Abstract
Immunoglobulin E (IgE) antibodies and mast cells have been so convincingly linked to the pathophysiology of anaphylaxis and other acute allergic reactions that it can be difficult to think of them in other contexts. However, a large body of evidence now suggests that both IgE and mast cells are also key drivers of the long-term pathophysiological changes and tissue remodeling that are associated with chronic allergic inflammation in asthma and other settings. Such potential roles include IgE-dependent regulation of mast-cell functions, actions of IgE that are largely independent of mast cells and roles of mast cells that do not directly involve IgE. In this review, we discuss findings supporting the conclusion that IgE and mast cells can have both interdependent and independent roles in the complex immune responses that manifest clinically as asthma and other allergic disorders.
People with allergic disorders such as atopic dermatitis (eczema), allergic rhinitis (hay fever), food allergy and allergic (or atopic) asthma can experience acute signs and symptoms of disease within minutes of exposure to the associated allergens. However, such individuals also typically develop long-term changes in the affected tissues, often called tissue remodeling, after repeated exposure to these allergens over periods of weeks to years. There is consensus that antigen-specific IgE antibodies, together with one of the major effector cells of allergy, the mast cell (Box 1), can be crucial for the development of the acute manifestations of these allergic disorders. But there is less agreement about the role of IgE and mast cells in the chronic, long-term tissue changes that account for much of the morbidity of these increasingly prevalent diseases.
Box 1 The basics of IgE antibodies and mast cells in allergy.
Antigen-dependent activation of tissue mast cells that have specific IgE bound to their surface is the central event in acute allergic reactions. IgE, the immunoglobulin isotype with by far the lowest concentration in the circulation, is unable to fix complement and has little ability to cross the placenta. Its plasma concentrations can be markedly elevated in some individuals with allergic diseases or parasite infections1. IgE is thought to mediate biological functions primarily by binding to FcεRI, CD23 and other receptors that are expressed on mast cells and other hematopoietic cells1,2. The binding of antigen-specific IgE to FcεRI sensitizes mast cells and other effector cells to release mediators in response to subsequent encounters with that specific antigen or with crossreactive antigens1–3. Binding of antigen-IgE immune complexes to CD23 or FcεRI can serve to amplify IgE-associated immune responses by facilitating antigen presentation through CD23 on B cells or by ‘antigen focusing’ through FcεRI on dendritic cells or other antigen-presenting cells, leading to the production of IgE to additional epitopes of the antigens that are contained in such immune complexes1,2.
However, it is thought that the most crucial function of IgE in allergic diseases is its ability to sensitize mast cells to release biologically active mediators in an antigen-specific manner. Mast cells are distributed throughout virtually all vascularized tissues in vertebrates, with relatively high numbers occurring near body surfaces, including the airway epithelium63,97 Along with dendritic cells, mast cells are one of the first immune cells to interact with allergens and other environmentally derived substances. Unlike granulocytes, mature mast cells do not ordinarily circulate in the blood; instead, hematopoietic stem cell–derived circulating mast cell precursors migrate to the peripheral tissues, where they complete their differentiation and maturation and take up residence79. Mast cells are potentially long-lived cells, and their number, distribution, phenotype and function can be regulated by many factors whose local concentrations can change at the sites of innate or adaptive immune responses78. In response to activation by IgE through FcεRI and specific antigens or by many other endogenous or exogenous substances, mast cells can produce diverse mediators that can promote or downregulate inflammation and influence tissue remodeling and function.
IgE1–3 and mast cells4–7 have each been the topic of recent reviews. We focus here on aspects of the biology of IgE and mast cells that we think are most relevant to their proven or potential roles in allergic disorders, especially asthma. We discuss evidence indicating that IgE and mast cells, acting either individually or in concert, can have both nonredundant and partially redundant roles in the pathogenesis of chronic and acute manifestations of asthma. We also describe some approaches that are being taken to exploit our understanding of the biology of IgE and mast cells to craft better ways to manage and treat people with allergic diseases.
Allergen sensitization and antigen-specific IgE production
The discovery and characterization of the antibody class now called IgE8, culminating in the independent descriptions of this class of antibodies by the Ishizakas9 and Johansson and Bennich10, arguably represents the most crucial advance in our understanding of the immunological basis of allergic disorders. Production of antigen-specific IgE requires that such antigens are taken up by dendritic cells, B cells or other antigen-presenting cells, which, in the presence of interleukin-4 (IL-4) or IL-13 provided early in the process by one or more cell types, present the processed antigens to cognate naive T cells that then acquire a T helper type 2 (TH2) cell phenotype11 (Fig. 1, left). TH2 cells both engage cognate B cells through B cell major histocompatibility complex (MHC) class II and co-stimulatory molecules and secrete IL-4 and IL-13, inducing B cells to undergo class-switch recombination (CSR), resulting in the variable, diverse, and joining (VDJ) segments that were initially linked to another constant (C) region in the immunoglobulin heavy chain locus (for example, Cμ or Cγ) to instead being linked to the Cε region (Fig. 1, right). CSR also can be induced by IL-4 and/or IL-13 that is derived from cells other than TH2 cells, which may include mast cells and basophils11–13.
Figure 1. Allergen sensitization and IgE production.
Initial allergen sensitization results in antigen-specific IgE production (left). In individuals not yet exposed to a new environmental allergen (designated here as an antigen (Ag)), the only IgE present (blue) does not have specificity for the new antigen(s). Such IgE can be bound to the αβγγ form of FcεRI on mast cells or to the αγγ form of FcεRI on the surface of macrophages, monocytes or dendritic cells or to CD23 on airway epithelial cells or other cells (not shown here). The new antigens (orange circles) are captured by dendritic cells or macrophages in the airway lumen or in the epithelium of the airway mucosa or gain access to submucosal dendritic cells through disrupted epithelium or, for some antigens with intrinsic protease activity, by disrupting epithelial cell tight junctions. Antigen-activated dendritic cells mature and migrate to regional lymph nodes or to sites in the local mucosa, where they present processed antigen epitopes to cognate T cells; in the presence of IL-4 or IL-13, which may be derived from a variety of potential cellular sources, this induces such T cells to become differentiated and activated TH2 cells. IL-4 and IL-13, which may be derived from TH2 cells (shown here), basophils, mast cells and/or other sources, also activate immunoglobulin heavy chain gene CSR for antigen-specific IgE production, designated here antigen-specific IgE (epitope A), in B cells. The antigen-specific IgE response is amplified by FAP and other mechanisms (right). Antigen-specific IgE can bind to multiple cell types through various IgE receptors. Antigen-induced aggregation of IgE bound to FcεRI stimulates mast cell degranulation and the release of mediators such as histamine, PGD2 and TNF, which promote recruitment of TH2 cells, the migration, maturation and activation dendritic cells and antigen presentation. IgE and antigen-IgE complexes can cross the epithelium by transcytosis mediated by CD23 on airway epithelial cells (1), allowing them to bind to and activate FcεRI on mast cells and dendritic cells. This process contributes to the perpetuation of allergic inflammation and, potentially, through promotion of IL-4 and/or IL-13 secretion by mast cells (2) and effects of activated mast cells on dendritic cells (3), to additional local IgE CSR and IgE production in B cells, either to additional epitopes of the original antigen (shown here) or to new antigens bound by dendritic cells (square blue symbols). Antigen presentation mediated by binding of antigen-IgE complexes to CD23 on B cells, followed by antigen presentation by these B cells to cognate T cells (not shown here), is called FAP (4), a process that can result in epitope spreading, with production of IgE recognizing new epitopes of the original antigen (for example, epitope B, shown here) or to epitopes of new antigens, if some IgE antibodies to that antigen already exist (not shown), and the subsequent exacerbation of allergic disorders. FcεRI αγγ trimers on other antigen-presenting cells (for example, dendritic cells, monocytes and macrophages) permit these cells to bind and internalize IgE that is bound to complex antigens; epitopes derived from such antigens, including those comprising epitopes for which there is not yet a specific IgE response, are then presented to cognate T cells, which, in the presence of IL-4 and/or IL-13, can become TH2 cells that in turn promote the production of IgE against these new epitopes by B cells (5). ICOS, inducible T cell co-stimulator; ICOSL, ICOS ligand; BCR, B cell receptor.
Antigen sensitization was previously thought to occur primarily in lymphoid germinal centers, but IgE-producing B cells that undergo clonal selection and affinity maturation also can be generated in the respiratory mucosa14 (Fig. 1, left). CSR resulting in production of IgE (in addition to IgA) also can occur in the gastrointestinal tract15, and patients with food allergy have higher concentrations of IgE in the gastrointestinal tract than healthy individuals15. Such evidence supports the conclusion that IgE can be produced locally by B cells in the gut- or airway-associated lymphoid tissue, as well as in the lymph nodes, of individuals with food allergy15, seasonal or perennial allergic rhinitis16 or atopic or nonatopic asthma14.
These observations suggest that much of the IgE responsible for ‘organ-specific’ allergic disorders may be produced locally in the affected anatomical sites, which also may be a survival niche for long-lived IgE-antibody–secreting plasma cells, and that IgE measured in the peripheral circulation may be primarily antibody that has escaped from the site of disease15. Indeed, concentrations of IgE in the peripheral blood are typically much lower than those of any other immunoglobulin isotype1. These findings also suggest that locally produced IgE may be pathogenic in at least some cases of so-called nonatopic asthma (that is, asthma in which IgE-dependent allergic mechanisms are not thought to have a key role) under circumstances in which it may be difficult to measure the amount of the crucial IgE antibodies systemically and in individuals in which no triggering antigen has been identified14.
Amplification of the IgE response to allergens through CD23
Once an individual has developed IgE antibodies to certain antigen epitopes, multiple mechanisms can lead to a more robust and diverse IgE responses to both the original as well as other antigens. Some of these mechanisms are mediated by CD23 (ref. 1), which can be expressed on cells such as epithelial cells, B cells and myeloid cells (Fig. 1). CD23 is a C-type lectin that can exist in a membrane-bound form that has three lectin domain “heads” separated from the membrane by a triple α-helix coiled-coil stalk, as well as in various soluble forms whose functions depend on whether these soluble forms are monomeric or trimeric1,17 (Table 1). The CD23 sheddase, ADAM metallopeptidase domain 10 (ADAM10), is the main protease that releases soluble CD23 from the membrane-associated form3,18. CD23 is sometimes called a low-affinity receptor for IgE, but when the three lectin head domains of CD23 interact with a single IgE molecule, the resulting affinity constant (Ka) (~108–109 M−1) approaches that of the high-affinity receptor for IgE, Fcε receptor I (FcεRI)(~1010 M−1)1,17.
Table 1.
Expression and major functions of IgE-binding receptors or molecules
| Receptors or molecules |
Cell typesa | Major functions |
|---|---|---|
| FcεRI (αβγγ or αγγ) | Mast38, basophils38, Langerhans38, dendritic38,41, monocytes38, eosinophils38,40, neutrophils39,40, platelets138,139, bronchial epithelial cells of asthmatics45 and airway smooth-muscle cells44,140. In mice: dendritic cells after Sendai virus infection41, neutrophils and eosinophils during Plasmodium infection40 and superior cervical ganglion and myenteric plexus neurons43; in rats: pinealocytes141. | αβγγ: immediate hypersensitivity2, parasite immunity2, enhanced cytokine production and survival in mast cells59 and MCp recruitment in the airway142. αγγ: antigen presentation1,38. |
| FcεRII (CD23) | B cells143, T cells, NK cells, monocytes, macrophages, follicular dendritic cells143, Langerhans cells, bone marrow stromal cells, neutrophils, eosinophils, platelets1,3,17 and airway20 and intestinal144,145 epithelial cells. | Regulation of IgE production1, killing of intracellular pathogens (Leishmania major146 and Toxoplasma gondii26) or tumor cells28, facilitated antigen presentation1 and transport of IgE and antigens across the epithelium19,144. |
| FcγRII and FcγIII[AU: OK?](in miceb) | Mast cells147 and macrophages147. | Cell activation147. |
| FcγRIV (only in mice) | Monocytes, neutrophils and macrophages148,149. | Phagocytosis, cytokine production and antigen presentation in macrophages148,149. |
| Gelactin-3 | Mast cells150,151, basophils150, neutrophils152, monocytes and macrophages153–156, eosinophils157, Langerhans158,159, T160, B161 and dendritic162. | Potentiate FcεRI activation151. |
The cell types listed in bold are expressed in both humans and mice.
Human FcγRIII has no affinity for IgE. However, human mast cells and basophils express the immunoreceptor tyrosine-based inhibitory motif–containing receptor, FcγRIIB, which can reduce activation of these effector cells through the FcεRI when it is co-ligated with the FcεRI127.
CD23 is thought to contribute to both positive and negative regulation of IgE production (Table 1), but the mechanisms responsible for this, and the role of CD23 in the pathology of allergic diseases, are not fully understood1. Moreover, some effects of CD23 on IgE production may be influenced by other CD23 binding partners, including CD21, which can permit CD23 to participate simultaneously in biological networks involving either the complement system or IgE, and various integrins1. However, certain functions of CD23 clearly have the potential to influence the biology of IgE-associated allergic disorders.
For example, CD23 on epithelial cells might amplify IgE responses by moving IgE and antigen-IgE complexes across the epithelium by transcytosis19,20 (‘1’ in Fig. 1, right), where they can bind to and activate FcεRI on mast cells, macrophages and dendritic cells, thereby promoting allergic inflammation, and, by inducing the secretion of IL-4 and/or IL-13 by mast cells, contribute to local IgE production (‘2’ in Fig. 1, right). IgE- and antigen-activated mast cells also release mediators such as histamine, tumor necrosis factor (TNF) and prostaglandin D2 (PGD2) that can, in turn, promote the maturation, functional activation and migration of dendritic cells, favoring the development of sensitization to additional antigens (‘3’ in Fig. 1, right).
IL-4 and IL-13 also can increase CD23 expression on B cells and myeloid cells21, thereby enhancing facilitated antigen presentation (FAP). In FAP, antigen-IgE complexes bound to the CD23 that is expressed on antigen-activated B cells can favor the presentation of such antigens to TH cells (‘4’ in Fig. 1, right and Table 1). The presentation of antigen-derived peptides initiated by the recognition of an antigen by membrane-associated B cell receptors is by definition effected only by interactions of cognate B cells with TH cells (Fig. 1, left). By contrast, FAP of antigens bound to secreted IgE can permit any antigen-activated, CD23-expressing B cells, regardless of the specificity of the cells’ B cell receptors, to present diverse peptides (from related or unrelated antigens) to cognate T cells (Fig. 1, right). Thus, FAP is an efficient mechanism for so-called epitope spreading, in which the presence of an antibody response to one epitope can ultimately result in the production of antibodies to other epitopes on the same or unrelated antigens1.
Epitope spreading is thought to contribute to the progressive development of allergies to multiple antigens in individuals with allergy. Epitope spreading may also contribute to the ‘atopic march’, wherein individuals who first present in early childhood with atopic dermatitis later develop allergic rhinitis and then atopic asthma22, and may help explain how genetic abnormalities affecting one epithelium (for example, the epidermis) can predispose to allergic disorders affecting other epithelia. Notably, a large fraction of Europeans and Asians with atopic dermatitis have loss-of-function mutations in the gene encoding filaggrin, which results in impaired barrier function of the skin23. Filaggrin is expressed in the epidermis of the skin and in other squamous epithelia but not in the airway or gastrointestinal mucosae24, however, individuals with filaggrin mutations that are associated with the development of atopic dermatitis are also at increased risk for the later development of atopic asthma25.
Additional functions of CD23 include the clearance of antigen-IgE complexes, the killing of pathogens by IgE-bearing monocytes and eosinophils26, involvement in IgE-independent27 and IgE-dependent28 monocyte-mediated toxicity against target cells and, through CD23 expressed on intestinal epithelial cells, the transportation of IgE and antigen-IgE complexes directly across the intestinal epithelium19,29, which may account for delivery of maternal IgE to the fetus by swallowed amniotic fluid30 (Table 1). By moving IgE in the opposite direction across the intestinal31,32 or respiratory20 epithelium, CD23 can favor the formation of antigen-IgE complexes in the lumen that can then be transported by CD23 back across the epithelium, resulting in the activation of local mast cells (or other FcεRI-bearing effector cells), a process that may both exacerbate allergic inflammation and impair mucosal epithelial function at the affected site.
It has been reported that local IgE synthesis and expression of CD23 can occur concurrently in the fetus30,33 and that a parental history of atopy and high titers of IgE in the cord blood are predictive of early atopy34. Moreover, CD23 gene polymorphisms have been associated with effects on the development of atopy in mice and humans35. It is thus tempting to attribute to CD23 at least some role in the development and, through epitope spreading and other mechanisms, the exacerbation of allergic disorders.
Amplification of the IgE response to allergens through FcεRI
IgE does not fix complement and has only limited ability to cross the placenta36, and it is thought that IgE’s main biological roles reflect its ability to bind to receptors on mast cells, basophils and a variety of other cell types. The high-affinity receptor for IgE, FcεRI, as expressed by mast cells and basophils, consists of an IgE-binding α chain, in which the two extracellular domains bind IgE, a β chain, which spans the plasma membrane four times and functions as a signal amplifier37, and two identical and largely intracellular γ chains38. The signaling motifs of this αβγγ form of FcεRI consist of immunoreceptor tyrosine-based activation motifs, one in the β chain and one in each of the γ chains38. An αγγ form of the receptor can be expressed on a variety of other cell types, including macrophages, dendritic cells, eosinophils, platelets and neutrophils38–40 (Table 1 and Fig. 1).
Although it was once thought that such widespread expression of the αγγ form of FcεRI among hematopoietic cells was more characteristic of humans than of mice, it is now known that mouse dendritic cells41,42, eosinophils40 and neutrophils40 can upregulate expression of this receptor during certain immune responses. Moreover, expression of the αβγγ form of FcεRI has been detected in mouse superior cervical ganglion and myenteric plexus neurons43 and in human airway smooth muscle cells44; FcεRI has also been reported on bronchial epithelial cells of asthmatic but not healthy individuals45 (Table 1). Although the in vivo importance of such non-hematopoietic expression of FcεRI has not yet been determined, these observations suggest that IgE and FcεRI may be able to induce functional changes in such structural cells directly rather than only indirectly through IgE-driven functions of FcεRI-bearing myeloid cells.
Interactions of IgE with FcεRI-bearing myeloid cells can have multiple effects with the potential to augment production of antigen-specific IgE. Antigens can be captured (or ‘focused’) by IgE bound to the αγγ form of FcεRI on the surface of cutaneous Langerhans cells and other dendritic cells, which then can migrate to regional lymph nodes or to local sites in the submucosa where they can present the processed antigens to cognate naive T cells, generating TH2 cells46 (‘5’ in Fig. 1, right). Although such Langerhans and dendritic cell functions can be expressed in the absence of mast cells, data from mast-cell–deficient mice47–50 and mast-cell–knockin mice (genetically mast-cell–deficient mice that have been engrafted with mast cells)47,48 indicate that mast cells have the potential to enhance these processes by promoting Langerhans and dendritic cell migration and, perhaps, by effects on the maturation and function of these two types of cells (‘3’ in Fig. 1, right).
In addition to immunologically ‘arming’ mast cells and basophils to undergo antigen- and IgE-mediated activation for mediator release, IgE binding to FcεRI also stabilizes the receptor’s expression on the surface of the cells51, thereby increasing the numbers of FcεRI on the cell surface52–54. This may largely account for observations showing that humans and mice with high circulating concentrations of IgE have large numbers of FcεRI on the surface of their basophils55,56 and mast cells54,57. IgE-dependent upregulation of mast-cell FcεRI surface expression allows the cells to bind more IgE, which can enable the cells to respond to a larger number of different antigens, to release mediators at lower concentrations of antigens and, perhaps, to secrete certain mediators that may not be detectably released by cells with lower numbers of surface FcεRI54,58,59. For example, when the cells were incubated for 4 d with IgE at 5 µg ml−, the numbers of FcεRI on mouse mast cells derived from bone marrow54 or human mast cells derived from umbilical cord blood58 increased approximately 30-fold or doubled, respectively. In the human mast cells, the increase in surface expression of FcεRI was associated with increases of 73% and 156% in the amount of histamine and leukotriene C4 (LTC4), respectively, secreted by the cells after challenge with antibodies to IgE58. Thus, IgE-dependent upregulation of mast-cell FcεRI surface expression is a potentially key positive amplification loop of allergic disease in individuals who develop increased concentrations of circulating or tissue IgE.
IgE and mast cells mediate an immediate hypersensitivity response
In an allergic person, whose tissue mast cells and other cell types already have antigen-specific IgE bound to FcεRI, re-exposure to the original or a crossreactive bivalent or multivalent antigen results in the crosslinking of adjacent FcεRI-bound IgE and the consequent aggregation of surface FcεRI. When the FcεRI aggregation is of sufficient strength and duration, it triggers mast cells and basophils to initiate complex signaling events that ultimately result in the secretion of a diverse group of biologically active products2,4,6,38,60–63. Some products, such as those stored preformed in the cells’ cytoplasmic granules, for example, histamine, serotonin (in rodents to a much greater extent than in humans), proteases such as tryptase, chymase and/or carboxypeptidase A3 and proteoglycans (heparin and/or chondroitin sulfates), as well as newly formed lipid-derived mediators, for example, PGD2, LTB4, LTC4, LTD4 and LTE4 and certain cytokines, are released by mast cells within minutes of antigen exposure. Others, including a diverse spectrum of cytokines, chemokines and growth factors, are produced in mast cells from new transcripts and are therefore secreted over a period of hours after the initial mast cell activation. Studies in mice suggest that, in addition to IgE, immunoglobulin light chains can also mediate antigen-specific mast cell activation, although the receptor responsible for this effect has been elusive64.
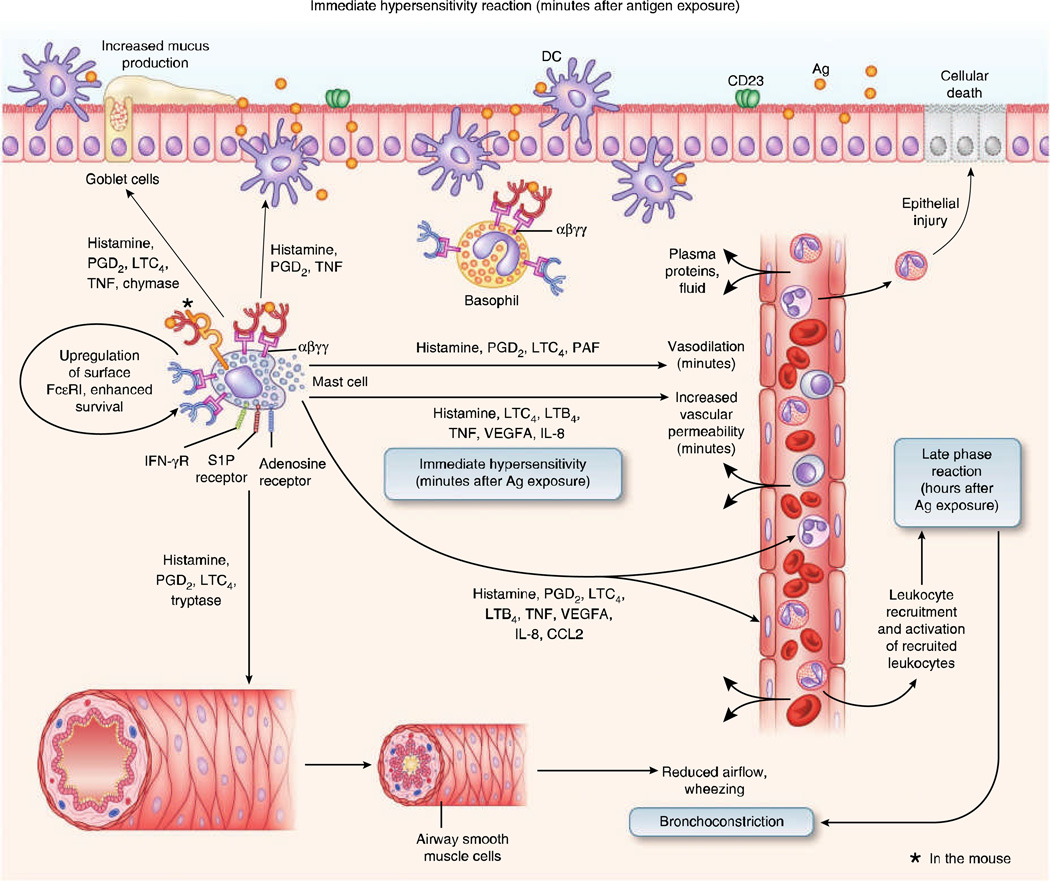
In aggregate, mediators released shortly after antigen- and IgE-induced mast cell degranulation induce a response termed an immediate hypersensitivity (or early phase) reaction within minutes of their release. If localized to the airways, this response is characterized by increased vascular permeability, contraction of the airway smooth muscle and enhanced secretion of mucus (Fig. 2), resulting in acutely reduced airflow and wheezing. If the response is systemic, it can result in anaphylaxis, a catastrophic immune response that can rapidly result in death if not properly treated65. The inflammation and functional changes associated with early phase responses to antigens typically resolve within a few hours. However, in some individuals, a second phase of inflammation, called the late phase reaction, develops at the site of antigen challenge, typically beginning a few hours after antigen exposure.
Figure 2. The early immediate hypersensitivity phase of antigen-induced airway inflammation.
The individual IgE molecules that are bound to FcεRI molecules on a single mast cell can be specific for different antigens (red and blue IgE symbols). Binding of IgE to FcεRI αβγγ on mast cells, which are normally located in airway tissues, and basophils, if they have been recruited from the blood to airway tissues, upregulates FcεRI surface expression and sensitizes these cells to respond when later exposed to specific antigens, and, in mast cells, some IgE molecules can enhance cytokine production and survival. The recognition of a particular bivalent or multivalent antigen by at least two IgE molecules bound to adjacent FcεRI molecules induces FcεRI aggregation, activating mast cells (and basophils, if they are present in airway tissues) to initiate an immediate hypersensitivity response by secreting preformed mediators and lipid mediators within minutes of antigen exposure. These mast cells also upregulate the production of many cytokines, chemokines and growth factors. Within minutes of exposure, the rapidly secreted mediators lead to bronchoconstriction, vasodilation, increased vascular permeability and increased mucus production. Mast cell mediators produced rapidly after antigen challenge can also promote dendritic cell migration, maturation and function and can contribute to the transition to the late phase reaction by promoting an influx of circulating leukocytes, both by upregulating adhesion molecules on vascular endothelial cells (for example, through TNF) and by secreting chemotactic mediators (such as LTB4 and PGD2) and chemokines (such as IL-8 and CC-chemokine ligand 2 (CCL2)). These recruited leukocytes can induce further inflammation and bronchoconstriction during the late phase reaction. PAF, platelet activating factor; IFN-γR, IFN-γ receptor; VEGFA, vascular endothelial growth factor
The IgE–mast-cell axis in chronic allergic inflammation
Mast cells activated by IgE and specific antigens produce mediators that drive early phase reactions (Fig. 2) and contribute to late phase reactions (Box 2), but these mast cells also secrete diverse cytokines, chemokines and growth factors that have the potential to influence airway remodeling4–7,63,66 (Fig. 3). Compared to wild-type mice, mice lacking the FcεRI α chain showed diminished airway inflammation, indicating decreased eosinophil concentrations, in an asthma model67. Studies in wild-type, genetically mast-cell–deficient and mast-cell–knockin mice indicated that activation of mast cells through the FcR γ chain (which in mice is required for mast cell activation by antigen-IgG1 immune complexes through FcγRIII, as well as for signaling through FcεRI) is required for the full development of many features of allergic inflammation and tissue remodeling in a model of chronic asthma68,69. However, many other effector cells also have the potential to contribute to these features of asthma, including antigen-specific effector T cells70, and the relative roles of mast cells compared to other effector cells in some of these settings is not fully resolved, particularly in human subjects.
Box 2 Late phase reactions and chronic antigen exposure lead to persistent allergic inflammation and tissue remodeling.
Mast cells previously activated during the early phase reaction secrete mediators that can orchestrate the recruitment, tissue infiltration and functional activation of circulating leukocytes, including granulocytes such as eosinophils, basophils and neutrophils, as well as monocytes and T cells66,80,163 (Fig. 2), which substantially increases the diversity of the cellular drivers of inflammation at the site of antigen challenge. Mast cells may therefore be a crucial source of mediators contributing to the initiation of late phase reactions164,165. Other sources include antigen-specific T cells, as well as myeloid cells activated by antigen-containing immune complexes if these cells are present at the site of antigen challenge, such as in individuals with ongoing airway inflammation163. Indeed, low levels of ongoing inflammation in the airways, as can be observed in individuals with asthma even during treatment, together with exposure to antigen peptides that are recognized by effector T cells—but not IgE antibodies—may account for the development of late phase responses in individuals without previous detectable early phasereactions163,166. As people afflicted with allergic asthma are usually exposed repeatedly to the antigens that elicit their allergic reactions, and this may occur over periods of years, their airways have experienced many antigen-induced early and late phase reactions. However, in addition to developing inflammation, the airways of individuals with asthma also show structural changes, called airway remodeling, which include increased numbers of mucus-producing goblet cells in the epithelium, evidence of repair responses at sites of epithelial injury, thickening of the smooth muscle layer and changes in connective tissues, blood and lymphatic vessels, mucus glands and nerves66,167. Persistent airway inflammation, a key feature of both allergic and non-allergic forms of asthma, probably contributes in a crucial way to tissue remodeling in asthma167,168. Evidence suggests that IgE and mast cells can substantially contribute to chronic airway inflammation and tissue remodeling in asthma by functioning both in a single pathway—interdependently through antigen- and IgE-dependent mast cell activation—and independently (Fig. 3).
Figure 3. Roles of IgE and mast cells in chronic airway inflammation and tissue remodeling.
In chronic allergic inflammation, repetitive or persistent exposure to allergens can result in both the production of IgE against multiple antigen epitopes of several different antigens (Fig. 1, right) and the development of long-term changes in the involved tissues (Box 2), including changes in mast cell number, tissue distribution (with mast cells in the epithelium and the smooth muscle layer, not shown here) and phenotype. Moreover, repetitive epithelial injury caused by chronic allergic inflammation can be exacerbated by exposure to pathogens such as viruses or bacteria or environmental factors, and the consequent repair response results in epithelial and mesenchymal changes that are thought to sustain TH2 cell–associated inflammation, promote sensitization to additional allergens or allergen epitopes (for example, epithelial-cell–derived TSLP can upregulate the expression of co-stimulatory molecules such as OX40, CD40 and CD80 by dendritic cells, not shown here) and regulate the airway remodeling process. These processes in turn result in many functionally relevant changes in the structure of the affected tissue. There is evidence that many of these changes can be influenced by IgE and mast cells, either acting in concert through the IgE–mast-cell axis or independently. For example, both soluble factors, such as INFγ, S1P, adenosine and IL-33, and cells present at the site, such as TH2 cells and Treg cells (which can interact with OX40L on mast cells) can modulate, or tune, IgE-dependent mast cell activation, and some pathogen-associated molecular patterns (PAMPs) and cytokines, including TSLP and IL-33, can activate mast cells independently of IgE to produce different spectra of cytokines or chemokines. Studies in mast-cell–knockin mice have indicated that some actions of mast cells, such as increasing the number of epithelial goblet cells, can occur in a model of chronic asthma by mast-cell–dependent mechanisms that do not require mast cell signaling through the FcεRI γ chain, whereas mast cells must express both the FcεRI γ chain and the IFN γ receptor 1 (IFN-γR1) to mediate increases in lung eosinophils, neutrophils and collagen (not shown here). Amplification of the IgE response by IgE, for example, by FAP (Fig. 1, right) and IgE- and antigen-dependent activation of basophils after their recruitment to the airways can occur independently of mast cells. PRR, pattern recognition receptor; GM-CSF, granulocyte-macrophage colony-stimulating factor
In addition to possible redundancy in the roles of mast cells and other cell types in the chronic changes associated with asthma, there is evidence that many factors can modify mast cell function in this setting. For example, in vitro58,71–76 and in vivo69,76,77 evidence indicates that the extent of antigen- and IgE-dependent mast cell activation may be influenced substantially (or ‘tuned’) by microenvironmental factors that affect the expression or function of surface receptors or signaling molecules that contribute to the positive or negative regulation of such responses4,6,7,78,80. Tuning factors that can be present locally at the sites of allergic inflammation, such as in the airways and other anatomical sites, include adenosine75, sphingosine-1 phosphate (S1P)76, certain chemokines77 and a variety of cytokines, such as IL-4 (refs. 58,71), IL-33 (refs. 72–74) and interferon γ (IFN-γ)69. Tuning also can be accomplished by cell-cell interactions. For example, interactions of mast cells and T cells can be bidirectional and complex79,80 and include the ability of IgE-activated mast cells to enhance proliferation and cytokine production in multiple T cell subsets81,82 and the ability of CD4+CD25+ regulatory T (Treg) cells to suppress IgE-dependent mast cell activation through interactions between tumor necrosis factor receptor superfamily, member 4 (OX40), either as expressed by Treg cells83 or in a soluble form84, and the OX40 ligand, OX40L expressed on mast cells (Fig. 3).
In addition to stabilizing expression of FcεRI on the mast cell surface and sensitizing mast cells to respond to specific antigens, IgE can have effects on mast cell survival or function59,85 that seem to be independent of the presence of the antigen for which the IgE has specificity. Such findings suggest that certain IgE antibodies might favor the expansion of mast cell numbers or have effects on mast cell function in vivo, including increasing their secretion of cytokines and chemokines, even in the absence of a specific antigen. In addition to binding to FcεRI or CD23, IgE, and FcεRI itself, can be bound by β-galactose–containing olilgosaccharide chains of galectin-3, permitting galectin-3 to activate mast cells and basophils through carbohydrate interactions that crosslink receptor-bound IgE, FcεRI or both86 (Table 1). Studies in mice lacking galectin-3 support the notion that galectin-3 can amplify the pathology that is observed in models of asthma87 or atopic dermatitis88.
Independent roles of IgE and mast cells in chronic allergic inflammation
By contrast, some potentially key effects of IgE or mast cells in allergic disease seem not to require direct interactions between these two effector elements. For example, IgE-dependent antigen focusing on dendritic cells and IgE-dependent FAP are thought to occur independently of mast cells (Fig. 1, right). Antigen- and IgE-dependent activation of other effector or immunoregulatory cells, including basophils that can produce a spectrum of mediators partially overlapping with those of mast cells89,90, does not require mast cells, except perhaps for helping to recruit such cells to sites of disease, and mast cells are not required for the actions of IgE that are mediated through CD23 on other cell types.
Mast-cell–mediator secretion can be directly activated by many stimuli independently of IgE and specific antigens, and many of these factors are known to be present locally at sites of allergic inflammation (Fig. 3). Different mast cell populations show different patterns of expression of receptors for pathogen-associated molecular patterns, including Toll-like receptors (TLRs)4–7, and activation of mast cells through different TLRs can induce these cells to secrete distinct patterns of cytokines or chemokines4–7. Many additional stimuli can directly activate mast cells and, in some cases, also enhance IgE-dependent mast cell activation, including adenosine75, S1P76, thymic stromal lymphopoietin (TSLP)91, IL-33 (refs. 72–74) and many other cytokines, as well as proteases, inflammatory mediators, products of complement activation and exogenous agents, including bacterial toxins4–7. Thus, diverse pathways, not just stimulation with IgE and specific antigens, can elicit mast cell activation and mediator release in individuals with asthma and other allergic disorders, and the interactions among these various activation pathways in the setting of allergic disease may be quite complex.
For example, evidence from mast-cell–knockin mice suggests that the marked increase in the number of airway goblet cells observed in a model of chronic asthma can occur by mechanisms that are mast-cell–dependent but that do not require that the mast cells express the FcεRI γ chain68,69 (Fig. 3). Although the relevance of these findings to human asthma has not been determined, gene set enrichment analyses have indicated that the changes in gene expression in the lung that are associated with this model of chronic asthma in mice69, in which many of the key features seem to require mast cells for full expression68,69, are similar to the mRNA changes observed in bronchial biopsy specimens obtained from a small group of patients with mild asthma92.
In a different model of asthma, evidence obtained using mice lacking the mast-cell–associated chymase, mast cell protease 4 (MCPT4), indicates that the local secretion of this protease by mast cells, presumably in response to their activation by IgE, antigens and/or other mechanisms, can diminish the airway hyperreactivity, airway inflammation and airway smooth muscle thickening that is associated with this model93. These provocative findings suggest that at least some mast-cell–derived mediators may have functions that can curtail the extent of pathology at the sites of chronic allergic disease.
However, defining the roles of mast cells in asthma and other disorders could well be characterized as a moving target. For example, analyses of airway epithelial brushings or endobronchial biopsies94–96, or lungs obtained at autopsy97,98, from different groups of patients have documented changes in the number, distribution and/or phenotype of mast cells in asthmatic airways (Fig. 3). Such changes include increased numbers of mast cells in the airway smooth muscle of some individuals with clinically mild asthma63,94, a significant trend to higher numbers of chymase-positive mast cells in the proximal airway epithelium of individuals with more severe asthma (such chymase-positive intraepithelial mast cells were rarely seen in healthy individuals)96 and a significant trend toward lower levels of submucosal mast cells in the proximal airways, but a significantly higher ratio of chymase-positive mast cells to total mast cells, with increasing severity of asthma96. In a group of individuals with mild to moderate asthma, those with high expression of IL-13–responsive genes in their epithelial brushings (‘TH2-high asthmatics’) had significantly higher numbers of intraepithelial mast cells than healthy controls or those individuals in the ‘TH2-low’ group, and these mast cells were positive by immunohistochemistry for tryptase and carboxypeptidase A3 (CPA3) but not chymase95. Morphological studies of autopsy specimens revealed evidence for higher levels of degranulation of mast cells in the airway smooth muscle of individuals with fatal asthma than in individuals without asthma or those with non-fatal asthma97, as well as increased numbers of neutrophils and degranulated mast cells in submucosal glands98. By altering the proximity of airway mast cell populations to key airway structural cells that are targets of mast-cell–derived mediators, and possibly by changing the reactivity of mast cells to various stimuli of mast cell activation, such perturbations of airway mast cell populations may influence the extent to which mast cells contribute to airway functional and structural changes in asthma63,94–98 (Fig. 3).
Why do such changes in mast cell populations occur in asthma? Many growth factors, cytokines and chemokines, some of which can be derived from mast cells, as well as other sources, can positively or negatively influence the number, phenotype and tissue distribution of mast cells, including the main survival and developmental factor for human and mouse mast cells, the c-KIT ligand stem-cell factor; many of these factors have been identified at sites of allergic inflammation in asthma and other allergic disorders4,6,7,62,63,79,99. However, which of these factors are most crucial in altering mast cell populations in the airways of patients with asthma in vivo has not yet been determined.
Therapeutic approaches targeting IgE or mast cells
Therapeutic approaches in allergic diseases have for many years included attempts to target particular mediators that can be derived from mast cells and, in many cases, also from other cells types100. In addition, corticosteroids, whose effects can suppress many proinflammatory pathways101, including some that may depend on mast cell functions such as cytokine production102–104, can ameliorate disease in many individuals with asthma and other allergic disorders101. However, although such approaches have been useful in many patients, there are major unmet therapeutic needs, particularly in asthma100.
Accordingly, given their role as key drivers of the pathology in allergic disorders, efforts are underway to target IgE and mast cells. Years of clinical experience with omalizumab, a humanized monoclonal antibody to IgE, have shown that it can provide benefit in some patients with moderate to severe asthma105,106, as well as in some individuals with intermittent (seasonal) and persistent (perennial) allergic rhinitis105, food allergy107 or atopic dermatitis108. Omalizumab also has been reported to provide clinical benefit in a small group of patients with chronic autoimmune urticaria (a disorder characterized by recurrent episodes of hives that are resistant to antihistamine treatment and in which many patients have autoantibodies to the FcεRI α chain or to IgE itself)109, in one patient with a severe case of apparently idiopathic cold-induced urticaria110 and in a multicenter, randomized, double-blind, placebo-controlled study of patients with chronic urticaria who had IgE autoantibodies against thyroperoxidase111. These findings strongly implicate IgE and/or activation of the mast cell FcεRI in these disorders.
Much of the benefit of omalizumab treatment is thought to reflect its ability to reduce the concentration of free IgE in the blood, which can in turn lower the expression of FcεRI on mast cells and basophils and perhaps other cell types56,57,112. Other effects, such as downregulation of IgE-committed B cells, may also contribute to the efficacy of the drug113. However, not all patients with asthma are helped by such treatment, whether because of difficulty in lowering IgE concentrations sufficiently at the sites of disease or for other reasons114, and such treatment is expensive; both of these factors limit the utility of omalizumab. Nevertheless, clinical experience with this agent supports the conclusion that IgE can have a key role in the pathology that is associated with asthma in some patients, fueling efforts to devise more effective and less costly ways to target IgE in allergic disease, including attempting to reduce or eliminate IgE using a synthetic IgE peptide vaccine115,116.
Efforts also are underway to interfere with the binding of IgE to FcεRI. Such work includes the design of small peptides that can block this binding117–120. Other blocking peptides have been identified using a combinatorial chemistry approach121, phage display122–124 or modifications of known IgE receptor agonists125,126. Some of these IgE-blocking peptides can inhibit mast cell activation in vitro120,121,126 and IgE-dependent passive cutaneous anaphylaxis reactions in vivo121. In addition, there is preclinical evidence that it may be possible to achieve therapeutic benefit in IgE-associated disorders by enhancing the negative regulation of signaling through the FcεRI, for example, through the use of fusion proteins that co-ligate the FcεRI with the immunoreceptor tyrosine-based inhibitory motif–containing receptor, FcγRIIB, that is expressed on both mast cells and basophils127.
Agents that target mast-cell–associated tyrosine kinases are also being investigated, including those with activity against c-KIT128–131 or spleen tyrosine kinase (SYK)132,133. Among the questions that need to be answered in future work targeting mast cells with these or other agents are: can mast cells be targeted with high specificity and perhaps locally at sites of disease, can mast cells be eliminated without activating the cells to release clinically relevant amounts of mediators134, and is there sufficient redundancy of the effector mechanisms in the beneficial innate and adaptive immune responses to which mast cells contribute such that mast cell numbers or functions can be safely ablated or reduced? As with any new therapeutic approach, careful studies will be required to assess whether the benefits of mast-cell–targeted treatments in selected patients outweigh any potential risks of adverse effects.
Conclusions
Despite many years of intense study, the roles of IgE and mast cells in asthma and other allergic diseases are not fully understood. This is particularly true regarding possible beneficial roles of certain mast cell products in these disorders. However, there is strong evidence that antigen-, IgE- and FcεRI-dependent activation of mast-cell–mediator secretion can influence the pathology of allergic disorders and that the consequences of this process can either directly affect structural cells residing in the affected tissues or influence the pathology indirectly through effects of mast cells on dendritic cells, T cells, B cells and other hematopoietic cell types. Other potentially key roles of IgE and mast cells in allergic disorders may reflect the actions of each of these two effectors that can be performed largely independently of the other, such as IgE-facilitated antigen presentation and epitope spreading and the effects of mast cells on certain aspects of tissue remodeling. However, IgE and mast cells are not the only contributors to pathology in allergic disease, and both clinical studies and analyses of animal models have indicated that there can be considerable redundancy in the innate and adaptive immune mechanisms that are at work in this setting.
We suggest that an appreciation of the complexity of allergic disorders, including the potential redundancy of the effector mechanisms that drive their pathology, has clear implications for efforts to prevent or treat such diseases. To the extent that it is possible to identify factors that can favor or suppress the development of allergic disorders, efforts should be made to avoid or reduce the effects of the former and exploit the latter to help genetically susceptible persons avoid becoming sensitized to allergens in the first place. For those individuals in whom these disorders have already developed, it would be useful to have reliable, objective criteria that have been independently confirmed in large cohorts of subjects and that can be used to subcategorize patients according to the most dominant pathways driving their disease, with particular attention being paid to those that are amenable to therapeutic manipulation. Such efforts to identify subsets of patients who may respond well to individualized pathway-targeted treatment are already underway for asthma135–137.
It seems to us that although efforts to define therapeutically relevant subsets of patients with allergic diseases are important and should continue to be pursued, it is probable that such work will confirm that IgE and mast cells contribute substantially to disease development, progression and organ-localized pathology in many people afflicted with asthma and other allergic disorders. In such individuals, we propose that targeting only IgE or mast cells may not produce as much benefit as effectively and judiciously targeting both. Such combination approaches would have the advantage of encompassing the pathological roles of IgE that do not involve mast cells and vice versa, as well as targeting the antigen-IgE-FcεRI–mast-cell axis that long has been considered a major driver of the pathology of allergic disease.
ACKNOWLEDGMENTS
We thank the members of the Galli lab and our collaborators and colleagues for their contributions to some of the work reviewed herein, apologize to the many contributors to this field whose work was not cited because of space limitations and acknowledge the support of US Public Health Service grants AI23990, AI070813 and CA72074 (to S.J.G.).
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
References
- 1.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 2.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol. Rev. 2011;242:128–143. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platzer B, Ruiter F, van der Mee J, Fiebiger E. Soluble IgE receptors—elements of the IgE network. Immunol. Lett. 2011;141:36–44. doi: 10.1016/j.imlet.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann AM, Abraham SN. New roles for mast cells in pathogen defense and allergic disease. Discov. Med. 2010;9:79–83. [PubMed] [Google Scholar]
- 6.Moon TC, et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010;3:111–128. doi: 10.1038/mi.2009.136. [DOI] [PubMed] [Google Scholar]
- 7.Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv. Exp. Med. Biol. 2011;716:2–12. doi: 10.1007/978-1-4419-9533-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanworth DR. The discovery of IgE. Allergy. 1993;48:67–71. doi: 10.1111/j.1398-9995.1993.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaka K, Ishizaka T, Hornbrook MM. Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J. Immunol. 1966;97:75–85. [PubMed] [Google Scholar]
- 10.Johansson SGO, Bennich H. Studies on a new class of immunoglobulin. I. Immunological properties. In: Kilander J, editor. Nobel Symposium 3. Gamma Globulins: Structure and Control of Biosynthesis; Almqvist and Wiksell; Stockholm. 1967. pp. 193–197. [Google Scholar]
- 11.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003;3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 12.Gauchat JF, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 13.Ryzhov S, et al. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J. Immunol. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- 14.Takhar P, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007;119:213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Coëffier M, Lorentz A, Manns MP, Bischoff SC. Epsilon germ-line and IL-4 transcripts are expressed in human intestinal mucosa and enhanced in patients with food allergy. Allergy. 2005;60:822–827. doi: 10.1111/j.1398-9995.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 16.KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur. Respir. J. 2000;15:491–497. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 17.Acharya M, et al. CD23/FcεRII: molecular multi-tasking. Clin. Exp. Immunol. 2010;162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibb DR, et al. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J. Exp. Med. 2010;207:623–635. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu Y, Perdue MH. CD23-mediated transport of IgE/immune complexes across human intestinal epithelium: role of p38 MAPK. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G532–G538. doi: 10.1152/ajpgi.00524.2005. [DOI] [PubMed] [Google Scholar]
- 20.Palaniyandi S, Tomei E, Li Z, Conrad DH, Zhu X. CD23-dependent transcytosis of IgE and immune complex across the polarized human respiratory epithelial cells. J. Immunol. 2011;186:3484–3496. doi: 10.4049/jimmunol.1002146. [DOI] [PubMed] [Google Scholar]
- 21.Punnonen J, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc. Natl. Acad. Sci. USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol. Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BE, Leung DY. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol. Res. 2012;4:12–16. doi: 10.4168/aair.2012.4.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying S, Meng Q, Corrigan CJ, Lee TH. Lack of filaggrin expression in the human bronchial mucosa. J. Allergy Clin. Immunol. 2006;118:1386–1388. doi: 10.1016/j.jaci.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 25.McLean WH, et al. Filaggrin variants confer susceptibility to asthma. J. Allergy Clin. Immunol. 2008;121:1294–1295. doi: 10.1016/j.jaci.2008.02.039. author reply 1295–1296. [DOI] [PubMed] [Google Scholar]
- 26.Vouldoukis I, et al. IgE mediates killing of intracellular Toxoplasma gondii by human macrophages through CD23-dependent, interleukin-10 sensitive pathway. PLoS ONE. 2011;6:e18289. doi: 10.1371/journal.pone.0018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plater-Zyberk C, Bonnefoy JY. Marked amelioration of established collagen-induced arthritis by treatment with antibodies to CD23 in vivo. Nat. Med. 1995;1:781–785. doi: 10.1038/nm0895-781. [DOI] [PubMed] [Google Scholar]
- 28.Karagiannis SN, et al. IgE-antibody–dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J. Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 29.Yu LC, et al. Enhanced transepithelial antigen transport in intestine of allergic mice is mediated by IgE/CD23 and regulated by interleukin-4. Gastroenterology. 2001;121:370–381. doi: 10.1053/gast.2001.26470. [DOI] [PubMed] [Google Scholar]
- 30.Thornton CA, et al. Fetal exposure to intact immunoglobulin E occurs via the gastrointestinal tract. Clin. Exp. Allergy. 2003;33:306–311. doi: 10.1046/j.1365-2222.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- 31.Tu Y, et al. CD23-mediated IgE transport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology. 2005;129:928–940. doi: 10.1053/j.gastro.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Berin MC, Li H, Sperber K. Antibody-mediated antigen sampling across intestinal epithelial barriers. Ann. NY Acad. Sci. 2006;1072:253–261. doi: 10.1196/annals.1326.002. [DOI] [PubMed] [Google Scholar]
- 33.Lima JO, et al. Early expression of iε, CD23 (FcεRII), IL-4Rα, and IgE in the human fetus. J. Allergy Clin. Immunol. 2000;106:911–917. doi: 10.1067/mai.2000.110228. [DOI] [PubMed] [Google Scholar]
- 34.Bergmann RL, et al. Predictability of early atopy by cord blood-IgE and parental history. Clin. Exp. Allergy. 1997;27:752–760. [PubMed] [Google Scholar]
- 35.Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Curr. Allergy Asthma Rep. 2007;7:331–337. doi: 10.1007/s11882-007-0050-y. [DOI] [PubMed] [Google Scholar]
- 36.Bønnelykke K, Pipper CB, Bisgaard H. Transfer of maternal IgE can be a common cause of increased IgE levels in cord blood. J. Allergy Clin. Immunol. 2010;126:657–663. doi: 10.1016/j.jaci.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Kraft S, Rana S, Jouvin MH, Kinet JP. The role of the FcεRI β-chain in allergic diseases. Int. Arch. Allergy Immunol. 2004;135:62–72. doi: 10.1159/000080231. [DOI] [PubMed] [Google Scholar]
- 38.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 39.Gounni AS, et al. Human neutrophils express the high-affinity receptor for immunoglobulin E (Fc ε RI): role in asthma. FASEB J. 2001;15:940–949. doi: 10.1096/fj.00-0378com. [DOI] [PubMed] [Google Scholar]
- 40.Porcherie A, et al. Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. J. Exp. Med. 2011;208:2225–2236. doi: 10.1084/jem.20110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grayson MH, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J. Exp. Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung DS, et al. Cutting edge: CD49d+ neutrophils induce FcεRI expression on lung dendritic cells in a mouse model of postviral asthma. J. Immunol. 2010;185:4983–4987. doi: 10.4049/jimmunol.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Kleij H, et al. Evidence for neuronal expression of functional Fc (ε and γ) receptors. J. Allergy Clin. Immunol. 2010;125:757–760. doi: 10.1016/j.jaci.2009.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redhu NS, et al. IgE induces transcriptional regulation of thymic stromallymphopoietin in human airway smooth muscle cells. J. Allergy Clin. Immunol. 2011;128:892–896. doi: 10.1016/j.jaci.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 45.Campbell AM, et al. Expression of the high-affinity receptor for IgE on bronchial epithelial cells of asthmatics. Am. J. Respir. Cell Mol. Biol. 1998;19:92–97. doi: 10.1165/ajrcmb.19.1.2648. [DOI] [PubMed] [Google Scholar]
- 46.Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366:421–428. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- 47.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J. Immunol. 2004;173:5275–5282. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 48.Suto H, et al. Mast cell–associated TNF promotes dendritic cell migration. J. Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 49.Dudeck A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Otsuka A, et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE. 2011;6:e25538. doi: 10.1371/journal.pone.0025538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface FcεRI. J. Immunol. 2001;167:1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 52.Furuichi K, Rivera J, Isersky C. The receptor for immunoglobulin E on rat basophilic leukemia cells: effect of ligand binding on receptor expression. Proc. Natl. Acad. Sci. USA. 1985;82:1522–1525. doi: 10.1073/pnas.82.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu C, MacGlashan D., Jr IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol. Lett. 1996;52:129–134. doi: 10.1016/0165-2478(96)02599-0. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi M, et al. IgE enhances mouse mast cell FcεRI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J. Exp. Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lantz CS, et al. IgE regulates mouse basophil FcεRI expression in vivo. J. Immunol. 1997;158:2517–2521. [PubMed] [Google Scholar]
- 56.MacGlashan DW, Jr, et al. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 57.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J. Allergy Clin. Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi M, et al. IgE enhances Fcε receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fcε receptor I expression and mediator release. J. Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- 59.Kashiwakura J, Otani IM, Kawakami T. Monomeric IgE and mast cell development, survival and function. Adv. Exp. Med. Biol. 2011;716:29–46. doi: 10.1007/978-1-4419-9533-9_3. [DOI] [PubMed] [Google Scholar]
- 60.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 61.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 62.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 63.Moiseeva EP, Bradding P. Mast cells in lung inflammation. Adv. Exp. Med. Biol. 2011;716:235–269. doi: 10.1007/978-1-4419-9533-9_13. [DOI] [PubMed] [Google Scholar]
- 64.Groot Kormelink T, Thio M, Blokhuis BR, Nijkamp FP, Redegeld FA. Atopic and non-atopic allergic disorders: current insights into the possible involvement of free immunoglobulin light chains. Clin. Exp. Allergy. 2009;39:33–42. doi: 10.1111/j.1365-2222.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 65.Ring J, Grosber M, Mohrenschlager M, Brockow K. Anaphylaxis: acute treatment and management. Chem. Immunol. Allergy. 2010;95:201–210. doi: 10.1159/000315953. [DOI] [PubMed] [Google Scholar]
- 66.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayr SI, et al. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J. Immunol. 2002;169:2061–2068. doi: 10.4049/jimmunol.169.4.2061. [DOI] [PubMed] [Google Scholar]
- 68.Yu M, et al. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu M, et al. Identification of an IFN-γ/mast cell axis in a mouse model of chronic asthma. J. Clin. Invest. 2011;121:3133–3143. doi: 10.1172/JCI43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. quiz 464. [DOI] [PubMed] [Google Scholar]
- 71.Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc. Natl. Acad. Sci. USA. 2000;97:10509–10513. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 73.Ho LH, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcεRI signals. J. Leukoc. Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 74.Iikura M, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 75.Hua X, Chason KD, Patel JY, Naselsky WC, Tilley SL. IL-4 amplifies the proinflammatory effect of adenosine in human mast cells by changing expression levels of adenosine receptors. PLoS ONE. 2011;6:e24947. doi: 10.1371/journal.pone.0024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olivera A, Rivera J. An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv. Exp. Med. Biol. 2011;716:123–142. doi: 10.1007/978-1-4419-9533-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beer F, et al. Role of β-chemokines in mast cell activation and type I hypersensitivity reactions in the conjunctiva: in vivo and in vitro studies. Immunol. Rev. 2007;217:96–104. doi: 10.1111/j.1600-065X.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 78.Galli SJ, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 79.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 81.Nakae S, et al. Mast cells enhance T cell activation: importance of mast cell–derived TNF. Proc. Natl. Acad. Sci. USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakae S, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 83.Gri G, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40–OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sibilano R, et al. Technical advance: soluble OX40 molecule mimics regulatory T cell modulatory activity on FcεRI-dependent mast cell degranulation. J. Leukoc. Biol. 2011;90:831–838. doi: 10.1189/jlb.1210651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bryce PJ, et al. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 86.Liu FT. Regulatory roles of galectins in the immune response. Int. Arch. Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- 87.Ge XN, et al. Allergen-induced airway remodeling is impaired in galectin-3–deficient mice. J. Immunol. 2010;185:1205–1214. doi: 10.4049/jimmunol.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saegusa J, et al. Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am. J. Pathol. 2009;174:922–931. doi: 10.2353/ajpath.2009.080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26:25–31. doi: 10.1016/j.it.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 90.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 91.Kaur D, et al. Mast cell-airway smooth muscle crosstalk: the role of thymic stromal lymphopoietin. [3 November 2011];Chest. doi: 10.1378/chest.11-1782. published online, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laprise C, et al. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genomics. 2004;5:21. doi: 10.1186/1471-2164-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waern I, et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J. Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 94.Brightling CE, et al. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 95.Dougherty RH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in TH2-high asthma. J. Allergy Clin. Immunol. 2010;125:1046–1053. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balzar S, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur. Respir. J. 2002;19:879–885. doi: 10.1183/09031936.02.00275802. [DOI] [PubMed] [Google Scholar]
- 98.Carroll NG, Mutavdzic S, James AL. Increased mast cells and neutrophils in submucosal mucous glands and mucus plugging in patients with asthma. Thorax. 2002;57:677–682. doi: 10.1136/thorax.57.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryan JJ, et al. Mast cell homeostasis: a fundamental aspect of allergic disease. Crit. Rev. Immunol. 2007;27:15–32. doi: 10.1615/critrevimmunol.v27.i1.20. [DOI] [PubMed] [Google Scholar]
- 100.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J. Allergy Clin. Immunol. 2011;128:495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 101.Barnes PJ. Glucocorticosteroids: current and future directions. Br. J. Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wershil BK, et al. Dexamethasone or cyclosporin A suppress mast cell-leukocyte cytokine cascades. Multiple mechanisms of inhibition of IgE- and mast cell–dependent cutaneous inflammation in the mouse. J. Immunol. 1995;154:1391–1398. [PubMed] [Google Scholar]
- 103.Matsuda K, et al. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J. Allergy Clin. Immunol. 2005;116:1357–1363. doi: 10.1016/j.jaci.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 104.Kato A, et al. Dexamethasone and FK506 inhibit expression of distinct subsets of chemokines in human mast cells. J. Immunol. 2009;182:7233–7243. doi: 10.4049/jimmunol.0801375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin. Exp. Allergy. 2005;35:408–416. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]
- 106.Pelaia G, et al. Update on optimal use of omalizumab in management of asthma. J. Asthma Allergy. 2011;4:49–59. doi: 10.2147/JAA.S14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rafi A, et al. Effects of omalizumab in patients with food allergy. Allergy Asthma Proc. 2010;31:76–83. doi: 10.2500/aap.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 108.Sheinkopf LE, Rafi AW, Do LT, Katz RM, Klaustermeyer WB. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc. 2008;29:530–537. doi: 10.2500/aap.2008.29.3160. [DOI] [PubMed] [Google Scholar]
- 109.Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK. Treatment of chronic autoimmune urticaria with omalizumab. J. Allergy Clin. Immunol. 2008;122:569–573. doi: 10.1016/j.jaci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J. Allergy Clin. Immunol. 2006;117:1415–1418. doi: 10.1016/j.jaci.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 111.Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J. Allergy Clin. Immunol. 2011;128:202–209. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 112.Prussin C, et al. Omalizumab treatment downregulates dendritic cell FcεRI expression. J. Allergy Clin. Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 113.Chang TW, Shiung YY. Anti-IgE as a mast cell–stabilizing therapeutic agent. J. Allergy Clin. Immunol. 2006;117:1203–1212. doi: 10.1016/j.jaci.2006.04.005. quiz 1213. [DOI] [PubMed] [Google Scholar]
- 114.MacGlashan D., Jr Therapeutic efficacy of omalizumab. J. Allergy Clin. Immunol. 2009;123:114–115. doi: 10.1016/j.jaci.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 115.Wang CY, et al. Synthetic IgE peptide vaccine for immunotherapy of allergy. Vaccine. 2003;21:1580–1590. doi: 10.1016/s0264-410x(02)00732-6. [DOI] [PubMed] [Google Scholar]
- 116.Peng Z, et al. Novel IgE peptide-based vaccine prevents the increase of IgE and down-regulates elevated IgE in rodents. Clin. Exp. Allergy. 2007;37:1040–1048. doi: 10.1111/j.1365-2222.2007.02741.x. [DOI] [PubMed] [Google Scholar]
- 117.McDonnell JM, et al. Structure based design and characterization of peptides that inhibit IgE binding to its high-affinity receptor. Nat. Struct. Biol. 1996;3:419–426. doi: 10.1038/nsb0596-419. [DOI] [PubMed] [Google Scholar]
- 118.McDonnell JM, et al. Structure-based design of peptides that inhibit IgE binding to its high-affinity receptor FcεRI. Biochem. Soc. Trans. 1997;25:387–392. doi: 10.1042/bst0250387. [DOI] [PubMed] [Google Scholar]
- 119.Sandomenico A, et al. IgE-binding properties and selectivity of peptide mimics of the FcvarεRI binding site. Mol. Immunol. 2009;46:3300–3309. doi: 10.1016/j.molimm.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 120.Sandomenico A, Monti SM, Palumbo R, Ruvo M. A new FcεRI receptor-mimetic peptide (PepE) that blocks IgE binding to its high affinity receptor and prevents mediator release from RBL 2H3 cells. J. Pept. Sci. 2011;17:604–609. doi: 10.1002/psc.1368. [DOI] [PubMed] [Google Scholar]
- 121.Rossi M, et al. Anti-allergic properties of a new all-D synthetic immunoglobulin-binding peptide. Mol. Immunol. 2008;45:226–234. doi: 10.1016/j.molimm.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura GR, Starovasnik MA, Reynolds ME, Lowman HB. A novel family of hairpin peptides that inhibit IgE activity by binding to the high-affinity IgE receptor. Biochemistry. 2001;40:9828–9835. doi: 10.1021/bi0109360. [DOI] [PubMed] [Google Scholar]
- 123.Nakamura GR, Reynolds ME, Chen YM, Starovasnik MA, Lowman HB. Stable “ζ” peptides that act as potent antagonists of the high-affinity IgE receptor. Proc. Natl. Acad. Sci. USA. 2002;99:1303–1308. doi: 10.1073/pnas.022635599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stamos J, et al. Convergent recognition of the IgE binding site on the high-affinity IgE receptor. Structure. 2004;12:1289–1301. doi: 10.1016/j.str.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 125.Buku A, Mendlowitz M, Condie BA, Price JA. Histamine-releasing activity and binding to the FcεRI alpha human mast cell receptor subunit of mast cell degranulating peptide analogues with alanine substitutions. J. Med. Chem. 2003;46:3008–3012. doi: 10.1021/jm0204892. [DOI] [PubMed] [Google Scholar]
- 126.Buku A, Keselman I, Lupyan D, Mezei M, Price JA. Effective mast cell degranulating peptide inhibitors of the IgE/FcεRI receptor interaction. Chem. Biol. Drug Des. 2008;72:133–139. doi: 10.1111/j.1747-0285.2008.00684.x. [DOI] [PubMed] [Google Scholar]
- 127.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fcγ-Fcε bifunctional fusion protein inhibits FcεRI-mediated degranulation. Nat. Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Akin C, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp. Hematol. 2003;31:686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 129.Gotlib J, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Juurikivi A, et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Ann. Rheum. Dis. 2005;64:1126–1131. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dubreuil P, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS ONE. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsubara S, et al. Inhibition of spleen tyrosine kinase prevents mast cell activation and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2006;173:56–63. doi: 10.1164/rccm.200503-361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rossi AB, et al. Identification of the Syk kinase inhibitor R112 by a human mast cell screen. J. Allergy Clin. Immunol. 2006;118:749–755. doi: 10.1016/j.jaci.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 134.Tebib J, et al. Masitinib in the treatment of active rheumatoid arthritis: results of a multicentre, open-label, dose-ranging, phase 2a study. Arthritis Res. Ther. 2009;11:R95. doi: 10.1186/ar2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szefler SJ, Dakhama A. New insights into asthma pathogenesis and treatment. Curr. Opin. Immunol. 2011;23:801–807. doi: 10.1016/j.coi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 136.Gonem S, Desai D, Siddiqui S, Brightling CC. Evidence for phenotype-driven treatment in asthmatic patients. Curr. Opin. Allergy Clin. Immunol. 2011;11:381–385. doi: 10.1097/ACI.0b013e328348a8f9. [DOI] [PubMed] [Google Scholar]
- 137.Szefler SJ. Advances in pediatric asthma in 2011: moving forward. J. Allergy Clin. Immunol. 2012;129:60–68. doi: 10.1016/j.jaci.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 138.Joseph M, et al. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur. J. Immunol. 1997;27:2212–2218. doi: 10.1002/eji.1830270914. [DOI] [PubMed] [Google Scholar]
- 139.Hasegawa S, et al. Functional expression of the high affinity receptor for IgE (FcεRI) in human platelets and it’s intracellular expression in human megakaryocytes. Blood. 1999;93:2543–2551. [PubMed] [Google Scholar]
- 140.Gounni AS, et al. Human airway smooth muscle cells express the high affinity receptor for IgE (FcεRI): a critical role of Fc ε RI in human airway smooth muscle cell function. J. Immunol. 2005;175:2613–2621. doi: 10.4049/jimmunol.175.4.2613. [DOI] [PubMed] [Google Scholar]
- 141.Ganguly S, et al. Neural adrenergic/cyclic AMP regulation of the immunoglobulin E receptor α-subunit expression in the mammalian pinealocyte: a neuroendocrine/immune response link? J. Biol. Chem. 2007;282:32758–32764. doi: 10.1074/jbc.M705950200. [DOI] [PubMed] [Google Scholar]
- 142.Dahlin JS, Ivarsson MA, Heyman B, Hallgren J. IgE immune complexes stimulate an increase in lung mast cell progenitors in a mouse model of allergic airway inflammation. PLoS ONE. 2011;6:e20261. doi: 10.1371/journal.pone.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maeda K, et al. Murine follicular dendritic cells and low affinity Fc receptors for IgE (FcεRII) J. Immunol. 1992;148:2340–2347. [PubMed] [Google Scholar]
- 144.Yu LC, et al. Intestinal epithelial CD23 mediates enhanced antigen transport in allergy: evidence for novel splice forms. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G223–G234. doi: 10.1152/ajpgi.00445.2002. [DOI] [PubMed] [Google Scholar]
- 145.Li H, et al. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131:47–58. doi: 10.1053/j.gastro.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 146.Vouldoukis I, et al. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the FcεRII/CD23 surface antigen. Proc. Natl. Acad. Sci. USA. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takizawa F, Adamczewski M, Kinet JP. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as FcγRII and FcγRIII. J. Exp. Med. 1992;176:469–475. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirano M, et al. IgEb immune complexes activate macrophages through FcγRIV binding. Nat. Immunol. 2007;8:762–771. doi: 10.1038/ni1477. [DOI] [PubMed] [Google Scholar]
- 149.Mancardi DA, et al. FcγRIV is a mouse IgE receptor that resembles macrophage FcεRI in humans and promotes IgE-induced lung inflammation. J. Clin. Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Craig SS, et al. Immunoelectron microscopic localization of galectin-3, an IgE binding protein, in human mast cells and basophils. Anat. Rec. 1995;242:211–219. doi: 10.1002/ar.1092420210. [DOI] [PubMed] [Google Scholar]
- 151.Chen HY, et al. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J. Immunol. 2006;177:4991–4997. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- 152.Truong MJ, et al. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/ε BP) of the S-type lectin family: role in IgE-dependent activation. J. Exp. Med. 1993;177:243–248. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ho MK, Springer TA. Mac-2, a novel 32,000 Mr mouse macrophage subpopulationspecific antigen defined by monoclonal antibodies. J. Immunol. 1982;128:1221–1228. [PubMed] [Google Scholar]
- 154.Sato S, Hughes RC. Control of Mac-2 surface expression on murine macrophage cell lines. Eur. J. Immunol. 1994;24:216–221. doi: 10.1002/eji.1830240134. [DOI] [PubMed] [Google Scholar]
- 155.Liu FT, et al. Expression and function of galectin-3, a β-galactoside-binding lectin, in human monocytes and macrophages. Am. J. Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 156.Novak R, Dabelic S, Dumic J. Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. [14 December 2011];Biochim. Biophys. Acta. doi: 10.1016/j.bbagen.2011.11.014. published online, [DOI] [PubMed] [Google Scholar]
- 157.Truong MJ, et al. IgE-binding molecules (Mac-2/ε BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur. J. Immunol. 1993;23:3230–3235. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- 158.Haines KA, Flotte TJ, Springer TA, Gigli I, Thorbecke GJ. Staining of Langerhans cells with monoclonal antibodies to macrophages and lymphoid cells. Proc. Natl. Acad. Sci. USA. 1983;80:3448–3451. doi: 10.1073/pnas.80.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smetana K, et al. Coexpression of binding sites for A(B) histo-blood group trisaccharides with galectin-3 and Lag antigen in human Langerhans cells. J. Leukoc. Biol. 1999;66:644–649. doi: 10.1002/jlb.66.4.644. [DOI] [PubMed] [Google Scholar]
- 160.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol. Rev. 2009;230:114–127. doi: 10.1111/j.1600-065X.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 161.Acosta-Rodríguez EV, et al. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during. Trypanosoma cruzi infection. J. Immunol. 2004;172:493–502. doi: 10.4049/jimmunol.172.1.493. [DOI] [PubMed] [Google Scholar]
- 162.Vray B, et al. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology. 2004;14:647–657. doi: 10.1093/glycob/cwh068. [DOI] [PubMed] [Google Scholar]
- 163.Kay AB. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 164.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-α. J. Clin. Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nagai H, et al. Role of mast cells in the onset of IgE-mediated late-phase cutaneous response in mice. J. Allergy Clin. Immunol. 2000;106:S91–S98. doi: 10.1067/mai.2000.106778. [DOI] [PubMed] [Google Scholar]
- 166.Haselden BM, Kay AB, Larche M. Immunoglobulin E–independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J. Exp. Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 168.Humbert M, et al. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol. Today. 1999;20:528–533. doi: 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]