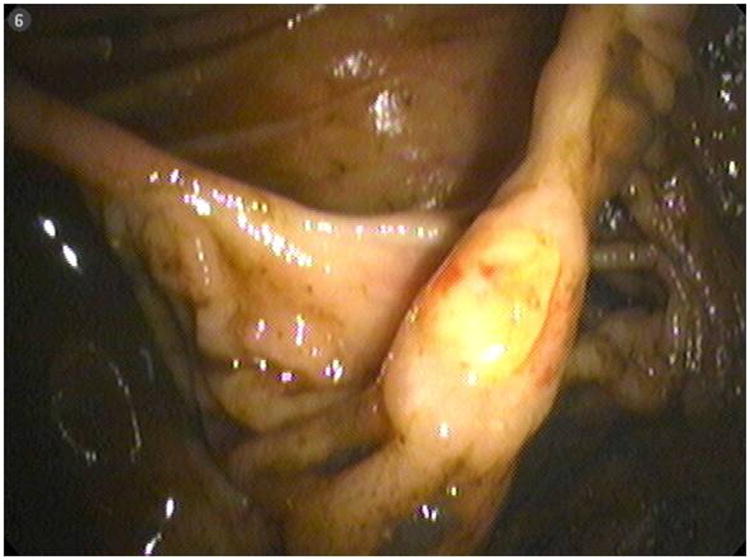
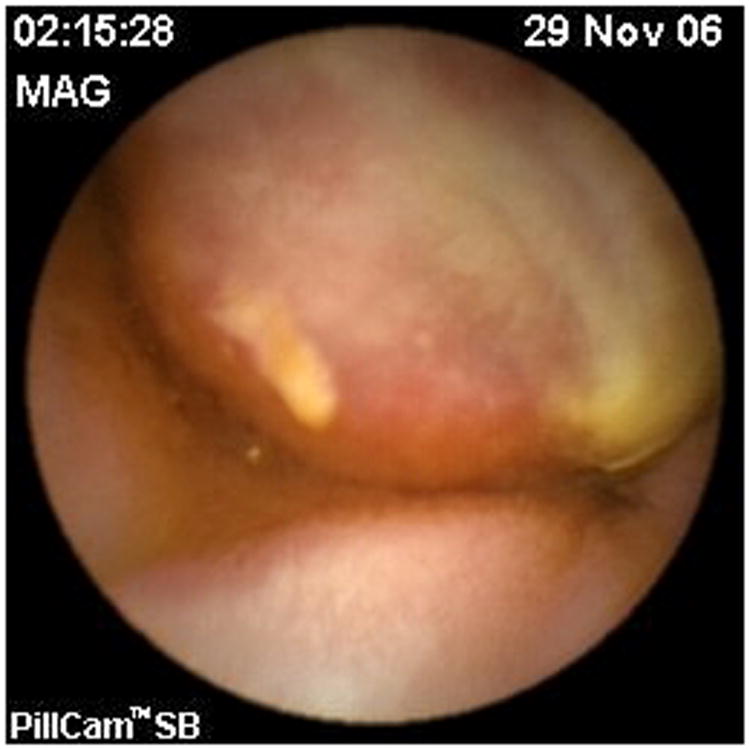
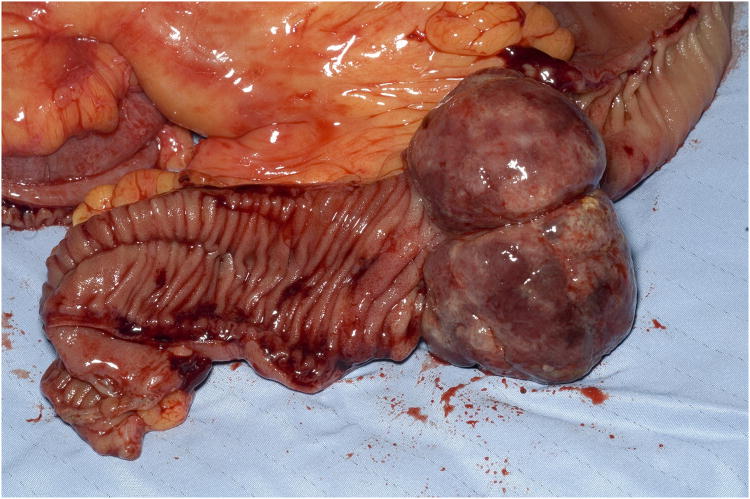
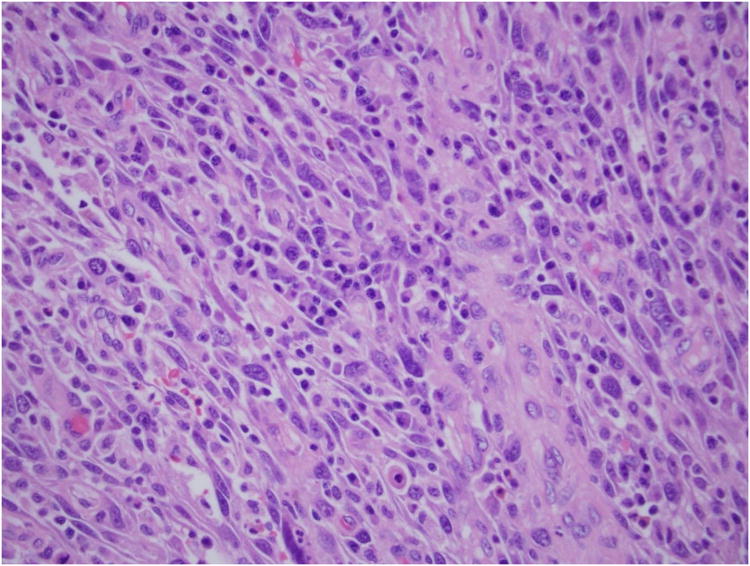
A 50-year-old female with a history of high-grade metastatic soft tissue sarcoma (STS; type not further classifiable), was referred for evaluation of GI bleeding. Initial diagnosis was made 4 years previously after resection of a left atrial mass. Subsequent disease sites included her buttocks, colon and adrenal gland. She had completed multiple courses of palliative treatment with chemotherapy (ifosfamide/etoposide and a clinical trial of bortezomib/vorinostat), radiotherapy, and surgical resections, including a right hemicolectomy. At the time of referral, her disease was felt to be stable. Clinical examination revealed pallor and a benign abdomen. Laboratory analysis showed iron deficiency anemia with hemoglobin 7.8 g/dL (reference range, 11.6 to 15.6 g/dL), hematocrit of 25% (reference range, 34% to 46%), iron of 10 mcg/dL (reference range, 50 to 160 mcg/dL), total iron-binding capacity of 182 mcg/dL (reference range, 250 to 450 mcg/dL) and iron saturation of 5% (reference range, 16% to 50%). The patient required two units of packed RBCs weekly to maintain a hematocrit of 25%. Initial procedures included upper endoscopy and small bowel push enteroscopy to mid jejunum, which were both normal. A colonoscopy revealed a small, linear, clean-based ulcer at the right hemicolectomy/ileocolonic anastomosis (Fig 1). Biopsies of the ulcer demonstrated recurrence of her sarcoma. However, with no stigmata of active, recent hemorrhage, this was not felt to be the source of her GI bleed. Computed tomography scan of her chest, abdomen, and pelvis showed no evidence of obvious disease recurrence, progression, or any small bowel or colonic abnormalities. Video capsule endoscopy (VCE; SB PillCAM; Given Imaging, Atlanta, GA) was performed to evaluate a possible source of obscure, occult GI bleed. This revealed two large ulcerated submucosal lesions, with active bleeding, in the suspected region of the distal jejunum to mid ileum (Fig 2). The video capsule endoscope remained in this region of the small bowel for approximately 1 hour 47 minutes before advancing into the distal ileum and across the ileocolonic anastomosis into the colon. The patient ultimately underwent exploratory laparotomy and surgical resection. Intraoperatively, an area of intussusception with two large intraluminal masses in the mid to distal ileum was identified approximately 1.5 feet proximal to the ileocolonic anastomosis (Fig 3). Surprisingly, the capsule endoscope was not retained in the area of intussuception. Intraoperative enteroscopy was performed to exclude any other proximal small bowel lesions. High-power view (×40) of hematoxylin and eosin-stained sections of the mass lesions demonstrated a proliferation of plump anaplastic spindle cells with frequent atypical mitoses (Fig 4), consistent histologically with her prior diagnosis of high-grade STS, type not otherwise classifiable. The differential diagnosis included GI stromal tumor, metastatic melanoma, leiomyosarcoma, and malignant fibrous histiocytoma. An S-100 immunohistochemistry stain was performed on the resected surgical specimen, and this revealed no tumor staining. In addition, the margins of the specimen were negative, and there were four benign lymph nodes. Prior tissue from our patient was negative for desmin, CD117, and S-100 by immunoperoxidase. Therefore, tests for desmin and CD117 in this particular ileocolonic surgical specimen were not performed. The patient survived for more than 2 years after resection of the small bowel metastasis but subsequently died 8 years after initial diagnosis as a result of left atrial recurrence.
Fig 1. Ulcer at ileocolonic anastomosis.
Fig 2. Metastatic sarcoma to small bowel on video capsule endoscopy.
Fig 3. Surgical intussusception specimen.
Fig 4. High-power (×40) H & E stain of small bowel mass.
STSs1 comprise a heterogeneous group of malignant tumors of mesenchymal origin and account for less than 1 % of annual cancer diagnoses.2 Histopathologic subtypes encompass a wide range as a result of maturation of mesenchymal cells into skeletal and smooth muscle, adipose, blood vessels, and fibrous tissue. In most patients, no cause is discernible. STSs commonly metastasize to the lung;3 however, as demonstrated here and described elsewhere,4-8 they may metastasize to many tissues, including small bowel.9, 10 The incidence of small bowel STS metastasis is unknown; however, on the basis of decades of experience at our comprehensive cancer center, it is likely rare. Small bowel VCE is performed to evaluate obscure, occult GI bleeding and can locate small bowel tumors.11 Presenting symptoms of primary and metastatic small bowel cancers are nonspecific and include abdominal pain, bowel obstruction, and obscure overt or occult GI bleeding. Among patients undergoing VCE, approximately 2.5% to 8.9% will have small bowel tumors identified.12 Case reports describe the use of VCE to find primary STS lesions.13-16 However, to our knowledge, we provide the first report of the use of VCE to detect occult small bowel STS metastasis. This case demonstrates the ability of VCE to diagnose cancer metastasis when other radiologic and endoscopic investigations were negative. VCE may benefit similar patients by permitting timely detection and improved clinical outcomes. One should consider occult small bowel metastasis when anemia and/or bleeding occur in patients with cancer believed to have stable or no active disease.
Acknowledgments
Support was provided by Physician Scientist Training in Cancer Medicine Grant No. T32 CA009614 from the National Institutes of Health (S.A.), and by T-32 Institutional Training Program Grant No. T32HS000083 from the Agency for Healthcare Research and Quality National Research Award (J.W.).
Footnotes
This work was presented in part at the 72nd Annual Scientific Meeting of the American College of Gastroenterology, October 12-17, 2007; Philadelphia, PA.
References
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. The New England journal of medicine. 2005;353(7):701–11. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Annals of surgery. 1999;229(5):602–10. doi: 10.1097/00000658-199905000-00002. discussion 10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behranwala KA, A'Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Annals of surgical oncology. 2004;11(7):714–9. doi: 10.1245/ASO.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Gupta T, Laskar S, Gujral S, Muckaden MA. Brain metastases in soft tissue sarcomas: case report and literature review. Sarcoma. 2005;9(3-4):147–50. doi: 10.1080/13577140500190921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehders A, Peiper M, Stoecklein NH, et al. Hepatic Metastasectomy for Soft-Tissue Sarcomas: Is It Justified? World journal of surgery. 2008 doi: 10.1007/s00268-008-9777-4. [DOI] [PubMed] [Google Scholar]
- 7.Vandergriff T, Krathen RA, Orengo I. Cutaneous metastasis of leiomyosarcoma. Dermatol Surg. 2007;33(5):634–7. doi: 10.1111/j.1524-4725.2007.33127.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwab JH, Boland P, Guo T, et al. Skeletal metastases in myxoid liposarcoma: an unusual pattern of distant spread. Annals of surgical oncology. 2007;14(4):1507–14. doi: 10.1245/s10434-006-9306-3. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian S, Kumar M, Thulkar S, Harsh K. Bowel metastases from primary leiomyosarcoma of the gluteal region. Singapore medical journal. 2008;49(3):e68–70. [PubMed] [Google Scholar]
- 10.Chiang KC, Yeh CN, Shih HN, Jan YY, Chen MF. Lower gastrointestinal bleeding due to small bowel metastasis from leiomyosarcoma in the tibia. Chang Gung medical journal. 2006;29(4):430–4. [PubMed] [Google Scholar]
- 11.Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40(6):488–95. doi: 10.1055/s-2007-995783. [DOI] [PubMed] [Google Scholar]
- 12.Urbain D, Van Laer W, Mana F. Capsule endoscopy for detection of small bowel malignancies. Surgical technology international. 2008;17:126–30. [PubMed] [Google Scholar]
- 13.Knop FK, Hansen MB, Meisner S. Small-bowel hemangiosarcoma and capsule endoscopy. Endoscopy. 2003;35(7):637. doi: 10.1055/s-2003-40224. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Ares D, Gonzalez-Conde B, Yanez J, et al. Jejunal leiomyosarcoma, a rare cause of obscure gastrointestinal bleeding diagnosed by wireless capsule endoscopy. Surgical endoscopy. 2004;18(3):554–6. doi: 10.1007/s00464-003-4255-4. [DOI] [PubMed] [Google Scholar]
- 15.Viazis N, Vlachogiannakos J, Georgiadis D, Rodias M, Noutsis C, Karamanolis DG. Classic Kaposi's sarcoma and involvement of the small intestine as shown by capsule endoscopy. Endoscopy. 2008;40(2):E209. doi: 10.1055/s-2008-1077467. [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman G, Quinn AM, Williams J, Hammadeh R. Clear cell sarcoma of the small bowel: a potential pitfall. Case report Apmis. 2005;113(10):716–9. doi: 10.1111/j.1600-0463.2005.apm_243.x. [DOI] [PubMed] [Google Scholar]