Abstract
Pharmacotherapy is effective in decreasing the incidence of osteoporotic fracture, morbidity, and mortality. This benefit is pronounced in patients at highest risk for fracture: those with prior osteoporotic fracture, very low bone mineral density, or receiving chronic corticosteroid treatment. We review the best pharmacotherapeutic options currently available for treating osteoporosis.
Introduction
Osteoporosis afflicts 10 million Americans, and another 30 million Americans have low bone mass that puts them at risk for development of osteoporosis1. The overall risk for a 50-year-old Caucasian or Asian woman to sustain an osteoporotic fracture during her lifetime is 50%. While men, African Americans, and Hispanics have lower risks, the health impact is still substantial. One-third of all hip fractures occur in men, and with our aging populace hip fracture incidence in men will eventually approach that of women1. A femoral neck or trochanteric fracture is associated with mortality of approximately 20%1. Of survivors, only one in three return to pre-fracture mobility1. Vertebral fractures deform the posture either with or without pain, and reduce musculoskeletal vitality. Ten year mortality for a woman with an incident vertebral fracture is increased two-fold, indicating that vertebral fracture is a harbinger of frailty1.
Fortunately, effective pharmacotherapies for treatment of osteoporosis in both women and men have been developed.2 Aminobisphosphonates were the first drugs unambiguously established to reduce hip and vertebral fracture, followed shortly thereafter by estrogen3 and denosumab4. Raloxifene – a selective estrogen receptor modulator (SERM)– and teriparatide – an anabolic PTH fragment – both decrease vertebral fracture and non-vertebral fracture without clearly impacting hip fracture3. Here we review the diagnosis of osteoporosis and how to match the needs of the osteoporotic patient with appropriate pharmacotherapy. Osteoporosis in children and hormone replacement will not be discussed, but the reader is referred to suitable reviews3,5.
Definitions and Diagnoses
Osteoporosis diagnostics can be complicated. The World Health Organization (WHO) defined osteoporosis as a systemic skeletal disease characterized by low bone mass with microarchitectural deterioration of bone tissue, thus increasing bone fragility and susceptibility to fracture1. For screening purposes, osteoporosis was defined by the WHO as a bone mineral density (BMD) at any site equal to or greater that 2.5 standard deviations below the fracture resistant mean peak bone mass of young adulthood.
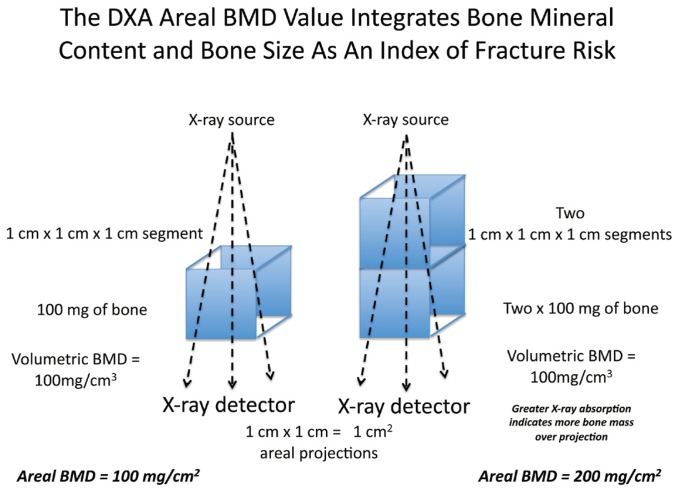
Denoted a “T score” rather than a “Z score” because the referent population for those at risk (older individuals) is a younger cohort, the utility of the T-score ≤ −2.5 has revolutionized our approach to fracture prevention. Screening by dual electron X-ray absorptiometry (DXA) identifies those without fracture who are at greatest risk for future fracture6. DXA evaluation is an “areal” BMD expressed as grams of bone mass/cm2, achieved by quantitative projection of bone content in three dimensions onto the two-dimensional plane of the X-ray detector (See Figure 1).
Figure 1.
Because larger bones have more total bone mass in all directions – including the height of bone perpendicular to the image projection plane of DXA analysis – areal BMD is greater for larger bones. Moreover, because larger bones are harder to break, the DXA value integrates bone mineral content and bone size into a single number that predicts fracture risk. A 10% reduction in BMD, or a −1 change in T score, doubles the risk for fracture7. DXA screening is recommended in women ≥ 65, men ≥ 70, and middle-aged adults at increased clinical risk for osteoporosis (prior fracture as an adult; family history of osteoporosis; chronic tobacco and/or corticosteroid use; low body weight) (http://www.nof.org/professionals/clinical-guidelines). Younger women with early menopause are also at risk. Benefits of DXA monitoring following initiation of therapy are more equivocal, but repeat DXA evaluation two years after the first screen provides sufficient time to detect a significant change (~ 3%).
Osteoporosis was a clinical diagnosis made well before DXA analysis was available. Low-energy fragility fractures, loss of height, and vertebral deformity with or without skeletal radiolucency on X-ray were all implemented as diagnostic criteria. Because of the greater prevalence of DXA-designated osteopenia vs. osteoporosis, the majority of fractures occur in individuals with osteopenia because of greater numbers of those at risk. Thus, therapeutic decisions must incorporate other clinical features that convey fracture risk in addition to the DXA value6. For example, the most significant risk factor for future fracture is a personal history of prior fracture as an adult. Prior fracture may be asymptomatic; a 15% deformation in anterior vertebral height on lateral x-ray or > 2 inch loss in maximum adult height represent fracture equivalents6. Even those individuals suffering fracture in motor vehicle accidents (MVAs) are also at increased risk for future low-impact osteoporotic fracture at any given bone mineral density8. Why might this occur? DXA provides information relevant to bone strength but not bone quality – the composite material properties that convey biomechanical toughness. Prior fracture in response to mechanical challenge brings “out of the woodwork” those individuals whose integrated bone size, bone mineral content, and bone quality convey fragility.
Recently, the WHO developed an algorithm for considering such clinical factors to calculate the 10-year risk for fracture6. The WHO Fracture Risk Assessment Tool, or FRAX, is available online at http://www.sheffield.ac.uk/FRAX/ and as an iPhone App. Age, gender, ethnicity, geographic local, height, weight, personal and parental fracture history, presence of rheumatoid arthritis, corticosteroid and tobacco use, alcohol consumption, and femoral neck areal BMD are integrated to estimate fracture risk. The National Osteoporosis Foundation recommends treatment in patients 50 or older with (a) a history of prior osteoporotic or vertebral fracture; (b) a DXA T score < −2.5 or lower at the femoral neck or spine; or (c) 10 year risk for hip fracture is > 3% or major osteoporotic fracture is >20% by FRAX in those with a T score between −1.0 and −2.59 (http://www.nof.org/professionals/clinical-guidelines).
Potential secondary causes of osteoporosis must be addressed along with nutrition (See Table 1). The 25-hydroxyvitamin D (25-OHD) level should be >30 ng/ml; if not, ergocalciferol 100,000 IU weekly for five to six weeks followed by repeat 25-OHD evaluation is required10. Thereafter, a total of 1000 IU vitamin D3 and 1200 mg elemental calcium daily are required in most adults11.
Table 1.
Important Contributors to Osteoporotic Fracture Risk
| Vitamin D deficiency or insufficiency | Renal calcium “leak”/idiopathic hypercalciuria |
| Hypercortisolism/Cushing’s syndrome and glucocorticoid therapy | Chronic gastrointestinal disease (celiac disease, Crohn’s disease, hepatobiliary disease, short gut syndrome, lactose intolerance) |
| Multiple myeloma or MGUS | Hyperthyroidism |
| Alcoholism, tobacco abuse | Hyperparathyroidism |
| Hypogonadism | Chronic immobilization, neuromuscular disease, stroke |
| Rheumatoid Arthritis | Type I > Type II diabetes |
| Chronic renal insufficiency | Chronic antidepressant, proton pump inhibitor, thiazolidinedione use |
| Hemochromatosis, metal intoxication/chronic low – level cadmium exposure | Hereditary collagen disorders |
Aminobisphosphonates
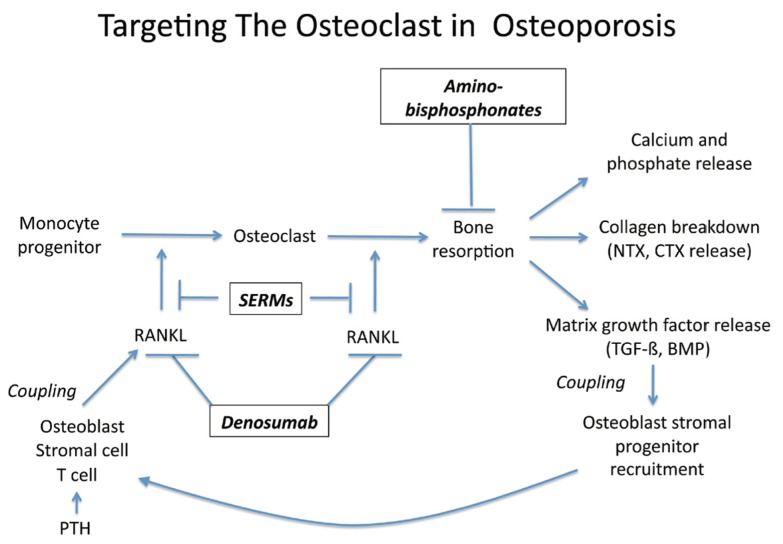
Alendronate, risedronate, ibandronate, and zoledronic acid12 reduce fracture risk by 50% within the first year of therapy in both genders. These aminobisphosphonates are pyrophosphate analogs that inhibit farnesyl diphosphate synthase, an enzyme that regulates G-proteins controlling osteoclast function12. Thus, aminobisphosphonates inhibit osteoclast-mediated bone resorption in vivo (See Figure 2). Aminobisphosphonates are orally poorly bioavailable, undergo renal clearance without metabolism, and deposited in bone3. The depot-like actions at this site allow IV formulations for zoledronic acid and ibandronate to be dosed every 12 months and 3 months, respectively12. Oral treatment with aminobisphosphonates requires the medicine to be taken on an empty stomach with an 8-ounce glass of water, avoiding recumbency and other oral intake for 30 minutes. Once weekly or once monthly oral regimens has improved adherence, but gastrointestinal upset may still limit use. IV ibandronate and zoledronic acid have improved the therapeutic landscape (See Table 2).
Figure 2.
Table 2.
Typical Pharmacotherapeutic Dosing Regimens For Treatment of Osteoporosis
| Alendronate | 10 mg PO q Day, 70 mg PO q Week |
| Risedronate | 5 mg PO q Day, 35 mg PO q Week, 150 mg PO q Month |
| Ibandronate | 150 mg PO q Month, 3 mg IV q 3 Months (15–30 second infusion) |
| Zoledronic acid | 5 mg IV q Year (15 minute infusion) (5 mg IV q 2 Years for osteoporosis prevention) |
| Raloxifene | 60 mg PO q Day |
| Denosumab | 60 mg SC q 6 Months |
| Teriparatide | 20 micrograms SC q Day, maximum of 24 months |
Contraindications include drug class hypersensitivity, hypocalcemia, renal insufficiency (GFR < 30 mL/min/1.73 m2), and the inability to stand or sit for 30 minutes (oral regimens). Potent IV aminobisphosphonates such as zoledronic acid are contraindicated in pregnancy, while oral compounds are classified as pregnancy category C. While children with osteogenesis imperfecta or secondary osteoporosis have been treated with bisphosphonates, this should only be implemented by a pediatric bone health specialist5. In children and pregnant women with osteoporosis, an expert in bone health should be consulted.
Complications may include gastrointestinal distress in about 10% of patients, and the rare but serious erosive esophagitis related to pill “reflux” is minimized by the above dosing recommendations3. Conflicting data address a questionable increase in risk for esophageal cancer13. This uncertain potential outcome is far outweighed by the benefits of osteoporosis pharmacotherapy, which decreases mortality and morbidity2. Oral aminobisphosphonates should not be used in patients with Barrett’s esophagus or gastroesophageal disease13. With IV bisphosphonates, a “flu-like” acute phase response may occur in 10% of patients, and pretreatment with 650 mg PO acetaminophen is helpful 12. Renal failure is a reportable complication of IV aminobisphosphonates14, and since the drugs cleared by the kidney, serum creatinine must be checked prior to each yearly dose of zoledronic acid or when pamidronate is given. For unclear reasons, this rare association is not seen with ibandronate. Once a GFR ≥30 ml/min as estimated from the initial serum creatinine has been established, repeated serum creatinine values are not currently required when dosing with IV ibandronate14.
A more concerning complication is osteonecrosis of the jaw15, which may be observed in 1/10,000 to 1/100,000 patients treated, related to concomitant oral bone trauma and infection. Initiation of aminobisphosphonate therapy should be postponed until elective oral surgery is performed, and oral surgery should be avoided when on these drugs15. Cumulative IV aminobisphosphonate administration conveys increased risk in the setting of malignancy, particularly multiple myeloma. A recent position statement provides details15.
Recently, an uncommon subtrochanteric fracture type has been potentially linked with aminobisphosphonate therapy, denoted “atypical femoral fracture.” While subtrochanteric fractures represent approximately 7% of all hip fractures, few exhibit the signs of brittle bone failure16. This atypical clinical scenario describes a non-traumatic, non-comminuted fracture of the subtrochanteric or diaphyseal femur. A history of femur or groin pain may precede an atypical fracture for months, with a periosteal reaction resembling a “stress fracture” or “dreaded black line” radiolucency on femoral radiographs. The explanation may relate to accumulation of “microtrauma” in at-risk individuals with abnormal bone quality. Many with atypical fracture will have other health issues, including diabetes or chronic glucocorticoid treatment.
Of note, ~6% of atypical femur fractures are observed in patients never treated with aminobisphosphonates. In addition, the incidence of femoral neck, trochanteric, and intertrochanteric hip fractures has declined following approval of aminobisphosphonate therapy, while that of subtrochanteric and femoral shaft fractures has not changed16. Thus, the pathobiology of the atypical femur fracture may be distinct from that of typical femur fractures -- and may not respond to aminobisphosphonate therapy in those at high risk for any type of femur fracture. If truly a complication of therapy, perhaps 1 atypical fracture arises for each 100 “routine” femur fractures prevented by aminobisphosphonate therapy16. While causality has not been established, increased awareness of this rare potential complication is appropriate, and FDA implemented a labeling change for these drugs in October 2010. In patients who lack severe osteoporosis or do not have severely reduced BMD (T ≤ −3.5), a drug holiday may be considered after five years of therapy. In previously compliant patients, a drug holiday of < 2 years appears acceptable and is not associated with significantly increased fracture risk16. In those at significantly increased risk for future fracture (prior osteoporotic fracture, T score < ≤ −3.5), benefits of continued therapy clearly outweigh risks. In patients on aminobisphosphonates with thigh or groin pain, plain radiography, MRI, or scintigraphy should be performed16. If periosteal reaction or “stress fracture” is apparent, therapy should be stopped and orthopedic nailing offered16. If an atypical fracture occurs, therapy should be stopped; if fracture healing after orthopedic stabilization is not evident in six to eight weeks, teriparatide (below) therapy should be considered17.
Selective Estrogen Receptor Modulators
Selective Estrogen Receptor Modulators (SERM) are ligands for estrogen receptors that induce these receptors to adopt unique conformations that differentially recruit transcriptional modulators. SERMs can exert agonist activities in one target tissue, but antagonist activities in another18. Currently, raloxifene is the only SERM approved for treatment of osteoporosis3. Raloxifene inhibits the formation and activation of the bone-resorbing osteoclast by mimicking estrogen’s suppression of RANKL signaling within the osteoclast. By contrast, raloxifene inhibits estrogen’s actions in the breast, and significantly reduces the risk for breast cancer. It does not increase risk for uterine malignancy. Unlike estrogen/progestin combinations, raloxifene does not increase the risk of cardiovascular disease19. Like estrogen, raloxifene does increase risk for venous thromboembolism, including deep vein thrombosis and fatal stroke. Hot flashes and leg cramps can occur. Contraindications include a history of thromboembolism or pregnancy, and raloxifene should not be used in children or in men outside of a clinical trial. Like aminobisphosphonates, raloxifene decreases the risk for vertebral and non-vertebral fractures. For unclear reasons raloxifene has not been demonstrated to decrease the risk for hip fracture3. Nevertheless, in post-menopausal women with osteoporosis, particularly those with increased breast cancer risk, raloxifene represents an important therapeutic option for osteoporosis treatment19.
Denosumab
The TNF superfamily member RANKL is absolutely essential for the development of osteoclasts from the monocyte lineage, and maintains osteoclast viability and bone-resorbing functions (See Figure 2). Denosumab is a humanized monoclonal antibody that binds to and inhibits RANKL function and is approved for treatment of osteoporosis. It reduces bone resorption, increases BMD, and reduces fracture risk – mimicking the role of an endogenous RANKL inhibitor called osteoprotegerin20. Denosumab can be dosed every six months, and is efficacious in men and women (See Table 2). Adjustment for renal insufficiency is not required. However, it should not be used indiscriminately in the setting of reduced renal function21. Because bone formation and bone resorption are generally very tightly coupled via osteoclast-mediated matrix turnover (See Figure 3), reductions in osteoclast formation with denosumab simultaneously decrease osteoblast-mediated bone formation. This effect was so significant that in 36% of patients, tetracycline labeling of bone surface, an index for bone formation, was completely absent21. Thus, the FDA indication for use of denosumab in osteoporosis is limited to those with severe disease and those that have failed other therapies. Back pain (30%), skin rashes (10%), lower extremity skin infections (0.4%) and pancreatitis (0.2%) may occur with denosumab therapy4.
Figure 3.
As for any of the potent antiresorptive therapies, contraindications include hypocalcemia and drug hypersensitivity. Osteonecrosis of the jaw has been reported15. Safety has not been established for treatment of children, and the drug is currently listed as pregnancy category C. Because breast development requires RANKL signaling in utero, denosumab should not be administered to pregnant individuals without input from a bone expert.
Teriparatide
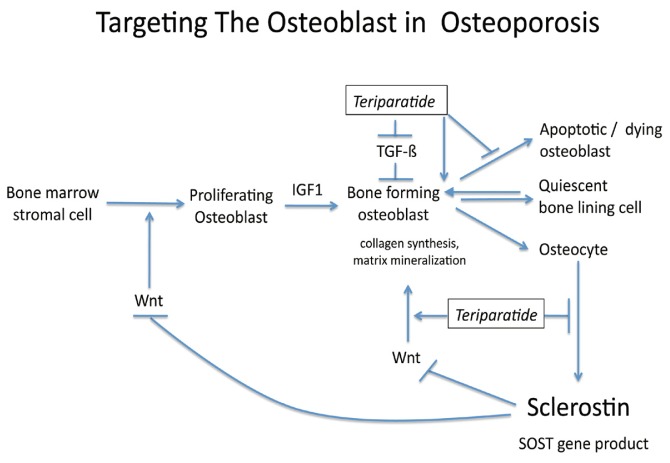
The only currently available anabolic agent in America for treating osteoporosis is teriparatide22, a synthetic peptide that mimics the action of PTH to maintain osteoblast longevity and bone synthetic function23. The mechanisms of teriparatide anabolism involve polypeptide hormones known as Wnts that promote bone formation by signaling through receptors known as LRP5 and LRP6. Osteocytes, the mature form of osteoblasts, control osteoblast formation by producing sclerostin, an inhibitor of Wnt-LRP5/6 signaling. Teriparatide downregulates sclerostin, thus interrupting the negative feedback mechanism whereby osteocytes control the formation of less mature but more synthetically active osteoblasts23. These same actions likely contribute to recruitment/reactivation of previously quiescent bone lining cells to adopt the synthetic osteoblast fate. Furthermore, teriparatide inhibits osteoblast apoptosis, thus prolonging the synthetic life-span of this bone forming cell (See Figure 3)23. Novel neutralizing antibodies are being developed that target/inhibit sclerostin activity as anabolic agents23.
Teriparatide is administered as a 20 μg daily subcutaneous injection (See Table 2). Improvements as great as 15% have been observed in the vertebral column in response to teriparatide within a six-month period. Side effects are few, and include transient flushing, mild nausea, and transient hypercalcemia. The drug best administered at night just prior to bedtime, which minimizes perception of nausea and orthostasis. Teriparatide carries a “black box” label because lifetime dosing of rats from birth to death over a two-year period increases the risk for osteosarcoma22. This has not been observed in adult humans, and is thought to arise in part due to the continuously growing skeleton of the rat (epiphyses never close). Thus, children or young adults with open epiphyses indicative of skeletal growth should not be treated with teriparatide. Moreover, adult patients at risk for osteosarcoma – those with history of radiation therapy, Paget’s disease, a prior history of osteosarcoma, or an unexplained elevated serum alkaline phosphatase -- should not be treated with teriparatide. Therapy should be limited to a total of 24 months of exposure. Terparatide use in pregnancy has not been well-studied, and this agent is pregnancy category C. Renal impairment (GFR < 30 cc/min) prolongs half-life by 75%, and may require dosing interval adjustment if hypercalcemia is noted > 12 hours after dosing once at steady state.
Glucocorticoid-induced osteoporosis (GIO) deserves special but brief consideration. The skeleton is exquisitely sensitive to the negative impact of glucocorticoids. Chronic (> 3 month) daily doses as low as 2.5 – 5.0 mg prednisolone equivalents double the risk for fracture24. Because glucocorticoids inhibit collagen synthesis in the bone remodeling unit – a feature exacerbated by aminobisphosphonates -- teriparatide outperforms aminobisphosphonates in restoration of vertebral bone mass and vertebral fracture prevention25. With severe GIO, teriparatide is preferred as the initial therapy without contraindication. However, aminobisphosphonates clearly reduce fracture risk in GIO. An aminobisphosphonate should be implemented immediately after cessation of teriparatide for GIO treatment, or implemented very early on when it is known that long-term (> 3 month) glucocorticoid therapy will be needed24.
Conclusions
Tremendous innovations in the treatment of osteoporosis have been achieved over the past two decades. However, with the advent of new therapies, new questions arise. While the benefits of therapy are clear – and far outweigh the risks for therapy for the vast majority of patients -- the accrual of rare, individual and time-dependent side effects are just now becoming evident. Although sequential anti-resorptive/anabolic pharmacotherapeutic cycles make biological sense, the role of any combination therapy in fracture reduction has not been established. Furthermore, treatment of osteoporosis in children and in the settings of malignancy, pregnancy, and chronic renal insufficiency present real concerns and vexing problems. Thankfully, novel therapies are on the horizon. The next decade holds even greater promise for delivering better care to our patients with osteoporosis.
Biography
Amy E. Riek, MD, is a Clinical Fellow and Dwight A. Towler MD, PhD, is Professor of Medicine, in the Division of Endocrinology, Metabolism, and Lipid Research at Washington University in St. Louis.
Contact: ariek@dom.wustl.edu


Footnotes
Disclosure and Acknowledgments
Dr. Towler is supported by NIH grants HL069229, HL088138, and HL088651, and the Barnes-Jewish Hospital Foundation. Dr. Reik is supported by NIH/NCRR Washington University-ICTS Grant Number UL1 RR024992. Contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. Dr. Towler is supported by the National Institutes of Health and Barnes-Jewish Hospital. He is also a consultant for Pfizer.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95(3):1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 3.Drugs for postmenopausal osteoporosis. Treat Guidel Med Lett. 2008;6(74):67–74. quiz 75–66. [PubMed] [Google Scholar]
- 4.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 5.Bachrach LK, Ward LM. Clinical review 1: Bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab. 2009;94(2):400–409. doi: 10.1210/jc.2008-1531. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. How to decide who to treat. Best Pract Res Clin Rheumatol. 2009;23(6):711–726. doi: 10.1016/j.berh.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli R, Slosman D, Bonjour JP. The role of dual energy X-ray absorptiometry of lumbar spine and proximal femur in the diagnosis and follow-up of osteoporosis. Am J Med. 1995;98(2A):33S–36S. doi: 10.1016/s0002-9343(05)80043-9. [DOI] [PubMed] [Google Scholar]
- 8.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298(20):2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, McCloskey EV, Johansson H, Oden A. Approaches to the targeting of treatment for osteoporosis. Nat Rev Rheumatol. 2009;5(8):425–431. doi: 10.1038/nrrheum.2009.139. [DOI] [PubMed] [Google Scholar]
- 10.Adams JS, Kantorovich V, Wu C, Javanbakht M, Hollis BW. Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab. 1999;84(8):2729–2730. doi: 10.1210/jcem.84.8.5899. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2008;624:55–71. doi: 10.1007/978-0-387-77574-6_5. [DOI] [PubMed] [Google Scholar]
- 12.A once-yearly IV bisphosphonate for osteoporosis. Med Lett Drugs Ther. 2007;49(1273):89–90. [PubMed] [Google Scholar]
- 13.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(1):89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 14.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74(11):1385–1393. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 15.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 16.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MC, Weinstein RS, Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010 doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 17.Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA. 2010;304(13):1480–1484. doi: 10.1001/jama.2010.1360. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010 doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 20.Rizzoli R, Yasothan U, Kirkpatrick P. Denosumab. Nat Rev Drug Discov. 2010;9(8):591–592. doi: 10.1038/nrd3244. [DOI] [PubMed] [Google Scholar]
- 21.Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, Maasalu K, Bolognese MA, Woodson G, Bone H, Ding B, Wagman RB, San Martin J, Ominsky MS, Dempster DW. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25(10):2256–2265. doi: 10.1002/jbmr.149. [DOI] [PubMed] [Google Scholar]
- 22.Teriparatide (forteo) for osteoporsis. Med Lett Drugs Ther. 2003;45(1149):9–10. [PubMed] [Google Scholar]
- 23.Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010;21(4):237–244. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 25.Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60(11):3346–3355. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]