Abstract
A novel, fast and sensitive 3200 QTRAP LC–MS/MS method was validated for rapamycin analysis in the rabbit eye following 0.2% administration of nanomicellar eye drop formulation. The LC–MS/MS technique was developed with electrospray ionization (ESI) in positive mode. Rapamycin was extracted from individual eye tissues and fluids by a simple protein precipitation method. Samples were reconstituted in 200 μL of 80% of acetonitrile in water containing 0.05% formic acid. Twenty microliter of the sample was injected on LC–MS/MS. Chromatographic separations was achieved on reversed phase C 8 Xterra column, 50 mm × 4.6 mm, 5 μm. Multiple reactions monitoring (MRM) transition m/z 936.6/409.3 for rapamycin and 734.4/576.5 for erythromycin were employed as internal standard. The calibration curves were linear r2 > 0.9998 over the concentration range from 2.3 ng/mL to 1000.0 ng/mL. Rapamycin was found to be stable in ocular tissue homogenates for 6 weeks at a refrigerated −80 °C and −20 °C temperatures. Rapamycin concentration was found to be 2260.7 ± 507.1 (mean ± S.D.) ng/g tissue and 585.5 ± 80.1 (mean ± S.D.) ng/g tissue in the cornea and iris ciliary muscle, respectively. This method has two advantages. First, a volatile base was used in the extraction procedure, which is easy to evaporate and generate consistent results. Second, the sodium adduct is employed that was stable in non-ammoniated mobile phase. The method demonstrates that absorption of rapamycin by a topical application of 0.2% rapamycin nanomicellar formulation generates therapeutically effective concentrations in the anterior segment of the eye.
Keywords: Rapamycin (Sirolimus), 3200 QTRAP LC–MS/MS bioanalytical method validation, Protein precipitation extraction, Rabbit anterior ocular tissue distribution study, 0.2% rapamycin nanomicellar formulation
1. Introduction
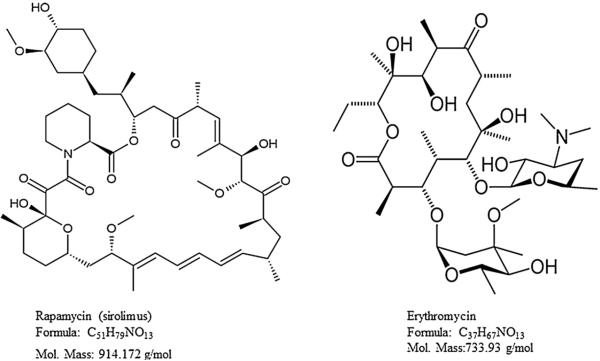
Rapamycin is an immunosuppressant drug and it was extracted from the bacterium Streptomyces hygroscopicus [1]. Rapamycin also known as sirolimus, was approved by the Food and Drug Administration as a mammalian target of rapamycin (mTOR) inhibitor. The mTOR pathway is involved in many biological processes usually in the development of cutaneous melanoma tumors. This pathway is an important target for anticancer drug development, which was approved for human use to treat advanced renal cell carcinoma [2]. Recently, rapamycin was investigated for an immunosuppressive treatment for the prevention of allograft rejections following corneal transplantation, as well as for chronic inflammatory disorders such as uveitis, corneal and choroidal neovascularization, and diabetic macular edema. It specially targets ocular surface diseases, including keratoconjunctivitis sicca, vernal conjunctivitis, or topical blepharitis [3]. Rapamycin also inhibits vascular endothelial growth factor (VEGF) production, and alters the response of endothelial cells to VEGF stimulation [4]. Usually, eye drops of glucocorticoids (e.g., prednisolone or dexamethasone) are commonly prescribed for this purpose. However, there are multiple pathologies where steroids remain ineffective, and induce intraocular pressure [5]. Therefore, there is a need to develop a novel topical formulation of rapamycin for ocular treatments. In order to evaluate the rapamycin absorption from this formulation and to estimate its concentrations in the rabbit eye, a sensitive, fast, and reliable bioanalytical method is required. The chemical structures of rapamycin and erythromycin as an internal standard (IS) are shown in Fig. 1.
Fig. 1.
Chemical structure, formula and molecular mass of rapamycin and erythromycin.
Various analytical techniques including HPLC, LC–MS and LC–MS/MS have been reported for the analysis of rapamycin in different cellular matrices with various extraction procedures [6]. The high performance liquid chromatography (HPLC) method was reported by liquid–liquid extraction with tertiary-butyl methyl ether and ethanol mixture. In this method, 1.0 mL of human whole blood sample volume was used with a linear concentration range 2–100 ng/mL [7]. But, this procedure requires a large aliquot volume and this procedure is not suitable for micro aliquot sample analysis. Later, rapamycin separation was achieved on octadecyl silyl (ODS)-silica gel followed by protein precipitation methods and these were lengthy procedures [8,9]. This method was further modified for the analysis of rapamycin in blood using C18 solid-phase extraction with 500 μL of blood sample. Quantitative analysis was performed with ammonium adduct [10] by electrospray ionization mass spectrometry. The method was linear over the range 0.2–100.0 μg/mL. This technique was expensive and tedious.
Recently, rapamycin LC/MS/MS method was also reported with a simple high-throughput procedure using online extraction with turbulent flow chromatography. In this method, 200 μL blood sample volume was used. Analyte was detected by APCI mass spectrometry in negative ion mode. This method was described to be linear over the calibration range 2.9–51.2 μg/L [11], but this method was rarely reported. Other reports were also described as sensitive and fast LC–MS method. In this manner rapamycin was separated on octadecyl silyl (ODS)-silica gel and extracted by protein precipitation technique. However, these procedures were laborious and lengthy. Similarly those methods were not able to reduce optimal ion suppression due to presence of zinc sulfates and phosphates that cause ion suppression [8,12]. So far, there is no LC–MS/MS method available in the literature for analysis of rapamycin in a rabbit eye tissue. Therefore, we have developed and validated a sensitive, robust and fast LC–MS/MS method in ocular matrices in order to quantify rapamycin in ocular tissue. The objective of this study is to present with a validated LC–MS/MS method. This technique was used successfully for rapamycin tissue distribution in the anterior segment of the rabbit eye by topical administration of 0.2% rapamycin nanomicellar formulation.
2. Experimental
2.1. Chemical and reagents
Rapamycin and erythromycin were purchased from LC laboratories USA and Sigma Chemicals (St. Louis, MO), respectively. HPLC grade methanol, acetonitrile, triethylamine, and formic acid were procured from Fisher Scientific (New Brunswick, NJ). Ultrapure water from MilliQ-system (Millipore, Molshecin France) was used through the study. All chemicals were of HPLC grade and used as received without further purification. Xterra reverse phase HPLC column procured from Waters Corporation USA.
2.2. Solutions and validation samples
Rapamycin and erythromycin as internal standard (IS) were dissolved in methanol to obtain, 1.0 mg/mL stock solutions, and were gradually diluted by the serial dilution method using calibrated pipettes (2–20 μL, 10–100 μL and 100–1000 μL), in order to obtain working stock dilutions at decreasing concentrations (10, 8.5, 7.5, 5.0, 2.5, 1.0, 0.1 and 0.023 μg/mL). These solutions were used for the preparation of mass spectrometry optimization, calibration curve and quality control standards in methanol and ocular tissue homogenates. These stock solutions and stock dilutions were stored at below 10 °C, and at −20 °C, respectively. For the preparation of calibration curve standards, aliquots of 200 μL of blank ocular tissue homogenates were spiked with 20 μL of each of the rapamycin working stock dilutions in order to obtain eight calibration curve standards at decreasing concentrations (1000, 850, 750.0, 500.0, 250.0, 100.0, 10.0 and 2.3 ng/mL). Similarly, quality control (QC) samples were independently prepared at four levels of concentration (800.0, 480.0, 10 and 2.3 ng/mL) in tissue homogenates from the working stock dilutions. Stock concentration was corrected by the formula below.
2.3. Mass spectrometry optimization
Mass spectrometry parameters optimization of rapamycin and erythromycin was carried out by a standard stock dilution (200.0 ng/mL) in methanol through direct infusion into the mass spectrometer with the inbuilt Harvard infusion pump. Data from rapamycin and erythromycin were first acquired in full scan from the range between m/z 50 and 1000 in order to identify the most suitable parent ion for MS/MS experiments. The sodium adduct [M+Na]+ at m/z 936.6 was selected as the parent ion for rapamycin and fragmented. The MS/MS parent ion was still preserved as a parent in the MS2 spectrum and it was together with two other daughter ions at m/z 345.5 and 409.3. Erythromycin was also detected by using the proton adduct [M+H]+ ion at m/z 734.4 as the parent (under the same instrumental parameters), daughter ions at m/z 158.2 and 576.5 were optimized.
2.4. LC–MS/MS chromatographic separation
LC–MS/MS chromatographic separation was achieved on reversed phase C-8 silica gel material. The column oven temperature was maintained at 40 °C. Extracted calibration and QC samples were reconstituted in 200 μL of mobile phase. Twenty microliters was injected on LC–MS/MS. Ionspray source temperature 350 °C and ionspray voltages 5500 V were optimized. Mass spectrometry data was acquired in positive ion mode and processed using Analyst software (version 1.4.2, AB Sciex). An LC–MS/MS analysis, rapamycin was eluted at retention time 3.011 min and erythromycin, as IS was at 1.318 min. The curtain gas (CUR) was at 40.0 psi, the nebulizer source gas 1 at 40.0 psi, and the turbo ion source gas 2 at 45.0 psi was utilized. Declustering potential 105.0 V, and entrance potential 10.0 V were optimized. Rapamycin fragmentation was induced by collisionally activated dissociation (CAD) with nitrogen gas. The collision gas pressure was set at 2.0 psi for MRM quantitation. The collision energy 75.0 V for rapamycin and 30.0 V for erythromycin and the collision cell exit potential 10.0 V for rapamycin and 8.0 V for erythromycin were utilized. The erythromycin scan was optimized by the proton adduct [M+H] + ion at m/z 734.4 as parent ion. The daughter ion at m/z 576.5 was selected. Dwell time 200 ms was employed. Chromatographic separation was carried out on a UFLC Shimadzu prominence system consisting of LC-20AD liquid chromatography low pressure gradient pump, SPD-M20A diode array detector, SIL-20AST auto sampler, and DGU-20As degasser (Shimadzu USA Manufacturing Inc., 3111 Lomita Boulevard, Torrance, California 90505, USA) with a reverse phase Xterra MS C 8 column 50 × 4.6 mm, i.d, 5 μm (Waters Corporation, 32 Maple Street, Massachusetts 01757-3696, USA). Isocratic mobile phase composed of 80% acetonitrile in water mixture containing 0.05% of formic acid at a flow rate of 0.25 mL/min was used. As sample injection volume of 20.0 μL was used, and total analytical run time was 6.0 min.
2.5. Sample preparation and extraction
Homogenates of rabbit ocular blank tissue were used for calibration curve and QC standards. A simple, clean and easy protein precipitation extraction technique was developed. Two hundred microliters aliquot of tissue homogenate sample was mixed with 25 μL (5 μg/mL) of internal standard. The mixture was vortex-mixed for 30 s, then 25 μL of a 50% triethyl amine in methanol was added, and the solution was vortex- mixed for additional 2.0 min. Then, 1 mL of methanol was added and this mixture was again vortex-mixed for 2 min in order to precipitate tissue protein. Then, the final mixture was centrifuged at 10000 rpm for 30 min at 4 °C. The supernatant was collected and dried under speed vacuum (Genevac DD-4X evaporator, Genevac Inc., 815 Route 208, Gardiner, NY 12525, USA) at 37 °C for 90 min. Dried samples were stored at −80 °C until further analysis. Samples were reconstituted in 200 μL of mobile phase and analyzed by LC–MS/MS.
2.6. The specificity and selectivity
The specificity and selectivity of the method were tested by analyzing six ocular blank samples. We processed LLOQ (n = 6) in order to assess the blank interference at the peak of interest. The percentage of blank interference was calculated by comparing mean peak area of LLOQ of the analyte for peak response obtained from the blank samples. Peak areas of blanks co-eluting with the analyte should not be more than 20% of the mean peak area at the LLOQ.
2.7. Precision and accuracy
Intra-day and inter-day precision and accuracy experiments were performed by analyzing extracted calibration curve and QC standards. The standard samples were prepared based on the procedure described in our previously published LC–MS/MS method [13]. Accuracy was reported as the percentage difference between the mean concentrations divided by the nominal concentration multiplied by 100. Accuracy of the method must be between 85% and 115% of the nominal value in all the standards, except at the lower limit of quantitation (LLOQ) level, which is 80–120% according to the guidance for industry bioanalytical method validation in Food and Drug Administration guidelines of May 2001(www.fda.gov). Precision was calculated using the coefficient of variation (CV) (standard deviation/mean concentration) multiplied by 100. Precision of the method should be within 15% of the nominal concentration except at the LLOQ, which is within 20%.
2.8. Matrix effect
Matrix effect was evaluated by analyzing of six replicates of each two sets at low, middle and high quality control standards of post-spiked (extracted blank tissue homogenates), and spiked in aqueous samples (represents no matrix effect) at the same concentration for rapamycin. One concentration of IS spiked in both the sets. Ion suppression was calculated by comparing mean peak area ratios of analyte and IS generated from the post-spiked quality controls of ocular tissue homogenate samples to aqueous spiked quality control standards. A relative matrix effect was estimated by comparing mean peak area ratios of the analytes to internal standard obtained from the post-spiked QC (blank ocular matrix samples versus aqueous standards). Thirty-six blank ocular matrix samples were processed and extracted for low, middle, and high QC standards according to the sample preparation and extraction procedure described in Section 2.6. Quality control standards, LQC, MQC and HQC were prepared in a blank reconstitution solution to obtain the same concentrations (800.0, 480.0 and 10.0 ng/mL).
2.9. Recovery
Extraction recovery of rapamycin was estimated by analysis of two sets of six replicates of each at three quality control standards of plasma spiked and post-spiked (representing 100% recovery) samples with the IS. Extraction recovery was measured by comparing mean peak area ratios of analyte and IS of ocular tissue/fluid homogenates to unextracted (post-spiked) quality control standards. Recovery quality control samples were made in a blank matrix and were extracted to get equal concentrations (800.0, 480.0 and 10.0 ng/mL).
2.10. Stability in ocular matrix
Stability samples were prepared in tissue homogenates and were stored at −80 °C and −20 °C for several weeks to estimate the degradation of analyte in the matrix. Stability QC samples were extracted along with the freshly spiked calibration curve standards and were tested on the same day. The extracted QC samples were stored for 2 days at 4 °C, and analyzed with the freshly spiked and processed calibration standards for in-injector and post-processed stability. The stability samples were frozen and stored for the next 6 weeks at −80 °C. Freeze–thaw stability QC samples were freezethawed for three cycles, and the analysis is summarized in Table 2. Stability experiments have shown that rapamycin had an adequate stability in matrix. These samples were stored for 6 weeks to anticipate the original sample’s storage conditions (Bench top, freeze storage, freeze thaw and processed storage stability) runs as described in our previous publication [13].
Table 2.
Ocular tissue homogenate stability results in anterior and posterior eye tissues.
| Nominal concentration (ng/mL) | Cornea |
Iris ciliary body |
Lens |
|||
|---|---|---|---|---|---|---|
| LQC 10 |
HQC 800 |
LQC 10 |
HQC 800 |
LQC 10 |
HQC 800 |
|
| Anterior chamber eye tissues | ||||||
| Freeze storage for 6 weeks at (−80 °C) | ||||||
| Mean calculated concentration (ng/mL) | 10.3 | 768 | 9.4 | 756 | 8.9 | 843 |
| CV (%) | 4.9 | 4.0 | 9.6 | 11.8 | 13.5 | 1.5 |
| Bias(%) | 2.9 | −4.2 | −6.4 | −5.8 | −12.4 | 5.1 |
| Freeze thaw (−80 °C) stability (3 cycles) | ||||||
| Mean calculated concentration (ng/mL) | 9.2 | 876 | 9.1 | 698 | 8.9 | 856 |
| CV (%) | 5.4 | 3.5 | 9.9 | 12.8 | 13.5 | 1.5 |
| Bias (%) | −8.7 | 8.7 | −9.9 | −14.6 | −12.4 | 6.5 |
| Autosampler in injector storage stability (2 days) | ||||||
| Mean calculated concentration (ng/mL) | 8.9 | 708 | 8.8 | 876 | 11.1 | 893 |
| CV (%) | 5.6 | 4.3 | 10.2 | 10.2 | 10.8 | 1.4 |
| Bias (%) | −12.4 | −13.0 | −13.6 | 8.7 | 9.9 | 10.4 |
| Post extracted samples storage stability (15 days) at (−80 °C) | ||||||
| Mean calculated concentration (ng/mL) | 8.5 | 850.9 | 9.1 | 688.0 | 8.4 | 890.0 |
| CV (%) | 5.9 | 3.6 | 9.9 | 12.9 | 14.3 | 1.4 |
| Bias (%) | −17.6 | 6.0 | −9.9 | −16.3 | −19.0 | 10.1 |
| Bench top stability (4 h) at 25 °C | ||||||
| Mean calculated concentration (ng/mL) | 8.6 | 879.0 | 8.9 | 765.0 | 9.9 | 901.0 |
| CV (%) | 5.8 | 3.5 | 10.1 | 11.6 | 12.1 | 1.4 |
| Bias (%) | −16.3 | 9.0 | −12.4 | −4.6 | −1.0 | 11.2 |
| Nominal concentration (ng/mL) | Aqueous humor |
Sclera |
Vitreous humor |
Retina choroid |
||||
|---|---|---|---|---|---|---|---|---|
| LQC 10 |
HQC 800 |
LQC 10 |
HQC 800 |
LQC 10 |
HQC 800 |
LQC 10 |
HQC 800 |
|
| Posterior chamber eye tissues | ||||||||
| Freeze storage for 6 weeks at (−80 °C) | ||||||||
| Mean calculated concentration (ng/mL) | 9.7 | 867.0 | 9.1 | 679.0 | 9.3 | 847.0 | 11.2 | 893.0 |
| CV (%) | 4.9 | 2.7 | 9.4 | 11.8 | 13.5 | 1.5 | 16.5 | 2.9 |
| Bias (%) | −3.1 | 7.7 | −9.9 | −17.8 | −7.5 | 5.5 | 10.7 | 10.4 |
| Freeze thaw (−80 °C) stability (3 cycles) | ||||||||
| Mean calculated concentration (ng/mL) | 8.9 | 908 | 9.1 | 809 | 10.2 | 870 | 11 | 805 |
| CV (%) | 5.6 | 2.3 | 9.9 | 11.0 | 11.8 | 1.4 | 15.5 | 2.8 |
| Bias (%) | 12.4 | 11.9 | −9.9 | 1.1 | 2.0 | 8.0 | 9.1 | 0.6 |
| Autosampler in injector storage stability (2 days) | ||||||||
| Mean calculated concentration (ng/mL) | 10.9 | 890 | 9.4 | 879 | 9.6 | 871 | 11.4 | 931 |
| CV (%) | 4.6 | 2.3 | 9.6 | 10.1 | 12.5 | 1.4 | 14.9 | 2.4 |
| Bias (%) | 8.3 | 10.1 | −6.4 | 9.0 | −4.2 | 8.2 | 12.3 | 14.1 |
| Post extracted samples storage stability (15 days) at (−80 °C) | ||||||||
| Mean calculated concentration (ng/mL) | 9.5 | 765.0 | 9.1 | 688.0 | 8.4 | 890.0 | 12.0 | 912.0 |
| CV (%) | 3.3 | 7.2 | 2.4 | 7.2 | 1.2 | 4.3 | 3.3 | 7.2 |
| Bias (%) | −5.3 | −4.6 | −9.9 | −16.3 | −19.0 | 10.1 | 16.7 | 12.3 |
| Bench top stability (4 h) at 25 °C | ||||||||
| Mean calculated concentration (ng/mL) | 12.0 | 765.0 | 9.1 | 688.0 | 10.3 | 809.0 | 9.4 | 912.0 |
| CV (%) | 3.3 | 7.2 | 2.4 | 7.2 | 1.2 | 4.3 | 3.3 | 7.2 |
| Bias (%) | 16.7 | −4.6 | −9.9 | −16.3 | 2.9 | 1.1 | −6.4 | 12.3 |
LQC: low quality control; HQC: high quality control, each QC samples were processed in quadruplicate.
2.11. Ocular tissue distribution study
The New Zealand White (NZW) Rabbit weighing approximately 2.5 kg were obtained from Myrtle’s Rabbitry (Thompson Station, TN). Animals were acclimated for 24 h in the UMKC animal facility. All the studies were conducted according to the Association for Research in Vision Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research. Animals were anesthetized prior to the experiment by means of ketamine HCl (35 mg/kg) and xylazine (3.5 mg/kg) administered intramuscularly. Anesthesia was maintained throughout the experiment. The Vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate) and Octoxynol-40 National Formulary (NF) grades were obtained from Eastman chemical company, USA to prepare nanmicellar solution. Two mg of rapamycin was loaded in 1 mL of Vitamin E TPGS and Octoxynol-40 nanomicellar solution in order to generate 0.2% (w/v) rapamycin nanomicellar formulation. Fifty microliter of this rapamycin nanomicellar formulation (0.2%) was instilled topically into the conjunctival sac of the left eye. One minute prior to the instillation of formulation, fifty microliters of buffer was instilled topically into the conjuctival sac of the right eye as control. After a period of 60 min, euthanasia was performed under deep anesthesia with an intravenous injection of sodium pentobarbital through the marginal ear vein. Following euthanasia, the eye balls were enucleated immediately and transferred to a beaker containing ice-cold phosphate buffer (pH 7.4). Enucleated eye balls were washed twice in cold phosphate buffer pH (7.4) to remove any drug adsorbed onto the surface. Aqueous humor (AH) was carefully withdrawn with 1 mL a tuberculin syringe by limbal paracentesis. Anterior ocular tissues (cornea, iris ciliary body and lens) were carefully dissected, dried with Kimwipes®, weighed and were homogenized in 500 μL chilled phosphate buffer (7.4) for about 4 min with a tissue homogenizer (Tissue Tearor, Model 985 370; Dremel Multipro, Racine, WI) in ice bath. Tissue samples (cornea, lens, iris ciliary body and AH) were processed and extracted according to the sample extraction protocol described in Section 2.6. These samples analyzed by the LC–MS/MS are shown in Table 3. The volume of buffer used for tissue homogenization (cornea, lens and iris ciliary body) was 500 μL. No dilution with buffer was made for aqueous humor.
Table 3.
Rapamycin distribution results in anterior segment of the rabbit eye after topical application of 0.2% rapamycin nanomicellar formulation along with the calibration curve standards (STD), and quality control (QC) standards precision & accuracy intra -day assay data, and retention time.
| Name | Nominal Conc. (ng/mL) | Retention time, min (mean, S.D.) | Cal. conc.(ng/mL) (Mean, S.D.) | (%) Accuracy | (%) Precision |
|---|---|---|---|---|---|
| CORNEA | |||||
| STD-1 | 2.3 | 3.104, 0.016 | 1.9, 0.33 | 82.6 | 17.4 |
| STD-2 | 10 | 3.017, 0.071 | 8.5, 1.37 | 85.0 | 16.1 |
| STD-3 | 100 | 3.012, 0.019 | 91.5, 7.03 | 91.6 | 7.7 |
| STD-4 | 250 | 3.018, 0.028 | 211.0, 26.0 | 85.6 | 12.7 |
| STD-5 | 500 | 3.054, 0.047 | 432.9, 62.03 | 86.6 | 14.4 |
| STD-6 | 750 | 3.030, 0.045 | 761.2, 123.0 | 101.5 | 16.2 |
| STD-7 | 850 | 3.024, 0.036 | 801.0, 124.33 | 94.6 | 15.6 |
| STD-8 | 1000 | 3.006, 0.011 | 899.9, 134.0 | 89.9 | 14.9 |
| LLOQQC | 2.3 | 3.024, 0.036 | 1.88, 0.24 | 81.6 | 12.8 |
| LQC | 10 | 3.016, 0.037 | 8.9, 1.31 | 89.0 | 14.7 |
| MQC | 480 | 3.022, 0.038 | 501.0, 71.33 | 104.3 | 14.2 |
| HQC | 800 | 3.041, 0.03 | 721.9, 81.33 | 90.1 | 7.4 |
| Results of 10 samples (mean ± S.D.): 2260.7 ± 507.1 ng/g tissue | |||||
| Iris ciliary muscle | |||||
| STD-1 | 2.3 | 3.002, 0.017 | 2.7, 0.36 | 117.4 | 13.3 |
| STD-2 | 10 | 3.000, 0.061 | 9.5, 0.90 | 95.0 | 9.5 |
| STD-3 | 100 | 3.102, 0.016 | 101.5, 5.6 | 101.5 | 5.5 |
| STD-4 | 250 | 2.995, 0.005 | 267.0, 30.0 | 106.8 | 11.2 |
| STD-5 | 500 | 3.007, 0.083 | 567.9, 32.03 | 113.6 | 5.6 |
| STD-6 | 750 | 3.028, 0.043 | 679.2, 102.0 | 90.5 | 15.0 |
| STD-7 | 850 | 3.012, 0.043 | 900.9, 125.0 | 105.6 | 13.9 |
| STD-8 | 1000 | 3.022, 0.018 | 1100.0, 124.0 | 110.0 | 12.2 |
| LLOQQC | 2.3 | 3.024, 0.036 | 2.5, 0.21 | 108.7 | 8.4 |
| LQC | 10 | 2.989, 0.061 | 10.9, 1.31 | 110.0 | 11.9 |
| MQC | 480 | 2.992, 0.034 | 550.0, 71.00 | 114.3 | 12.2 |
| HQC | 1000 | 3.001, 0.01 | 908.0, 101.33 | 113.1 | 11.4 |
| Results of 10 samples (mean ± S.D.): 585.5 ± 80.1 ng/g tissue | |||||
| Aqueous humor | |||||
| STD-1 | 2.3 | 32000, 0.002 | 1.99, 0.22 | 86.5 | 11.1 |
| STD-2 | 10 | 3.042, 0.061 | 10.9, 0.80 | 109.0 | 7.3 |
| STD-3 | 100 | 3.012, 0.019 | 111.5, 10.60 | 111.5 | 9.5 |
| STD-4 | 250 | 3.018, 0.028 | 245.0, 12.0 | 98.0 | 4.9 |
| STD-5 | 500 | 3.024, 0.047 | 570.0, 23.03 | 114.0 | 4.0 |
| STD-6 | 750 | 3.031, 0.041 | 789.2, 102.0 | 105.5 | 12.9 |
| STD-7 | 850 | 3.024, 0.037 | 906.0, 111.00 | 106.6 | 12.3 |
| STD-8 | 1000 | 3.006, 0.011 | 1113.9, 104.0 | 111.3 | 12.1 |
| LLOQQC | 2.3 | 3.004, 0.016 | 2.6, 0.32 | 89.0 | 10.1 |
| LQC | 10 | 3.018, 0.039 | 8.9, 0.90 | 89.7 | 10.1 |
| MQC | 480 | 3.032, 0.018 | 421.0, 2.60 | 87.3 | 0.6 |
| HQC | 1000 | 3.045, 0.031 | 709.9, 65.00 | 88.6 | 9.2 |
| Results of 10 samples (mean ± S.D.): below limit of quantitation (< 2.3 ng/mL) ng/mg protein | |||||
| LENS | |||||
| STD-1 | 2.3 | 2.958, 0.042 | 2.6, 0.11 | 113.0 | 4.2 |
| STD-2 | 10 | 2.958, 0.048 | 9.9, 0.89 | 99.0 | 9.0 |
| STD-3 | 100 | 2.959, 0.014 | 114.0, 8.90 | 114.0 | 7.8 |
| STD-4 | 250 | 2.952, 0.023 | 230.0, 11.0 | 92.0 | 4.7 |
| STD-5 | 500 | 2.956, 0.024 | 532.0, 20.03 | 106.2 | 3.8 |
| STD-6 | 750 | 2.979, 0.016 | 700.2, 103.0 | 93.3 | 14.7 |
| STD-7 | 850 | 2.969, 0.055 | 768.9, 102.33 | 90.6 | 13.3 |
| STD-8 | 1000 | 3.009, 0.058 | 1089.9, 112.0 | 108.9 | 10.3 |
| LLOQQC | 2.3 | 2.986, 0.065 | 2.03, 0.30 | 88.3 | 114.8 |
| LQC | 10 | 2.949, 0.036 | 9.9, 1.10 | 99.0 | 11.1 |
| MQC | 480 | 2.966, 0.019 | 521.0, 2.40 | 108.5 | 0.5 |
| HQC | 1000 | 2.984, 0.050 | 721.9, 81.33 | 113.6 | 7.8 |
| Results of 10 samples (mean ± S.D.): below limit of quantitation (<2.3 ng/mL) ng/mg protein | |||||
Ten samples of each cornea, iris ciliary muscle, lens, and aqueous humor were analyzed against each calibration curve STD and QC standards separately. BLOQ: below limit of quantitation, Low, middle and High QC: LQC, MQC and HQC, respectively.
3. Results and discussion
3.1. Specificity, selectivity, limit of quantification and linearity of calibration standards
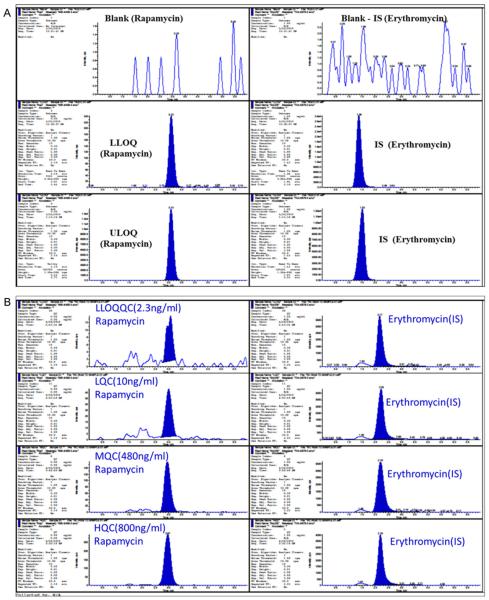
Rapamycin and erythromycin both were clearly extracted and separated from endogenous peaks originating from the blank matrix. The assay condition had adequate specificity for rapamycin, while no interfering peaks were observed at its retention time as shown in Fig. 4A. Erythromycin was used as an IS in this method because it is readily available in our laboratory and has structural similarity. It is also economical compared to other drugs which were used as IS such as deuterated rapamycin (rapamycin 13Cd3), 27-demethoxy-sirolimus, 32-demethoxy rapamycin, tacrolimus and secomycin. However, it also shows consistent recovery and served for our purpose of research. The lower limit of quantitation (LLOQ) for rapamycin was set to 2.3 ng/mL. The chromatographic resolution has been increased significantly by modifying mobile phase composition and the column heater temperature as shown in Fig. 4A. The chromatogram represents that the peak response of rapamycin was proportional to the concentration from LLOQ to ULOQ. In Fig. 4A, we have shown one of the best illustrations of an extracted chromatogram at both the LLOQ and ULOQ level with IS along with the extracted blank. The signal to noise ratio (S/N) of rapamycin was determined at LLOQ level, and it was greater than 50. The S/N ratio explains the extraction efficiency and capability that removes all interfering endogenous components which are usually present in the biological matrix. Fig. 4B shows the extracted quality control standard chromatograms in ocular matrix at four different concentration levels of LLOQ, LQC, MQC and HQC.Fig. 4B exhibits the response correlation which is corresponding with the increasing concentration of the quality control standards. This chromatogram also illustrates there is no background intervention in the quality control standard peak and as well as in IS peak windows.
Fig. 4.
(A) Integration algorithmic typical MRM chromatograms for selected extracted samples at lower and upper limit of quantitation (LLOQ and ULOQ) with IS. It shows endogenous peak in extracted blank sample. (top), Signal to noise ratio (S/N) for rapamycin peak at LLOQ level was greater than 50. LLOQ, (2.3 ng/mL), and ULOQ, 800.0 ng/mL) in the left column from top to bottom, erythromycin as an internal standard (IS) blank, at LLOQ level and ULOQ level in the right column from top to bottom in ocular matrix. (B) A typical example of extracted LLOQ (2.3 ng/mL), Low QC (10 ng/mL), middle QC (480 ng/mL) and high QC (800 ng/mL) Rapamycin (analyte) (m/z) [M+Na]+: 936.6/409.3, Erythromycin (internal standard): (m/z) [M+H]+ 734.4/576.5 MRM quantitation chromatograms in ocular tissue matrices.
3.2. Accuracy and precision
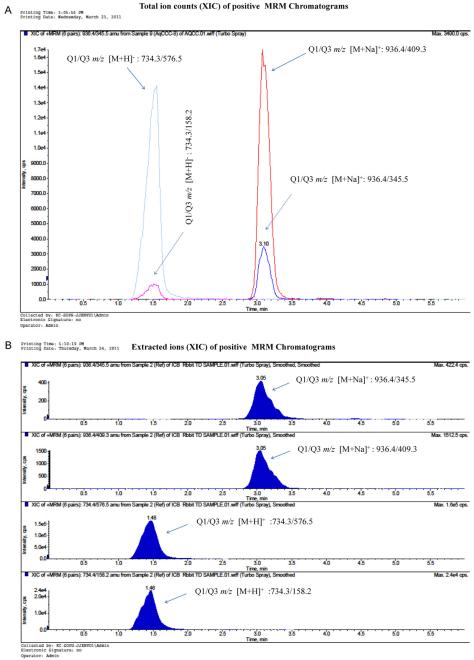
The best linear fit and least-square residuals for the calibration curve were achieved with a 1/X2 weighing factor, giving a mean linear regression equation for the calibration curve. The assay was linear over the range 2.3–1000 ng/mL with r2 (n = 6) ≤0.993 with a mean and standard deviation (S.D.). A regression equation for rapamycin, y = 0.0208 (0.0016) x + 0.0043 (0.0073) was obtained. Inter-and intra-day performance of validation quality control results are summarized in Table 1, showing both accuracy and precision for the drug concentrations of 2.3–800 ng/mL. The lower limit of quantitation (LLOQ) with inter-day coefficients of variation ranged between 0.3% and 17.2% for cornea, iris ciliary body, lens, aqueous humor, sclera, vitreous humor, and retina choroid as shown in Table 1. Regression constant describes the relationship between expected results and analyzed results and was linear, r2 ≤ 0.9998 for rapamycin, in the entire tissue matrix. Fig. 4B. represents an example of extracted validation quality control of LLOQQC (2.3 ng/mL), LQC (10 ng/mL), MQC (480 ng/mL) and HQC (800 ng/mL) chromatogram peak responses in ocular matrix. The chromatogram result explains the methods robustness and accuracy. Fig. 4B shows the correlation of results for all the tissue homogenates derived by the method. Fig. 3(A) and (B) MRM chromatogram demonstrates the total ion (XIC) counts and extracted ion (XIC) counts in positive mode. These Figures have shown the peak response with two different transitions have the same retention times for rapamycin and erythromycin but vary in the response.
Table 1.
Ocular tissue homogenate inter day precision validation results, quality control each (n = 6 of 1 of 3 batches).
| LLOQC) (2.3 ng/mL) | LQC (10 ng/mL) | MQC (480 ng/mL) | HQC (800 ng/mL) | |
|---|---|---|---|---|
| Cornea | ||||
| Mean concentration (ng/mL) | 2.1 | 10.7 | 499.0 | 829.0 |
| Inter run % CV | 10.3 | 8.5 | 5.7 | 1.8 |
| Inter run % Bias | −10.9 | 6.8 | 3.9 | 3.6 |
| Iris ciliary body | ||||
| Mean concentration (ng/mL) | 2.0 | 10.3 | 472.1 | 847.4 |
| Inter run % CV | 10.9 | 3.8 | 2.0 | 2.8 |
| Inter run % Bias | −15.2 | 2.9 | −1.6 | 5.9 |
| Lens | ||||
| Mean concentration (ng/mL) | 2.1 | 9.8 | 562.1 | 807.4 |
| Inter run % CV | 17.2 | 7.9 | 1.0 | 4.0 |
| Inter run % Bias | −10.9 | −2.2 | −3.7 | 0.9 |
| Aqueous humor | ||||
| Mean concentration (ng/mL) | 2.0 | 10.3 | 494.7 | 824.2 |
| Inter run % CV | 7.1 | 4.0 | 4.6 | 3.0 |
| Inter run % bias | −13.0 | 2.8 | 3.1 | 3.0 |
| Sclera | ||||
| Mean concentration (ng/mL) | 2.2 | 9.7 | 432.1 | 797.4 |
| Inter run % CV | 9.9 | 8.2 | 10.9 | 2.3 |
| Inter run % bias | −6.5 | −3.3 | −10.0 | −0.3 |
| Vitreous humor | ||||
| Mean concentration (ng/mL) | 2.6 | 10.2 | 452.1 | 825.4 |
| Inter run % CV | 0.0 | 11.1 | 16.7 | 2.6 |
| Inter run % bias | 13.0 | 2.4 | −5.8 | 3.2 |
| Retina choroid | ||||
| Mean concentration (ng/mL) | 2.4 | 9.5 | 409.6 | 840.4 |
| Inter run % CV | 15.0 | 14.0 | 3.7 | 0.0 |
| Inter run % bias | 2.2 | −4.9 | −14.7 | 5.0 |
LLOQC: lower limit of quality control; LQC: low quality control; MQC: middle quality control; HQC: high quality control.
Fig. 3.
A Total ion count (XIC) of positive scan MRM chromatograms of rapamycin with precursor ion [M+Na]+/product ion (m1) Q1/Q3 m/z: 936.6/409.3 and precursor ion [M+Na]+ product ion (m2) Q1/Q3 m/z: 936.6/345.3 (right side peaks at retention time 3.1 min), Erythromycin with precursor ion [M+H]+ product ion (m1) Q1/Q3 m/z: 734.4/576.3 and precursor ion [M+H]+ product ion (m2) Q1/Q3 m/z: 734.4/158.2 (left side peaks at retention time 1.5 min) in ocular matrix. (B) Extracted ion count (XIC) of positive scan MRM chromatograms of rapamycin with precursor ion [M+Na]+ product ion (m1) Q1/Q3 m/z: 936.6/409.3 and precursor ion [M+Na]+ product ion (m2) Q1/Q3 m/z: 936.6/345.3 (top two columns); Erythromycin with precursor ion [M+H]+ product ion (m1) Q1/Q3 m/z: 734.4/576.3 and precursor ion [M+H]+ product ion (m2) Q1/Q3 m/z: 734.4/158.2 (bottom two columns).
3.3. Mass spectrometry characterization MS/MS
Most reported LC–MS/MS methods have used ammonium acetate or formate buffers in the mobile phase system. Eventually, they have selected ammonium adducts of rapamycin for analysis. Usually, ammoniated buffer mobile phase systems will enhance ionization of analytes, but at the same time which may generally clog the peak tubes, pump seals, and rapamycin might also degrade faster at high buffer concentrations at pH 7.4. Therefore, we optimized rapamycin with a sodium adduct [M+Na]+ in positive ion mode in a formic acid mobile phase system. Formic acid containing mobile phase mixtures has the advantage that they never clog the peak tubes and the seals.
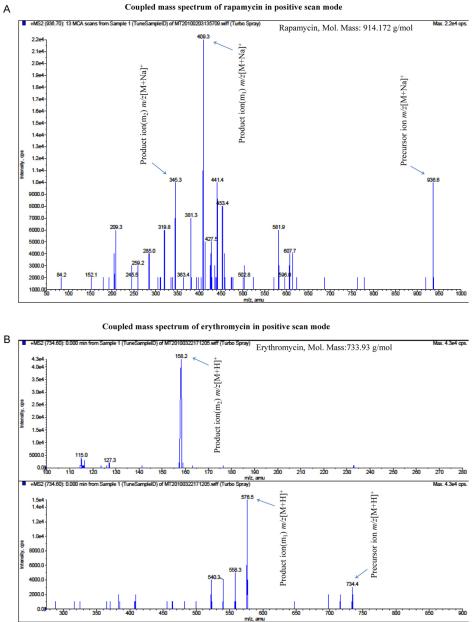
The instrument parameters were determined monthly on a routine basis as per our in-house protocol by calibration with polypropylene glycol standards as recommended by the manufacturer. The orifice voltage was adjusted to obtain maximum signal intensity and CAD experiments were carried out after obtaining maximum signal intensity using the orifice voltage as described in Section 2. High pure liquid nitrogen gas was employed as CAD and collision gas with the collision energy. Molecular ion full-scan spectrums were acquired for both the rapamycin and erythromycin in positive ion mode as shown in Fig. 2(A) and (B). The spectrum has shown in Fig. 2A. The MS2 spectrum with sodium adducts were also matched with the previously reported method [14]. The two most abundant fragment ions [M+Na]+ m/z at 409.3 and 345.3 were selected for MRM analysis. In which, the m/z 936.3 → 409.3 transition has shown higher intensity and reproducible results than the 936.3 → 345.5 ion pair. Eventually, the 936.3 → 409.3 ion was selected for entire MRM quantitative analysis as shown in Fig. 3A. Earlier reports also described how rapamycin sodium adduct [M+Na]+ show relatively greater response than the proton adduct [M+H]+ in positive mode with the electrospray ionization [15,16]. We also observed that sodium adducts of rapamycin has exhibited minimal matrix effect with the optimal response in all the ocular tissue matrices. Transition pairs were selected for erythromycin in which 734.4 → 158.5 have higher intensity than 734.4 → 576.5 in MRM Fig. 3(A) and (B).
Fig. 2.
(A) Coupled mass spectrum of rapamycin in Electrospray Ionization (ESI) positive scan mode with sodium adduct, precursor ion mass to charge ration (m/z) [M+Na]+: 936.6 Da, product ions m/z (m1) 409.3 and (m2) 345.3 Da. (B) Coupled mass spectrum of erythromycin in Electrospray Ionization (ESI) positive scan mode with proton adduct, precursor ion mass to charge ration (m/z) [M−H]−: 734.4, product ion m/z (m1) 576.3 and (m2) 158.2 Da.
3.4. Recovery
Recovery of rapamycin was high and reproducible in this extraction method. Several extraction techniques such as protein precipitation, liquid–liquid extraction and its combination of procedures with different solvent and solvent systems such as ethyl acetate, methyl (t)-butyl ether, dichloromethane, chloroform, cyclohexane, hexane, ether, ethanol, 2-propanol, perchloric acid, triethyl amine, acetonitrile and methanol were tested. All the tissue proteins were precipitated with the methanol, and triethyl amine digested all the tissues and broke their integrity. By using this procedure we got the highest recovery of rapamycin. Mean recovery of rapamycin was found between 85% and 90% for the cornea, 89–91% for iris ciliary body, 81–93% for lens, 78–87% for aqueous humor, 82–93% for vitreous humor 81–94% for sclera and 86–90% for retina choroid at LQC, MQC and HQC levels. The mean recovery of IS in tissue homogenate was found between 65% and 70% for cornea, 69–75% for Iris ciliary body, 45–60% for Lens, 54–73% for aqueous humor, 64–70% for vitreous humor 71–78% for Sclera and 76–80% retina choroid.
3.5. Matrix effect
Ion suppression expresses the matrix effect on ionization of analytes of interest. Usually, most results obtained from LC–MS/MS analysis are inconsistent because of ineffective sample preparation and extraction techniques. These problems may be overcome by modifying sample extraction procedure [17]. Liquid–liquid and solid-phase extraction are generally the most effective approach, but they are expensive and laborious. The elimination of undesirable water-soluble compounds, including non-volatile materials like phosphates and sulfates are important in electro spray ionization.
Reconstitution solution also plays a key role in ionization of analytes. We processed six replicates of LLOQ and tested for its matrix effect. We found the ionization and peak response was increased when it was reconstituted in acetonitrile:water:formic acid mixture (80:20:0.05) compared to other reconstitution solutions such as acetonitrile, methanol and its mixtures. In this technique, triethylamine and methanol were able to generate clear and clean samples from the water-soluble compounds, such as phosphates and zinc sulfates. The zinc sulfate and phosphate were inhibiting the ionization of analytes during the ESI [18,19]. The percentage of matrix was calculated by relative peak areas ratios was found 16%, 20% and 26% at HQC, MQC, and LQC levels, respectively.
3.6. Stability evaluation in ocular matrices
All stability experiments of rapamycin were performed in all the anterior and posterior segment of rabbit eye tissues separately. These stability studies were produced satisfactory linear regression and correlation co-efficient in each tissue. Both linear regression and correlation co-efficient parameters are calculated with the calibration curve and it was obtained from a constructed eight point standard curve. The percentage of accuracy and coefficient of variation (CV) of each stability experiment in each tissue of anterior and posterior segment of the eye results are shown in the Table 2. Rapamycin was stable for three freeze–thaw cycles at −80 °C and six weeks at freezer storage temperature in all tissue homogenates such as cornea, lens, sclera, vitreous humor, aqueous humor, iris ciliry muscle and retina choroid. It was also stable for four hours on bench top at 25 °C 2 days for in-injector storage, and 15 days for post extracted storage at −80 °C. All the stability results of anterior and posterior tissue are presented in Table 2. An LC–MS/MS analysis, extraction procedure, pH, and reconstitution solvent are important parameters to obtain better chromatographic separation, reproducible and reliable results. Rapamycin was estimated in the anterior eye tissues such as cornea, lens, iris ciliary body and aqueous humor. The calibration curve accuracy and precision, and retention times, and ten individual samples of each anterior eye tissue such as cornea, iris ciliary muscle, lens, and aqueous humor results are presented in the Table 3.
3.7. Sample analysis of tissue distribution study in the rabbit eye
Rapamycin was measured in ocular tissues post 1 h of topical administration of 0.2% rapamycin mixed nanomicellar formulation. Rapamycin was found to be at 2260.7 ± 507.1 ng/g tissues in the cornea, and 585.5 ± 80.1 ng/g tissues in the iris ciliary body. Rapamycin concentration found by mass spectrometry analysis in the cornea, lens, and iris ciliary muscle was normalized with the volume (500 μL) used for homogenization and tissue (0.11 g approx) weight. In the lens and aqueous humor, rapamycin was found to be below the limit of quantitation(i.e. <2.3 ng/mg protein). The results of rapamycin distribution in the eye are shown in Table 3. The distribution coefficient between hydrophilic and lipophilic phases is also important for estimation of drug distribution in the eye. Rapamycin is practically insoluble in water (2.6 μg/mL) because of its hydrophobicity and high molecular weight (MW 914 Da). It does not have certain functional groups which are ionizable at pH range between 1 and 10 to permeate easily into the cell. Therefore, it is less effective to enter in the cell to maintain sufficient therapeutic concentration at the site of action. However, rapamycin entrapped in a micelle at 0.2% can able to permeate into the tissue. That is what we observed in our study that 0. 2% rapamycin formulation can invade into the cornea and iris ciliary muscle and is a capable system for a topical treatment of corneal allograft rejection, as well as autoimmune uveitis [20].
A novel 0.2% nanomicellar formulation of rapamycin showed therapeutic concentration in the anterior segment of the eye. The results of rapamycin found in the posterior eye tissues such as sclera, vitreous humor, and retina choroid will be published later.
4. Conclusions
The LC–MS/MS method is a simple, cost-effective, and reliable technique for quantitative evaluation of rapamycin in ocular matrices. In addition, a 200 μL sample volume was used for each tissue in this procedure which is much lower. Low aliquot volume may help to minimize the cost of the studies related to rapamycin analysis. An LC–MS/MS method validation in ocular matrices by using sodium adducts [M+Na]+ has not been reported previously. Sodium adducts are more stable in non-ammoniated organic mobile phases in positive electrospray ionization. With the sodium adduct in electrospray ionization of mass spectrometry and triethylamine in the extraction method have reduced the matrix effect. The quantitative estimation of rapamycin generated consistent values with high accuracy and precision. The peak area of rapamycin continued to reproduce upon repeated injection, which shows the ruggedness of the method. All these advantages render the method suitable for systematic investigation of rapamycin in the rabbit eye.
An LC–MS/MS method was successfully utilized for the estimation of rapamycin in the cornea, iris ciliary body, lens and aqueous humor. This method demonstrated that a novel 0.2% rapamycin nanomicellar formulation can successfully deliver therapeutic concentrations in the eye. This formulation could be a promising therapeutic tool for the immunomodulatory treatment of ocular surface disorders, such as keratoconjunctivitis sicca, vernal conjunctivitis, and blepharitis. The results showed that the highest rapamycin concentration observed was in the cornea compared to the iris ciliary muscle, lens, and aqueous humor. Rapamycin entrapped in a nanomicellar core and the hydrophilic heads of micelles which transported the drug into the cornea and iris ciliary muscle, but very negligible contents were noted in the lens and aqueous humor.
Acknowledgments
This research was supported by the National Institutes of Health grants R01 EY 09171-12 and R01 EY 10659-10, and Lux Biosciences Inc. Ltd. New Jersey, USA.
References
- [1].Sehgal SN, Baker H, Vezina C. J. Antibiot. (Tokyo) 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- [2].Populo H, Soares P, Rocha AS, Silva P, Lopes JM. Melanoma Res. 2010;20:107–117. doi: 10.1097/CMR.0b013e32832ccd09. [DOI] [PubMed] [Google Scholar]
- [3].Bertelmann E, Pleyer U. Ophthalmologica. 2004;218:359–367. doi: 10.1159/000080937. [DOI] [PubMed] [Google Scholar]
- [4].Stahl A, Paschek L, Martin G, Gross NJ, Feltgen N, Hansen LL, Agostini HT. FEBS Lett. 2008;582:3097–3102. doi: 10.1016/j.febslet.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [5].Erdurmus M, Cohen EJ, Yildiz EH, Hammersmith KM, Laibson PR, Varssano D, Rapuano CJ. Cornea. 2009;28:759–764. doi: 10.1097/ICO.0b013e3181967318. [DOI] [PubMed] [Google Scholar]
- [6].Napoli KL, Kahan BD, Chromatogr J. B: Biomed. Appl. 1994;654:111–120. doi: 10.1016/0378-4347(93)e0456-z. [DOI] [PubMed] [Google Scholar]
- [7].Lee JH, Cha KH, Cho W, Park J, Park HJ, Cho Y, Hwang SJ. J. Pharm. Biomed. Anal. 2010;53:1042–1047. doi: 10.1016/j.jpba.2010.06.030. [DOI] [PubMed] [Google Scholar]
- [8].Mano N, Nozawa M, Sato M, Mori M, Yamaguchi H, Kanda K, Nogami M, Goto J, Shimada M, Chromatogr J. B: Analyt. Technol. Biomed. Life Sci. 2011;879:968–974. doi: 10.1016/j.jchromb.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [9].Serkova N, Jacobsen W, Niemann CU, Litt L, Benet LZ, Leibfritz D, Christians U. Br. J. Pharmacol. 2001;133:875–885. doi: 10.1038/sj.bjp.0704142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor PJ, Johnson AG, Chromatogr J. B: Biomed. Sci. Appl. 1998;718:251–257. doi: 10.1016/s0378-4347(98)00371-5. [DOI] [PubMed] [Google Scholar]
- [11].Salm P, Taylor PJ, Rooney F. Ther. Drug Monit. 2008;30:292–300. doi: 10.1097/FTD.0b013e3181771feb. [DOI] [PubMed] [Google Scholar]
- [12].Mano N, Sato M, Nozawa M, Matsumoto Y, Mori M, Yamaguchi H, Goto J, Shimada M, Chromatogr J. B: Analyt. Technol. Biomed. Life Sci. 2011;879:987–992. doi: 10.1016/j.jchromb.2011.03.013. [DOI] [PubMed] [Google Scholar]
- [13].Earla R, Boddu SH, Cholkar K, Hariharan S, Jwala J, Mitra AK. J. Pharm. Biomed. Anal. 2010;52:525–533. doi: 10.1016/j.jpba.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Napoli KL. Clin. Chem. 2009;55:1250–1252. doi: 10.1373/clinchem.2009.126508. [DOI] [PubMed] [Google Scholar]
- [15].Streit F, Christians U, Schiebel HM, Napoli KL, Ernst L, Linck A, Kahan BD, Sewing KF. Clin. Chem. 1996;42(9):1417–1425. [PubMed] [Google Scholar]
- [16].Hallensleben K, Raida M, Habermehl G. J. Am. Soc. Mass Spectrom. 2000;11:516–525. doi: 10.1016/S1044-0305(00)00123-9. [DOI] [PubMed] [Google Scholar]
- [17].Jiang H, Cao H, Zhang Y, Fast DM, Chromatogr J. B: Analyt. Technol. Biomed. Life Sci. 2012;891–892:71–80. doi: 10.1016/j.jchromb.2012.02.031. [DOI] [PubMed] [Google Scholar]
- [18].Clavijo C, Strom T, Moll V, Betts R, Zhang YL, Christians U, Bendrick-Peart J, Chromatogr J. B: Analyt. Technol. Biomed. Life Sci. 2009;877:3506–3514. doi: 10.1016/j.jchromb.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bogusz MJ, Enazi EA, Hassan H, Abdel-Jawaad J, Ruwaily JA, Tufail MA, Chromatogr J. B: Analyt. Technol. Biomed. Life Sci. 2007;850:471–480. doi: 10.1016/j.jchromb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- [20].Buech G, Bertelmann E, Pleyer U, Siebenbrodt I, Borchert HH. J. Ocul. Pharmacol. Ther. 2007;23:292–303. doi: 10.1089/jop.2006.130. [DOI] [PubMed] [Google Scholar]