelial cells in CL-1 gels, and that this association is required for branching morphogenesis. Our preliminary experiments with integrin β3 (Itgb3) indicated that this integrin is also involved in the reciprocal association between Mmp14 and Itgb1 in MECs (data not shown), suggesting that the protein complex of Mmp14/Itgb1 might be only a piece of a larger complex of proteins that also includes other integrins and possibly growth factor receptors. We will be addressing the other molecular partners of Mmp14 in future experiments.
Having demonstrated how Mmp14 signals even in the absence of its proteolytic activity during branching morphogenesis, we examined which domain is required for signaling to Itgb1. To address this issue unambiguously, we silenced endogenous Mmp14 in MECs and then re-expressed FLAG-tagged full-length MMP14 or its deletion mutants. Surprisingly, whereas the full-length MMP14 and the extracellular domain-deleted mutant restored the level of Itgb1 and branching, the catalytic domain-deleted mutant did not. These results indicated that the transmembrane/cytoplasmic (TM/CP) domain of MMP14 has a key function in signaling, but that the hemopexin domain has an inhibitory effect, but only when its catalytic domain is deleted. Previous studies had demonstrated a naturally derived cleaved form of catalytic domain of MMP14 can act as a dominant-negative regulator of wild-type MMP14 proteolytic activity (Itoh et al., 2001; Lehti et al., 2002). Here, we demonstrate that when the catalytic domain is absent, the hemopexin domain has an inhibitory effect on both branching and activation of Itgb1 in sparse CL-1 gels. The catalytic domain-deleted mutant may have differences in protein structure or binding partners that inhibit the association with Itgb1 and branching. Combining the present findings with the previous reports suggests that cells use the different domains of MMP14 not only for controlling the proteolytic actions of MMP14, but also for regulating integrin function, and signaling in a collagenous microenvironment.
We demonstrated previously that the interaction between Mmp14 hemopexin domain and CD44 determined the motility of mammary epithelial cells resulting in the sorting of cells to the branching initiation points (Mori et al., 2009). We have also demonstrated that the proteolytic activity of Mmp14 is required for branching in dense ECM (Alcaraz et al., 2011). These reports suggest that mammary epithelial tissue recruits the Mmp14-expressing cells to the tip of the branching bud to degrade the local collagen in order to clear a path for penetration. We show here that once this is accomplished, the catalytic activity is dispensable. However, the Mmp14 molecule is still required for branching, and the TM/CP domain of Mmp14 is the minimum required domain for controlling both the level of Itgb1 and branching in a sparse collagen microenvironment. Whereas during cell sorting the hemopexin domain is used for associating with CD44 (Mori et al., 2009), this domain can exert an inhibitory activity on Itgb1 levels leading the decreased signaling and branching in endogenously silenced Mmp14 mammary epithelial cells, as shown in this study (see scheme in Fig. 6C).
In conclusion, we posit that the Mmp14-dependent Itgb1 regulation, in combination with increased expression of Mmp14 observed at the tips of invading mammary end buds, may constitute a signaling module and dynamics relevant to the invasive fronts of branching tissues. We show that, under specific conditions, non-catalytic domains of Mmp14 play crucial roles in the ability of mammary epithelial cells to invade into stroma during development. Because these same developmental programs are subverted in malignant cells during tumor progression, our results may shed light on why MMP inhibitors that only targeted the catalytic domains failed so dramatically in clinical trials (Overall and Kleifeld, 2006a; Overall and Kleifeld, 2006b). We also suggest that domains of MMP14 other than its catalytic domain could be targets for controlling cellular invasion in cancer.
Acknowledgments
We thank Ren Xu, Aaron T. Boudreau and Ramray Bhat for insightful discussions.
Footnotes
Funding
The work in M.J.B.’s laboratory is supported by grants from the US Department of Energy, Office of Biological and Environmental Research and Low Dose Scientific Focus Area [DE-AC02-05CH1123]; by National Cancer Institute [R37CA064786, R01CA057621, R01CA140663, U54CA112970, U01CA143233 and U54CA143836 – Bay Area Physical Sciences – Oncology Center, University of California, Berkeley, CA, USA]; by the US Department of Defense [W81XWH0810736]; by the Susan G. Komen Breast Cancer Foundation [02-1591 to H.M.]; and by a Glenn T. Seaborg Postdoctoral Fellowship from Lawrence Berkeley National Laboratory (to C.M.G.). M.J.B. is the recipient of an Innovator Award from the Department of Defense Breast Cancer Research Program. We dedicate this paper to Charles Daniel, whose pioneering studies of mammary gland and its end bud have inspired us all. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.084236/-/DC1
References
- Alcaraz J., Buscemi L., Grabulosa M., Trepat X., Fabry B., Farré R., Navajas D. (2003). Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 84, 2071–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J., Mori H., Ghajar C. M., Brownfield D., Galgoczy R., Bissell M. J. (2011). Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr. Biol. (Camb.) 3, 1153–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. J., Holmbeck K., Yamada S., Birkedal-Hansen H., Parks W. C., Senior R. M. (2005). Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Dev. Dyn. 232, 1079–1090 [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Matrisian L. M. (2002). Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 3, 207–214 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Foroutan H., Sachs M., Weidner K. M., Birchmeier W. (1995). Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J. Cell Biol. 131, 1573–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun T. H., Hotary K. B., Sabeh F., Saltiel A. R., Allen E. D., Weiss S. J. (2006). A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125, 577–591 [DOI] [PubMed] [Google Scholar]
- Chuong C.-M. (1998). Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: R.G. Landes; [Google Scholar]
- Endo K., Takino T., Miyamori H., Kinsen H., Yoshizaki T., Furukawa M., Sato H. (2003). Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 278, 40764–40770 [DOI] [PubMed] [Google Scholar]
- Fata J. E., Werb Z., Bissell M. J. (2004). Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 6, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J. E., Mori H., Ewald A. J., Zhang H., Yao E., Werb Z., Bissell M. J. (2007). The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 306, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster T. (2012). Energy migration and fluorescence. 1946. J. Biomed. Opt. 17, 011002 [DOI] [PubMed] [Google Scholar]
- Hirai Y., Lochter A., Galosy S., Koshida S., Niwa S., Bissell M. J. (1998). Epimorphin functions as a key morphoregulator for mammary epithelial cells. J. Cell Biol. 140, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Kajita M., Kinoh H., Mori H., Okada A., Seiki M. (1999). Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J. Biol. Chem. 274, 34260–34266 [DOI] [PubMed] [Google Scholar]
- Itoh Y., Takamura A., Ito N., Maru Y., Sato H., Suenaga N., Aoki T., Seiki M. (2001). Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 20, 4782–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda E., Litos G., Grünert S., Downward J., Beug H. (2002). Oncogenic Ras/Her-2 mediate hyperproliferation of polarized epithelial cells in 3D cultures and rapid tumor growth via the PI3K pathway. Oncogene 21, 5148–5159 [DOI] [PubMed] [Google Scholar]
- Kajita M., Itoh Y., Chiba T., Mori H., Okada A., Kinoh H., Seiki M. (2001). Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 153, 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand F., Rishi K., Werb Z. (2002). Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J. Cell Sci. 115, 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Laing M., Muller W. (2005). c-Src-null mice exhibit defects in normal mammary gland development and ERalpha signaling. Oncogene 24, 5629–5636 [DOI] [PubMed] [Google Scholar]
- Knäuper V., Will H., López-Otin C., Smith B., Atkinson S. J., Stanton H., Hembry R. M., Murphy G. (1996). Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J. Biol. Chem. 271, 17124–17131 [DOI] [PubMed] [Google Scholar]
- Lehti K., Lohi J., Juntunen M. M., Pei D., Keski-Oja J. (2002). Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J. Biol. Chem. 277, 8440–8448 [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Werb Z. (2008). Patterning mechanisms of branched organs. Science 322, 1506–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. N., Schwesinger C., Bush K. T., Stuart R. O., Rose D. W., Shah M. M., Vaughn D. A., Steer D. L., Nigam S. K. (2004). Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev. Biol. 275, 44–67 [DOI] [PubMed] [Google Scholar]
- Mori H., Tomari T., Koshikawa N., Kajita M., Itoh Y., Sato H., Tojo H., Yana I., Seiki M. (2002). CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 21, 3949–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Gjorevski N., Inman J. L., Bissell M. J., Nelson C. M. (2009). Self-organization of engineered epithelial tubules by differential cellular motility. Proc. Natl. Acad. Sci. USA 106, 14890–14895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Borowsky A. D., Bhat R., Ghajar C. M., Seiki M., Bissell M. J. (2012). Laser scanning-based tissue autofluorescence/fluorescence imaging (LS-TAFI), a new technique for analysis of microanatomy in whole-mount tissues. Am. J. Pathol. 180, 2249–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A. W., Daugherty P. S. (2005). Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat. Biotechnol. 23, 355–360 [DOI] [PubMed] [Google Scholar]
- Ohuchi E., Imai K., Fujii Y., Sato H., Seiki M., Okada Y. (1997). Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 272, 2446–2451 [DOI] [PubMed] [Google Scholar]
- Overall C. M., Kleifeld O. (2006a). Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br. J. Cancer 94, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall C. M., Kleifeld O. (2006b). Tumour microenvironment – opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 6, 227–239 [DOI] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 [DOI] [PubMed] [Google Scholar]
- Petersen O. W., Rønnov-Jessen L., Howlett A. R., Bissell M. J. (1992). Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 89, 9064–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Keely P. J. (2009). Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28, 4326–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann E., Ball R., Groner B., Friis R. R. (1989). New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J. Cell Biol. 108, 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins K. S., Mernaugh G., Su Y., Quaranta V., Koshikawa N., Seiki M., Pozzi A., Zent R. (2010). MT1-MMP-mediated basement membrane remodeling modulates renal development. Exp. Cell Res. 316, 2993–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. (1994). A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370, 61–65 [DOI] [PubMed] [Google Scholar]
- Shaner N. C., Steinbach P. A., Tsien R. Y. (2005). A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 [DOI] [PubMed] [Google Scholar]
- Simian M., Hirai Y., Navre M., Werb Z., Lochter A., Bissell M. J. (2001). The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 128, 3117–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffgen K., Dufraux K., Hathaway H. (2002). Enhanced branching morphogenesis in mammary glands of mice lacking cell surface beta1,4-galactosyltransferase. Dev. Biol. 244, 114–133 [DOI] [PubMed] [Google Scholar]
- Sympson C. J., Talhouk R. S., Alexander C. M., Chin J. R., Clift S. M., Bissell M. J., Werb Z. (1994). Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 125, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabova L., Yamada S. S., Birkedal-Hansen H., Holmbeck K. (2005). Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J. Cell. Physiol. 205, 123–132 [DOI] [PubMed] [Google Scholar]
- Taddei I., Deugnier M. A., Faraldo M. M., Petit V., Bouvard D., Medina D., Fässler R., Thiery J. P., Glukhova M. A. (2008). Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R. S., Chin J. R., Unemori E. N., Werb Z., Bissell M. J. (1991). Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development 112, 439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Weaver V. M., Petersen O. W., Larabell C. A., Dedhar S., Briand P., Lupu R., Bissell M. J. (1998). Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 95, 14821–14826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Daniel C. W. (1983). Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 97, 274–290 [DOI] [PubMed] [Google Scholar]
- Wiseman B. S., Sternlicht M. D., Lund L. R., Alexander C. M., Mott J., Bissell M. J., Soloway P., Itohara S., Werb Z. (2003). Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 162, 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Cukierman E. (2007). Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 [DOI] [PubMed] [Google Scholar]
- Yana I., Sagara H., Takaki S., Takatsu K., Nakamura K., Nakao K., Katsuki M., Taniguchi S., Aoki T., Sato H., et al. (2007). Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci. 120, 1607–1614 [DOI] [PubMed] [Google Scholar]