Abstract
Objective
We hypothesized that BMI and DNA variants would predict age at menarche in PCOS. Subjects: Subjects with PCOS defined by the NIH criteria (n=522) and controls with regular menstrual cycles and no hyperandrogenism (n=472); aged 18 to 45 years were studied.
Methods
Age at menarche was compared between PCOS cases and controls, and examined as a function of multiple parameters.
Results
There was a strong inverse relationship between BMI and age at menarche in PCOS (r=−0.32; p=5×10−11). The chromosome 6 rs7759938-T variant was associated with earlier age at menarche in women with PCOS (12.60±0.09 vs. 13.41±0.23 years; genotype TT vs. CC; p=0.006). Age at menarche was predicted by PCOS status (β=0.512; p<0.001), reported weight group at 10 to 14 years (β= −0.432; p<0.001), current BMI (β= −0.0202; p=0.01) and genotype (β=0.169; p=0.02).
Conclusions
Age at menarche in women with PCOS is influenced by BMI and genetic variants near LIN28B.
Trial Registration
Keywords: BMI, birthweight, breastfeeding, puberty
Introduction
Girls with PCOS exhibit a wider range in age at menarche than control subjects.1 Therefore, the timing of menarche may provide supporting information to a clinician attempting to make a diagnosis of PCOS in adolescence. However, the age at menarche in women with PCOS may be earlier or later than controls based on several factors that have opposing influence. The waves of progressive follicle development that occur during puberty,2 which are accompanied by estradiol increases, withdrawal bleeding and eventual ovulatory follicle development may be delayed if the follicle arrest characteristic of PCOS begins in adolescence, resulting in a later age at menarche despite some breast development from estradiol production by the arrested follicles. As such, girls who are later diagnosed with PCOS can present with primary amenorrhea.3 On the contrary, girls who develop PCOS may experience premature pubarche and in a subset of these girls, specifically those born small for gestational age, menarche may be earlier.4 Further, higher weight is associated with earlier age at menarche5 and many girls who go on to develop PCOS are overweight or obese at adolescence.6 Evaluating the age at menarche in large groups of women with PCOS may help dissect these influences.
In addition to overweight and arrested follicle development, age at menarche in women with PCOS may be regulated by the genetic variants involved in normal menarche. The first large genome-wide association studies identified two chromosomal regions that are associated with age at menarche.7–10 Variants in or near LIN28B on chromosome 6 (rs314276-C, rs314277-C and rs7759938-T), and on chromosome 9 (rs2090409-A) in a region in which a candidate gene has not yet been identified7–10 have been associated with an earlier age at menarche. The role of genetic variants associated with age at menarche has not been examined in women with PCOS.
We hypothesized that women with PCOS would have an altered age at menarche compared to control subjects, influenced not only by weight but also by genetic variants. The hypothesis was tested in a large group of women with PCOS and controls of European ancestry.
Methods
Subjects
All subjects were U.S. women of European ethnicity, screened at the same research hospital and between the ages of 18 and 45 years. Subjects with PCOS (n=522) had oligomenorrhea (< 9 menstrual periods/yr) and clinical and/or biochemical evidence of hyperandrogenism, fulfilling the NIH criteria.11;12 Clinical hyperandrogenism was defined by: 1) an elevated Ferriman Gallwey score > 9;13 or 2) acne on the face or back. Biochemical hyperandrogenism was defined as testosterone >2.8 nmol/L (63 ng/dL), DHEAS >1.16 μmoL/L (430 μg/dL) or androstenedione levels >13.3 nmol/L (3.8 ng/mL).12 Control subjects had regular menstrual cycles, 21 to 35 days, and no physical exam or biochemical evidence of hyperandrogenism (n=472). Subjects were excluded for a personal history or evidence of late onset congenital adrenal hyperplasia.12 All subjects had normal thyroid function and prolactin levels and a follicular phase FSH level in the premenopausal range. Subjects were on no hormonal medication, except for stable thyroid hormone replacement.
Protocol
The study was approved by the Institutional Review Board of the Massachusetts General Hospital and all subjects gave written informed consent. All PCOS subjects were studied ≥10 days after their last menstrual period and after a 12 hour fast.12 Subjects underwent a detailed history using a standardized questionnaire; physical exam including measurement of waist circumference at the umbilicus and hip circumference at the widest diameter; a pelvic ultrasound (ATL HDI 1500, 5 MHz convex array transducer); and blood samples for lipids, glucose, insulin, gonadotropin and sex-steroid levels. An oral glucose tolerance test was performed, with blood sampling 2 hours after a 75 gram glucose load.
Age at menarche was reported by recall, along with other milestones of puberty, birth and development information. Subjects were able to speak with parents if they did not know the answer to questions about birth or the neonatal period. The correlation between recorded age at menarche and recalled age after 23–33 years was high in previous studies (r=0.79; p<0.001),14 which is a longer interval than that for a majority of subjects in the current study. Weight during adolescence was reported in weight groups; a previously validated questionnaire parameter.15
Assays
Serum LH and FSH were measured using a 2-site monoclonal non-isotopic system (Axsym, Abbott Laboratories, Abbott Park, IL) and expressed in IU per liter as equivalents of the Second International Reference Preparation 71/223 of human menopausal gonadotropins. Serum testosterone and androstenedione were measured using a RIA (Coat-a-Count, Diagnostic Products Corporation, Los Angeles, CA). 17-OH progesterone was measured by liquid chromatography-tandem mass spectrometry (Mayo Medical Laboratories, New England). SHBG and insulin were measured using an immunochemiluminescent assay (Immulite 2000, DPC).
Genotyping
Variants previously associated with age at menarche at 6q21 rs314276, rs314277 and rs77599387–9 and 9q31.2 rs209040910 were selected. A panel of 152 markers informative for European, African American and Latin ancestry were genotyped to control for false associations related to population stratification.16–18 Patient DNA was isolated from whole blood. Genotyping was performed by primer extension of multiplex products with detection by MALDI-TOF mass spectroscopy using a Sequenom platform. One single nucleotide polymorphism (SNP), rs314276, failed with an ability to determine the genotype (call rate) in <90%. Forty-four PCOS cases and 29 control samples failed with a call rate of <90%. The remaining three age at menarche SNPs showed no departure from Hardy-Weinberg Equilibrium (p > 0.001).
Statistical Analysis
χ2 analysis was used to compare the proportion of women with PCOS and control subjects reporting categorical variables. Continuous data were log transformed for analysis. Phenotypic characteristics were compared between PCOS cases and controls using ANCOVA adjusting for age and BMI or two way ANOVA to maintain BMI as a variable. χ2 analysis was used to test association of three age at menarche SNPs with PCOS in cases and controls. Log-transformed quantitative traits were compared by ANOVA across three genotype classes for each variant tested. Data were adjusted for age, BMI and ancestry16–18 and results are reported after correction for multiple testing by permutation analysis using 5000 permutations. A p value <0.05 was considered significant after corrections.
Results
Neonatal Parameters
Women with PCOS were more likely to report an early or late birth compared with control subjects (p=0.005) (Table 1). There was no difference in reported birth order or birth weight, even when subjects with early or late birth were removed (p=0.3), between PCOS and control subjects. Women with PCOS were less likely to have been breast fed (62% vs. 75%; p<0.001; PCOS vs. controls, respectively).
Table 1.
Childhood and Puberty Parameters in Women with PCOS and Controls
| PCOS | Ctl | P value | |
|---|---|---|---|
| Birth Timing | |||
| Early (at or before the 37th week) | 32 (9) a | 17 (6) | 0.005 |
| On Time (38th–42nd week) | 249 (74) | 259 (84) | |
| Late (after 42nd week) | 57 (17) | 32 (10) | |
| Birth Weight | |||
| Low (≤ 2500 gms) | 34 (12) | 31 (11) | 0.29 |
| Medium (2750 to 4250 gms) | 240 (82) | 240 (85) | |
| High (>4500 gms) | 20 (7) | 11 (4) | |
| Birth Order | |||
| First Born | 170 (47) | 148 (44) | 0.4 |
| Second | 107 (30) | 121 (36) | |
| Third | 46 (13) | 40 (12) | |
| Fourth | 20 (6) | 15 (4) | |
| Fifth | 5 (1) | 3 (1) | |
| Sixth or later | 10 (3) | 6 (2) | |
| Pubertal Development | |||
| Early | 40 (11) | 16 (5) | 0.001 |
| On Time | 255 (72) | 286 (85) | |
| Late | 61 (17) | 33 (10) | |
| Age at Menarche (yrs) | |||
| ≤10 | 27 (6) | 24 (7) | 0.01 |
| 11 | 55 (13) | 41 (12) | |
| 12 | 104 (25) | 111 (32) | |
| 13 | 115 (27) | 103 (30) | |
| 14 | 57 (14) | 45 (13) | |
| ≥15 | 61 (15) | 23 (7) | |
| Age at Pubarche | 11.37±0.10 | 11.87±0.09 | 0.01 |
| Age at Growth Spurt | 11.91±0.12 | 12.31±0.10 | 0.9 |
| Age at Thelarche | 11.64±0.10 | 11.93±0.10 | 0.7 |
| Age at Menarche | 12.72±0.07 | 12.50±0.07 | 0.12 |
| Cycles/year before age 18 yrs | 8.47±0.21 | 12.61±0.09 | <0.001 |
Total number (%)
Mean±SE; adjusted for age and BMI
Puberty Parameters
Women with PCOS were less likely to report development on time and more likely to report earlier and later pubertal development compared to peers. There were more women with PCOS than control subjects who reported age at menarche of 15 years or greater (Table 1). Within the PCOS group, there was an inverse correlation between current BMI and age at menarche (r=−0.32; p=5×10−11), pubarche (−0.13; p=0.04), thelarche (−0.33; p=1×10−9) and growth spurt (r=−0.23; p<0.001), but not in control women (all p>0.05). Therefore, data were adjusted for age and BMI. Women with PCOS reported an earlier age at pubarche on average than control subjects (Table 1). Women with PCOS were also more likely to report irregular menses (≤9 menses per year) before the age of 18 years (79 vs. 15%; p<0.001) and a lower number of cycles per year than control subjects (Table 1). There was an inverse correlation between the number of menses per year and age at menarche (−0.21; p=1×10−4).
Pubertal Weight and Age at Menarche
Women with PCOS who were currently overweight (≥25 to 26.9 kg/m2) or obese (≥ 27 kg/m2) were more likely to report that they were slightly or very overweight compared to peers at ages 5 to 9 (42 vs. 17%; current BMI ≥ 25 kg/m2 vs. < 25 kg/m2, respectively), 10 to 14 (66 vs. 22%) and 15 to 19 (70 vs. 18%) than women with PCOS whose BMI was currently < 25 kg/m2 (all p<0.001). Control women who were currently overweight or obese were also more likely to report that they were slightly or very overweight compared to peers at ages 5 to 9 (28 vs. 11%), 10 to 14 (50 vs. 16%) and 15 to 19 (52 vs. 11%) than women whose BMI was currently < 25 kg/m2 (all p<0.001). However, the proportion of control women who reported overweight and the overall number of women who were currently overweight or obese was lower than in women with PCOS (25 vs. 64%; p<0.001).
Women with PCOS were more likely than controls to report that their weight was slightly higher or much higher than their peers at ages 5 to 9, 10 to 14 and 15 to 19 years (Table 2; all p<0.01), and the proportion of subjects reporting their weight as much higher than their peers increased from the 5 to 9 to the 10 to 14 year old age group. In contrast, there was no difference in reported height compared to peers at the same ages between PCOS and control subjects (data not shown). Both current BMI (β= −0.03; p=0.009) and weight category at age 10 to 14 years (β= −0.4; p<0.001) correlated with age at menarche in women with PCOS. The weight category at age 10 to 14 years also predicted age at menarche in controls (β= −0.5; p<0.001), although current BMI did not (β= −0.003; p=0.9). Women with PCOS and controls who reported being thin compared to their peers at age 10 to 14 years had later menarche at age 14 or ≥ 15 years, while those who weighed slightly or much more than their peers at 10–14 years had a younger age at menarche (Figure 1).
Table 2.
Reported Weight Compared to Peers
| Weight Groupa | Less Than Peers | Similar to Peers | Slightly Higher than Peers | Much Higher than Peers |
|---|---|---|---|---|
| Age 5 to 9 years | ||||
| PCOS | 53 (14) | 195 (53) | 94 (26) | 25 (7) |
| Control | 68 (20) | 215 (65) | 44 (13) | 6 (2) |
| Age 10 to 14 years | ||||
| PCOS | 48 (13) | 132 (36) | 127 (35) | 55 (15) |
| Control | 72 (22) | 176 (53) | 71 (22) | 10 (3) |
| Age 15 to 19 years | ||||
| PCOS | 40 (11) | 136 (37) | 106 (29) | 81 (22) |
| Control | 67 (20) | 190 (58) | 61 (19) | 11 (3) |
p<0.001 between PCOS and controls in all age categories
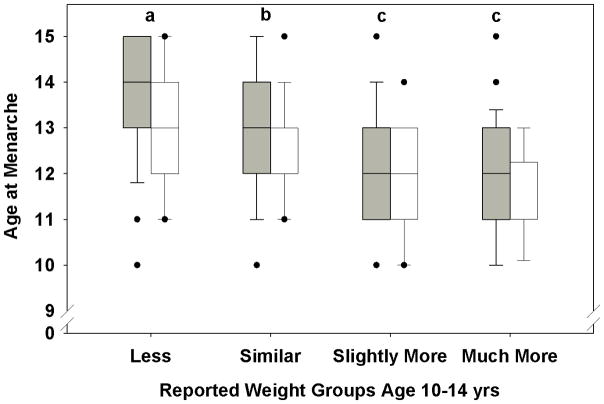
Figure 1.
Age at menarche in women with PCOS (gray boxes) and controls (white boxes) as a function of reported weight group at ages 10 to 14 years. The line represents the median, boxes the 25th and 75th percent confidence limits and the black dots the age at menarche for outliers. The age at menarche was later in women with PCOS and controls whose weight was less than their peers (a vs. b; p<0.001) and lower in those whose weight was more than their peers (c vs. b; p<0.001) than in the group with a similar weight to that of their peers.
The expected differences in BMI, androgen and gonadotropin levels, and metabolic parameters were demonstrated between women with PCOS and controls (Table 3; asterisk p<0.001 between PCOS and control). In addition, control subjects were taller than women with PCOS. Overall, earlier menarche was associated with shorter stature, higher BMI, waist and hip circumference, lower SHBG and HDL, higher Ferriman Gallwey score, fasting insulin levels and measurements of insulin resistance in women with PCOS and controls, and with lower HbA1C in control women (Table 3). These relationships with age at menarche disappeared when controlled for BMI, with the exception of shorter height (p<0.001), larger waist circumference (p=0.02) and higher Ferriman Gallwey score (p<0.01). There was no relationship between current androgen and gonadotropin levels, ovarian volume or follicle number and age at menarche (Table 3).
Table 3.
Current Phenotype as a Function of Age at Menarche in Women with PCOS and Controls
| Age at Menarche (yrs) | ≤10 | 11 | 12 | 13 | 14 | ≥15 | p value1 | |
|---|---|---|---|---|---|---|---|---|
| Current Age (yrs) | P2 | 27.8±1.2 | 27.7±0.8 | 27.7±0.6 | 27.6±0.7 | 29.7±1.1 | 29.8±1.1 | 0.2 |
| C | 27.9±1.5 | 26.8±1.1 | 26.9±0.6 | 27.7±0.7 | 28.5±1.0 | 28.2±1.7 | ||
| Height (cm) | P | 1.62±0.01a, 3 | 1.63±0.01a | 1.64±0.01a | 1.64±0.01a | 1.64±0.01 | 1.66±0.01 b | <0.001 |
| C*,4 | 1.63±0.01a | 1.65±0.01 a | 1.65±0.01 a | 1.66±0.01 | 1.66±0.01 | 1.70±0.02 b | ||
| BMI (kg/m2) | P | 37.3±1.4a | 31.8±1.2 b | 32.4±0.8 b | 30.3±0.7 b | 26.4±0.9 c | 27.2±1.0 c | <0.0015 |
| C* | 24.8±1.1 | 25.0±0.8 | 23.3±0.3 | 23.6±0.4 | 22.8±0.5 | 23.2±0.6 | ||
| Waist Circ (cm) | P | 109±3.0a | 99.4±2.9b | 99.8±1.9b | 96.2±1.8b | 88.4±2.c | 88.3±2.4c | <0.0015 |
| C* | 81.1±2.7 | 83.7±2.5 | 78.7±0.9 | 81.9±1.1 | 79.3±1.5 | 81.0±1.8 | ||
| Hip Circ (cm) | P | 118.8±2.0 a | 111.2±2.7 a,b | 112.5±1.7 a,b | 108.6±1.6 b | 101.3±1.9 c | 101.1±2.1 c | <0.0015 |
| C* | 100.3±3.1 | 101.5±2.1 | 97.0±0.8 | 99.2±0.9 | 97.3±1.1 | 99.1±1.8 | ||
| WHR | P | 0.92±0.02 | 0.89±0.01 | 0.89±0.01 | 0.88±0.01 | 0.87±0.01 | 0.87±0.01 | 0.5 |
| C* | 0.81±0.02 | 0.82±0.01 | 0.81±0.01 | 0.83±0.01 | 0.81±0.01 | 0.82±0.01 | ||
| SBP (mmHg) | P | 113±4 | 112±2 | 116±2 | 113±2 | 115±2 | 110±2 | 0.5 |
| C* | 111±4 | 110±2 | 108±1 | 107±1 | 108±2 | 106±2 | ||
| DBP (mmHg) | P | 70±3 | 71±2 | 72±1 | 71±1 | 71±2 | 70±2 | 1.0 |
| C | 72±3 | 71±2 | 70±1 | 69±1 | 69±1 | 69±2 | ||
| T (ng/dL) | P | 78.5±16.0 | 59.7±4.4 | 66.8±3.6 | 64.8±3.8 | 59.8±10.6 | 58.1±5.1 | 0.3 |
| C* | 38.2±2.9 | 36.2±3.5 | 34.4±1.6 | 36.7±2.0 | 35.8±2.8 | 33.8±3.7 | ||
| SHBG (nmol/L) | P | 25.0±2.7 | 33.9±2.5 | 40.6±3.1 | 41.1±2.6 | 45.1±3.7 | 51.1±4.1 | 0.02 |
| C* | 65.1±7.5 | 64.2±5.0 | 64.3±3.0 | 66.0±3.2 | 70.0±5.8 | 65.6±7.5 | ||
| Adione (ng/mL) | P | 3.5±0.4 | 3.9±0.2 | 3.6±0.2 | 3.7±0.2 | 3.2±0.2 | 3.5±0.2 | 0.9 |
| C* | 2.9±0.3 | 3.1±0.3 | 3.1±0.1 | 3.2±0.2 | 3.2±0.3 | 3.1±0.3 | ||
| DHEAS (μg/dL) | P | 183±22 | 214±17 | 210±17 | 208±12 | 194±25 | 168±13 | 0.7 |
| C | 180±17 | 222±21 | 202±10 | 219±12 | 203±14 | 226±27 | ||
| LH (IU/L) | P | 22.2±2.5 | 27.7±2.7 | 26.1±2.2 | 28.4±2.3 | 22.9±2.7 | 26.7±2.8 | 0.7 |
| C* | 22.5±6.6 | 12.1±0.9 | 16.3±1.7 | 13.9±0.9 | 14.9±1.5 | 11.8±1.0 | ||
| FSH (IU/L) | P | 10.4±0.5 | 9.9±0.4 | 10.7±0.4 | 10.3±0.3 | 10.4±0.6 | 10.7±0.6 | 0.8 |
| C | 11.2±1.4 | 9.7±0.4 | 10.7±0.4 | 10.6±0.4 | 10.6±0.5 | 10.2±0.7 | ||
| LH:FSH | P | 2.2±0.2 | 2.9±0.3 | 2.5±0.2 | 2.7±0.2 | 2.1±0.2 | 2.6±0.3 | 0.7 |
| C* | 1.9±0.3 | 1.3±0.1 | 1.6±0.1 | 1.4±0.1 | 1.4±0.1 | 1.2±0.1 | ||
| FG Score | P | 17.6±1.4a | 13.9±1.2 | 13.1±0.7 | 12.5±0.8b | 11.0±1.2b | 11.2±1.0b | 0.01 |
| C* | 4.2±0.6 | 3.8±0.4 | 4.1±0.2 | 3.9±0.2 | 4.4±0.4 | 3.5±0.4 | ||
| HbA1C (%) | P | 5.4±0.1 | 5.3±0.1 | 5.3±0.04 | 5.3±0.05 | 5.3±0.04 | 5.3±0.05 | 0.75 |
| C* | 5.0±0.07 | 5.2±0.05 | 5.2±0.03 | 5.2±0.03 | 5.2±0.06 | 5.2±0.05 | ||
| Fasting Glucose (mg/dL) | P | 89.2±2.5 | 86.0±1.8 | 84.8±1.0 | 84.9±1.2 | 84.7±1.6 | 84.4±1.5 | 0.5 |
| C* | 82.1±2.8 | 80.2±1.1 | 80.8±0.6 | 81.7±0.9 | 81.6±1.0 | 79.1±1.3 | ||
| Glucose 120 min (mg/dL) | P | 111.9±6.2 | 105.5±4.0 | 104.1±3.5 | 103.4±4.3 | 98.7±6.0 | 91.4±3.9 | 0.3 |
| C* | 81.8±4.7 | 86.1±4.4 | 84.2±2.4 | 84.9±2.1 | 92.2±2.8 | 80.2±4.0 | ||
| Fasting Insulin (μIU/m L) | P | 13.8±2.1 | 11.4±1.2 | 11.3±0.9 | 9.7±0.9 | 10.9±2.3 | 8.9±1.5 | 0.02 |
| C* | 4.9±0.5 | 5.6±0.6 | 5.5±0.5 | 5.3±0.3 | 4.5±0.4 | 4.6±0.4 | ||
| Insulin 120 min (μIU/m L) | P | 89.1±22.9 | 61.4±7.4 | 62.8±6.8 | 56.3±6.1 | 58.4±10.1 | 49.2±9.0 | 0.07 |
| C* | 25.9±3.8 | 28.2±4.6 | 27.3±1.8 | 23.9±1.6 | 24.1±2.2 | 20.9±2.4 | ||
| HOMA-IR | P | 3.2±0.5 | 2.5±0.3 | 2.5±0.2 | 2.1±0.2 | 2.4±0.5 | 2.0±0.4 | 0.02 |
| C* | 0.9±0.1 | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 | 0.9±0.09 | 0.9±0.09 | ||
| Glucose/Insulin | P | 9.6±1.5 | 11.4±1.1 | 12.8±1.1 | 15.6±1.1 | 17.7±2.1 | 17.3±1.5 | 0.02 |
| C* | 22.3±2.9 | 19.1±1.7 | 19.0±0.9 | 19.7±1.0 | 23.1±1.7 | 19.7±1.6 | ||
| Chol (mg/dL) | P | 196±9 | 173±5 | 183±5 | 186±4 | 188±6 | 187±5 | 0.1 |
| C* | 180±6 | 171±5 | 174±3 | 177±4 | 174±4 | 160±4 | ||
| Triglyc (mg/dL) | P | 126±15 | 116±15 | 104±7 | 94±6 | 91±10 | 93±9 | 0.06 |
| C* | 72±7 | 63±3 | 66±2 | 71±3 | 61±3 | 63±5 | ||
| HDL (mg/dL) | P | 45±3 | 46±2a | 52±2 | 54±2 | 59±3b | 57±3 | 0.00 |
| C* | 64±4 | 59±2 | 62±2 | 61±1 | 64±2 | 60±2 | ||
| LDL (mg/dL) | P | 128±7 | 106±5 | 113±5 | 113±3 | 111±5 | 112±5 | 0.1 |
| C* | 101±6 | 94±4 | 96±3 | 99±4 | 95±4 | 82±3 | ||
| Ovarian Volume (mL) | P | 15.8±2.0 | 14.9±1.1 | 14.6±0.8 | 14.2±0.6 | 13.7±0.9 | 15.2±1.0 | 0.4 |
| C* | 10.1±0.9 | 8.1±0.6 | 9.4±0.4 | 8.9±0.5 | 9.8±0.7 | 10.1±0.9 | ||
| Follicle Number | P | 13.4±0.6 | 13.5±0.6 | 13.2±0.5 | 14.5±0.4 | 12.9±0.6 | 13.7±0.5 | 0.3 |
| C* | 9.6±0.6 | 9.0±0.5 | 9.3±0.3 | 9.5±0.3 | 9.4±0.5 | 10.3±0.6 |
p value for age at menarche
p value adjusted for BMI
P-PCOS, C-Control, Circ-circumference, SBP-systolic blood pressure, DBP-diastolic blood pressure, T-testosterone, Adione-androstenedione, FG-Ferriman Gallwey score.
Values with different superscripts are different than each other in Holm-Sidak post-hoc testing.
Asterisk indicates differences in women with PCOS compared to controls (p<0.001).
p<0.001 for the interaction between PCOS vs. control and age at menarche
p<0.05 for the interaction between PCOS vs. control and age at menarche
There was no relationship between genetic loci associated with menarche and PCOS status, including variants on chr 6 rs7759938 (OR 1.02 [0.8;1.3]; p=0.9) and rs314277 (OR 0.9 [0.7;1.2]; p=0.5) and chr 9 rs2090409 (OR 1.13 [0.9;1.4]; p=0.3). The rs7759938 T variant was associated with earlier age at menarche in all subjects and in women with PCOS, alone (Table 4). The A variant at rs2090409 on 9q31.2 was associated with smaller waist circumference in control subjects (Table 4). Taken together, age at menarche was predicted by a combination of PCOS status (β=0.512; p<0.001), current BMI (β= −0.0202; p=0.01), reported weight group at age 10 to 14 years (β= −0.432; p<0.001) and genotype at rs7759938 (β=0.169; p=0.02).
Table 4.
Relationship Between Genotype Associated with Age at Menarche and PCOS Phenotypic Traits in Women with PCOS and Controls
| Gene | SNP | Group | Allele | Trait | Value | P value |
|---|---|---|---|---|---|---|
| LIN28B | rs7759938 | All Subjects | CC | Age at Menarche (yrs) | 13.01±0.16a | 0.006 |
| CT | 12.66±0.07 | |||||
| TT | 12.47±0.07b | |||||
| LIN28B | rs7759938 | PCOS | CC | Age at Menarche (yrs) | 13.41±0.23a | |
| CT | 12.71±0.10b | |||||
| TT | 12.60±0.09b | |||||
| LIN28B | rs7759938 | Controls | CC | Age at Menarche (yrs) | 12.61±0.23 | |
| CT | 12.62±0.11 | |||||
| TT | 12.35±0.10 | |||||
| Chr9q31.2 | rs2090409 | Controls | CC | Waist Circum (cm) | 81.84±0.86 | 0.04 |
| CA | 79.44±0.87 | |||||
| AA | 79.38±2.06 |
Values with different superscripts are different than each other in post-hoc testing.
Discussion
Women with PCOS were more likely to report early and late development compared to their peers. The difference was explained by reported earlier pubarche and earlier or later menarche in women with PCOS. The timing of menarche was influenced by BMI, reported low or high weight compared to peers at ages 10–14 years and genotype at variant rs7759938 near LIN28B. The data suggest that age at menarche in women with PCOS is associated with identified genetic variants controlling age at menarche and genetic/environmental factors, such as BMI.
While it was previously demonstrated that overweight girls experience earlier pubarche, thelarche and menarche than those with a normal BMI,5,19 there is little data examining age at menarche in girls who develop PCOS. The only exception is in a subset of girls with PCOS born small for gestational age, which later develop early pubarche and menarche.4 Our data demonstrate a strong inverse relationship between reported weight group during the menarchal age window and age at menarche, consistent with data in the population.5,19 Thus, girls with PCOS who report overweight in the pubertal window have an earlier age at menarche, whereas those who are thin have a later age at menarche. Interestingly, our PCOS subjects did not have a low birth weight, consistent with previous studies.6;20 However, they did have an earlier reported pubarche independent of current BMI. Thus, weight at both the high and low end during puberty is a predictor of menarchal age in girls who develop PCOS, while pubarche appears to be early regardless of weight.
The reported weight group tended to increase from ages 5–9 to ages 10–14 years and then stayed higher in age group 15–19 years in both the PCOS and control groups. The current rate of obesity was also much greater in the subjects who were overweight from age 10 through age 19 years. These results are consistent with population data indicating that adolescent obesity predicts adult obesity.21;22 These findings are also consistent with previous suggestions that overweight in PCOS starts in adolescence or with weight gain from adolescence to adulthood.6;23 They also provide validation for the reported weight group in adolescence. Of note, women with PCOS were less likely to have been breast fed, which has been associated with a decreased risk of obesity in epidemiological studies.24
As the age at menarche was associated with both reported weight group during puberty and with current BMI, it was possible to examine differences in current phenotype in women with PCOS who had an early versus a late menarche. Interestingly, despite the higher current weight in women with PCOS who had an earlier age at menarche compared to those with a later age at menarche, there was no difference in current reproductive phenotype as measured by androgen levels, gonadotropin levels and ovarian morphology. In contrast, metabolic parameters including weight, HDL and markers of insulin resistance were less favorable in women with PCOS and earlier menarche. The data suggest that there may be two menarchal scenarios in which PCOS develops. The first may be associated with early menarche related to overweight and obesity starting or worsening in puberty. The second may be associated with thinner stature, later age at menarche and fewer menstrual cycles, reflecting mainly the arrested follicle development and possibly less estradiol than in the overweight adolescents. Of note, the relationship between later age at menarche and taller stature was demonstrated, consistent with later closure of the epiphyses and possibly lower estradiol levels in puberty. Similarly control subjects were slightly taller than PCOS subjects, perhaps related to the lower BMI and therefore estradiol levels.
Even when controlled for BMI, age at menarche in women with PCOS was associated with variant rs7759938, located upstream from LIN28B. Of note, other variants in LIN28B are associated with adult height.25 Further, rs7759938 is associated with growth spurt during early puberty.26 Lin-28, the C. elegans homolog of human LIN28B, appears to regulate genes that execute stage-specific and cell-specific development and over expression results in developmental abnormalities.27;28 Taken together, age at menarche in women with PCOS is influenced by the same genetic variant that influences age at menarche and pubertal growth in large population studies.7–9 The genetic effect is independent of weight in girls with PCOS and may reflect the somatic growth suggested by previous studies.
We did not demonstrate a relationship between the other LIN28B variant, rs314277, or the chromosome 9q31.2 variant rs2090409 and age at menarche,7;10 perhaps related to the power of the current study. Nevertheless, the A allele of rs2090409 was associated with a smaller waist circumference in control subjects. The effect is the opposite one would expect based on the younger age at menarche conferred by the A allele7;10 and the expected relationship of obesity to younger age at menarche.5;19 These findings suggest that the genetic contribution of rs2090409 and waist size may be acting through a mediator different than adiposity.
The study is limited by the retrospective acquisition of the age at menarche, along with weight group at ages 9–16 years, birthweight, gestational age and breastfeeding history. Menarche recall is not as remote as in previous studies in which the correlation between documented and recalled menarchal age 30 to 40 years later is as high as r=0.78. The correlation between reported weight compared to peers at ages 9–16 years and documented weight, a validated question, is also high among middle-aged women.15,29 Further, the correlation between current weight and reported weight group at menarche is very high, as would be expected based on the relationship between adolescent and adult weight.6,21–23 Finally, others demonstrate a strong correlation between recalled and actual birthweight and breastfeeding.20,30 The study is also limited by numbers. Nevertheless, the study had 80% power to detect a relationship between LIN28B and age at menarche at a p<0.05 assuming that the OR for the effect is the same as that for the population. We did not examine variants associated with BMI and age at menarche,31 as previous studies have not demonstrated a relationship between PCOS and variants associated with BMI.32 However, we have demonstrated that rs9939609 in FTO, the variant most closely associated with BMI in Europeans,33 was not associated with PCOS (unpublished data).
In summary, age at menarche in women with PCOS is influenced by both low and high weight compared to peers during puberty but is also subject to genetic influence observed in population studies. Specifically, those women with PCOS who are lean or of normal weight and carry the C allele at rs7759938 are more likely to have late age at menarche, while those who were overweight starting at or before puberty and carry the T allele at rs7759938 are more likely to have an early menarche compared to controls. The findings suggest the possibility that there are two menarchal scenarios resulting in PCOS, one influenced by overweight at the time of puberty and another in which weight is lower and menarche later. Further studies are needed to document lifestyle and estradiol or other hormonal differences in these two states at the time at the time of puberty.
Acknowledgments
The project described was supported by Award Number R01HD065029 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development, Award Number 1 UL1 RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources and award 1-10-CT-57 from the American Diabetes Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development, National Center For Research Resources, the National Institutes of Health or the American Diabetes Association.
Reference List
- 1.Dahlgren E, Johansson S, Lindstedt G, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57(3):505–513. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- 2.Macklon NS, Fauser BCJM. Aspects of ovarian follicle development throughout life. Horm Res. 1999;52:161–170. doi: 10.1159/000023456. [DOI] [PubMed] [Google Scholar]
- 3.Guzick D. Polycystic ovary syndrome: symptomatology, pathophysiology, and epidemiology. Am J Obstet Gynecol. 1998;179(6 Pt 2):S89–S93. doi: 10.1016/s0002-9378(98)70238-8. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez L, Jimenez R, de Zegher F. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics. 2006;117(1):117–121. doi: 10.1542/peds.2005-0664. [DOI] [PubMed] [Google Scholar]
- 5.Stark O, Peckham CS, Moynihan C. Weight and age at menarche. Arch Dis Child. 1989;64(3):383–387. doi: 10.1136/adc.64.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laitinen J, Taponen S, Martikainen H, et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 0 AD;27(6):710–715. doi: 10.1038/sj.ijo.0802301. [DOI] [PubMed] [Google Scholar]
- 7.He C, Kraft P, Chen C, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724–128. doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulem P, Gudbjartsson DF, Rafnar T, et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet. 2009;41:734–738. doi: 10.1038/ng.383. [DOI] [PubMed] [Google Scholar]
- 9.Ong KK, Elks CE, Li S, et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009;41:729–733. doi: 10.1038/ng.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry JR, Stolk L, Franceschini N, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009;41(6):648–650. doi: 10.1038/ng.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 12.Welt CK, Arason G, Gudmundsson JA, et al. Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab. 2006;91:4361–4368. doi: 10.1210/jc.2006-1191. [DOI] [PubMed] [Google Scholar]
- 13.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 14.Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672–679. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 15.Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol. 1991;18(2):155–166. doi: 10.1080/03014469100001492. [DOI] [PubMed] [Google Scholar]
- 16.Price AL, Butler J, Patterson N, et al. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet. 2008;4(1):e236. doi: 10.1371/journal.pgen.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MW, Patterson N, Lautenberger JA, et al. A high-density admixture map for disease gene discovery in african americans. Am J Hum Genet. 2004;74(5):1001–1013. doi: 10.1086/420856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price AL, Patterson N, Yu F, et al. A genomewide admixture map for Latino populations. Am J Hum Genet. 2007;80(6):1024–1036. doi: 10.1086/518313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 20.Legro RS, Roller RL, Dodson WC, Stetter CM, Kunselman AR, Dunaif A. Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab. 2010;95(2):789–799. doi: 10.1210/jc.2009-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80(3):569–575. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- 22.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 23.Apter D, Butzow T, Laughlin GA, Yen SS. Metabolic features of polycystic ovary syndrome are found in adolescent girls with hyperandrogenism. J Clin Endocrinol Metab. 1995;80(10):2966–2973. doi: 10.1210/jcem.80.10.7559882. [DOI] [PubMed] [Google Scholar]
- 24.Gillman MW, Rifas-Shiman SL, Camargo CA, Jr, et al. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285:2461–2467. doi: 10.1001/jama.285.19.2461. [DOI] [PubMed] [Google Scholar]
- 25.Lettre G, Jackson AU, Gieger C, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40(5):584–591. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widen E, Ripatti S, Cousminer DL, et al. Distinct Variants at LIN28B Influence Growth in Height from Birth to Adulthood. The American Journal of Human Genetics. 2010;86(5):773–782. doi: 10.1016/j.ajhg.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Chen Y, Ito H, et al. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Moss EG, Lee RC, Ambros V. The Cold Shock Domain Protein LIN-28 Controls Developmental Timing in C. elegans and Is Regulated by the lin-4 RNA. Cell. 1997;88(5):637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 29.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–572. [PubMed] [Google Scholar]
- 30.Troy LM, Michels KB, Hunter DJ, et al. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol. 1996;25(1):122–127. doi: 10.1093/ije/25.1.122. [DOI] [PubMed] [Google Scholar]
- 31.Elks CE, Perry JR, Sulem P, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42(12):1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewens KG, Jones MR, Ankener W, et al. FTO and MC4R gene variants are associated with obesity in polycystic ovary syndrome. PLoS ONE. 2011;20;6(1):e16390. doi: 10.1371/journal.pone.0016390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]