Abstract
Metastasis involves the spread of cancer cells from the primary tumor to surrounding tissues and to distant organs and is the primary cause of cancer morbidity and mortality. In order to complete the metastatic cascade, cancer cells must detach from the primary tumor, intravasate into the circulatory and lymphatic systems, evade immune attack, extravasate at distant capillary beds, and invade and proliferate in distant organs. Currently, several hypotheses have been advanced to explain the origin of cancer metastasis. These involve an epithelial mesenchymal transition, an accumulation of mutations in stem cells, a macrophage facilitation process, and a macrophage origin involving either transformation or fusion hybridization with neoplastic cells. Many of the properties of metastatic cancer cells are also seen in normal macrophages. A macrophage origin of metastasis can also explain the long-standing “seed and soil” hypothesis and the absence of metastasis in plant cancers. The view of metastasis as a macrophage metabolic disease can provide novel insight for therapeutic management.
Keywords: systemic metastasis, VM mouse, stem cells, Warburg, macrophage, myeloid cells, cell fusion, phagocytosis, mitochondria, seed and soil
I. THE METASTATIC CASCADE
Metastasis is the general term used to describe the spread of cancer cells from the primary tumor to surrounding tissues and to distant organs and is the primary cause of cancer morbidity and mortality.1-8 It is estimated that metastasis is responsible for about 90% of cancer deaths.9 This estimate has changed little in more than 50 years.10,11 Metastasis involves a series of sequential and interrelated steps. In order to complete the metastatic cascade, cancer cells must detach from the primary tumor, intravasate into the circulatory and lymphatic systems, evade immune attack, extravasate at distant capillary beds, and invade and proliferate in distant organs.1-4,7,12,13 Metastatic cells also establish a microenvironment that facilitates angiogenesis and proliferation, resulting in macroscopic, malignant secondary tumors. Although systemic metastasis is responsible for about 90% of cancer deaths, most research in cancer does not involve metastasis in the in vivo state.5,14 That about 1,500 people continue to die each day from cancer further attests to the failure in managing the disease once it disseminates through the body.14
II. MODELS OF METASTASIS
A difficulty in characterizing the cellular origin of metastasis comes, in large part, from a dearth of animal models that show systemic metastasis involving bone marrow and multiple organ systems.5,14 Tumor cells that are naturally metastatic should not require intravenous injection to initiate the metastatic phenotype. The key phenotype of metastasis is that the tumor cells spread naturally from the primary tumor site to secondary locations. Systemic metastasis occurs for the VM-M3 tumor from any implantation site when grown in its natural immunocompetent and syngeneic VM mouse host (Fig. 1). Numerous investigators, however, use intravenous tumor cell injection models to study metastasis.14 While these models can provide information on tumor cell survival in the circulation, it is not clear if this information is relevant to survival of naturally metastatic tumor cells. If the tumor cells evaluated in animal models are not naturally metastatic, it is not clear why they would be used as models of metastasis in the first place.14 Unnatural models of cancer metastasis can provide misinformation on the nature of the disease.14
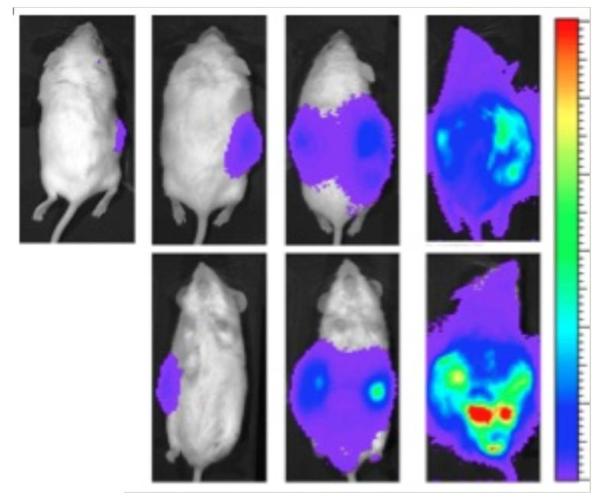
FIGURE 1.
Systemic metastasis of the VM-M3/Fluc tumor cells grown in the inbred VM mouse. Whole body view of bioluminescence from metastatic VM-M3 tumor cells. VM-M3 tumor cells, containing the firefly luciferase gene, were implanted subcutaneously on the flank of a syngeneic VM mouse on day 0 as we described in (223). Bioluminescent signal from the metastatic cells was measured in live mice using IVIS Lumina system (Caliper LS). Bioluminescence appeared throughout the mouse after 23 days indicative of widespread systemic dissemination of metastatic cells. The mouse is shown in prone position at 3, 10, 17 and 23 days (left to right) after subcutaneous flank implantation of VM-M3/Fluc tumor cells. The bottom row shows the mouse in supine position at those days. Bioluminescent cells were also detected ex vivo in multiple organ systems of the VM mouse host.223 Source: Reprinted with modification from223.
According to Yuri Lazebnik, much of what is known about metastasis comes from model systems that have more in common with benign tumors than with metastatic carcinomas.5 If the models used to understand the nature of metastases do not accurately model the phenomenon, then the lack of progress in managing metastases should not be surprising.14 The in vitro models have shortcomings in that they do not replicate all of the steps required for systemic metastasis in vivo. Migration of cells into scratches or in Boyden chambers might or might not be related to the phenomenon seen in vivo.14 Few investigators have linked the migratory behavior of tumor cells seen in the in vitro invasion assays with the invasive and metastatic behavior of these cells in the natural in vivo host. We found that the invasive behavior of the CT-2A mouse glioma seen in vitro was not associated with wide-spread invasion or metastasis when grown in vivo.14,15 Although the major steps of metastasis are well documented, the process by which metastatic cells arise from within populations of non-metastatic cells of the primary tumor is largely unknown.3,7,16,17 It would therefore be helpful to highlight current views on the cellular origin of metastasis.
III. ORIGIN OF METASTATIC CANCER CELLS
A. Epithelial to Mesenchymal Transition (EMT)
The EMT posits that metastatic cells arise from either epithelial stem cells or differentiated epithelial cells through a step-wise accumulation of gene mutations that eventually transform the epithelial cell into a tumor cell with mesenchymal features.8,9,14,18-22 This idea comes from findings that many cancers arise in epithelial tissues where abnormalities in cell-cell and cell-matrix interactions occur during tumor progression. Eventually, neoplastic cells emerge that appear as mesenchymal cells which lack cell-cell adhesion, are dysmorphic in shape, and eventually spread to distant organs.7,18,19 How does this extremely complicated phenomenon actually happen?
Jean Paul Thiery provided a comprehensive overview of how EMT might contribute to metastasis (Fig. 2). Recent studies also suggest that misplaced (ectopic) co-expression of only two genes might be all that is necessary to facilitate EMT in some gliomas, though the process is highly complex.23 However, considerable controversy surrounds the EMT hypothesis of metastasis, as EMT is not often detected in tumor pathological preparations.1,24,25 The EMT is primarily considered a phenomenon of the in vitro environment.7 It remains debatable whether this in vitro model of metastasis has an in vivo counterpart.
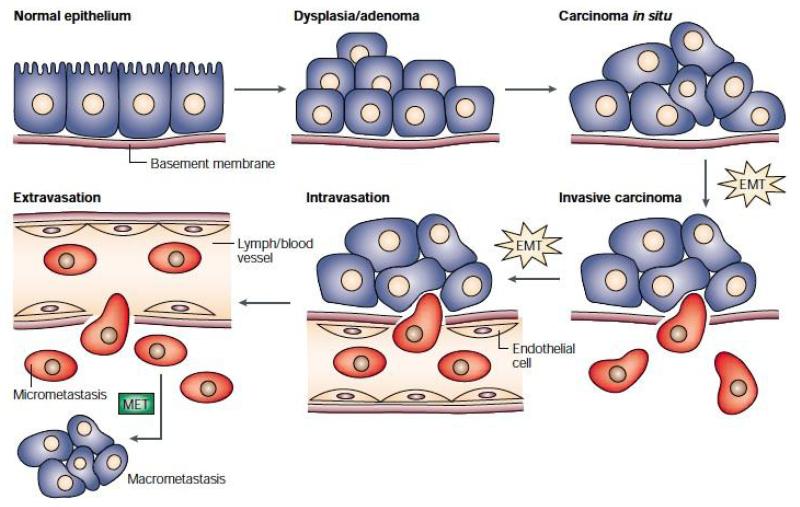
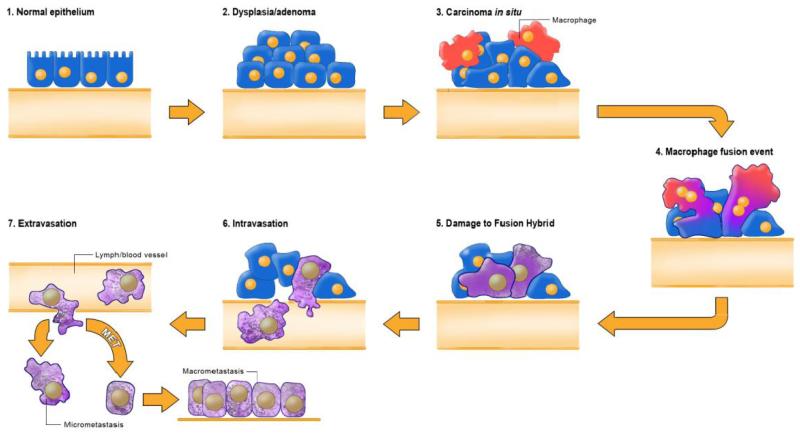
FIGURE 2.
The epithelial-mesenchymal transition and mesenchymal-epithelial transition (MET) model of tumor metastasis. According to Jean Paul Thiery, normal epithelia lined by a basement membrane can proliferate locally to give rise to an adenoma. Further transformation by epigenetic changes and genetic alterations leads to a carcinoma in situ, still outlined by an intact basement membrane. Further alterations can induce local dissemination of carcinoma cells, possibly through an EMT, as the basement membrane becomes fragmented. The invasive carcinoma cells (red) then intravasate into lymph or blood vessels, allowing their passive transport to distant organs. At secondary sites, solitary carcinoma cells extravasate, remain solitary (micrometastasis), or form a new carcinoma through an MET. Reprinted with permission from18.
The idea for the EMT arose from attempts to draw parallels between the behavior of normal cells during metazoan morphogenesis and the behavior of cancer cells during tumor progression.9,14,18 Adaptation of the EMT into the gene theory of cancer suggested that metastasis is the endpoint of a series of genomic alterations and clonal selection. This then provided the neoplastic cells with a growth advantage over normal cells.19,22,26,27 It is difficult to understand how a collection of gene mutations, many of which are random and deleterious, could produce cells with the capacity to detach from the primary tumor, intravasate into the circulation and lymphatic systems, evade immune attack, extravasate at distant capillary beds, and recapitulate epithelial characteristics following invasion and proliferation in distant organs. This would be quite a feat for a cell with a disorganized genome.14
The recapitulation of epithelial characteristics at distant secondary sites is referred to as the mesenchymal epithelial transition (MET) and is thought to involve a reversal of the changes responsible for the EMT.9,18,19 No clear explanation has appeared on how the genomic instability and multiple-point mutations and chromosomal rearrangements responsible for the neoplastic mesenchymal phenotype could be reversed or suppressed when the tumor cells recapitulate the epithelial phenotype at distant sites.14 If many of these genomic changes are not reversed, how is it possible that they could be responsible for EMT in the first place?
Our recent studies in the VM mouse model of systemic metastasis suggest that random mutations and EMT are not required for the origin of metastasis.14,28 The massive complexity associated with the EMT hypothesis is largely man-made, especially in attempting to describe the phenomenon as a gene driven process.9,14,18,19,23,29 If one looks closely, many of the gene expression profiles observed in metastatic cancers are similar to those associated with the function of macrophages or other fusogenic cells of the immune system.7,30,31 Moreover, many gene changes associated with EMT can also be found in most non-metastatic benign tumors.5,29 Accumulating evidence suggests that cancer is not a genetic disease, but is rather a metabolic disease involving respiratory insufficiency with compensatory fermentation.14 The genomic instability seen in tumor cells arises as a downstream epiphenomenon of the underlying metabolic defects. Credible mechanisms of cancer metastasis must therefore be framed in light of the underlying origin of cancer as a mitochondrial respiratory disease.14 The EMT/MET hypothesis has yet to do this.
B. Stem Cell Origin of Metastatic Tumor Cells
Several investigators hold that metastatic cancer cells arise from populations of tissue stem cells.32-34 Most tissues contain cells in semi-differentiated states that can replace dead or damaged cells due to natural wear and tear.14 These undifferentiated or semi-differentiated cells are often referred to as tissue stem cells and are considered by many to be the origin of metastatic cancers.23,32,35,36 Similarities in gene expression and biological characteristics are often seen in stem cells and cancer cells.37 Observations that tumor cells express characteristics of undifferentiated stem cells come from the fact that embryonic stem cells and tumor cells can use anaerobic energy (fermentation) for metabolism.14 Telomerase activity, which is generally higher in tumor cells than in normal cells, is also linked to fermentation energy.14 It is therefore not surprising that numerous genetic and biochemical phenotypes are shared between tumor cells and stem cells, as most tumor cells also use energy from fermentation for their survival and growth.
As stem cells are known for their ability to proliferate and migrate during tissue morphogenesis and differentiation, it was reasonable to assume that genetic damage to stem cells could give rise to metastatic cancers in various tissues.20,32,37 However, many tumor cells with stem cell properties do not express systemic metastasis. Indeed, many of the chemically-induced brain tumors we developed in mice over the years express stem cell properties, but do not display extensive invasion or metastasis when implanted in subcutaneous locations or in various organs.14,15,38,39 Many of the human xenograft tumor models rarely show systemic metastasis when grown in the immune compromised mouse host despite expressing several of the Hanahan and Weinberg cancer hallmarks.14,40 The origin of glioblastoma from stem cells alone is now questioned.41 While metastatic cancers can express properties of stem cells, expression of stem cell properties is not synonymous with expression of distant invasion and metastasis. Tumors derived from hematopoietic stem cells, however, may be an exception.33 Hematopoietic stem cells can give rise to myeloid cells, which we consider the origin of most metastatic cancers.28,39 We found that only those mouse tumor cells expressing characteristics of macrophages showed systemic metastasis.14,28,39
C. Macrophage Facilitation of Metastasis
It has long been recognized that many malignant tumors contain significant numbers of macrophages and other cells of the stroma.37,42-49 The macrophages present in tumors are generally referred to as tumor-associated macrophages (TAM). TAM can establish the pre-metastatic niche, while enhancing tumor inflammation and angiogenesis.50-52 In other words, TAM facilitate the metastatic cascade (Fig. 3). While gene mutations are still thought to initiate neoplasia under this model, it is the stromal macrophages acting as cellular chaperones that facilitate tumor development, progression, and the eventual seeding of metastasis.37,42,44,50,53,54 The stromal TAM are viewed as essential participants in all phases of metastasis, but are not considered neoplastic themselves. However, we recently reviewed evidence showing that many human metastatic tumors also contain neoplastic cells with macrophage properties.14,28,55 It is not easy to distinguish neoplastic from non-neoplastic macrophages in the inflamed tumor microenvironment, as both cells are similar in gene expression, morphology, and function.14,55,56 In contrast to the view that macrophages serve as accessories or cellular chaperones for the metastatic cascade of neoplastic stem cells, we consider the metastatic cells themselves as derived from macrophages or other similar cells of myeloid origin.
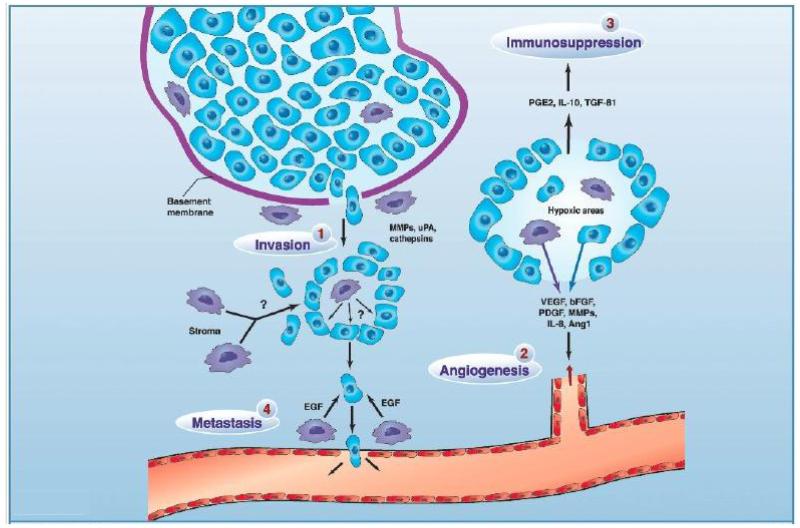
FIGURE 3.
The role of different TAM subpopulations in tumor progression. 1, invasion: TAM secrete a variety of proteases to breakdown the basement membrane around areas of proliferating tumor cells (e.g., ductal carcinoma in situ in the breast), thereby prompting their escape into the surrounding stroma where they show deregulated growth. 2, angiogenesis: In areas of transient (avascular) and chronic (perinecrotic) tumor hypoxia, macrophages cooperate with tumor cells to induce a vascular supply for the area by up-regulating a number of angiogenic growth factors and enzymes. These diffuse away from the hypoxic area and, together with other pro-angiogenic stimuli in the tumor microenvironment, stimulate endothelial cells in neighboring, vascularized areas to migrate, proliferate, and differentiate into new vessels. 3, immunosuppression: Macrophages in hypoxic areas secrete factors that suppress the antitumor functions of immune effectors within the tumor. 4, metastasis: A subpopulation of TAM associated with tumor vessels secretes factors like epidermal growth factor (EGF) to guide tumor cells in the stroma toward blood vessels where they then escape into the circulation. TAM secrete growth factors in the stromal compartment to stimulate tumor cell division and/or undefined factors that promote tumor cell motility. Reprinted with permission from reference42.
D. Myeloid Cell Origin of Metastasis
According to our hypothesis, metastatic cancers arise from respiratory insufficiency in myeloid cells or in their lineage descendants, e.g., macrophages, dendritic cells, or lymphocytes.14 Chronic inflammation in the microenvironment can damage mitochondrial respiration in activated macrophage.14,57,58 Many metastatic cancers express aerobic glycolysis (Warburg effect), which can be detected in PET scans.14,59 Aerobic glycolysis in tumor cells arises ultimately from insufficient respiration.14 Fusion hybridization between macrophages and non-metastatic cancer stem cells also blurs the boundaries between the nuclear and cytoplasmic contribution to the metastatic phenotype.14,31 Before tackling these issues, it would be good to first consider the evidence that metastatic cancer cells can arise from myeloid cells
As an alternative or complimentary hypothesis to the view that normal macrophages facilitate the metastatic spread of neoplastic stem cells; the myeloid hypothesis of metastasis suggests that metastatic cancer cells arise directly from cells of myeloid origin or from hybrid cells following fusion between macrophages and non-metastatic stem cells.14,28,39,55 The myeloid cell origin of metastasis would also encompass the macrophage fusion hypothesis of metastatic cancer, since it is the properties of macrophages that contribute to the metastatic cascade.60,61 Myeloid cells are already mesenchymal cells and would not, therefore, require the complicated genetic mechanisms proposed for the EMT in order to metastasize. Macrophages arise from the myeloid lineage and have long been considered the origin of human metastatic cancer.17,28,60,62-64 Macrophages can fuse with epithelial cells within the inflamed microenvironment thus manifesting properties of both the epithelial cell and the macrophage in the fusion hybrids.31,65,66 The origin of metastatic cancer from hematopoietic stem cells or yolk sac-derived macrophages is also consistent with the myeloid cell hypothesis.67 The uncontrolled growth of these cells can arise following respiratory damage in their homotypic fusion hybrids. In his recent review on metastasis, David Tarin states: “..it would appear that tumour metastasis first appears in the lower chordates in parallel with the origin of lymphocytes and this may indicate that metastasis cannot occur until an organism has evolved the genes for lymphocyte trafficking.”1 According to our hypothesis, it is hematopoietic stem cells themselves or their lineage descendants that become the metastatic cells either through direct transformation in the inflamed microenvironment or through their fusion with neoplastic tumor cells.
It is important to mention that metastatic cells of macrophage origin are generally not found in rodent tumor transplant models. Most macrophages seen in chemically-induced tumors are derived from TAM, as we previously showed in experimental mouse brain tumors grown either orthotopically or subcutaneously in the flank.68, 69 We have suggested that rodent tissues respond to tumor implants as if they were an acute infection or wound.14 This would involve invasion of TAM and activation of local macrophages. It is also possible that fusion hybrids would form between tumor cells and host macrophages, but this might not cause damage to the macrophage mitochondria. In contrast to the acute situation in mice, neoplastic transformation is a protracted process in humans. Murine myeloid cells respond acutely to the tumor implant, whereas human myeloid cells in the inflamed microenvironment respond chronically to the tumor initiating insult. It is not clear why highly metastatic carcinomas, similar to those seen in humans, are rarely found in experimental rodent tumors. There are, however, some exceptions. It is possible under some circumstances to create chronic environments through repeated transplantations that can give rise to fusion hybridizations with metastasis as an eventual outcome.39,70
IV. MACROPHAGES AND METASTASIS
Macrophages are among the most versatile cells of the body with respect to their ability to migrate, to change shape, and to secrete growth factors and cytokines.14,39,71-73 These macrophage behaviors are also the recognized behaviors of metastatic cells.14 Macrophages manifest two distinct polarization phenotypes: the classically activated (M1 phenotype) and the alternatively activated (M2 phenotype). Macrophages acquire the M1 phenotype in response to pro-inflammatory molecules and release inflammatory cytokines, reactive oxygen species, and nitric oxide.30,46,48,74-76 In contrast, macrophages acquire the M2 phenotype in response to anti-inflammatory molecules such as IL-4, IL 13, IL-10 and to apoptotic cells.46,77 M2 macrophages promote tissue remodeling and repair, but are immunosuppressive and poor antigen presenters.48 Although the M1 and the M2 macrophages play distinct roles during tumor initiation and malignant progression, macrophage-epithelial cell fusions can involve either activation state.
M1 macrophages facilitate the early stages of tumorigenesis through the creation of an inflammatory microenvironment that can produce nuclear and mitochondrial damage.50,78 However, TAM can also undergo a phenotypic switch to the M2 phenotype during tumor progression.46,78 The TAM population comprising M2 macrophages scavenge cellular debris, promote tumor growth, and enhance angiogenesis. M2 macrophages also fuse with tumor cells, thus, expressing characteristics of both cell types. It has always been difficult to know for certain, however, whether TAM are part of the normal stroma or are part of the malignant cell population.28,55 This is especially the case in human cancers.56
Increasing evidence suggests that many of the myeloid/macrophage cells seen within human tumors are also part of the malignant cell population. Aichel first proposed over a century ago that tumor progression involved fusion between leukocytes and somatic cells.1-5 Several human metastatic cancers express multiple molecular and behavioral characteristics of macrophages including phagocytosis, cell-cell fusion, and antigen expression (Table 1). Tarin also considers the expression of osteopontin and CD44 as important for the regulatory gene group/network associated with metastasis.1 This is interesting as there is strong evidence that both osteopontin and CD44 are expressed in monocytes and macrophages under various physiological and pathological states.79-81 We argued that an origin of metastatic cancer from myeloid cells could account for many mesenchymal properties of metastatic cancers.14,28 It is not, therefore, necessary to invoke an EMT to account for metastasis.
TABLE 1.
Tumors expressing macrophage characteristics
| Tumor | Phagocytosis | Fusogenicity | Gene Expression |
|---|---|---|---|
| Bladder | 224 | 56 | 56 |
| Brain | 39, 116, 117, 225-231 | 145, 232, 233 | 39, 55, 231 |
| Breast | 118-120, 124, 234-237 | 238-242 | 138, 140, 153 |
| Carcinoma of unknown primary |
175 | 176 | |
| Endometrial | 243 | ||
| Fibrosarcoma | 237 | ||
| Gall bladder | 244 | ||
| Liver | 245 | ||
| Lung | 114, 120, 121, 246, 247 | 239 | 152, 155, 156 |
| Lymphoma/Leukemia | 248-250 | 125, 251, 252 | |
| Melanoma/Skin | 93, 122, 165, 253, 254 | 64, 141 | 254-257 |
| Meth A Sarcoma | 147 | 147 | 147 |
| Multiple myeloma | 258 | 259 | |
| Ovarian | 237, 260 | 261 | |
| Pancreatic | 115, 262 | 263 | 262 |
| Rectal/Colon | 31, 157 | 137 | |
| Renal | 264 | 142, 143 | 264 |
| Rhabdomyosarcoma | 265, 266 | ||
| Reviews | 91, 267-269 | 17, 60, 62, 63, 66, 132, 134, 146, 242, 251, 270 |
This table is updated from that previously published in28.
Interestingly, macrophages express most hallmarks of metastatic tumor cells when responding to tissue injury or disease. For example, monocytes (derived from hematopoietic bone marrow cells) extravasate from the vasculature and are recruited to the wound via cytokines released from the damaged tissue.28,30 Within the wound, monocytes differentiate into alternatively-activated macrophages and dendritic cells where they release a variety of pro-angiogenic molecules including vascular endothelial growth factor, fibroblast growth factor, and platelet derived growth factor.30,82,83 M2 macrophages also actively phagocytize dead cells and cellular debris.30,73 On occasion, macrophages undergo homotypic fusion resulting in multinucleated giant cells with increased phagocytic capacity.63,84,85 Following these wound-healing activities, macrophages intravasate back into the circulation where they travel to the lymph nodes to participate in the immune response.73,86,87
Some phagocytic macrophages also migrate to lymph nodes and differentiate into dendritic cells.88 These findings indicate that normal macrophages are capable of expressing all hallmarks of metastatic cancer cells including tissue invasion, release of pro-angiogenic molecules/cytokines, survival in hypoxic and necrotic environments, intravasation into the circulatory/lymphatic systems, and extravasation from these systems at distant locations. An EMT is not necessary to explain these behaviors, as they are already the evolutionary programmed behaviors of macrophages.
A. Phagocytosis is a Shared Behavior of Macrophages and Many Metastatic Cancer Cells
Phagocytosis involves the engulfment and ingestion of extracellular material, and is a specialized behavior of M2 macrophages and other professional phagocytes.73,89 This process is essential for maintaining tissue homeostasis by clearing apoptotic cells, cellular debris, and invading pathogens. Like M2 macrophages, many malignant tumor cells are phagocytic both in vitro and in vivo (Table 1). Tumor cell phagocytosis was first described from histopathological observations of foreign cell bodies within the cytoplasm of cancer cells, which displayed crescent-shaped nuclei.60 This cellular phenotype resulted from the ingested material pushing the nucleus to the periphery of the phagocytic cell. These cells were commonly referred to as either “birds-eye” or “signet-ring” cells.90,91 While this phagocytic/cannibalistic phenomenon is commonly seen in feeding microorganisms, cell cannibalism is also seen in malignant human tumor cells.90-93 Fais and colleagues provided dramatic evidence of tumor cell phagocytosis in showing how malignant melanoma cells eat T-cells.91 This is remarkable as T-cells are thought to target and kill tumor cells.
There is also evidence that some tumor cells can eat natural killer cells.92 If macrophage-derived metastatic cells can eat T-cells and possibly natural killer cells, then it is possible that immune therapies involving these cells might not be effective for long-term management of some metastatic cancers. Indeed, cancer immunotherapies have had little impact in reducing the yearly death rate from advanced metastatic cancers.14 It is not often mentioned how potential immunotherapies for melanoma and other cancers might deal with the issue of phagocytic metastatic macrophages.14,94
Melanocytes are the resident macrophages of the skin. Expression of cathepsins B and D are elevated in the phagocytic melanoma cells just as they are in malignant melanomas.93 These tumor cell phagocytic/cannibalistic behaviors are not to be confused with autophagy; a cellular self-digestion process often associated with starvation conditions.90,95,96 Many human cancers and some murine cancers can phagocytize other tumor cells, erythrocytes, leukocytes, platelets, and dead cells, as well as extracellular particles (Table 1). Hence, phagocytosis appears similar in resident skin macrophages and in malignant melanoma.
1. Cancers with Phagocytic Behavior
Numerous reports have described the phagocytic behaviors seen in aggressive human cancers (Table 1). We previously identified two spontaneous invasive/metastatic murine brain tumors (VM-M2 and VM-M3) that express many macrophage characteristics including phagocytosis.14,39 These metastatic tumor cells engulf fluorescent beads. One of the more interesting features of these natural mouse brain tumors was their metastatic behavior when grown outside the central nervous system. The cells spread to multiple organ systems following implantation into most extracranial sites. While extracranial metastasis of central nervous system tumors is not common, many gliomas, especially glioblastoma multiforme (GBM), are highly metastatic if the tumor cells gain access to extraneural tissue.14,55 Indeed, several investigators have documented the metastatic behavior of malignant brain cancers, especially GBM.39,55,97-102 The VM-M2 and VM-M3 tumors replicate this feature of GBM behavior.
One report showed that recipients of organs from a donor with GBM developed metastatic cancer.103 This indicates that neoplastic cells from this GBM metastasized from the brain and infiltrated extraneural tissues without detection. As extraneural tissues often are not examined in patients dying from GBM, it is not clear if this was a rare event or was part of a more general phenomenon.14 Brent Reynolds, a leader in the stem cell field, mentioned that circulating metastatic cells are not uncommon in GBM patients (personal communication). Moreover, extracranial metastasis of brain tumors portends an extremely poor survival, with the vast majority of patients surviving less than 6 months from the diagnosis of metastatic GBM disease.104 The widely held view that metastasis does not occur for GBM should be reevaluated.14,102 Many GBM patients often die prior to detection of systemic metastasis. Thus, GBM patients should not donate their organs for transplantation.
While it might be difficult to prove a myeloid origin of invasive GBM cells, substantial evidence shows that subpopulations of neoplastic GBM cells display the phagocytic behavior of macrophages/microglia.14,55 As microglia are the resident macrophages of the brain, we considered that some of the cells in these tumors could arise from neoplastic microglia/macrophages.37,45,55 GBM, like many advanced metastatic human cancers, contain mixtures of numerous neoplastic cell types, many of which have mesenchymal properties and are of unknown cellular origin.105-107 Indeed, the original nineteenth century observations of Virchow (1863/1865) described glioblastomas as gliosarcomas of mesenchymal origin.108 While numerous mesenchymal cells are frequently seen in GBM, the specific classification of all tumor cell types within human GBM remains ambiguous at best.14,55,105,109-111
According to our hypothesis, many of the neoplastic mesenchymal cells seen in GBM arise from transformed macrophages or microglia that fuse with neoplastic stem cells.14,55 Such hybrid cells would represent the most invasive neoplastic cells within the tumor. It also appears that bevacizumab (Avastin®) selects for the most invasive cells within GBM.14,112 This could account for why tumor recurrence following bevacizumab therapy is universally fatal.14,113 Complicated EMT explanations for the mesenchymal properties of GBM are unnecessary once it becomes recognized that the same properties can arise from neoplastic transformation of microglia/macrophages.
Phagocytic behaviors have been reported for many human cancers including skin, breast, lymphoma, lung, brain, ovarian, pancreatic, renal, endometrial, rhabdomyosarcoma, myeloma, fibrosarcoma and bladder (Table 1). For most of these tumors, the phagocytic phenotype was restricted primarily to those cells that were also highly invasive and metastatic.39, 93, 114-122 Hence, the most potentially deadly cells within tumors are those with macrophage properties.
Lugini and co-workers measured the phagocytic behavior of cell lines derived from primary human melanomas and metastatic lesions.122 The phagocytic behavior in all of the cell lines derived from metastatic lesions was similar to that of the macrophage controls, whereas phagocytic behavior was not found in any of the cell lines derived from primary melanomas.122 Histological examination of in vivo metastatic melanoma lesions confirmed the presence of phagocytic tumor cells.93 The phagocytic properties of tumor cells suggest an origin from macrophages or other professional phagocytes.28
Numerous phagocytic tumor cells were identified within metastatic breast cancer lesions and were not observed within the primary tumor of the same patient.119 This is similar to the appearance of phagocytic signet ring cells observed in secondary metastatic lesions in other breast cancer patients.123 Additionally, the number of phagocytic tumor cells present within the tumor stroma correlates with breast cancer malignancy and grade.124 Hence, phagocytosis is a common macrophage phenotype seen in many metastatic human cancers.14,28
B. Metastatic Behavior of the RAW 264.7 Mouse Macrophages
RAW 264.7 cells are considered a normal mouse macrophage cell line and are widely used to study a broad range of macrophage properties.14 RAW 264.7 cells were transformed with Abelson leukemia virus and derived from BALB/c mice. It is known that viruses damage mitochondrial function, which is the initial event in the transformation of a normal cell to a neoplastic cell.14 We used the RAW 264.7 cells as a control cell line for our metastatic VM-M2 and VM-M3 metastatic cancer cells.39 Using fluorescent microspheres, we found that the phagocytic activity of the metastatic VM-M2 and VM-M3 tumor cells was similar to that of the RAW 264.7 macrophage cell line.39 Not only were there similarities between the RAW cells and the metastatic VM tumor cells for phagocytic behavior, but also these cells were similar in their morphology, gene expression, and lipid composition.39
How was it possible that the RAW macrophages could be so similar to the metastatic VM cells, yet be considered normal cells by some investigators? To determine whether the RAW cells were tumorigenic or non-tumorigenic, we implanted the RAW 264.7 cells subcutaneously into the flanks of immunodeficient BALB/SCID mice. The RAW cells not only formed tumors, but also showed systemic metastasis.14 We discovered that the RAW 264.7 macrophage cell line is highly metastatic following intracerebral and subcutaneous transplantation into SCID mice. The metastatic properties of the RAW 264.7 cells are similar to those of our VM-M2 and VM-M3 cell lines in forming metastases in lung, liver, spleen, and kidney.14, 39
The metastatic behaviors of the RAW cells also appear similar to the metastatic behaviors of the tumors described by Kerbel and colleagues.125,126 Like the VM-M2 and VM-M3 tumor cell lines, the RAW 264.7 cells express little ganglioside GM3 and metastasize to multiple organ systems (liver, spleen, kidney, lung, and brain) when grown subcutaneously outside the brain.39 Ganglioside GM3 inhibits angiogenesis and blocks tumor cell invasion.39,127 These findings provide further evidence showing that cells with macrophage properties can give rise to metastatic cancer regardless of how the cells are classified.
C. Fusogenicity is a Shared Behavior of Macrophages and Many Metastatic Cancer Cells
Fusogenicity is the ability of a cell to fuse with another cell through the merging of their plasma membranes.31,84,85 This process can arise in vitro as is seen with the formation of antibody-producing hybridomas. However, fusion in human cells is a highly regulated process that is essential for fertilization (sperm and egg) and skeletal muscle (myoblasts) and placenta (trophoblast) formation.14,128 Outside of these developmental processes, cell-to-cell fusion is normally restricted to differentiated cells of myeloid origin.129 During differentiation, subsets of macrophages fuse with each other to form multinucleated osteoclasts in bone or multinucleated giant cells in response to foreign bodies.63,84 Osteoclasts and giant cells have increased cell volume that facilitates engulfment of large extracellular materials.63
Macrophages are also thought to fuse with damaged somatic cells during the process of tissue repair.31,63,84,85,129,130 In addition to homotypic fusion, macrophages are known to undergo heterotypic fusion with tumor cells.17,31,63,65,131,132 Aichel first suggested that fusion between somatic cells and leukocytes could induce aneuploidy resulting in tumors with increased malignancy.64 Mekler and Warner later proposed that fusion of committed tumor cells with host myeloid cells would produce tumor hybrids capable of migrating throughout the body and invading distant organs.28,133,134 Recent studies from Wong and co-workers described how macrophages fuse with tumor epithelial cells.31,135 Besides inflammation, radiation also increases the fusion hybrid process.135 It possible that decreased long-term survival in some irradiated cancer patients results from enhanced production of macrophage-epithelial fusion hybrids.14 We have stated that the human brain should rarely if ever be irradiated.14,136 It is our opinion that radiation therapy will contribute to recurrence of brain tumors and possibly to the recurrence of other cancers including those from breast, rectum, and bladder.14,56,61,137,138
Pawelek and colleagues strongly favor the fusion hypothesis for the origin of metastatic cancers.60,64,139-144 They provide compelling evidence showing that fusion hybrids could account for the diversity of cell phenotypes observed within tumors. Fusion between neoplastic tumor cells and myeloid cells, with subsequent nuclear fusion, could produce new phenotypes in the absence of new mutations, as the hybrids would express genetic and functional traits of both parental cells.64 These neoplastic hybrids would express the macrophages characteristics to intravasate, extravasate, and migrate to distant organs while also possessing the unlimited proliferative potential of the cancer cells. Since myeloid cells are part of the immune system, it would be easy to see how tumor hybrids would also be able to evade immune surveillance.14
1. Fusogenic Tumor Cells
Many tumor cells are fusogenic.132 Fusogenic tumor cells are found in a wide-variety of cancer types including, melanoma, breast, renal, liver, gall bladder, lymphoma and brain (Table 1). Tumor cell hybrids can form either in vitro or in vivo from fusions between two tumor cells or between a tumor cell and a normal somatic cell. One of the first reports of tumor cell fusion hybrids showed that human glioma cells, when implanted within the cheeks of hamsters, spontaneously fused with non-tumorigenic host cells, resulting in metastatic hybrid humanhamster tumor cells.145 Goldenberg and co-workers recently described how several genes including that for CXCR4 could be transferred horizontally from glioblastoma cells to non-tumor cells of the hamster leading to enhanced metastasis and tumor progression.70 This is interesting in light of our findings that CXCR4 is expressed in our highly metastatic VM-M2 macrophage/microglial tumor cells.15,39 Many of the early reports for fusogenic cancers described fusions between lymphomas and myeloid cells. For example, spontaneous in vivo fusion between the non-metastatic murine MDW4 lymphoma and host bone marrow cells resulted in aneuploid metastatic tumor cells.28,125 Seyfried recently proposed that the horizontal transfer of information from one cell to another during tumor progression was an example of Lamarckian inheritance and that the evolutionary concepts of Lamarck could better explain tumor progression than those of Darwin.14
Munzarova, et al., recognized that numerous traits expressed in macrophages were also expressed in metastatic melanoma cells and suggested that the tumor metastasis could result from fusions between tumor cells and macrophages.62,146 Pawelek and co-workers showed that the majority of macrophage-melanoma hybrids displayed increased metastatic potential when grown in vivo.64 Further studies revealed that Cloudman S91 melanoma cells underwent spontaneous fusion with the murine host cells in vivo resulting in secondary lesions that were comprised mostly of tumor-host cell hybrids. The fusion of tumor cells with host myeloid cells was a compelling explanation.141
Artificial fusions of human monocytes and mouse melanoma cells revealed that the resulting hybrids expressed both human and mouse genes.64 Other investigators also showed that the macrophage-specific antigens F4/80 and Mac-1 were expressed in murine Meth A sarcoma cells after spontaneous in vivo fusion with host cells. Interestingly, latex bead phagocytosis was also expressed in the Meth A sarcoma-host cell fusion hybrids.147 Since these fusion hybrids expressed genotypes and phenotypes of both parental cells, it appears that the non-metastatic tumor cells could acquire an invasive/metastatic phenotype without new mutations. Such findings are at odds with the somatic mutation theory of cancer and with the EMT hypothesis of metastasis.
It is well documented that tumor-associated macrophages promote tumor progression through the release of cytokines, and pro-angiogenic and pro-metastatic molecules.37,42,50,51 However, the fusion of cancer cells with tissue macrophages could also accelerate tumor progression. Fusion among tumor cells in human solid tumors is difficult to detect. Several reports provide evidence for fusions between tumor cells and myeloid cells in human bone marrow transplant (BMT) recipients.142,143 Such fusions would accelerate tumor progression as illustrated from the recent work of Goldenberg and colleagues.70
Wong and colleagues recently conducted parabiosis experiments, where one mouse is surgically attached to another mouse, to show how bone marrow-derived cells of one mouse fuse with intestinal tumor cells of the other mouse.14,31 Moreover, they identified the macrophage as the driver for this process. They also showed that the fused hybrid cells retained a transcriptome identity characteristic of both parental derivatives, while also expressing unique transcripts.31 These findings show how fusions between macrophages and tumor cells, within the inflamed wound environment, could give rise to the metastatic phenotype of cancer cells thus enhancing tumor progression.
It is important to recognize that both radiation therapy and immuno-suppression can increase the incidence of metastatic cancers.148 DNA analysis of micro-dissected metastatic cells from a child diagnosed with renal cell carcinoma after a bone marrow transplant revealed DNA from both the BMT donor and the recipient in the metastatic cells.143 Bone marrow and tumor cell hybrids were also identified in a female who developed renal carcinoma after receiving a BMT from a male donor.142 These reports provided further compelling genetic evidence that spontaneous fusions can occur between human myeloid cells and tumor cells. It should not therefore be surprising that radiation therapy might exacerbate disease progression and metastasis in some cancer patients.14,61,137,149
Macrophage-macrophage fusions could also induce aneuploidy in the fused hybrids.144,150 The numerous in vitro studies and in vivo reports suggest that myeloid hybrids could be responsible for the metastatic progression of numerous cancers.14 Multinucleated giant cells, a signature of hybrid formation, are frequently seen in human cancers suggesting that cell fusions are not rare events (Table 1). Regardless of the mechanism, metastatic cells express numerous behaviors of mesenchymal/myeloid cells and, if exploited, could generate novel therapeutic strategies for managing metastatic cancers as we recently described.14
D. Myeloid Biomarkers Expressed in Tumor Cells
Myeloid cells express a wide variety of biomarkers that are unique to their ontogeny and function.151 Routine histological and immuno-histochemical analyses are often preformed to assess tumor type and grade. Since TAMs are often correlated with a poor patient prognosis, tumor biopsies are frequently evaluated for macrophage markers. Although TAM are generally thought to comprise the macrophage antigen-expressing cells observed within the tumor stroma, several reports show that macrophage-specific antigens and biomarkers are also expressed on a wide variety of human cancer cells (Table 1).
One of the more interesting studies was that of Ruff and Pert, who demonstrated that several macrophage antigens (CD26, C3bi and CD11b) were expressed on the tumor cells from small cell lung carcinoma (SCLC).28,152 Levels of expression were comparable to that seen in the monocyte controls. It is important to note that the macrophage antigens were also expressed in the cultured tumor cells themselves. This tumor cell expression was confirmed from in vivo tissue preparations. This eliminated the possibility that the antigen expression was derived from TAM. These investigators concluded that the SCLC tumor cells in their specimens were not of lung epithelial origin, but rather were of “myeloid origin”. A malignant transformation of recruited myeloid cells, from smoking-related tissue damage, was offered as an explanation for the origin of tumor cells with myeloid/macrophage properties,152 although this interpretation was controversial.153,154 The authors provided additional data and a convincing argument supporting a macrophage origin.155 The findings of Ruff and Pert that myeloid-cell phenotypes were expressed in SCLC were confirmed in other independent studies of this tumor type.154,156 In light of the above discussion, it is also possible that the myeloid properties of the SCLC were derived from fusions of macrophages and neoplastic lung epithelial cells.
Besides SCLC, myeloid-associated antigens (CD14 and CD11b) were also expressed in five metastatic breast cancer cell lines.153 None of the breast cancer cell lines, however, expressed markers for B-cells or T-cells.153 The authors suggested that common antigen sharing between different cell types could be related to common cellular interactions.153 Further evidence for a mesenchymal origin of metastatic cancer comes from tissue microarray analysis of 127 breast cancer patients.137 The CD163 macrophage scavenger receptor was expressed on the tumor cells of 48% of the patients, while MAC387 macrophage marker was expressed on the tumor cells of 14% of the patients.137 Pathology confirmed that the staining was localized to the tumor cells and not solely to the tumor infiltrating macrophages. Interestingly, cancers that contain CD163-expressing tumor cells have a more advanced histological grade, enhanced metastasis, and reduced patient survival.137 This report demonstrated, for the first time, that tumor cells expressing macrophage antigens could be identified in more than half of breast cancer patients.
Similar studies were conducted on patients with bladder and rectal cancer.56,137 As in breast cancer patients, CD163 was expressed on tumor cells in many patients with bladder and rectal cancer.56,137 Moreover, CD163 expression was found in 31% of the rectal tumors from patients in the preoperative irradiation group, but was expressed in only 17% in the non-irradiation group. Prognosis was also worse for those patients with CD163-positive cancer cells than in those patients with CD163-negative cancer cells. Inflammation and radiation is known to enhance formation of macrophage epithelial cell fusion hybrids.157 In addition to these studies on human cancers, Maniecki and co-workers showed that expression of CD163 could be a common phenotype of many metastatic cancers arising from heterotypic cell fusions between tumor cells and macrophages.56 The findings in these metastatic cancers are consistent with the origin of metastatic cells from transformed macrophages or from macrophage fusion hybrid cells, which are increased from radiation and inflammation.
These findings provide additional evidence that radiation therapy can be counter productive to long-term survival of patients.14,61 Although radiation therapy can help some cancer patients, radiation therapy will also enhance mitochondrial damage and fusion hybridization thus potentially making the disease much worse. These findings are consistent with the role of radiation in inducing tumor cell-macrophage fusions and in exacerbating the metastatic properties of some cancers.61,137,157 It also appears that some anti-angiogenic drugs like bevacizumab and cediranib actually increase the number of invasive cells with macrophage properties in brain tumors.14,49,112,158 In light of the findings presented here, we suggest that these drugs select for invasive tumor cells with macrophage properties.14 This would not be beneficial to patients. Viewed together, these studies demonstrate that macrophage antigens, which are associated with enhanced metastasis and poor prognosis, are expressed on the tumor cells of patients with breast, bladder, rectal cancers, and brain cancers.
1. Cathepsins, Ezrin, and E-Cadherin
Macrophages express high levels of lysosomal-enriched cathepsins, which facilitate the digestion of proteins ingested following phagocytosis or pinocytosis.14,159,160 This is interesting since lysosomal cathepsins D and B are viewed as prognostic factors in cancer patients.93,160 Indeed, a high content of these enzymes in tumors of the head and neck, breast, brain, colon, or endometrium was considered a sign for high malignancy, high metastasis, and overall poor prognosis.160 Besides the cathepsins, activated macrophages also express ezrin as part of a protein complex with radixin and moesin.161 The ezrin-radixin-moesin is a family of molecules that play essential roles in tissue remodeling by linking the cell surface with the actin cytoskeleton and facilitating signal-transduction pathways.14,162 There is increasing awareness that ezrin is also expressed in metastatic cancer cells suggesting an important role in metastatic phenotype of cancer cells.91,163-166 The transition from the epithelial to the mesenchymal phenotype is associated with downregulation of the cell adhesion molecule, E-cadherin.18 It is important to recognize that E-cadherin is either unexpressed or expressed in low levels in macrophages.167,168 Viewed collectively, these findings provide further evidence linking macrophage phenotypes with the properties of metastatic cancers.
2. Anemia and Increased Hepcidin in Metastatic Cancer
Iron deficient anemia is a co-morbid trait in many patients with metastatic cancers.169,170 Hepcidin is a key regulator of iron metabolism and plasma iron levels by controlling the efflux of iron from enterocytes, hepatocytes, and macrophages and by internalizing and degrading the iron exporter, ferroportin.171 Hepcidin might contribute to the systemic anemia in colorectal cancer patients by acting at the level of the macrophage.169 Activated macrophages express IL-6, which induces expression of hepcidin. Macrophages are the major cell type responsible for systemic iron recycling.169,172 The Ward, et al., findings are therefore consistent with our hypothesis that metastatic cancer is a disease of myeloid cells especially macrophages.14,28,55 Many characteristics of metastatic cancers can be explained once it becomes recognized that metastatic cancer is a macrophage metabolic disease. Hence, iron deficient anemia should not be unexpected for metastatic cancers derived from transformed macrophages or macrophage fusion hybrids.
E. Carcinoma of Unknown Primary Origin
Carcinoma of unknown primary (CUP) is a systemic metastatic disease without an identifiable primary tumor and is often associated with poor prognosis. Approximately 5% of all newly diagnosed cancers are classified as CUP.173,174 These cancers are often classified as adenocarcinomas, squamous cell carcinomas, poorly differentiated carcinoma, and neuroendocrine carcinomas.173,174 It is thought that these cancers metastasize before the primary tumor has had time to develop into a macroscopic lesion.174 Signet-ring cells were found in some CUP indicating that subsets of these cancers exhibit phagocytic behavior like other metastatic cancers.175 Interestingly, aneuploidy was identified in 70% of CUP adenocarcinomas, but was not found in about 30% of the tumors.176 Aneuploidy can arise in part from cell fusion events.125 Survival was better in patients with aneuploid tumors than with diploid tumors showing that patients with diploid tumors do not have a more favorable prognosis. This is interesting and is consistent with findings that aneuploidy actually slows cell growth.14,177,178 Due to their high aggressiveness, we suggested that some CUPs could arise from macrophage fusion hybrids.14,28
F. Many Metastatic Cancers Express Multiple Macrophage Properties
The evidence presented here and in our recent review show that many metastatic cancers express multiple myeloid characteristics (Table 1). For instance, many phagocytic or fusogenic tumors also express myeloid antigens further supporting a myeloid origin of these metastatic cancers. It is important to mention that the myeloid properties are expressed in the tumor cells themselves and should not be confused with myeloid properties expressed in TAM, which are also present in the tumors but are not part of the neoplastic cell population.14 The Pawelek, Lazebnik, and Wong groups have amassed compelling evidence that cell fusion events involving macrophages can give rise to cells that metastasize.14,31,132,144,179,180 In contrast to the EMT/MET explanation of metastasis, the macrophage cell fusion explanation of metastasis does not require the induction and reversion of extremely complicated gene regulatory systems. The macrophage origin of metastasis can account for most observations related to the disease.14
V. LINKING METASTASIS TO MITOCHONDRIAL DYSFUNCTION
Substantial evidence now indicates that nearly all cancers are a type of mitochondrial disease arising from respiratory insufficiency.14,181 This damage leads to fermentation as a compensatory source of energy according to the original theory of Warburg.14,182,183 When permanent respiratory damage occurs in cells of myeloid origin including hematopoietic stem cells and their fusion hybrids, metastasis would be a potential outcome.14 It is not necessary to blame mutations or to invent complicated genetic regulatory systems to explain the phenomenon of metastasis.
Numerous studies indicate that mitochondria from a broad range of metastatic cancers are abnormal and incapable of generating energy through normal respiration.14,184,185 Energy through fermentation is the single most common hallmark of all cancer cells including those with metastatic potential. This phenotype arises from mitochondrial dysfunction.181 Mitochondrial damage can arise in any cell within the inflammatory microenvironment of the incipient tumor including TAM, homotypic fusion hybrids of hematopoietic cells, or heterotypic fusion hybrids of macrophages and neoplastic epithelial cells.14 The end result would be cells with metastatic potential. Although metastatic cells will differ in their morphology from one organ system to the next, they all suffer from the common malady of insufficient respiration. The origin of metastatic cancer from myeloid cells and fusion hybrids can explain the substantial morphological and genetic diversity seen among different tumor types.132 Metastasis can arise in macrophage fusion hybrids that sustain irreversible mitochondrial damage (Fig. 4).
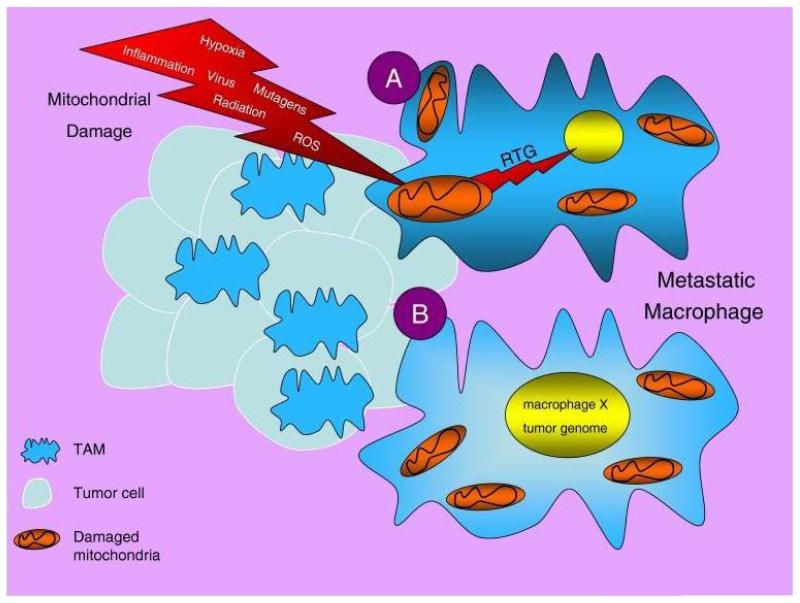
FIGURE 4.
Proposed mechanisms of macrophage transformation and metastasis. The tumor microenvironment consists of numerous mitochondria damaging elements, which could impair mitochondria energy production in TAM and tissue macrophages. This would eventually produce genetic instability through the mitochondrial stress or retrograde signaling (RTG) response (A).14,181 Fusions between macrophages or between macrophages and cancer stem cells could result in cells expressing both the tumor and macrophage genomes (B). The end result would be cells that can survive in hypoxic environments, can proliferate, and can spread to multiple sites through the circulation. Source: Reprinted with permission from28.
A. Respiratory Damage in Macrophage Fusion Hybrids
Substantial evidence indicates that normal mitochondrial function suppresses tumorigenesis.14 Cytoplasm containing mitochondria with normal respiratory function can suppress tumorigenicity despite the continued presence of the tumor cell nucleus.14 These findings indicate that nuclear gene mutations alone cannot account for the origin or progression of cancer. How do these findings relate to the origin of metastatic cancer cells following macrophage fusions with other cells? If normal macrophages fuse with neoplastic stem cells, it might be anticipated that normal respiratory function of the macrophages would suppress tumorigenicity in the fused hybrid.14 Although normal respiration would initially suppress tumorigenicity in fused hybrids, persistent or recurrent inflammation in the microenvironment will eventually damage the majority of mitochondria in the fused hybrids, thus initiating the path to metastasis.14 As macrophages evolved to survive in hypoxic and inflammatory environments, considerable time and iterative damage to respiration would be necessary to initiate tumorigenesis in the fusion hybrids. It is also noteworthy that radiation exposure would not only enhance fusion hybrid formation, but would also damage respiration thus leading to compensatory fermentation and the onset of tumorigenesis. It should not be surprising why long-term survival is reduced or why more aggressive tumors recur in many patients that receive radiation to treat their cancers.14
As respiration is responsible for maintaining genomic stability and the differentiated state, respiratory insufficiency will eventually induce the default state of unbridled proliferation and genomic instability.14, 181 If this occurs in cells of myeloid origin like macrophages, then emergence of cells with enhanced metastatic potential would be a predicted outcome. Macrophages are genetically programmed to exist in the circulation and to enter and exit tissues.186 The dysregulated behavior of these cells through corrupted energy metabolism would have dire consequences. Oncogene activation and tumor suppressor-gene inactivation are required to maintain energy production through fermentation following irreversible injury to oxidative phosphorylation.14 Enhanced glucose uptake seen in metastatic lesions under PET scanning is indicative of enhanced glycolysis and abnormal energy metabolism.
VI. THE “SEED AND SOIL” HYPOTHESIS IN LIGHT OF THE MACROPHAGE ORIGIN OF METASTASIS
It is well documented that metastatic tumor cells do not invade distant organs randomly. Rather, metastatic cancer cells invade in a non-random pattern with lung, liver, and bone as primary sites of metastases.2,3 The English surgeon, Stephen Paget, was the first to record this phenomenon in his “seed and soil” hypothesis of breast cancer metastasis.3,187 He proposed that certain tumor cells (the seed) have a preferential affinity to invade certain organs (the soil).187 None of the current models of metastasis, i.e., the EMT/MET, stem cells, or TAM accessory models, have addressed Paget’s “seed and soil” hypothesis. The origin of metastasis as a macrophage disease, however, can address this phenomenon.14
Although the non-random dissemination of metastatic cancer cells has engaged the attention of numerous investigators for decades, no credible genetic mechanism has been able to account for the phenomenon.2,3,188 The seed and soil hypothesis is extremely difficult to explain if cancer is viewed as a genetic disease.14,123,188 There are simply no clear connections between the non-random invasion of distant organs and the genetic abnormalities found in metastatic cells.14 On the other hand, a credible explanation of the seed and soil hypothesis emerges if cancer is viewed as a macrophage metabolic disease.
Basically, respiratory insufficiency in cells of myeloid origin can explain the seed and soil phenomenon.14 This comes from findings showing that mature cells of monocyte origin (macrophages) enter and engraft tissues in a non-random manner.189 Macrophages are genetically programmed to exist in the circulation and to preferentially enter various tissues during wound healing and the replacement of resident myeloid cells.186,189 Some macrophage populations in liver are regularly replaced with bone marrow derived monocytic cells, whereas other macrophage populations are more permanent and require fewer turnovers.190 It is reasonable to assume that metastatic cancer cells derived from macrophages or fusions of monocytic cells with epithelial cells will also preferentially home to those tissues that naturally require regular replacement of resident macrophages.
This prediction comes from findings that many metastatic cells express characteristics of macrophages.14,31 Macrophage turnover should be greater in tissues like liver and lung where the degree of bacterial exposure and the wear-and-tare on the resident macrophage populations is considerable.191 This could explain why these organs are a preferred soil of many metastatic cancer cells. Bone marrow should also be a common target of metastatic cells because this site is the origin of the hematopoietic stem cells, which give rise to myeloid cells. Liver, lung, and bone are also preferential sites for metastatic spread for the VM mouse tumor cells.14,39 This is one reason why the natural tumors in the VM mouse, which preferentially home to these tissues, are an excellent model for metastatic cancer.14,39
Because the metastatic cells express insufficient respiration with compensatory fermentation, these cells will enter their default state of proliferation, as would any neoplastic cell.14,192 In addition to those organs receiving high macrophage turnover, macrophages also target sites of inflammation and injury.191 This is interesting in light of findings showing that metastatic cancer cells from lung and breast can appear in the mouth following recent tooth extraction or along needle tracts following biopsy.193-195 An unhealed wound is an ideal “soil” for macrophage infiltration.191,196 This phenomenon is referred to as inflammatory “oncotaxis” and can explain in part the seed and soil hypothesis.197 If metastasis were a metabolic disease of myeloid cells, then the appearance of metastatic cells in recent tooth extraction or wounds would not be unexpected.14 While the mechanistic details of these phenomena will require further examination, the general principle is clear. The non-random pattern of metastasis to visceral organs, bone marrow, and wounds (the soil) is consistent with a macrophage (the seed) origin of metastasis.
VII. REVISITING THE MESENCHYMAL EPITHELIAL TRANSITION (MET)
In contrast to the EMT, the MET involves proliferation and re-expression of epithelial characteristics following extravasation, invasion, and proliferation at distant cites (Fig. 2). The MET is considered a reversibility of the EMT.19 It is not clear how a series of somewhat random somatic mutations could orchestrate the sophisticated series of behaviors associated the EMT, and then have these mostly reversed during the MET. However, a myeloid cell origin of metastasis provides a more credible explanation of metastasis than the MET/MET. Metastatic cells arising from myeloid cell fusions would retain the genetic architecture necessary for entering and exiting the circulation at recognized sites. It is not necessary to construct complicated mutation based regulatory systems to explain these phenomena. Macrophages naturally enter and exit the circulatory and lymphatic systems. The circulatory system is not a “hostile” environment for cells in the macrophage lineage, as macrophage precursors, i.e., monocytes, exist naturally in the circulation. These cells also express the cell-surface adhesion molecules (selectins) necessary for extravasation at designated organs.14 They already express the batteries of metalloproteases necessary for degradation of basement membranes and invasion. When these capabilities occur together with impaired respiration, dysregulated proliferation would be an expected outcome.14 While these properties certainly implicate myeloid cells as the origin of metastatic cells, the fusogenic properties of myeloid cells can also explain how metastatic cells can recapitulate the epithelial characteristics of the primary tumor at secondary growth sites (Fig. 5).
FIGURE 5.
Fusion hybrid hypothesis of cancer cell metastasis. According to our hypothesis, metastatic cancer cells arise following direct transformation or following fusion hybridization between neoplastic epithelial cells and myeloid cells (macrophages). Macrophages are known to invade in situ carcinoma as if it were an unhealed wound. This creates a protracted inflammatory microenvironment leading to fusion hybridization between the neoplastic epithelial cell and the macrophage. Fusion hybridization can explain the phenomenon of EMT without invoking new mutations. Inflammation damages mitochondria leading to enhanced fermentation and acidification of the microenvironment. Mitochondrial damage leading to respiratory insufficiency becomes the driver for the neoplastic transformation of the epithelial cell and of the fusion hybrids (Figure 4). As macrophages are already mesenchymal cells that naturally possess the capability to enter (intravasate) and exit (extravasate) the circulation, the neoplastic fusion hybrid will behave as a rogue macrophage. The fusogenic properties of macrophage cells can also explain how metastatic cells can recapitulate the epithelial characteristics of the primary tumor at secondary micro-metastatic growth sites. This process can explain the phenomenon of MET without invoking a mutation suppression mechanism. Reprinted with permission from Seyfried and Ling.14
Previous studies of fusion hybrids showed that functional hepatocytes could be derived from bone marrow derived macrophages or myelomonocytic cells following cell fusions.198 Rizvi, et al., also showed that expression of epithelial characteristics were found in fusion hybrids between bone marrow-derived cells and either normal epithelium or neoplastic intestinal epithelium.179 More recently, Wong and colleagues showed how macrophage/epithelial cell hybrids could recapitulate phenotypes of epithelial cells while retaining the properties of macrophages.31 It is clear that phenotypes of epithelial cells and macrophages can be maintained in fusion hybrids of macrophages and intestinal epithelial tumor cells. Moreover, these characteristics are passed on to daughter cells through somatic inheritance.
Fusions of activated macrophages with epithelial cells in the primary tumor microenvironment will bestow the capability of the fused cells to degrade basement membranes, to enter and exit the circulatory and lymphatic systems, and to recapitulate the epithelial characteristics of the primary tumor at distant secondary sites. The dysregulated growth at secondary sites is the consequence of damaged respiration in theses cells.14 Hence, the origin of metastatic cells from macrophage fusion hybrids with dysfunctional mitochondria can explain the phenomenon of metastasis (Fig. 5).
VIII. GENETIC HETEROGENITY IN CANCER METASTASIS
Considerable genetic heterogeneity is observed in comparing tumor tissue from primary growth sites with tissue from distant metastases.16,123,188,199 Genetic heterogeneity is seen not only between patients with similar tumor histopathology, but also for the tumors growing at different sites within the same patient.14 Almost every type of genetic heterogeneity imaginable from point mutations to major genomic rearrangements can be found in metastatic and highly invasive cancers including those from breast, brain, and pancreas.123,188,199-201 The mostly non-uniform distribution of mutations in these tumors is consistent with findings that each neoplastic cell within a given tumor can have a profile of changes uniquely different from any other cell within the tumor.14,202 Moreover, if the spread of metastatic cells to some organs (like liver and lung) occurs earlier than spread to other organs, it is possible that genetic heterogeneity would be greater in these organs than in organs that receive metastatic cells later in the disease progression. This is expected if the number of divisions is greater for tumor cells that arrive earlier in these organs than for tumor cells that arrive later in other organs. This could explain why genomic heterogeneity is more diverse in some organs than in other organs or in the primary tumor.188 These complications can obscure attempts to accurately define the clonal origin of tumor cells.
In their analysis of the genomic heterogeneity observed in pancreatic cancer, Campbell and colleagues conclude that, “the biological pathways underlying these forms of genomic instability remain unclear.”188 As genomic stability is dependent on normal mitochondrial function,14 it should not be surprising that there is a “richness of genetic variation in cancer” as Campbell and co-workers describe.188 The richness is the likely consequence of damaged respiration with compensatory fermentation in populations of fusion hybrids that differ from each other in genetic architecture. A non-uniform or random distribution of mutations can arise from the migration of these hybrid cells to other organs. The gene mutations also arise as downstream epiphenomena of respiratory insufficiency coupled with compensatory fermentation.14 As the linkage of genomic instability to mitochondrial dysfunction was not discussed in any of the cancer genome studies mentioned above, we can only assume that the investigators were unaware of this linkage. It is unfortunate that so many industrious investigators focus so much attention on the genomic instability of tumors, which is largely irrelevant to the disease. Real progress in cancer management will be realized only after the cancer field breaks its addiction to the gene theory and recognizes the centrality of mitochondrial damage in the origin and progression of the disease.14
IX. TRANSMISSIBLE METASTATIC CANCERS
Transmissible cancers are those that can be passed from one animal to another through physical contact. The best known is the canine transmissible venereal tumors and the Tasmanian Devil Tumor Disease (DFTD).203,204 These tumors often spread from the primary site of contact to distant organs. The metastatic behavior of these transmissible tumors is basically the same as that seen for the non-transmissible human metastatic cancers. Previous studies indicate that the canine transmissible tumors share several features with histocytes, a type of macrophage.205,206 Indeed, many of the tumors express characteristics of both macrophages and epithelial cells. Such observations would suggest a clonal origin from a macrophage-epithelial cell fusion hybrid.14 Murchison and colleagues recently showed that DFTD originated from cells expressing Schwann cell and epithelial characteristics.204 It is important to mention that hematopoietic bone marrow cells can elaborate Schwann cell-like phenotypes in injury conditions.207 It is also possible that these transmissible metastatic cancers arise as fusion hybrids involving myeloid cells and epithelial cells. Further studies will be necessary to determine if these metastatic cancers arise from similar mechanisms responsible for the origin of metastasis from macrophage-epithelial fusion hybrids.
Like all cancers, mitochondrial dysfunction and respiratory insufficiency would be the expected driver phenotype of these transmissible cancers. However, Tasmanian Devils living in the Western part of the island are resistant to the disease. It appears that the resistance results from a unique DNA polymorphism in the mitochondria of these animals.204 This is interesting in light of findings showing that transmissible cancers will occasionally acquire mitochondria from the host.208 This is another example of horizontal gene transfer and Lamarckian inheritance.14 Is it possible that properties of mitochondria determine the origin of transmissible cancers? Further studies are needed to evaluate the linkage between fusion hybridization and role of mitochondria in the origin of transmissible cancers.
X. THE ABSENCE OF METASTASES IN CROWN-GALL PLANT TUMORS
The crown-gall disease in plants shares many features with tumors in animals.209-211 Crown-gall tumors arise form bacterial infections that enter damaged areas of the plant leading to plant cell proliferation. The mechanisms by which bacteria induce crown-gall disease in plants are similar to those by which viruses induce tumors in animals.14,211 Robinson first suggested that Warburg’s cancer theory might account for the abnormal cell proliferation in crown-gall tumors following bacterial damage to respiration in the affected plant cells.209 Indeed, defects in mitochondrial morphology and energy metabolism were later described in crown-gall tumors.212-214
It is interesting that the crown-gall tumors express four of the Hanahan and Weinberg hallmarks of cancer, i.e., self-sufficiency in growth signals, insensitivity to growth inhibitory (anti-growth) signals, evasion of programmed cell death (apoptosis), and limitless replicative potential.14 However, these tumors do not express invasion or metastasis.211 With the exception of metastasis and invasion, the abnormalities in growth and physiology are similar in crown-gall disease and in animal tumors.14 If metastasis arises from damaged respiration in macrophages or in their fusion hybrids as we discussed above, then it becomes clear why the grown-gall tumors do not display invasion or metastasis despite expressing other hallmarks of tumors. The crown-gall tumors do not metastasize because they do not have macrophages or myeloid cells as part of their immune system.215 The findings in crown-gall tumors are also consistent with the Tarin’s hypothesis, “that metastasis cannot occur until an organism has evolved the genes for lymphocyte trafficking.”1,14 Plants have not evolved these genes as far as we know. According to our hypothesis, metastasis occurs predominantly in cells that express properties of macrophages.
XI. MANAGING METASTATIC CANCERS
As a metabolic disease, most if not all cancers can be managed by targeting those fuels necessary for their proliferation and survival. The goal is to first transition energy metabolism of all normal cells of the body to ketone bodies, which tumor cells cannot effectively use for energy.14 It is well documented that most tumor cells require glucose for energy through glycolysis.216-218 Glutamine is also a major metabolic fuel for many cells of the immune system including macrophages.181,219,220 We recently showed that the simultaneous targeting of glucose and glutamine under calorie restriction could significantly reduce systemic metastatic cancer in the VM-M2 mouse model.221,222 Indeed, targeting these fuels was more effective in blocking metastasis than was using the well-known toxic chemotherapies methotrexate or cisplatin.223 Once metastatic cancer becomes recognized as a metabolic disease, new and less toxic solutions will emerge for effective management.14
XII. CONCLUDING REMARKS
A transition from an epithelial cell to a mesenchymal cell is considered an underlying characteristic of metastasis. However, it is improbable that random mutations acquired through a Darwinian selection process could account for all of the myeloid-cell behaviors necessary for the completion of the metastatic cascade. As an alternative to a series of gain-of-function mutations and clonal selection, we propose that the metastatic mesenchymal phenotype arises initially from respiratory damage in macrophages or in epithelial-macrophage fusion hybrids, which is then followed by compensatory fermentation. This would produce the metastatic lesion images seen on PET. Inflammation and radiation damage enhances hybridization while also damaging mitochondrial function over time. It is our opinion that the myeloid origin of metastasis is the most compelling explanation for the origin of metastasis and tumor progression.14 We anticipate major advances in management of metastatic cancer once this explanation becomes more widely recognized.
ACKNOWLEDGMENTS
This work was supported, in part, by the National Institutes of Health [grant numbers HD-39722, NS-55195 and CA-102135], a grant from the American Institute of Cancer Research, and the Boston College Expense Fund.
ABBREVIATIONS
- BMT
bone marrow transplant
- CUP
carcinoma of unknown primary
- EMT
epithelial mesenchymal transition
- GBM
glioblastoma multiforme
- MET
mesenchymal epithelial transition
- SCLC
small cell lung carcinoma
- TAM
tumor associated macrophage
REFERENCES
- 1.Tarin D. Cell and tissue interactions in carcinogenesis and metastasis and their clinical significance. Sem Cancer Biol. 2011;21(2):72–82. doi: 10.1016/j.semcancer.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer. 2002;2(8):563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Rev Cancer. 2003;3(6):453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 4.Welch DR. Defining a cancer metastasis. Amer Assoc Cancer Res. Education Book. 2006:111–5. [Google Scholar]
- 5.Lazebnik Y. What are the hallmarks of cancer? Nature Rev Cancer. 2010;10(4):232–3. doi: 10.1038/nrc2827. [DOI] [PubMed] [Google Scholar]
- 6.Tarin D. Comparisons of metastases in different organs: biological and clinical implications. Clin Cancer Res. 2008;14(7):1,923–5. doi: 10.1158/1078-0432.CCR-07-5259. [DOI] [PubMed] [Google Scholar]
- 7.Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol. 2008;3:221–47. doi: 10.1146/annurev.pathmechdis.3.121806.151523. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1,559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 10.Sporn MB. The war on cancer. Lancet. 1996;347(9012):1,377–81. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 11.Faguet G. The War on Cancer: An Anatomy of a Failure, A Blueprint for the Future. Springer; Dordrecht, The Netherlands: 2008. [Google Scholar]
- 12.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214(3):283–93. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 13.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nature Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 14.Seyfried TN. Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons; Hoboken: 2012. p. 432. [Google Scholar]
- 15.Shelton LM, Mukherjee P, Huysentruyt LC, Urits I, Rosenberg JA, Seyfried TN. A novel pre-clinical in vivo mouse model for malignant brain tumor growth and invasion. Journal Neuro-oncology. 2010;99(2):165–76. doi: 10.1007/s11060-010-0115-y. [DOI] [PubMed] [Google Scholar]
- 16.Steeg PS. Heterogeneity of drug target expression among metastatic lesions: lessons from a breast cancer autopsy program. Clin Cancer Res. 2008;14(12):3,643–5. doi: 10.1158/1078-0432.CCR-08-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawelek JM. Cancer-cell fusion with migratory bone-marrow-derived cells as an explanation for metastasis: new therapeutic paradigms. Future Oncology (London, England) 2008;4(4):449–52. doi: 10.2217/14796694.4.4.449. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg RA. The Biology of Cancer. Garland Science; New York: 2007. p. 796. [Google Scholar]
- 20.Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119(6):1,417–9. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21(3):497–503. doi: 10.1093/carcin/21.3.497. [DOI] [PubMed] [Google Scholar]
- 22.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 23.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, Califano A, Iavarone A. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–25. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart IR. New evidence for tumour embolism as a mode of metastasis. J Pathol. 2009;219(3):275–6. doi: 10.1002/path.2616. [DOI] [PubMed] [Google Scholar]
- 25.Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst. 2008;100(4):232–3. doi: 10.1093/jnci/djn032. [DOI] [PubMed] [Google Scholar]
- 26.Nowell PC. Tumor progression: A brief historical perspective. Semin Cancer Biol. 2002;12(4):261–6. doi: 10.1016/s1044-579x(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 27.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 28.Huysentruyt LC, Seyfried TN. Perspectives on the mesenchymal origin of metastatic cancer. Cancer Metastasis Rev. 2010;29(4):695–707. doi: 10.1007/s10555-010-9254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7,443–54. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15(11):599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, Wong MH. Fusion between Intestinal Epithelial Cells and Macrophages in a Cancer Context Results in Nuclear Reprogramming. Cancer Res. 2011;71(4):1,497–505. doi: 10.1158/0008-5472.CAN-10-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trosko JE. Review paper: cancer stem cells and cancer nonstem cells: from adult stem cells or from reprogramming of differentiated somatic cells. Vet Pathol. 2009;46(2):176–93. doi: 10.1354/vp.46-2-176. [DOI] [PubMed] [Google Scholar]
- 33.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 34.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70(1):6–22. [PubMed] [Google Scholar]
- 36.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9,392–400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 37.Seyfried TN. Perspectives on brain tumor formation involving macrophages, glia, and neural stem cells. Perspect Biol Med. 2001;44(2):263–82. doi: 10.1353/pbm.2001.0035. [DOI] [PubMed] [Google Scholar]
- 38.Binello E, Qadeer ZA, Kothari HP, Emdad L, Germano IM. Stemness of the CT-2A immunocompetent mouse brain tumor model: Characterization in vitro. J Cancer. 2012;3:166–74. doi: 10.7150/jca.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huysentruyt LC, Mukherjee P, Banerjee D, Shelton LM, Seyfried TN. Metastatic cancer cells with macrophage properties: Evidence from a new murine tumor model. Inter J Cancer. 2008;123(1):73–84. doi: 10.1002/ijc.23492. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Siebzehnrubl FA, Reynolds BA, Vescovi A, Steindler DA, Deleyrolle LP. The origins of glioma: E Pluribus Unum? Glia. 2011;59(8):1,135–47. doi: 10.1002/glia.21143. [DOI] [PubMed] [Google Scholar]
- 42.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 43.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Rev Cancer. 2009;9(4):239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J of Leukocyte Biol. 2008;84(3):623–30. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50(3):305–11. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 46.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: Regulation by distinct molecular mechanisms. J Immunol. 2008;180(4):2,011–7. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 47.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56(20):4,625–9. [PubMed] [Google Scholar]
- 48.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 49.di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, Hedley-Whyte ET, Sorensen AG, Batchelor TT, Jain RK. Glioblastoma recurrence after cediranib therapy in patients: Lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71(1):19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Peinado H, Rafii S, Lyden D. Inflammation joins the “niche.”. Cancer Cell. 2008;14(5):347–9. doi: 10.1016/j.ccr.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26(3-4):373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 54.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 55.Huysentruyt LC, Akgoc Z, Seyfried TN. Hypothesis: are neoplastic macrophages/microglia present in glioblastoma multiforme? ASNNeuro. 2011;3(4):art:e00064. doi: 10.1042/AN20110011. doi:10.1042/AN20110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maniecki MB, Etzerodt A, Ulhoi BP, Steiniche T, Borre M, Dyrskjot L, Orntoft TF, Moestrup SK, Moller HJ. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Inter J Cancer. 2012 doi: 10.1002/ijc.27506. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 57.Navarro A, Boveris A. Hypoxia exacerbates macrophage mitochondrial damage in endotoxic shock. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R354–5. doi: 10.1152/ajpregu.00726.2004. [DOI] [PubMed] [Google Scholar]
- 58.Frost MT, Wang Q, Moncada S, Singer M. Hypoxia accelerates nitric oxide-dependent inhibition of mitochondrial complex I in activated macrophages. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R394–400. doi: 10.1152/ajpregu.00504.2004. [DOI] [PubMed] [Google Scholar]
- 59.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1,029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawelek JM. Tumour cell hybridization and metastasis revisited. Melanoma Res. 2000;10(6):507–14. doi: 10.1097/00008390-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Seyfried TN, Shelton LM, Mukherjee P. Does the existing standard of care increase glioblastoma energy metabolism? Lancet Oncol. 2010;11(9):811–3. doi: 10.1016/S1470-2045(10)70166-2. [DOI] [PubMed] [Google Scholar]
- 62.Munzarova M, Kovarik J. Is cancer a macrophage-mediated autoaggressive disease? Lancet. 1987;1(8539):952–4. doi: 10.1016/s0140-6736(87)90295-9. [DOI] [PubMed] [Google Scholar]
- 63.Vignery A. Macrophage fusion: Are somatic and cancer cells possible partners? Trends Cell Biol. 2005;15(4):188–93. doi: 10.1016/j.tcb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Chakraborty AK, de Freitas Sousa J, Espreafico EM, Pawelek JM. Human monocyte × mouse melanoma fusion hybrids express human gene. Gene. 2001;275(1):103–6. doi: 10.1016/s0378-1119(01)00647-3. [DOI] [PubMed] [Google Scholar]
- 65.Van den Bossche J, Bogaert P, van Hengel J, Guerin CJ, Berx G, Movahedi K, Van den Bergh R, Pereira-Fernandes A, Geuns JM, Pircher H, Dorny P, Grooten J, De Baetselier P, Van Ginderachter JA. Alternatively activated macrophages engage in homotypic and heterotypic interactions through IL-4 and polyamine-induced E-cadherin/catenin complexes. Blood. 2009;114(21):4,664–74. doi: 10.1182/blood-2009-05-221598. [DOI] [PubMed] [Google Scholar]
- 66.Pawelek JM. Tumour-cell fusion as a source of myeloid traits in cancer. Lancet Oncol. 2005;6(12):988–93. doi: 10.1016/S1470-2045(05)70466-6. [DOI] [PubMed] [Google Scholar]
- 67.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. Epub 2012/03/24. [DOI] [PubMed] [Google Scholar]
- 68.Seyfried TN, el-Abbadi M, Ecsedy JA, Bai HW, Yohe HC. Influence of host cell infiltration on the glycolipid content of mouse brain tumors. J Neurochem. 1996;66(5):2,026–33. doi: 10.1046/j.1471-4159.1996.66052026.x. [DOI] [PubMed] [Google Scholar]
- 69.Ecsedy JA, Yohe HC, Bergeron AJ, Seyfried TN. Tumor-infiltrating macrophages influence the glycosphingolipid composition of murine brain tumors. J Lipid Res. 1998;39(11):2,218–27. [PubMed] [Google Scholar]
- 70.Goldenberg DM. Horizontal transmission of malignancy by cell-cell fusion. Expert Opin Biol Ther. 2012 doi: 10.1517/14712598.2012.671807. Epub 2012/04/17. [DOI] [PubMed] [Google Scholar]
- 71.Stossel TP. Mechanical responses of white blood cells. In: Snyderman JIGaR., editor. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams & Wilkins; New York: 1999. pp. 661–79. [Google Scholar]
- 72.Gordon S. Development and distribution of mononuclear phagocytes: Relevance to inflammation. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams & Wilkins; New York: 1999. pp. 35–48. [Google Scholar]
- 73.Burke B, Lewis CE. The Macrophage. 2nd ed. Oxford University Press; New York: 2002. [Google Scholar]
- 74.Sica A, Saccani A, Mantovani A. Tumor-associated macrophages: A molecular perspective. Int Immunopharmacol. 2002;2(8):1,045–54. doi: 10.1016/s1567-5769(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 75.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 77.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2003;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Underhill CB, Nguyen HA, Shizari M, Culty M. CD44 positive macrophages take up hyaluronan during lung development. Develop Biology. 1993;155(2):324–36. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- 80.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Amer J Pathol. 1998;152(2):353–8. [PMC free article] [PubMed] [Google Scholar]
- 81.Culty M, O’Mara TE, Underhill CB, Yeager H, Jr., Swartz RP. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J Leukocyte Biol. 1994;56(5):605–11. doi: 10.1002/jlb.56.5.605. [DOI] [PubMed] [Google Scholar]
- 82.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukocyte Biol. 1994;55(3):410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 83.Chettibi S, Ferguson MWJ. Wound repair: An overview. In: Snyderman JIGaR., editor. Inflamation: Basic Principles and Clinical Correlates. Lippincott Williams & Wilkins; New York: 1999. pp. 865–81. [Google Scholar]
- 84.Sica A, Mantovani A. Macrophage fusion cuisine. Blood. 2009;114(21):4,609–10. doi: 10.1182/blood-2009-09-242800. [DOI] [PubMed] [Google Scholar]
- 85.Vignery A. Osteoclasts and giant cells: macrophage-macrophage fusion mechanism. International J Exper Patholo. 2000;81(5):291–304. doi: 10.1111/j.1365-2613.2000.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1,191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 87.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: Inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157(6):2,577–85. [PubMed] [Google Scholar]
- 88.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11(6):753–61. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 89.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 90.Akst J. It’s a cell-eat-cell world. The Scientist. 2011;25(August):44–9. [Google Scholar]
- 91.Fais S. Cannibalism: a way to feed on metastatic tumors. Cancer Letters. 2007;258(2):155–64. doi: 10.1016/j.canlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 92.Wang S, Guo Z, Xia P, Liu T, Wang J, Li S, Sun L, Lu J, Wen Q, Zhou M, Ma L, Ding X, Wang X, Yao X. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009;19(12):1,350–62. doi: 10.1038/cr.2009.114. [DOI] [PubMed] [Google Scholar]
- 93.Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, Gentile M, Luciani F, Parmiani G, Rivoltini L, Malorni W, Fais S. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66(7):3,629–38. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 94.Couzin-Frankel J. Immune therapy steps up the attack. Science. 2010;330(6003):440–3. doi: 10.1126/science.330.6003.440. [DOI] [PubMed] [Google Scholar]
- 95.Klionsky DJ. Cell biology: regulated self-cannibalism. Nature. 2004;431(7004):31–2. doi: 10.1038/431031a. [DOI] [PubMed] [Google Scholar]
- 96.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1,069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoffman HJ, Duffner PK. Extraneural metastases of central nervous system tumors. Cancer. 1985;56(7 Suppl):1,778–82. doi: 10.1002/1097-0142(19851001)56:7+<1778::aid-cncr2820561309>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 98.Taha M, Ahmad A, Wharton S, Jellinek D. Extracranial metastasis of glioblastoma multiforme presenting as acute parotitis. Br J Neurosurg. 2005;19(4):348–51. doi: 10.1080/02688690500305506. [DOI] [PubMed] [Google Scholar]
- 99.Laerum OD, Bjerkvig R, Steinsvag SK, de Ridder L. Invasiveness of primary brain tumors. Cancer Metastasis Rev. 1984;3(3):223–36. doi: 10.1007/BF00048386. [DOI] [PubMed] [Google Scholar]
- 100.Rubinstein LJ. Tumors of the central nervous system. Armed Forces Institute of Pathology; Washington, D.C.: 1972. p. 400. [Google Scholar]
- 101.Gotway MB, Conomos PJ, Bremner RM. Pleural Metastatic Disease From Glioblastoma Multiforme. J Thorac Imaging. 2011;26(2):W54–8. doi: 10.1097/RTI.0b013e3181d449d6. [DOI] [PubMed] [Google Scholar]
- 102.Kalokhe G, Grimm SA, Chandler JP, Helenowski I, Rademaker A, Raizer JJ. Metastatic glioblastoma: case presentations and a review of the literature. J Neuro-Oncol. 2012;107(1):21–7. doi: 10.1007/s11060-011-0731-1. [DOI] [PubMed] [Google Scholar]
- 103.Armanios MY, Grossman SA, Yang SC, White B, Perry A, Burger PC, Orens JB. Transmission of glioblastoma multiforme following bilateral lung transplantation from an affected donor: case study and review of the literature. Neuro-oncology. 2004;6(3):259–63. doi: 10.1215/S1152851703000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ng WH, Yeo TT, Kaye AH. Spinal and extracranial metastatic dissemination of malignant glioma. J Clin Neurosci. 2005;12(4):379–82. doi: 10.1016/j.jocn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Strojnik T, Kavalar R, Zajc I, Diamandis EP, Oikonomopoulou K, Lah TT. Prognostic impact of CD68 and kallikrein 6 in human glioma. Anticancer Res. 2009;29(8):3,269–79. [PubMed] [Google Scholar]
- 106.Duffy PE. Astrocytes: Normal, Reactive, and Neoplastic. Raven Press; New York: 1983. p. 224. [Google Scholar]
- 107.Han SJ, Yang I, Otero JJ, Ahn BJ, Tihan T, McDermott MW, Berger MS, Chang SM, Parsa AT. Secondary gliosarcoma after diagnosis of glioblastoma: Clinical experience with 30 consecutive patients. J Neurosurg. 2010 May;112(5):990–6. doi: 10.3171/2009.9.JNS09931. [DOI] [PubMed] [Google Scholar]
- 108.Scherer HJ. A critical review: The pathology of cerebral gliomas. J Neurol Neuropsychiat. 1940;3:147–77. doi: 10.1136/jnnp.3.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tso CL, Shintaku P, Chen J, Liu Q, Liu J, Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM, Nelson SF. Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res. 2006;4(9):607–19. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- 110.Fan X, Salford LG, Widegren B. Glioma stem cells: Evidence and limitation. Sem Cancer Biol. 2007;17(3):214–8. doi: 10.1016/j.semcancer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 111.Yates AJ. An overview of principles for classifying brain tumors. Mol Chem Neuropathol. 1992;17(2):103–20. doi: 10.1007/BF03159986. [DOI] [PubMed] [Google Scholar]
- 112.de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neurooncology. 2010;12(3):233–42. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, DeAngelis LM, Lassman AB. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1,200–6. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molad Y, Stark P, Prokocimer M, Joshua H, Pinkhas J, Sidi Y. Hemophagocytosis by small cell lung carcinoma. Am J Hematol. 1991;36(2):154–6. doi: 10.1002/ajh.2830360218. [DOI] [PubMed] [Google Scholar]
- 115.Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullopancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol. 2005;18(11):1,504–11. doi: 10.1038/modpathol.3800460. [DOI] [PubMed] [Google Scholar]
- 116.Bjerknes R, Bjerkvig R, Laerum OD. Phagocytic capacity of normal and malignant rat glial cells in culture. J Natl Cancer Inst. 1987;78(2):279–88. [PubMed] [Google Scholar]
- 117.Youness E, Barlogie B, Ahearn M, Trujillo JM. Tumor cell phagocytosis. Its occurrence in a patient with medulloblastoma. Arch Pathol Lab Med. 1980;104(12):651–3. [PubMed] [Google Scholar]
- 118.Ghoneum M, Gollapudi S. Phagocytosis of Candida albicans by metastatic and non metastatic human breast cancer cell lines in vitro. Cancer Detect. Prev. 2004;28(1):17–26. doi: 10.1016/j.cdp.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 119.Marin-Padilla M. Erythrophagocytosis by epithelial cells of a breast carcinoma. Cancer. 1977;39(3):1,085–9. doi: 10.1002/1097-0142(197703)39:3<1085::aid-cncr2820390312>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 120.Spivak JL. Phagocytic tumour cells. Scand J Haematol. 1973;11(3):253–6. doi: 10.1111/j.1600-0609.1973.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 121.Falini B, Bucciarelli E, Grignani F, Martelli MF. Erythrophagocytosis by undifferentiated lung carcinoma cells. Cancer. 1980;46(5):1,140–5. doi: 10.1002/1097-0142(19800901)46:5<1140::aid-cncr2820460511>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 122.Lugini L, Lozupone F, Matarrese P, Funaro C, Luciani F, Malorni W, Rivoltini L, Castelli C, Tinari A, Piris A, Parmiani G, Fais S. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: A key role of ezrin. Lab Invest. 2003;83(11):1,555–67. doi: 10.1097/01.lab.0000098425.03006.42. [DOI] [PubMed] [Google Scholar]
- 123.Wu JM, Fackler MJ, Halushka MK, Molavi DW, Taylor ME, Teo WW, Griffin C, Fetting J, Davidson NE, De Marzo AM, Hicks JL, Chitale D, Ladanyi M, Sukumar S, Argani P. Heterogeneity of breast cancer metastases: Comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14(7):1,938–46. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abodief WT, Dey P, Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology. 2006;17(5):304–5. doi: 10.1111/j.1365-2303.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 125.Kerbel RS, Lagarde AE, Dennis JW, Donaghue TP. Spontaneous fusion in vivo between normal host and tumor cells: possible contribution to tumor progression and metastasis studied with a lectin-resistant mutant tumor. Mol Cell Biol. 1983;3(4):523–38. doi: 10.1128/mcb.3.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kerbel RS, Twiddy RR, Robertson DM. Induction of a tumor with greatly increased metastatic growth potential by injection of cells from a low-metastatic H-2 heterozygous tumor cell line into an H-2 incompatible parental strain. Inter J Cancer. 1978;22(5):583–94. doi: 10.1002/ijc.2910220513. [DOI] [PubMed] [Google Scholar]
- 127.Seyfried TN, Mukherjee P. Ganglioside GM3 is antiangiogenic in malignant brain cancer. J Oncol. 2010;;2010:961243. doi: 10.1155/2010/961243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581(11):2,181–93. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 129.Camargo FD, Chambers SM, Goodell MA. Stem cell plasticity: from transdifferentiation to macrophage fusion. Cell Prolif. 2004;37(1):55–65. doi: 10.1111/j.1365-2184.2004.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J Clin Invest. 2004;113(9):1,266–70. doi: 10.1172/JCI21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paris S, Sesboue R. Metastasis models: the green fluorescent revolution? Carcinogenesis. 2004;25(12):2,285–92. doi: 10.1093/carcin/bgh219. [DOI] [PubMed] [Google Scholar]
- 132.Duelli D, Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell. 2003;3(5):445–8. doi: 10.1016/s1535-6108(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 133.Mekler LB, Drize OB, Osechinskii IV, Shliankevich MA. Transformation of a normal differentiated cell of an adult organism, induced by the fusion of this cell with another normal cell of the same organism but with different organ or tissue specificity. Vestn Akad Med Nauk USSR. 1971;26(4):75–80. [PubMed] [Google Scholar]
- 134.Warner TF. Cell hybridizaiton: An explanation for the phenotypic diversity of certain tumours. Med Hypoth. 1975;1(1):51–7. doi: 10.1016/0306-9877(75)90042-0. [DOI] [PubMed] [Google Scholar]
- 135.Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC, Swain JR, Wong MH. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology. 2010;139(6):2,072–82 e5. doi: 10.1053/j.gastro.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim Biophysic Acta. 2010;1807(6):577–94. doi: 10.1016/j.bbabio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Shabo I, Olsson H, Sun XF, Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Inter J Cancer. 2009;125(8):1,826–31. doi: 10.1002/ijc.24506. [DOI] [PubMed] [Google Scholar]
- 138.Shabo I, Stal O, Olsson H, Dore S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Inter J Cancer. 2008;123(4):780–6. doi: 10.1002/ijc.23527. [DOI] [PubMed] [Google Scholar]
- 139.Rachkovsky M, Pawelek J. Acquired melanocyte stimulating hormone-inducible chemotaxis following macrophage fusion with Cloudman S91 melanoma cells. Cell Growth Differ. 1999;10(7):517–24. [PubMed] [Google Scholar]
- 140.Handerson T, Camp R, Harigopal M, Rimm D, Pawelek J. Beta1,6-branched oligosaccharides are increased in lymph node metastases and predict poor outcome in breast carcinoma. Clin Cancer Res. 2005;11(8):2,969–73. doi: 10.1158/1078-0432.CCR-04-2211. [DOI] [PubMed] [Google Scholar]
- 141.Chakraborty AK, Sodi S, Rachkovsky M, Kolesnikova N, Platt JT, Bolognia JL, Pawelek JM. A spontaneous murine melanoma lung metastasis comprised of host × tumor hybrids. Cancer Res. 2000;60(9):2,512–9. [PubMed] [Google Scholar]
- 142.Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D, Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: Visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005;35(10):1,021–4. doi: 10.1038/sj.bmt.1704939. [DOI] [PubMed] [Google Scholar]
- 143.Chakraborty A, Lazova R, Davies S, Backvall H, Ponten F, Brash D, Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34(2):183–6. doi: 10.1038/sj.bmt.1704547. [DOI] [PubMed] [Google Scholar]
- 144.Pawelek JM, Chakraborty AK. The cancer cell--leukocyte fusion theory of metastasis. Adv Cancer Res. 2008;101:397–444. doi: 10.1016/S0065-230X(08)00410-7. [DOI] [PubMed] [Google Scholar]
- 145.Goldenberg DM, Pavia RA, Tsao MC. In vivo hybridisation of human tumour and normal hamster cells. Nature. 1974;250(5468):649–51. doi: 10.1038/250649a0. [DOI] [PubMed] [Google Scholar]
- 146.Munzarova M, Lauerova L, Capkova J. Are advanced malignant melanoma cells hybrids between melanocytes and macrophages? Melanoma Res. 1992;2(2):127–9. doi: 10.1097/00008390-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 147.Busund LT, Killie MK, Bartnes K, Seljelid R. Spontaneously formed tumorigenic hybrids of Meth A sarcoma cells and macrophages in vivo. Inter J Cancer. 2003;106(2):153–9. doi: 10.1002/ijc.11210. [DOI] [PubMed] [Google Scholar]
- 148.Ades L, Guardiola P, Socie G. Second malignancies after allogeneic hematopoietic stem cell transplantation: new insight and current problems. Blood Rev. 2002;16(2):135–46. doi: 10.1054/blre.2002.0010. [DOI] [PubMed] [Google Scholar]
- 149.Tebeu P-M, Verkooijen HM, Bouchardy C, Ludicke F, Usel M, Major AL. Impact of external radiotherapyon survivalafter stage I endometrial cancer: Results from a population based study. J Cancer Sci Ther. 2011;3(2):41–6. [Google Scholar]
- 150.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nature Rev Cancer. 2008;8(5):377–86. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 151.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukocyte Biol. 2004;75(3):388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 152.Ruff MR, Pert CB. Small cell carcinoma of the lung: macrophage-specific antigens suggest hemopoietic stem cell origin. Science. 1984;225(4666):1,034–6. doi: 10.1126/science.6089338. [DOI] [PubMed] [Google Scholar]
- 153.Calvo F, Martin PM, Jabrane N, De Cremoux P, Magdelenat H. Human breast cancer cells share antigens with the myeloid monocyte lineage. Brit J Cancer. 1987;56(1):15–9. doi: 10.1038/bjc.1987.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gazdar AF, Bunn PA, Jr., Minna JD, Baylin SB. Origin of human small cell lung cancer. Science. 1985;229(4714):679–80. doi: 10.1126/science.2992083. [DOI] [PubMed] [Google Scholar]
- 155.Ruff MR, Pert CB. Origin of human small cell lung cancer. Science. 1985;229(4714):680. doi: 10.1126/science.229.4714.680. [DOI] [PubMed] [Google Scholar]
- 156.Bunn PA, Jr., Linnoila I, Minna JD, Carney D, Gazdar AF. Small cell lung cancer, endocrine cells of the fetal bronchus, and other neuroendocrine cells express the Leu-7 antigenic determinant present on natural killer cells. Blood. 1985;65(3):764–8. [PubMed] [Google Scholar]
- 157.Davies PS, Powell AE, Swain JR, Wong MH. Inflammation and proliferation act together to mediate intestinal cell fusion. PloS one. 2009;4(8):e6, 530. doi: 10.1371/journal.pone.0006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, Miletic H, Wang J, Stieber D, Stuhr L, Moen I, Rygh CB, Bjerkvig R, Niclou SP. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci. 2011;108(9):3,749–54. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Diment S, Leech MS, Stahl PD. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988;263(14):6,901–7. [PubMed] [Google Scholar]
- 160.Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, Maier-Borst W, Heene DL. Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol Hematol. 1997;26(2):77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 161.Moon Y, Kim JY, Choi SY, Kim K, Kim H, Sun W. Induction of ezrin-radixin-moesin molecules after cryogenic traumatic brain injury of the mouse cortex. Neuroreport. 2011;22(6):304–8. doi: 10.1097/WNR.0b013e3283460265. [DOI] [PubMed] [Google Scholar]
- 162.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: The role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11(4):276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krishnan K, Bruce B, Hewitt S, Thomas D, Khanna C, Helman LJ. Ezrin mediates growth and survival in Ewing’s sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clinical Exper Metastasis. 2006;23(3-4):227–36. doi: 10.1007/s10585-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 164.Park HR, Cabrini RL, Araujo ES, Paparella ML, Brandizzi D, Park YK. Expression of ezrin and metastatic tumor antigen in osteosarcomas of the jaw. Tumori. 2009;95(1):81–6. doi: 10.1177/030089160909500113. [DOI] [PubMed] [Google Scholar]
- 165.Fais S. A role for ezrin in a neglected metastatic tumor function. Trends Molec Med. 2004;10(6):249–50. doi: 10.1016/j.molmed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 166.Hunter KW. Ezrin, a key component in tumor metastasis. Trends Molec Med. 2004;10(5):201–4. doi: 10.1016/j.molmed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 167.Bobryshev YV, Lord RS, Watanabe T, Ikezawa T. The cell adhesion molecule E-cadherin is widely expressed in human atherosclerotic lesions. Cardiovas Res. 1998;40(1):191–205. doi: 10.1016/s0008-6363(98)00141-2. [DOI] [PubMed] [Google Scholar]
- 168.Armeanu S, Buhring HJ, Reuss-Borst M, Muller CA, Klein G. E-cadherin is functionally involved in the maturation of the erythroid lineage. J Cell Biol. 1995;131(1):243–9. doi: 10.1083/jcb.131.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ward DG, Roberts K, Brookes MJ, Joy H, Martin A, Ismail T, Spychal R, Iqbal T, Tselepis C. Increased hepcidin expression in colorectal carcinogenesis. World J Gastroenterol. 2008;14(9):1,339–45. doi: 10.3748/wjg.14.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leonard RC, Untch M, Von Koch F. Management of anaemia in patients with breast cancer: role of epoetin. Ann Oncol. 2005;16(5):817–24. doi: 10.1093/annonc/mdi161. [DOI] [PubMed] [Google Scholar]
- 171.Kamai T, Tomosugi N, Abe H, Arai K, Yoshida K. Increased serum hepcidin-25 level and increased tumor expression of hepcidin mRNA are associated with metastasis of renal cell carcinoma. BMC Cancer. 2009;9:270. doi: 10.1186/1471-2407-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ganz T. Hepcidin--a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18(2):171–82. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 173.Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP) Crit Rev Oncol Hematol. 2009;69(3):271–8. doi: 10.1016/j.critrevonc.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 174.Carlson HR. Carcinoma of unknown primary: searching for the origin of metastases. Jaapa. 2009;22(8):18–21. doi: 10.1097/01720610-200908000-00006. [DOI] [PubMed] [Google Scholar]
- 175.Heidemann J, Gockel HR, Winde G, Herbst H, Domschke W, Lugering N. Signet-ring cell carcinoma of unknown primary location. Metastatic to lower back musculature - remission following FU/FA chemotherapy. Z Gastroenterol. 2002;40(1):33–6. doi: 10.1055/s-2001-19634. [DOI] [PubMed] [Google Scholar]
- 176.Hedley DW, Leary JA, Kirsten F. Metastatic adenocarcinoma of unknown primary site: abnormalities of cellular DNA content and survival. Eur J Cancer Clin Oncol. 1985;21(2):185–9. doi: 10.1016/0277-5379(85)90171-3. [DOI] [PubMed] [Google Scholar]
- 177.Torres EM, Williams BR, Tang YC, Amon A. Thoughts on aneuploidy. Cold Spring Harbor Symposia on Quantitative Biology. 2010;75:445–51. doi: 10.1101/sqb.2010.75.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Torres EM, Williams BR, Amon A. Aneuploidy: cells losing their balance. Genetics. 2008;179(2):737–46. doi: 10.1534/genetics.108.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA. 2006;103(16):6,321–5. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lazova R, Chakraborty A, Pawelek JM. Leukocyte-cancer cell fusion: Initiator of the Warburg effect in malignancy? Adv Exper Med Biol. 2011;714:151–72. doi: 10.1007/978-94-007-0782-5_8. [DOI] [PubMed] [Google Scholar]
- 181.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutrit & Metabol. 2010;7(1):7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 183.Warburg O. On the respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 184.Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 186.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 187.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 188.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague Jw, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1,109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci USA. 1998;95(25):14,944–9. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: Functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110(12):4,077–85. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25(3):231–43. doi: 10.1053/ejso.1998.0634. [DOI] [PubMed] [Google Scholar]
- 192.Soto AM, Sonnenschein C. The somatic mutation theory of cancer: Growing problems with the paradigm? Bioessays. 2004;26(10):1,097–107. doi: 10.1002/bies.20087. [DOI] [PubMed] [Google Scholar]
- 193.Hirshberg A, Leibovich P, Horowitz I, Buchner A. Metastatic tumors to postextraction sites. J Oral Maxillofac Surg. 1993;51(12):1,334–7. doi: 10.1016/s0278-2391(10)80138-7. [DOI] [PubMed] [Google Scholar]
- 194.Cho E, Kim MH, Cha SH, Cho SH, Oh SJ, Lee JD. Breast cancer cutaneous metastasis at core needle biopsy site. Ann Dermatol. 2010;22(2):238–40. doi: 10.5021/ad.2010.22.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993;22(9):385–90. doi: 10.1111/j.1600-0714.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 196.Jones FS, Rous P. On the Cause of the localization of secondary tumors at points of Injury. J Exp Med. 1914;20(4):404–12. doi: 10.1084/jem.20.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Walter ND, Rice PL, Redente EF, Kauvar EF, Lemond L, Aly T, Wanebo K, Chan ED. Wound healing after trauma may predispose to lung cancer metastasis: Review of potential mechanisms. Am J Respir Cell Mol Biol. 2011;44(5):591–6. doi: 10.1165/rcmb.2010-0187RT. [DOI] [PubMed] [Google Scholar]
- 198.Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10(7):744–8. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 199.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1,801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100(12):2,235–41. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1,807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Salk JJ, Fox EJ, Loeb LA. Mutational heterogeneity in human cancers: Origin and consequences. Annu Rev Pathol. 2010;5:51–75. doi: 10.1146/annurev-pathol-121808-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Park MS, Kim Y, Kang MS, Oh SY, Cho DY, Shin NS, Kim DY. Disseminated transmissible venereal tumor in a dog. J Vet Diagn Invest. 2006;18(1):130–3. doi: 10.1177/104063870601800123. [DOI] [PubMed] [Google Scholar]
- 204.Murchison EP, Tovar C, Hsu A, Bender HS, Kheradpour P, Rebbeck CA, Obendorf D, Conlan C, Bahlo M, Blizzard CA, Pyecroft S, Kreiss A, Kellis M, Stark A, Harkins TT, Marshall Graves JA, Woods GM, Hannon GJ, Papenfuss AT. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science. 2010;327(5961):84–7. doi: 10.1126/science.1180616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mozos E, Mendez A, Gomez-Villamandos JC, Martin De Las Mulas J, Perez J. Immunohistochemical characterization of canine transmissible venereal tumor. Vet Pathol. 1996;33(3):257–63. doi: 10.1177/030098589603300301. [DOI] [PubMed] [Google Scholar]
- 206.Marchal T, Chabanne L, Kaplanski C, Rigal D, Magnol JP. Immunophenotype of the canine transmissible venereal tumour. Vet Immunol Immunopathol. 1997;57(1-2):1–11. doi: 10.1016/s0165-2427(96)05757-1. [DOI] [PubMed] [Google Scholar]
- 207.Zhao FQ, Zhang PX, He XJ, Du C, Fu ZG, Zhang DY, Jiang BG. Study on the adoption of Schwann cell phenotype by bone marrow stromal cells in vitro and in vivo. Biomed Environ Sci. 2005;18(5):326–33. [PubMed] [Google Scholar]
- 208.Rebbeck CA, Leroi AM, Burt A. Mitochondrial capture by a transmissible cancer. Science. 2011;331(6015):303. doi: 10.1126/science.1197696. [DOI] [PubMed] [Google Scholar]
- 209.Robinson W. Some features of crown-gall in plants in reference to comparisons with cancer. Proc Roy Soc Med. 1927;20:1,507–9. doi: 10.1177/003591572702000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nester EW, Gordon MP, Amasino RM. Crown gall: A molecular and physiological analysis. Ann Rev Plant Physiol. 1984;35:387–413. [Google Scholar]
- 211.Levine M. Plant tumores and their relationship to cancer. Botanical Rev. 1936;2:439–55. [Google Scholar]
- 212.Tamaoki T, Hildebrandt AC, Burris RH, Riker AJ, Hagihara B. Respiration and phosphorylation of mitochondria from normal and crown-gall tissue cultures of tomato. Plant Physiol. 1960;35(6):942–7. doi: 10.1104/pp.35.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Klein RM. Nitrogen and Phosphorus Fractions, Respiration, and structure of normal and crown gall tissues of tomato. Plant Physiol. 1952;27(2):335–54. doi: 10.1104/pp.27.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fogelberg SO, Struckmeyer E, Roberts RH. Morphological variations of mitochondria in the presence of plant tumors. Amer J Botany. 1957;44(5):454–9. [Google Scholar]
- 215.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 216.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: A comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Marsh J, Mukherjee P, Seyfried TN. Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet. Nutrit & Metabol. 2008;5:33. doi: 10.1186/1743-7075-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Marsh J, Mukherjee P, Seyfried TN. Akt-dependent proapoptotic effects of dietary restriction on late-stage management of a phosphatase and tensin homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma. Clin. Cancer Res. 2008;14(23):7,751–62. doi: 10.1158/1078-0432.CCR-08-0213. [DOI] [PubMed] [Google Scholar]
- 219.Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36(2):153–63. doi: 10.1590/s0100-879x2003000200002. [DOI] [PubMed] [Google Scholar]
- 220.Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutrition. 2001;131(9 Suppl):2515S–22S. doi: 10.1093/jn/131.9.2515S. Discussion 23S-4S. [DOI] [PubMed] [Google Scholar]
- 221.Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Inter J Cancer. 2010;127(10):2,478–85. doi: 10.1002/ijc.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shelton LM, Huysentruyt LC, Mukherjee P, Seyfried TN. Calorie restriction as an anti-invasive therapy for malignant brain cancer in the VM mouse. ASN Neuro. 2010;2(3):e00038. doi: 10.1042/AN20100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huysentruyt LC, Shelton LM, Seyfried TN. Influence of methotrexate and cisplatin on tumor progression and survival in the VM mouse model of systemic metastatic cancer. Inter J Cancer. 2010;126(1):65–72. doi: 10.1002/ijc.24649. [DOI] [PubMed] [Google Scholar]
- 224.Kojima S, Sekine H, Fukui I, Ohshima H. Clinical significance of “cannibalism” in urinary cytology of bladder cancer. Acta Cytol. 1998;42(6):1,365–1,369. doi: 10.1159/000332169. [DOI] [PubMed] [Google Scholar]
- 225.Zimmer C, Weissleder R, Poss K, Bogdanova A, Wright SC, Jr., Enochs WS. MR imaging of phagocytosis in experimental gliomas. Radiology. 1995;197(2):533–8. doi: 10.1148/radiology.197.2.7480707. [DOI] [PubMed] [Google Scholar]
- 226.van Landeghem FK, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, Thiesen B, Bruck W, von Deimling A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30(1):52–7. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 227.Persson A, Englund E. The glioma cell edge--winning by engulfing the enemy? Med Hypotheses. 2009;73(3):336–7. doi: 10.1016/j.mehy.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 228.Nitta T, Okumura K, Sato K. Lysosomal enzymic activity of astroglial cells. Pathobiology. 1992;60(1):42–4. doi: 10.1159/000163695. [DOI] [PubMed] [Google Scholar]
- 229.Chang GH, Barbaro NM, Pieper RO. Phosphatidylserine-dependent phagocytosis of apoptotic glioma cells by normal human microglia, astrocytes, and glioma cells. Neuro-oncology. 2000;2(3):174–83. doi: 10.1093/neuonc/2.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jeyakumar M, Norflus F, Tifft CJ, Cortina-Borja M, Butters TD, Proia RL, Perry VH, Dwek RA, Platt FM. Enhanced survival in Sandhoff disease mice receiving a combination of substrate deprivation therapy and bone marrow transplantation. Blood. 2001;97(1):327–9. doi: 10.1182/blood.v97.1.327. [DOI] [PubMed] [Google Scholar]
- 231.Leenstra S, Das PK, Troost D, de Boer OJ, Bosch DA. Human malignant astrocytes express macrophage phenotype. J Neuroimmunol. 1995;56(1):17–25. doi: 10.1016/0165-5728(94)00128-b. issn: 0165-5728. [DOI] [PubMed] [Google Scholar]
- 232.Takeuchi H, Kubota T, Kitai R, Nakagawa T, Hashimoto N. CD98 immunoreactivity in multinucleated giant cells of glioblastomas: An immunohistochemical double labeling study. Neuropathology. 2008;28(2):127–31. doi: 10.1111/j.1440-1789.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 233.Deininger MH, Meyermann R, Trautmann K, Morgalla M, Duffner F, Grote EH, Wickboldt J, Schluesener HJ. Cyclooxygenase (COX)-1 expressing macrophages/microglial cells and COX-2 expressing astrocytes accumulate during oligodendroglioma progression. Brain Res. 2000;885(1):111–6. doi: 10.1016/s0006-8993(00)02978-4. [DOI] [PubMed] [Google Scholar]
- 234.Ghoneum M, Matsuura M, Braga M, Gollapudi S. S. cerevisiae induces apoptosis in human metastatic breast cancer cells by altering intracellular Ca2+ and the ratio of Bax and Bcl-2. International Journal of Oncology. 2008;33(3):533–9. [PubMed] [Google Scholar]
- 235.Coopman PJ, Do MT, Thompson EW, Mueller SC. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin Cancer Res. 1998;4(2):507–15. [PubMed] [Google Scholar]
- 236.Ghoneum M, Wang L, Agrawal S, Gollapudi S. Yeast therapy for the treatment of breast cancer: a nude mice model study. In Vivo. 2007;21(2):251–8. [PubMed] [Google Scholar]
- 237.Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clinical & Exper Metastasis. 2007;24(8):587–97. doi: 10.1007/s10585-007-9114-6. [DOI] [PubMed] [Google Scholar]
- 238.Bjerregaard B, Holck S, Christensen IJ, Larsson LI. Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci. 2006;63(16):1,906–11. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124(2):687–94. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 240.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68(6):835–53. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- 241.Athanasou NA, Wells CA, Quinn J, Ferguson DP, Heryet A, McGee JO. The origin and nature of stromal osteoclast-like multinucleated giant cells in breast carcinoma: implications for tumour osteolysis and macrophage biology. Brit J Cancer. 1989;59(4):491–8. doi: 10.1038/bjc.1989.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Acebo P, Giner D, Calvo P, Blanco-Rivero A, Ortega AD, Fernandez PL, Roncador G, Fernandez-Malave E, Chamorro M, Cuezva JM. Cancer abolishes the tissue type-specific differences in the phenotype of energetic metabolism. Transl Oncol. 2009;2(3):138–45. doi: 10.1593/tlo.09106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chandrasoma P. Polymorph phagocytosis by cancer cells in an endometrial adenoacanthoma. Cancer. 1980;45(9):2,348–51. doi: 10.1002/1097-0142(19800501)45:9<2348::aid-cncr2820450919>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 244.Caruso RA, Muda AO, Bersiga A, Rigoli L, Inferrera C. Morphological evidence of neutrophil-tumor cell phagocytosis (cannibalism) in human gastric adenocarcinomas. Ultrastruct Pathol. 2002;26(5):315–21. doi: 10.1080/01913120290104593. [DOI] [PubMed] [Google Scholar]
- 245.Hu J, La Vecchia C, Negri E, Chatenoud L, Bosetti C, Jia X, Liu R, Huang G, Bi D, Wang C. Diet and brain cancer in adults: a case-control study in northeast China. Inter J Cancer. 1999;81(1):20–3. doi: 10.1002/(sici)1097-0215(19990331)81:1<20::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 246.DeSimone PA, East R, Powell RD., Jr Phagocytic tumor cell activity in oat cell carcinoma of the lung. Hum Pathol. 1980;11(5 Suppl):535–9. [PubMed] [Google Scholar]
- 247.Richters A, Sherwin RP, Richters V. The lymphocyte and human lung cancers. Cancer Res. 1971;31(3):214–22. [PubMed] [Google Scholar]
- 248.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117(2):326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Radosevic K, van Leeuwen AM, Segers-Nolten IM, Figdor CG, de Grooth BG, Greve J. Occurrence and a possible mechanism of penetration of natural killer cells into K562 target cells during the cytotoxic interaction. Cytometry. 1995;20(4):273–80. doi: 10.1002/cyto.990200402. [DOI] [PubMed] [Google Scholar]
- 250.Koren HS, Handwerger BS, Wunderlich JR. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975;114(2 pt 2):894–7. [PubMed] [Google Scholar]
- 251.Larizza L, Schirrmacher V, Pfluger E. Acquisition of high metastatic capacity after in vitro fusion of a non-metastatic tumor line with a bone marrow-derived macrophage. J Exp Med. 1984;160(5):1,579–84. doi: 10.1084/jem.160.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.De Baetselier P, Roos E, Brys L, Remels L, Feldman M. Generation of invasive and metastatic variants of a non-metastatic T-cell lymphoma by in vivo fusion with normal host cells. Inter J Cancer. 1984;34(5):731–8. doi: 10.1002/ijc.2910340522. [DOI] [PubMed] [Google Scholar]
- 253.Breier F, Feldmann R, Fellenz C, Neuhold N, Gschnait F. Primary invasive signet-ring cell melanoma. J Cutan Pathol. 1999;26(10):533–6. doi: 10.1111/j.1600-0560.1999.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 254.Monteagudo C, Jorda E, Carda C, Illueca C, Peydro A, Llombart-Bosch A. Erythrophagocytic tumour cells in melanoma and squamous cell carcinoma of the skin. Histopathology. 1997;31(4):367–73. doi: 10.1046/j.1365-2559.1997.2670867.x. [DOI] [PubMed] [Google Scholar]
- 255.Brocker EB, Suter L, Sorg C. HLA-DR antigen expression in primary melanomas of the skin. J Invest Dermatol. 1984;82(3):244–7. doi: 10.1111/1523-1747.ep12260181. [DOI] [PubMed] [Google Scholar]
- 256.Facchetti F, Bertalot G, Grigolato PG. KP1 (CD 68) staining of malignant melanomas. Histopathology. 1991;19(2):141–5. doi: 10.1111/j.1365-2559.1991.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 257.Munzarova M, Rejthar A, Mechl Z. Do some malignant melanoma cells share antigens with the myeloid monocyte lineage? Neoplasma. 1991;38(4):401–5. [PubMed] [Google Scholar]
- 258.Savage DG, Zipin D, Bhagat G, Alobeid B. Hemophagocytic, non-secretory multiple myeloma. Leuk Lymphoma. 2004;45(5):1,061–4. doi: 10.1080/10428190310001623856. [DOI] [PubMed] [Google Scholar]
- 259.Andersen TL, Soe K, Sondergaard TE, Plesner T, Delaisse JM. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Brit J Haematol. 148(4):551–61. doi: 10.1111/j.1365-2141.2009.07980.x. [DOI] [PubMed] [Google Scholar]
- 260.Yasunaga M, Ohishi Y, Nishimura I, Tamiya S, Iwasa A, Takagi E, Inoue T, Yahata H, Kobayashi H, Wake N, Tsuneyoshi M. Ovarian undifferentiated carcinoma resembling giant cell carcinoma of the lung. Pathol Int. 2008;58(4):244–8. doi: 10.1111/j.1440-1827.2008.02218.x. [DOI] [PubMed] [Google Scholar]
- 261.Talmadge JE, Key ME, Hart IR. Characterization of a murine ovarian reticulum cell sarcoma of histiocytic origin. Cancer Res. 1981;41(4):1,271–80. [PubMed] [Google Scholar]
- 262.Kern HF, Bosslet K, Sedlacek HH, Schorlemmer HU. Monocyte-related functions expressed in cell lines established from human pancreatic adenocarcinoma. II. Inhibition of stimulated activity by monoclonal antibodies reacting with surface antigens on tumor cells. Pancreas. 1988;3(1):2–10. doi: 10.1097/00006676-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 263.Imai S, Sekigawa S, Ohno Y, Yamamoto H, Tsubura Y. Giant cell carcinoma of the pancreas. Acta Pathol Jpn. 1981;31(1):129–33. doi: 10.1111/j.1440-1827.1981.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 264.Chetty R, Cvijan D. Giant (bizarre) cell variant of renal carcinoma. Histopathology. 1997;30(6):585–7. doi: 10.1046/j.1365-2559.1997.5560789.x. [DOI] [PubMed] [Google Scholar]
- 265.Etcubanas E, Peiper S, Stass S, Green A. Rhabdomyosarcoma, presenting as disseminated malignancy from an unknown primary site: a retrospective study of ten pediatric cases. Med Pediatr Oncol. 1989;17(1):39–44. doi: 10.1002/mpo.2950170108. [DOI] [PubMed] [Google Scholar]
- 266.Tsoi WC, Feng CS. Hemophagocytosis by rhabdomyosarcoma cells in bone marrow. Am J Hematol. 1997;54(4):340–2. [PubMed] [Google Scholar]
- 267.Matarrese P, Ciarlo L, Tinari A, Piacentini M, Malorni W. Xeno-cannibalism as an exacerbation of self-cannibalism: a possible fruitful survival strategy for cancer cells. Cur Pharma Design. 2008;14(3):245–52. doi: 10.2174/138161208783413239. [DOI] [PubMed] [Google Scholar]
- 268.Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9(10):796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 269.Gupta K, Dey P. Cell cannibalism: diagnostic marker of malignancy. Diag Cytopathol. 2003;28(2):86–7. doi: 10.1002/dc.10234. Epub 2003/02/01. [DOI] [PubMed] [Google Scholar]
- 270.Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nature Rev Cancer. 2007;7(12):968–76. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]