Abstract
Reversing brain degeneration and trauma lesions will depend on cell therapy. Our previous work identified neural precursor cells derived from the skeletal muscle of Nestin-GFP transgenic mice, but their identity, origin, and potential survival in the brain are only vaguely understood. In this work, we show that Nestin-GFP+ progenitor cells share morphological and molecular markers with NG2-glia, including NG2, PDGFRα, O4, NGF receptor (p75), glutamate receptor-1(AMPA), and A2B5 expression. Although these cells exhibit NG2, they do not express other pericyte markers, such as α-SMA or connexin-43, and do not differentiate into the muscle lineage. Patch-clamp studies displayed outward potassium currents, probably carried through Kir6.1 channels. Given their potential therapeutic application, we compared their abundance in tissues and concluded that skeletal muscle is the richest source of predifferentiated neural precursor cells. We found that these cells migrate toward the neurogenic subventricular zone displaying their typical morphology and nestin-GFP expression two weeks after brain injection. For translational purposes, we sought to identify these neural progenitor cells in wild-type species by developing a DsRed expression vector under Nestin-Intron II control. This approach revealed them in nonhuman primates and aging rodents throughout the lifespan.
Keywords: Nestin-expressing neural progenitors, NG2-glia, Stem cells, Skeletal muscle, Progenitor cells, Nestin
Introduction
Central nervous system disorders are the leading cause of disability worldwide [1]. While whole-organ transplantation is not viable, grafting neural precursor cells is one strategy to regenerate nerve tissue damaged by age, trauma, or disease [2]. However, problems with histocompatibility, inadequate tissue supplies, and the ethics of using embryonic stem cells and neural tissue isolated from the brain call for another source of neural progenitor cells. Adult stem cells from tissues apart from the central nervous system (CNS) are under study as an alternative source of cells capable of neural differentiation [3–5]. This strategy would enable the patient to be both donor and host.
Muscle tissue is one of the largest organs in the body, comprising nearly 50% of total body mass [6], and should be an abundant, accessible, and safe source of stem cells compared to embryonic tissues. Autologous cell transplantation circumvents the immunological and ethical concerns delaying cell therapy. Previous studies have discovered a population of multipotent skeletal muscle cells that differentiate into mesodermal lineages, including myogenic [7,8], adipogenic [9], osteogenic [10], chondrogenic [11], endothelial [12], and under certain conditions, they can break germ-layer commitment and differentiate into ectodermal lineages, including neural cells [3,13–17].
We recently isolated neural progenitor cells from the skeletal muscle cultures of adult Nestin-GFP transgenic mice and demonstrated their expression of specific neural progenitor markers, replicative capacity, ability to form neurospheres, and functional response to neurotransmitter [17]. Different cell types may express nestin in the skeletal muscle. Reports have identified Nestin-GFP transgene expression in quiescent satellite cells [17,18], capillary networks between myofibers [18], and mesenchymal stem cells in many different tissues [19]. For this reason, we used various markers to better characterize the skeletal muscle-derived neural progenitors.
Various multipotent neural progenitor cells, including radial glia cells [20], NG2-glia [21], astrocyte-like subtypes [22], microglia-like subtypes [23], and ependymal cells [24], have been identified in the CNS. They differ in their tendency to differentiate into neurons, astrocytes, and/or oligodendrocytes [25], their role in CNS repair after injury [26], and their therapeutic potential [27]. Whether skeletal muscle-derived neural progenitor cells correspond to or resemble any of them is unknown. Although skeletal muscle-derived cells express some markers of neural progenitors, their precise expression profile remains incomplete, and whether they are exclusively committed to the neural lineage is still unknown.
We used transgenic mouse models in which fluorescence expression is controlled by the regulatory elements of specific genes (Nestin-GFP; β-actin-DsRed, Nestin-GFP/β-actin-DsRed, and NG2-DsRed), immunocytochemistry, electrophysiology, stereotactic brain injection, expression vector, and fluorescent-activated cell sorting (FACS) to demonstrate that skeletal muscle-derived neural progenitor cells resemble a specific class of neural progenitors, NG2-glia (NG2+, PDGFRα+, O4+, NGF receptor (p75)+, AMPA (Glutamate receptor 1)+, A2B5+ and rectifier K+ channels expression) from the brain. They have similar expression profiles, and we confirmed their commitment to the neural lineage and their inability to transdifferentiate into muscle cells. When injected into the brain striatum of adult mice, they migrated toward neurogenic areas.
We suggest that they can be isolated from a variety of young-adult and older adult species, including nonhuman primates. We engineered a plasmid that can be used to identify cells similar to Nestin-GFP+ neural progenitors from nontransgenic nonhuman primates. Based on cell markers, functional properties, sources, and abundance, we propose that Nestin-GFP+ progenitor cells could be used to treat an array of pathologies affecting the central nervous system, including trauma, degeneration, and neoplasias.
Methods
Animals
Nestin-GFP transgenic mice (our colony) were maintained homozygous for the transgene on the C57BL/6 genetic background [28]. C57BL/6 wild-type mice (our colony) were used as controls. Aging FVB (Friend Virus B) mice (our colony) have long been used as a model of aging skeletal muscle in our laboratory [29–31]. NG2-DsRed transgenic mice expressing DsRed-T1 under the control of the NG2 promoter [32] and β-actin-DsRed trans-genic mice expressing red fluorescent protein variant DsRed.MST under the control of the chicken β-actin promoter coupled with the cytomegalovirus (CMV) immediate early enhancer [33] were purchased from the Jackson Laboratory (Bar Harbor, Maine). All tissues of β-actin-DsRed transgenic mice fluoresce red [33]. Nestin-GFP mice were crossbred with β-actin-DsRed mice to generate Nestin-GFP/β-actin-DsRed double-transgenic mice. All mouse colonies were housed at Wake Forest School of Medicine (WFSM) in a pathogen-free facility of the Animal Research Program under 12:12-h light/dark cycle and fed ad libitum. Both male and female homozygous mice were used, and their ages ranged from 3 to 5 months. African Green vervet monkeys were housed in the WFSM Primate Center. A piece of their vastus lateralis muscle was surgically excised immediately after euthanasia. Animal handling and procedures were approved by the WFSM Animal Care and Use Committee.
Flexor digitorum brevis (FDB) culture preparation
FDB muscle from Nestin-GFP transgenic, Nestin-GFP/β-actin transgenic, aging FVB and C57BL/6 wild-type mice were used for cell culture. FDB muscle was preferred over more traditional muscles for most experiments because of it is small and flat, allowing more complete dissociation by trituration in a single step, shortening the experiment significantly. Methods for FDB culture preparation have been described [17]. Briefly, muscles were carefully dissected away from the surrounding connective tissue and minced, then digested by gentle agitation in 0.2% (w/v) type-2 collagenase (Worthington, Lakewood, NJ) in Krebs solution at 37 °C for 2 h. They were resuspended in growth medium and dissociated by gentle trituration. The growth medium used to plate cell cultures consisted of Dulbecco’s modified Eagle medium (DMEM)-high glucose (Invitrogen, Carls-bad, CA), supplemented with 2% L-glutamine (Invitrogen), 50 U/ml penicillin (Invitrogen), 50 mg/ml streptomycin (Invitrogen), 10% (v/v) horse serum (Invitrogen), and 0.5% (v/v) CEE (Gemini Bio-products, West Sacramento, CA). It supported both proliferation and differentiation of myogenic cells [34]. Finally, cells were plated on 35-mm dishes (Fisher Scientific, Pittsburgh, PA) pre-coated with 10 μg/ml laminin (Invitrogen), following the company’s protocol, at 2–3 × 104 cells/cm2.
Isolation of neural progenitor cells from FDB muscles
To isolate Nestin-GFP+ neural progenitor cells, fluorescence-activated cell sorting (FACS) experiments were performed 7–14 days after muscle dissociation. Cultured FDB-derived cells were washed with phosphate-buffered saline (PBS) and treated with 0.25% trypsin/0.05% EDTA (Invitrogen) to isolate them in a suspension. When all cells were detached, the enzymatic reaction was stopped with the growth medium described above. We applied mechanical trituration using fire-polished glass pipettes to increase cell dissociation. Cells were centrifuged at 1000 rpm for 5 min, and the pellet was resuspended in DMEM (Invitrogen) at 106 cells/ml. Aggregates were removed by passing them through a 40-μm cell strainer (BD Biosciences, Mississauga, Ontario, Canada) prior to sorting.
Fluorescence-activated cell sorting (FACS)
FACS was carried out on a BD FACS (Aria Sorter, San Jose, CA) at 4 °C and a pressure of 20 psi, using a laser at the 488-nm line, a 530/30 band pass filter, a 100-μm sorting tip, and a 34.2 kHz drive frequency, sterilized with 10% bleach. This instrument allowed us to characterize cells by size as well as fluorescence. Low flow rate improved sorting purity. Data acquisition and analyses were performed using BD FACS Diva 5.0.3 software, gated for a high level of GFP expression. The clear separation of GFP+ from GFP− cells explains the ease of sorting [17]. Sorted cells were re-analyzed to confirm that all were GFP+ [17].
Isolation and culture of Nestin-GFP+ cells from adipose tissue, skeletal muscle, and bone marrow
Adipose tissue
Cells were obtained from the inguinal or epididymal fat of 3–4-month-old Nestin-GFP transgenic mice. Adipose tissue was removed by sterile dissection, weighted, pooled, finely minced with scissors, and digested in a buffer containing collagenase (2 mg/ml) (Worthington) and hyaluronidase (0.5 mg/ml) (Worthing-ton) in Krebs solution at 37 °C for 2 h. The cells were resuspended in 0.25% trypsin/0.05% EDTA (Invitrogen) in PBS for 15 min at 37 °C, dissociated, centrifuged, and the supernatant discarded. The pellet was resuspended in growth medium, dissociated, and triturated. Cells were seeded on laminin-precoated dishes and incubated at 37 °C and 5% CO2 in a humidified incubator.
Skeletal muscle
Methods for skeletal muscle preparation have been described [17]. Briefly, muscles were carefully dissected away from the surrounding connective tissue, weighed, and minced, then digested by gentle agitation in 0.2% (w/v) type-2 collagenase (Worthington) in Krebs solution at 37 °C for 2 h, dissociated by trituration, and resuspended in 0.25% trypsin/0.05% EDTA (Invitrogen) in PBS for 15 min at 37 °C. After centrifuging at 1500 rpm for 5 min, the supernatant was removed, and the pellet resuspended in growth medium. Aggregates were removed by passing them through a 40-μm cell strainer (BD Biosciences) prior to culture.
Bone marrow
We obtained bone marrow by aseptically flushing the femur medullary cavity with 0.25% trypsin/0.05% EDTA (Invitrogen) in PBS. The cells were dissociated; the cell suspension was centrifuged, and the supernatant discarded to measure tissue weight. Cells were resuspended in growth medium and dissociated by trituration, then seeded and incubated as described above.
The number of Nestin-GFP+ progenitor cells with neural morphology was counted at day 10 in culture. The number of Nestin-GFP+ cells derived from fat, skeletal muscle, or bone marrow was normalized to the dissociated tissue weight (mg) and number of nuclei (%). Only GFP+ cells with two or more processes were counted.
Neurosphere culture
Mouse E14.5 striata neural stem cells (neurospheres) were purchased from StemCell Technologies (Vancouver, Canada) and cultured according to the manufacturer’s protocols [35]. Briefly, primary cells were seeded at a density of 1 × 105 in T-25 cm2 flasks, and neurosphere growth monitored under the microscope. Neurospheres were maintained in Complete NeuroCult Proliferation Medium (StemCell Technologies) consisting of mouse NeuroCult NSC Basal Medium plus mouse NeuroCult NSC Proliferation Supplemented with 20 ng/ml recombinant human epidermal growth factor (rh EGF) (StemCell Technologies) per the manufacturer’s instructions. Neurospheres were subcultured no more than once per collection. Cell passaging was performed by dissociating neurospheres using NeuroCult Chemical Dissociation kit (StemCell Technologies). Differentiation was initiated by plating the neurospheres on laminin-coated plates in complete NeuroCult differentiation medium (StemCell Technologies) consisting of mouse NeuroCult NSC Basal Medium plus mouse NeuroCult NSC Differentiation Supplement without cytokines in the absence of rh EGF, per the manufacturer’s instructions.
C2C12 myoblast culture
The mouse skeletal muscle cell line C2C12 (ATCC, Manassas, VA) was cultured as described previously [36]. Briefly, C2C12 myo-blasts were plated on tissue culture dishes in proliferation medium consisting of DMEM (1 g/l glucose) (Invitrogen), containing 10% FBS (Atlanta Biologicals, Atlanta, GA) and 2 mM Glutamax (Invitrogen).
Reverse transcription polymerase chain reaction (RT-PCR)
To detect the mRNA expression in cells, total RNA was isolated using TRIZOL reagent (Life Technologies, Carlsbad, CA), RNA was dissolved in sterile, RNase-free water (Invitrogen) and quantitated spectrophotometrically at 260 nm. RT-PCR was performed in accordance with the manufacturer’s instructions using the SuperScript III First-Strand synthesis system for RT-PCR system (Invitrogen). For each experiment, equivalent amounts of intact RNA (0.1 to 0.2 μg) were used. As negative controls, the RT reactions were performed in the absence of RNA (only water) or reverse transcriptase. The cDNA was amplified by PCR using the primers included in Table 1. PCR Master Mix was purchased from Promega (Fitchburg, WI). Each PCR reaction contained 1 × Promega PCR Master Mix, 1 μM of each primer, and the cDNA of the cells used in each case (Nestin-GFP+, NG2 glia, or C2C12 cells). The volume of each reaction was brought up to 50 μl with water. DNA amplification was carried out as follows: denaturation at 94 °C for 2 min, followed by 35 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 2 min. After 35 cycles, the reactions were incubated at 72 °C for 7 min to increase the yield of amplification. PCR products were verified with DNA 2% agarose gel electrophoresis.
Table 1.
Genes, GenBank accession numbers, coding regions, primers.
| Gene | GenBank Accession numbers | Coding regions | Forward primer and positions | Reverse primer and positions |
|---|---|---|---|---|
| Nestin | NM_016701.3 | CDS: 111-5705 | AGGACCAGGTGCTTGAGAGA (2248–2267) | TCCTCTGCGTCTTCAAACCT (2383–2364) |
| NG2 | NM_139001.2 | CDS: 87-7070 | GCACGATGACTCTGAGACCA (3020–3039) | AGCATCGCTGAAGGCTACAT (3242–3223) |
| PDGFRα | NM_011058.2 | CDS: 180-3449 | TGGCATGATGGTCGATTCTA (2870–2889) | CGCTGAGGTGGTAGAAGGAG (3021–3002) |
| AMPA | NM_001113325.2 | CDS: 417-3140 | ACCACTACATCCTCGCCAAC (1093–1112) | TCACTTGTCCTCCACTGCTG (1237–1218) |
| NGFR | NM_033217.3 | CDS: 141-1424 | CAACCAGACCGTGTGTGAAC (326–345) | GGAGAACACGAGTCCTGAGC (560–541) |
| MyoD | NM_010866.2 | CDS: 200-1156 | AGTGAATGAGGCCTTCGAGA (571–590) | GCATCTGAGTCGCCACTGTA (792–773) |
| GAPDH | NM_008084.2 | CDS: 51-1052 | GTGGCAAAGTGGAGATTGTTGCC (118–140) | GATGATGACCCTTTTGGCTCC (407–387) |
DsRed Nestin-Intron II plasmid
We constructed a DsRed-Nestin-Intron II plasmid by replacing luciferase with DsRed cDNA in the pNES2In32/1628 plasmid (kindly donated by Dr. Naihe Jing, Laboratory of Molecular Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China). This plasmid contains 1597 bp of the second Nestin Intron, and the insert is in position 7–1603 [37,38]. The DsRed cDNA was obtained by PCR using DsRed2-N1 (Clontech, Mountain View, CA) as the template and primers: Forward, 5′-GCAAAAAGCTTGGCATTCCGGTACTGTTGGTAAAGCCA-CCATGGCCTCCTCCGAGAACGTCATCACC-3′; Reverse, 5′-GACTCT-AGAATTACAGGAACAGGTGGTGGCGGC-3′. To ensure that the original sequence in front of the luciferase cDNA start codon in pNES2In32/1628 was not modified by the replacement, a forward primer was designed to add the DsRed sequence (in bold) between HindIII and the start codon. The PCR product was gel- purified, digested with HindIII (Promega) and XbaI (Promega) (underlined in the primers), and ligated to HindIII and XbaI-digested pNES2In32/ 1628 backbone vector. Plasmid sequence was confirmed by sequencing. As positive control for transfection we used a DsRed plasmid (DsRed2-N1) (Clontech).
Isolation and transfection of African Green vervet monkey interstitial cells
Cells derived from monkey vastus lateralis muscle were cultured in the same conditions used for the mouse muscle. Prior to plasmid transfection, the growth medium was replaced with 2 ml of OPTI-MEM transfection medium (Invitrogen), and 1 μg of plasmid DNA was diluted into 100 μl of OPTI-MEM transfection medium mixed with 4 μg of Lipofectamine 2000 reagent (1 mg/ml) (Invitrogen). Cells were incubated for 15 min prior to dilution in OPTI-MEM to a final DNA concentration of 0.5 μg/ml. The transfection medium was replaced with growth medium after 6 h. DsRed expression was assessed 24–96 h posttransfection, and results recorded after 96 h.
Cell eletrophysiology
Ionic currents were recorded in the whole-cell configuration of patch-clamp using an Axopatch 200B and pClamp 10 software (Molecular Devices Inc., Sunnyvale, CA) as described [39,40,17]. Signals were digitized and filtered at 5 kHz using Digidata 1440A (Molecular Devices, Sunnyvale, CA). Electrophysiological recordings were carried out at room temperature in an extracellular recording solution containing (in mM): 145NaCl, 3KCl, 10HEPES, 3CaCl2 · 2H2O, 8glucose and 2MgCl2, and pH 7.3 was reached with NaOH. The pipette solution contained D-gluconic acid 136.5, 17.5 KCl, 9NaCl, 1MgCl2, 10HEPES, and 0.2 of EGTA, and pH 7.3 was reached with KOH. Pipette resistance was 2–3 MΩ. Primary neurons and Nestin-GFP+ neural progenitor cells were identified by their distinct neural morphology, characterized by a small cell body, long, uneven processes, and the absence or presence of GFP fluorescence, respectively. Their identity was confirmed by immunostaining with Tuj1 (class III β tubulin) antibody in parallel cultures.
Nestin-GFP+ neural progenitor cells co-cultured with C2C12 myoblasts
Nestin-GFP+, Nestin-GFP+/β-actin-DsRed+, and Nestin-GFP−/ β-actin-DsRed+ cells were sorted from 7-day-old FDB cultures derived from Nestin-GFP or Nestin-GFP/β-actin-DsRed mice, respectively. Afterward, they were cultured alone or with C2C12 cells (ATCC) [41] on dishes precoated with laminin in proliferation medium [DMEM (Invitrogen) containing 2-mM L-glutamine (Invitrogen) and 1% penicillin/streptomycin (Invitrogen), supplemented with 10% FBS (Atlanta Biologicals)] for 3 days, and then differentiation medium [DMEM (Invitrogen) containing 2-mM L-glutamine (Invitrogen) and 1% penicillin/ streptomycin (Invitrogen), supplemented with 2% HS (Invitrogen)] for 7 days. They were then fixed in 4% paraformaldehyde PFA and stained with MyoD, MF 20, and Hoechst to identify myogenic cells, myotubes, and nuclei, respectively.
Isolation of brain-derived NG2 progenitors
NG2-DsRed cells were isolated from 3 to 5 month-old mice. The brain was isolated, cut into small pieces, enzymatically digested using 20 units/ml papain and 0.05 mg/ml DNase (Papain Dissociation kit, Worthington) at 37 °C for 30 min, and mechanically triturated with fire-polished Pasteur pipettes of various tip sizes. Cells were collected by centrifugation (300 × g for 7 min), and the pellet resuspended in buffer containing ovomucoid (Papain Dissociation kit), centrifuged again, and resuspended in growth medium. NG2-DsRed+ cells were selected by FACS.
Brain injection
To evaluate cell survival in the brain, 50,000 cells were injected in the striatum, and the mouse sacrificed 2 weeks later. Mice were anesthetized with a ketamine/xylazine mixture (114/17 mg/kg), and a 0.5-mm burr hole was made 3 mm right of the midline and 1.5 mm posterior to the bregma through a scalp incision. Cells were injected at a concentration of 5 × 104 cells in 5-μl growth medium. For stereotaxic injection, we used a 10-μl syringe (Hamilton, Reno, NV) with a 30-gauge needle, inserted through the burr hole to a depth of 3 mm, mounted on a Just For Mice stereotaxic apparatus (Harvard Apparatus, Holliston, MA) at a rate of 2 μl/min. Mice were monitored for body weight and ambulatory, feeding, and grooming activities afterward. Two weeks after cell injection, mice were anesthetized again and transcardially perfused with PBS followed by perfusion with 4% paraformaldehyde solution in PBS. After decapitation, brains were rapidly dissected out, removed from the skull, postfixed for 24 h in the same fixative solution, and cryoprotected with 30% sucrose in PBS for 2 days. The brains were then placed in embedding cryomolds, covered with tissue embedding medium (O.C.T. Compound; Tissue-Tek; Sakura Finetek, Tokyo, Japan), and snap-frozen in liquid nitrogen.
Brain histological processing
Frozen brains were sectioned transversely into serial 20-μm thick coronal sections using a cryostat (Zeiss Microm HM 500, Oberkochen, Germany) at −20 °C, mounted on SuperFrost Plus Microscope Slides in series of six (Fisher Scientific), and stored at −20 °C before processing for immunocytochemistry. Sections were dried at room temperature for 1 h, rehydrated in PBS, and permeabilized with 0,5% Triton X-100 (Sigma, St. Louis, MO) in PBS solution and blocked to saturate nonspecific antigen sites using 5% (v/v) goat serum/PBS (Jackson Immunoresearch Labs, West Grove, PA) at 4 °C, overnight. The next day, the sections were incubated with primary antibodies at room temperature for 4 h and visualized using appropriate species-specific secondary antibodies. Hoechst 33342 was used as a nuclear marker. The sections were mounted on slides using Fluorescent Mounting Medium (DakoCytomation, Carpinteria, CA) and examined with fluorescence microscopy.
Immunocytochemistry
Cultured cells were fixed with 4% PFA for 30 min, then permeabilized in 0.5% Triton X-100 (Sigma), and blocked to saturate nonspecific antigen sites using 5% (v/v) goat serum/PBS (Jackson Immunoresearch Labs) overnight at 4 °C. The next day, the cells were incubated with primary antibodies at room temperature for 4 h and visualized using appropriate species-specific secondary antibodies conjugated with Alexa Fluor 488, 568, or 647 at 1:1000 dilution (Invitrogen). They were counterstained with Hoechst 33342 reagent at 1:2000 dilution (Invitrogen) to label the DNA and mounted on slides for fluorescent microscopy with Fluorescent Mounting Medium (DakoCytomation).
Primary antibodies
Table 2 shows the antibodies, their dilution, and source.
Table 2.
Antibodies, concentration, and source.
| Antibody | Dilution | Source | Location |
|---|---|---|---|
| Rabbit monoclonal anti-Tuj1 | 1:800 | Covance | Princeton, NJ |
| Mouse monoclonal anti-MyoD | 1:800 | BD Pharmingen | San Diego, CA |
| Rat monoclonal anti-mouse CD31 | 1:200 | BD Pharmingen | San Diego, CA |
| Mouse monoclonal anti-α-smooth muscle actin | 1:1000 | Sigma | St. Louis, MO |
| Rabbit polyclonal anti-NG2 Chondroitin Sulfate | 1:100 | Chemicon-Millipore | Temecula, CA |
| Rabbit anti-PDGFRα | 1:250 | Dr. W. Stallcup | Sanford-Burnham Medical Research Institute, CA |
| Rabbit monoclonal anti-NGF receptor (p75) | 1:200 | Epitomics | Burlingame, CA |
| Rabbit polyclonal anti-Glutamate receptor 1 | 1:200 | GeneTex | Irvine, CA |
| Mouse monoclonal anti-Glial Fibrillary Acidic Protein (GFAP) | 1:200 | Chemicon-Millipore | Temecula, CA |
| Mouse monoclonal anti-oligodendrocyte marker O4 | 1:50 | Chemicon-Millipore | Temecula, CA |
| Mouse monoclonal anti-connexin 43 (Cx43IF1) | 1:100 | Fred Hutchinson Cancer Research Center | Seattle, WA |
| Rabbit polyclonal anti-Kir6.1 | 1:100 | Alomone Labs | Jerusalem, Israel |
| Mouse monoclonal anti-4D4 (A2B5-like) | 1:100 | Developmental Studies Hybridoma Bank, University of Iowa | Iowa City, IA |
| Mouse anti-Pax7 | 1:100 | Developmental Studies Hybridoma Bank, University of Iowa | Iowa City, IA |
Microscopy, cell imaging, and counting
An inverted motorized fluorescent microscope (Olympus, IX81, Tokyo, Japan) with an Orca-R2 Hamamatsu CCD camera (Hama-matsu, Japan) was used for image acquisition. Camera drive and acquisition were controlled by a MetaMorph Imaging System (Olympus). Ten arbitrary microscopic fields were counted in each immunostained plate, and values pooled from parallel duplicates per time point and individual experiment.
Statistical analysis
Results are expressed as the mean±SEM. Statistical significance was assessed using analysis of variance (ANOVA) followed by Student’s t-test using GraphPad Prism (GraphPad Software, San Diego, CA). P<0.05 was considered significant.
Results
Isolation of Nestin-GFP+ progenitor cells with neural morphology from adipose tissue, skeletal muscle, and bone marrow
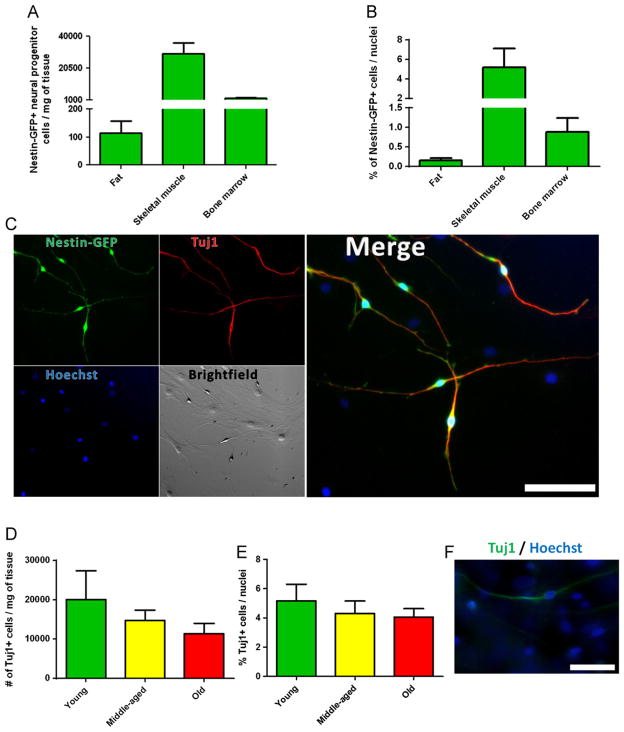
To explore the abundance and extraction efficiency of large pools of Nestin-GFP+ progenitor cells in readily accessible tissues, we dissociated and cultured equivalent masses of fat tissue, skeletal muscle, and bone marrow from Nestin-GFP transgenic mice. After 10 days, we examined Nestin-GFP+ progenitor cells exhibiting neural morphology derived from each (Fig. 1A and B). All Nestin-GFP+ cells are neural progenitors and express Tuj1 (class III β tubulin), a neural progenitor marker [42], at this culture time [17]. Our analysis may not reflect the relative abundance of the various cell populations in the tissue but it provides this information at day 10 in culture, which is relevant for therapy. We did not isolate Nestin-GFP+ neural precursors from freshly dissociated muscle because other cells (i.e., satellite cells), are Nestin-GFP+ as well [17,18]. The number was expressed per tissue weight (mg) and number of nuclei (%). More cells were derived from skeletal muscle (29,517±6370 cells/mg; n = 5) than from bone marrow (1905±585 cells/mg; n = 5) or adipose tissue (113±43 cells/mg; n = 5) for the same tissue mass (Fig. 1A), which corresponded to 5.2±2.0, 0.9±0.4, and 0.2±0.1% of Nestin-GFP+ cells/nuclei, respectively (Fig. 1B). All Nestin-GFP+ cells stained positive for Tuj1 (Fig. 1C). These data indicate that Nestin-GFP+ progenitor cells are more abundant in skeletal muscle than bone marrow or fat tissue.
Fig. 1.
Nestin-GFP+ progenitor cells derive from adipose tissue, skeletal muscle, and bone marrow and can be isolated from the skeletal muscle of young, middle-aged, and old mice. (A) and (B) Number of Nestin-GFP+ progenitor cells at day 10 in culture was normalized to tissue weight (A) and number of nuclei (B). Only GFP+ cells with neural morphology exhibiting 2 or more processes were counted (n = 5 preparations). Nestin-GFP+ neural progenitors stained positive for Tuj1 (C). (D) and (E) Number of FDB muscle-derived Tuj1+ cells from young (3-month), middle-aged (12-month), and old (24-month) FVB mice. Cells were fixed at day 10 and stained for Tuj1. The percent of Tuj1+ cells derived from each FDB muscle was counted and normalized to the weight of starting tissue (D) or the number of nuclei (E) (n = 3 preparations). (D) Representative Tuj1+ cell derived from the skeletal muscle of an old mouse. (C) Scale bar = 100 μm, (F) Scale bar = 20 μm.
Nestin-GFP+ progenitor cells can be extracted from the skeletal muscle of aging mice
To determine whether the probability of extracting Nestin-GFP+ progenitor cells declines with age, we analyzed the cells derived from FDB muscles from young-adult (3-month), middle-aged (12-month), and old (24-month) FVB mice (Fig. 1D–F). Because Tuj1+ neural progenitor cells exhibiting neural morphology are not present in muscle cross sections [17], these cells were identified by Tuj1 immunostaining after 7 days in culture [17]. The number of cells was expressed per weight of starting muscle tissue (Fig. 1D) and number of nuclei (Fig. 1E). To reduce the variation associated with enzymatic preparation, samples from different age groups were processed in parallel (n = 3). The number of skeletal muscle-derived Tuj1+ cells/mg of tissue in young-adult, middle-aged, and old mice was 20,033±7350, 14,736±2600, and 11,393±2605 cells/mg of tissue, respectively (Fig. 1D), which corresponds to 5.2±1.1, 4.3±0.8, and 4.1±0.6% of Tuj1+ cells/nuclei, respectively (Fig. 1E). Although the number tended to decline with aging, we observed no statistically significant difference in the cells’ yield across ages (Fig. 1D and E).
DsRed-Nestin-Intron II plasmid helps to identify neural progenitors in skeletal muscle cultures from various wild-type species
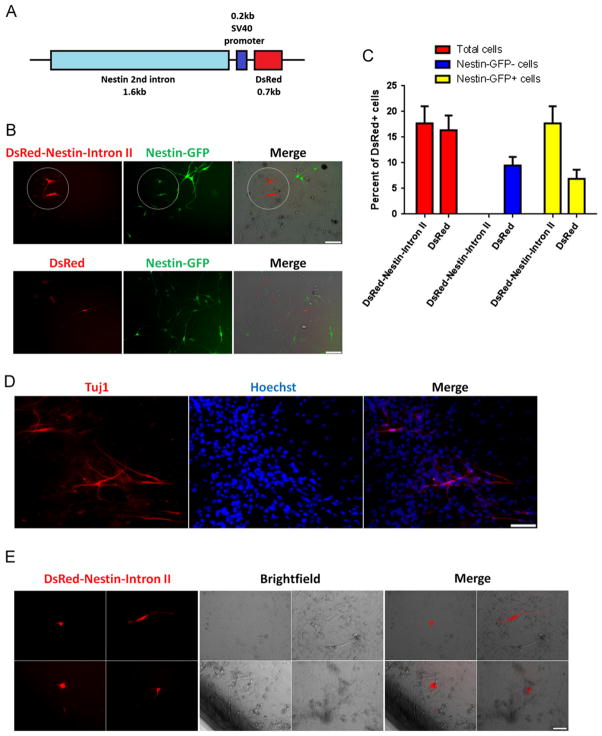
We constructed a DsRed-Nestin-Intron II plasmid to identify neural progenitors in myogenic cultures from nontransgenic mice (Fig. 2A). To test its specificity, we transfected it into 7 day-old FDB cultures derived from Nestin-GFP mice and found that all DsRed+ cells were also Nestin-GFP+ (17.7±3.3% of total cells) (Fig. 2B and C), meaning that the plasmid mimics Nestin-GFP expression in muscle-derived neural progenitors. However, not all Nestin-GFP+ cells were DsRed+, even after transfected with a DsRed plasmid, which is explained by the suboptimal transfection efficiency (Fig. 2B and C).
Fig. 2.
The DsRed-Nestin-Intron II plasmid is useful in identifying neural progenitor cells in wild-type species. (A) DsRed-Nestin-Intron II plasmid construct. DsRed was placed under control of the 1.6 kb second Intron Nestin gene. (B) Muscle-derived Nestin-GFP+ progenitor cells after DsRed-Nestin-Intron II or DsRed plasmid transfection at day 7 in culture. DsRed-Nestin-Intron II overlaps with GFP fluorescence (circle). (C) Total percent of cells transfected with either DsRed-Nestin-Intron II or DsRed plasmids (red), percent of Nestin-GFP− cells (blue), and Nestin-GFP+ progenitor cells (yellow) expressing DsRed. All DsRed-Nestin-Intron II cells overlap with Nestin-GFP+ progenitor cells. No Nestin-GFP− cells expressing DsRed-Nestin-Intron II were found, while both Nestin-GFP− and Nestin-GFP+ cells express the DsRed plasmid. (D) Tuj1+ neural progenitors derived from the vastus lateralis muscle of African vervet monkeys cultured for 10 days under conditions similar to those described for muscles from Nestin-GFP transgenic mice (see the Methods section). (E) Vastus lateralis muscle-derived cells from a monkey biopsy cultured for 10 days, transfected with DsRed-Nestin-Intron II plasmid, and imaged 2 days later (n = 3 preparations). ((B), (D), and (E)) Scale bar = 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To investigate whether neural progenitors can be extracted from the skeletal muscle of different species, we dissociated and cultured skeletal muscle biopsies from African Green vervet monkeys. After 10 days, we found that 3.3±1.1% of the cells were Tuj1+ with long, thin processes, similar to those of Nestin-GFP+ progenitor cells (Fig. 2D), which corresponds to 18660 Tuj1+ cells/mg (n = 4). To identify these cells in monkey skeletal muscle culture, we transfected it with the DsRed-Nestin-Intron II plasmid and found that all DsRed+ cells exhibited neural morphology similarly to Nestin-GFP+ progenitor cells (Fig. 2E) (n = 3 preparations from three monkeys) and co-expressed Tuj1 (data not shown).
Nestin-GFP+ progenitor cells differ from myoblasts, Schwann cells, endothelial cells, macrophages/microglia, pericytes, smooth muscle cells, and fibroblasts in the skeletal muscle cultures
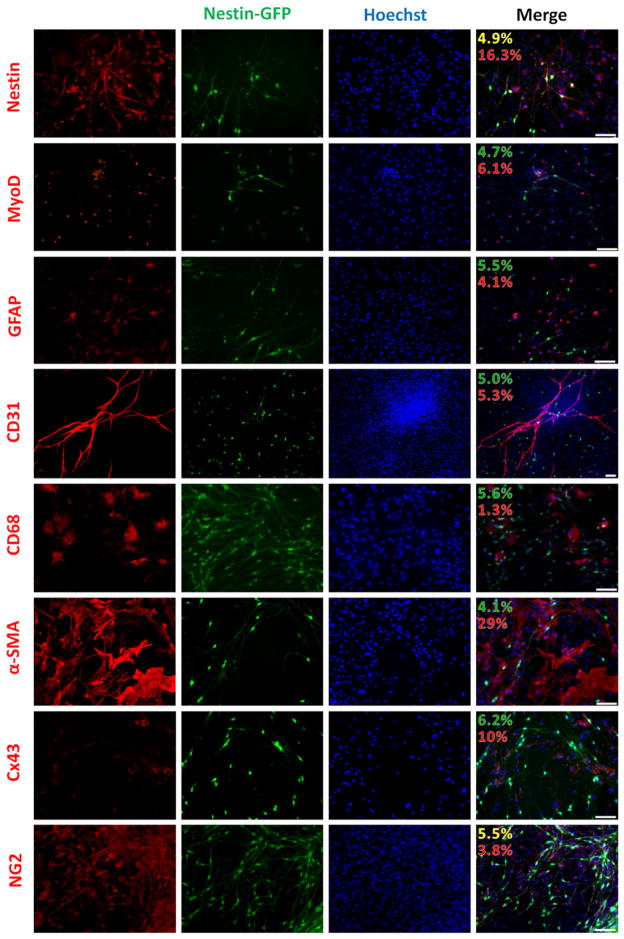
Nestin-GFP+ progenitor cells are obtained from a pool of skeletal muscle interstitial cells with poorly understood properties [17]. To define their relationship to different mesenchymal cells in the skeletal muscle interstitium, we examined their marker-expression profile, using FDB culture from Nestin-GFP mice. After 7 days in culture, two populations of cells expressing Nestin protein were detected, GFP+ (4.9±1.0%) and GFP− (16.3±3.0%) (Fig. 3). Notice that all GFP− cells expressing nestin protein were negative for Tuj1 [17].
Fig. 3.
FDB-derived Nestin-GFP+ progenitor cells, cultured for 7 days, do not exhibit markers of myoblasts, Schwann cells, endothelial cells, macrophage/microglia, or pericytes. Immunostaining of Nestin-GFP mouse FDB-derived cells fixed at day 7 in culture. (A) Nestin antibody (Rat 401) stained all nestin-GFP+ progenitor cells as well as a population that expressed nestin protein but not GFP. Antibodies against MyoD, GFAP, CD31, CD68, α-SMA, and Connexin-43 were used but did not overlap with Nestin-GFP+ progenitor cells. NG2, a marker of brain NG2-glia, was found in both pericytes and Nestin-GFP+ progenitor cells (n = 4 preparations). Nuclei were stained with Hoechst. The percentage of cells expressing various markers in Nestin-GFP+ (yellow) or Nestin-GFP− (red) cells is shown in the merge panel. The percentage of Nestin-GFP+ cells that did not express the marker is in green. Note that Nestin-GFP+ cells differ from pericytes (α-SMA+/Connexin43+), but express NG2 proteoglycan, which is also expressed in pericytes (Suppl. Fig. 1). Scale bar = 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Nestin protein expression has been reported in several tissues and cell types [38,43–64]. This relatively wide spectrum of nestin expression reflects the presence of various regulatory elements in the nestin gene. GFP may not be detected in some cells displaying Nestin protein because the Nestin-GFP transgenic mice were designed using only the second intron of the nestin gene, which is known to drive expression in neural stem and progenitor cells [28,38]. The first and second introns of the nestin gene consistently direct reporter gene expression to developing muscle or neural precursors, respectively [38]. Therefore, some muscle cells may express nestin protein but not GFP. The 5 kb promoter region is also included in the transgene construct, although its regulatory function is still unclear [65].
We found a way to distinguish Nestin-GFP+ progenitor cells from the other cells in culture. They did not exhibit markers of myoblast (MyoD), Schwann (GFAP), endothelial (CD31), or microglia/macrophage (CD68). Positive cells to these markers represented: MyoD, 6.1±2.5%; GFAP: 4.1±1.3%; CD31: 5.3±1.8%; and CD68: 1.3±0.4%, respectively (Fig. 3). The culture conditions used here may not favor myogenic growth, even though previous studies claim that they support both proliferation and differentiation of myogenic cells [34]. The paucity of MyoD+ nuclei (~6%) and absence of Pax7+ cells after 7 days in our cultures (data not shown) support this conclusion. Cultured cells may secrete growth factors and cytokines, such as TGF-β, VEGF, PDGF-BB, and BMP [66], that inhibit or decrease myogenesis.
Their morphological properties – small cytoplasm and thin, multipolar extensions – differ from those of fibroblastoid pericytes [67]. They were negative to the pericyte markers connexin 43 (Cx43) and α-SMA (Fig. 3 and Suppl. Fig. 1). Pericytes expressing Cx43 represented 10±2.0% of cells in culture. Note that the Cx43 antibody used here also stained fibroblasts and/or myocytes [68]. The α-SMA antibody stained vascular smooth muscle cells in addition to pericytes; thus, the total number of these two cell types accounted for 29±5.8% (Fig. 3 and Suppl. Fig. 1). Although Nestin-GFP+ progenitor cells shared nerve/glia antigen-2 (NG2) proteogly-can expression with pericytes [69] (Fig. 3 and Suppl. Fig. 1), NG2 is also expressed on NG2-glia cells’ surface [70]. NG2-glia cells function as neural progenitors in the CNS [71].
Nestin-GFP+ progenitor cells express markers of NG2-glia cells
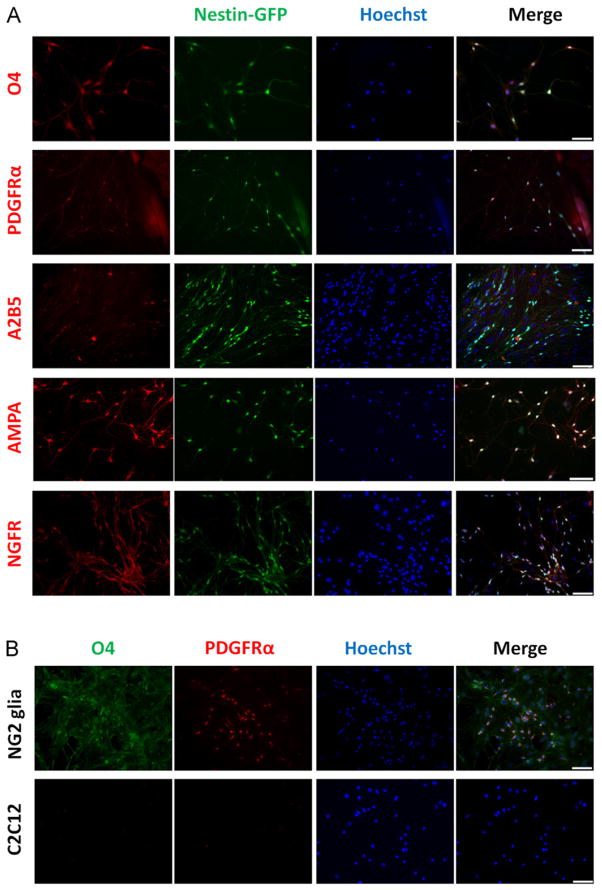
Because neural progenitor cells derived from skeletal muscle differ from pericytes (α-SMA-/Cx43-) and express NG2 proteo-glycan, we tested for the expression of other typical markers of NG2-glia cells in Nestin-GFP+ progenitor cells. All Nestin-GFP+ cells expressed NG2-glia markers, such as oligodendrocyte antigen O4 [72], platelet-derived growth factor receptor α subunit (PDGFRα) [73], cell-surface ganglioside A2B5 [74], glutamate receptor 1 (AMPA receptor) [75], and NGF receptor (p75) [76] (Fig. 4A and Suppl. Fig. 2). While no immunostaining was observed in a myoblast culture used as a negative control, O4 and PDGFRα immunostaining was positive in primary NG2-glia progenitors derived from neurospheres (Fig. 4B). To confirm our results, we performed RT-PCR analysis of Nestin-GFP+ cells using NG2-glia progenitors and myoblasts as controls and examined the expression of Nestin, NG2, PDGFRα, AMPA, NGFR, and MyoD (n = 3). We found that, like NG2-glia progenitors, Nestin-GFP+ progenitor cells expressed all these markers except for MyoD (Suppl. Fig. 2).
Fig. 4.
FDB-derived Nestin-GFP+ progenitor cells exhibit NG2-glia markers. FDB-derived Nestin-GFP+ cells sorted after day 7 in culture were plated and fixed after 4 days. (A) The first column shows immunostaining for O4, PDGFRα, A2B5, glutamate receptor-1 (AMPA), and NGF receptor (p75), while the second and third show their corresponding Nestin-GFP and Hoechst nuclear fluorescence (n = 4 preparations). (B) Neurosphere-derived NG2-glia cells, cultured in differentiation medium, and C2C12 myoblasts were used as positive and negative controls, respectively, for O4 and PDGFRα antibodies. Scale bar = 100 μm.
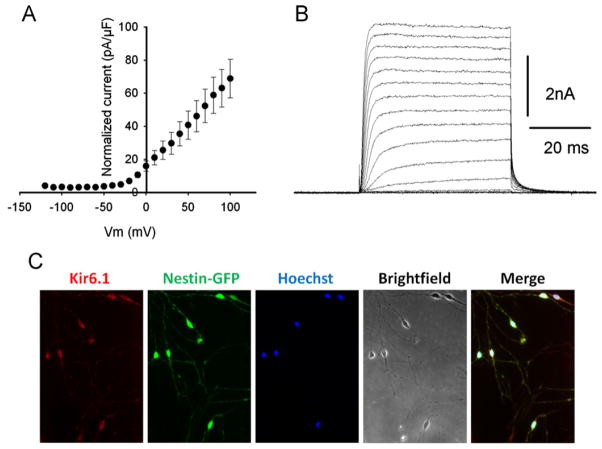
Skeletal muscle-derived neural progenitor cells exhibit K+ outward currents
To determine whether Nestin-GFP+ progenitor cells derived from adult skeletal muscle are excitable, we performed patch-clamp recordings to look for K+ and Na+ currents. Cells were sorted from 7-day-old FDB cultures and grown for 4 days. We observed K+ outward currents in both neurosphere-derived neurons and Nestin-GFP+ progenitor cells (n = 35) (Fig. 5A and B), but Na+ inward current was detected only in the neurosphere-derived neurons (n = 18) (data not shown). Nestin-GFP+ progenitor cells were unable to fire action potentials (n = 17) (data not shown), and, like brain NG2-glia cells (oligodendrocyte progenitors) [74], they expressed the inward rectifier Kir6.1 potassium channel (Fig. 5C), which peripheral pericytes, characterized by PDGFRβ, Desmin, α-SMA, and NG2+ expression, did not [77].
Fig. 5.
Nestin-GFP+ progenitor cells express Kir6.1 potassium channels and inward rectifying potassium currents. Nestin-GFP+ progenitor cells were sorted from Nestin-GFP mouse FDB cells cultured for 7 days and grown in laminin coated dishes for 4 days. K+ currents: voltage relationship (A) and illustration of a typical set (B). Currents normalized to membrane capacity were elicited by a series of command pulses between −120 and +100 mV in 10 mV increments from a holding potential of −80 mV. Capacitative and leakage currents were subtracted off-line. (C) FDB-derived Nestin-GFP+ progenitor cells were cultured for 7 days, sorted, grown for another 4 days, fixed, and immunostained for Kir6.1.
Nestin-GFP+ progenitor cells are committed to the neural lineage and do not differentiate into muscle cells in vitro
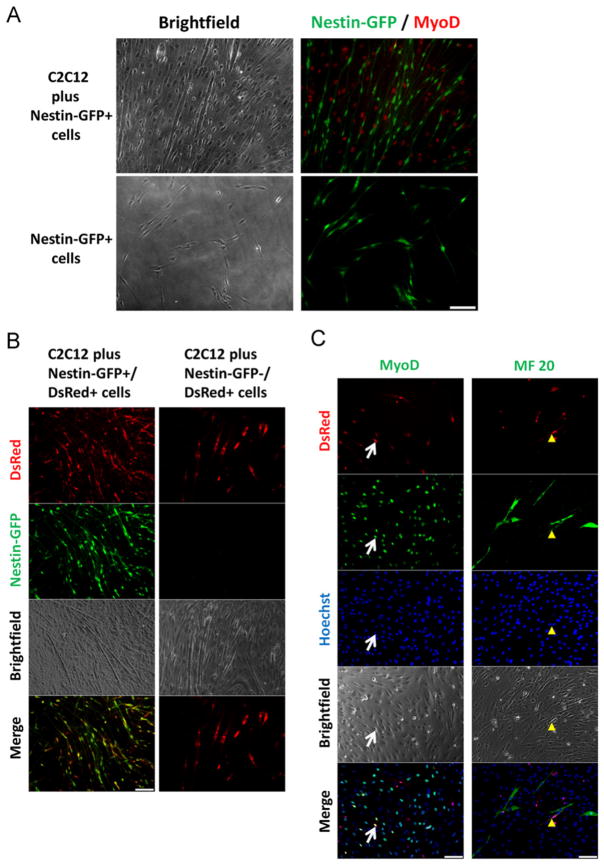
Here we examined the potential differentiation of Nestin-GFP+ cells into a myogenic lineage by co-culturing them with C2C12 myoblasts based on the myogenic inductive potency of this muscle cell line [66]. To test whether Nestin-GFP+ cells are committed to neural lineage or can transdifferentiate into muscle cells as reported for pericytes [66,78,79], we cultured Nestin-GFP+ progenitor cells sorted from 7-day-old FDB cultures derived from Nestin-GFP mice in myogenic differentiation medium or with C2C12 myoblasts. We observed that after 7 days in differentiation medium, they retain their neural morphology and Nestin-GFP expression and do not differentiate into muscle cells, even in co-culture with myoblasts, which form MyoD+ myotubes (Fig. 6A) (n = 3 preparations for only Nestin-GFP+ progenitor cells or Nestin-GFP+ progenitor cells plus myoblasts).
Fig. 6.
Nestin-GFP+ progenitor cells are committed to the neural lineage and do not differentiate into muscle cells in vitro. (A) Nestin-GFP+ progenitor cells were sorted from 7 day-old FDB cultures from Nestin-GFP mice, co-cultured with C2C12 cells for 3 days in proliferation medium, and followed for 7 days in differentiation medium. Immunocytochemical analysis revealed that Nestin-GFP+ progenitor cells sustain their GFP expression but do not express MyoD (visualized by Alexa 568 fluorescence red) when cultured alone in myogenic differentiation medium or co-cultured with C2C12 myoblasts (n = 3 preparations). (B) FDB cultures from Nestin-GFP/β-actin-DsRed mice, in which all the cells express DsRed fluorescence, were cultured for 7 days. Nestin-GFP+/DsRed+ and Nestin-GFP−/DsRed+ cells were sorted and co-cultured with C2C12 cells. Nestin-GFP−/DsRed+ cells but not Nestin-GFP+/DsRed+ fused with C2C12 cells in which some DsRed+ myotubes were detected (n = 3 preparations). (C) Nestin-GFP−/DsRed+ cells were co-cultured with C2C12 myoblasts. DsRed+, MyoD+, and MF 20+ cells were detected. A Nestin-GFP−/ DsRed+ cell co-expressing MyoD is indicated with a white arrow, and a MF 20+ myotube derived from a Nestin-GFP−/DsRed+ cell with a yellow arrow (n = 3 preparations). (A)–(C) Scale bars = 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To prove that, in contrast to Nestin-GFP− cells (i.e., pericytes) [78–80], Nestin-GFP+ progenitor cells sorted from FDB cultures after 7 days are committed to the neural lineage, we used Nestin-GFP/β-actin-DsRed transgenic mice. All the cells of these mice express DsRed fluorescent protein, enabling us to track their fate. Nestin-GFP+/β-actin-DsRed+ progenitor cells and Nestin-GFP−/ β-actin-DsRed+ cells were sorted from 7-day-old FDB cultures and cultured with C2C12 myoblasts to test their myogenic capability. After 7 days in differentiation medium, all the Nestin-GFP+ progenitor cells retained their neural morphology and co-expressed DsRed. No DsRed+ cells were detected beside the Nestin-GFP+ cells (Fig. 6B). All myotubes were DsRed-, which means that they do not derive from Nestin-GFP+ progenitor cells (Fig. 6B). In contrast, Nestin-GFP− cells fused with myoblasts to form cells similar to myotubes DsRed+ (Fig. 6B). To confirm that the DsRed+ cells were myotubes and that some of the Nestin-GFP− cells retained their myogenic capacity in vitro, we analyzed the expression of muscle lineage markers (MyoD and MF 20) in the co-culture between Nestin-GFP−/β-actin-DsRed+ and C2C12 myoblasts. We found that, after 7 days in differentiation medium, many DsRed+ cells expressed MyoD, and some MF 20+ myotubes DsRed+ were detected (Fig. 6C). These experiments support our hypothesis that Nestin-GFP+ progenitor cells are predifferentiated for neural lineage [17] and unlikely to participate in muscle formation, distinguishing them from such other cell types as pericytes [78–80] and satellite cells [81] present in skeletal muscle-derived cultures.
Brain-injected Nestin-GFP+ progenitor cells migrate toward the neurogenic subventricular zone (SVZ) in adult mice
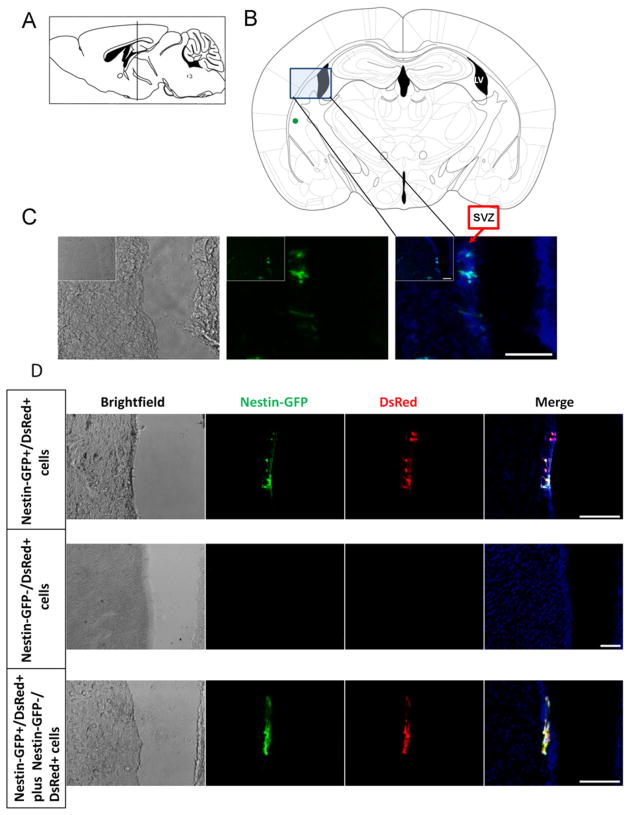
Because multipotent NG2+ cells have been identified in the subventricular zone (SVZ) of early postnatal and adult animals [71,82,83] and can migrate long distances [84], we examined whether Nestin-GFP+ progenitor cells retain neural progenitor characteristics and migrate toward neurogenic areas. Injected into the striatum of 3-month-old mice (50,000 cells/5 μl), Nestin-GFP+ progenitor cells (n = 2) were detected in the SVZ two weeks later (Fig. 7A–C; Suppl. Figs. 3 and 4A) similarly to brain-derived NG2-DsRed progenitors (Suppl. Fig. 4B). To demonstrate that this migration is specific, we used the Nestin-GFP/β-actin-DsRed transgenic mice described above. Nestin-GFP+/β-actin-DsRed+ progenitor cells (n = 2) or Nestin-GFP−/β-actin-DsRed+ cells (n = 2) were sorted from 7-day-old FDB cultures and injected (50,000 cells/5 μl) into the same location for Nestin-GFP+ progenitor cells (Fig. 7A, B, and D). To test the viability of these cells after sorting, Nestin-GFP+/β-actin-DsRed+ progenitor cells and Nestin-GFP−/β-actin-DsRed+ cells were cultured for 7 days; viable cells were observed in vitro for both populations (data not shown). Two weeks after injection, Nestin-GFP+/β-actin-DsRed+ progenitor cells, but no Nestin-GFP−/β-actin-DsRed+ cells, were detected in the SVZ (Fig. 7A, B, and D). Nestin-GFP−/ β-actin-DsRed+ cells remained at the place of injection (data not shown). To confirm these results, a mixture of Nestin-GFP+/ β-actin-DsRed+ progenitor cells and Nestin-GFP−/β-actin-DsRed+ cells was injected in the striatum (n = 2). After 2 weeks, only Nestin-GFP+/β-actin-DsRed+ progenitor cells were detected in the SVZ; no Nestin-GFP−/β-actin-DsRed+ cells were detected lining the lateral ventricle (Fig. 7A, B, and D). This migration route, similar to that reported for a subpopulation of neural progenitors in the striatum [85], supports the inherent capacity of Nestin-GFP+ progenitor cells to integrate with neural precursors in the brain [85]. Whether these cells engraft and form part of a neuronal circuit remains unknown.
Fig. 7.
Sorted Nestin-GFP+ progenitor cells survive and migrate in the adult mouse striatum two weeks after stereotactic injection. Mouse brain sagittal (A) and coronal (B) sections (modified from George Paxinos and Keith B.J. Franklin, 2001, with permission) illustrating where cells were injected (green dot). (A) The vertical line indicates the location of the coronal section, 1.5 mm caudal to the bregma. (B) The region selected in the shaded box and outlined in blue shows the area magnified in images (C) and (D). (C) Representative brain coronal section from a 3-month-old mouse showing Nestin-GFP+ progenitor cells 2 weeks postinjection (n = 2 mice). Lower magnification (white box) of the same area shows the route followed by Nestin-GFP+ progenitor cells. Note that they migrate medially approximately 600 μm from the core of the injection site toward the subventricular zone (SVZ) and seem to line the lateral ventricle (LV). Merged images of GFP fluorescence with Hoechst 33342 are shown. (D) Sorted Nestin-GFP+/DsRed+ and Nestin-GFP−/DsRed+ cells were injected into the striatum. Representative brain coronal section from a 3-month-old mouse showing DsRed+ cells in the SVZ lining the lateral ventricle 2 weeks postinjection (n = 2 mice). Nestin-GFP+/DsRed+ but not Nestin-GFP−/DsRed+ cells migrate toward the SVZ. After injecting Nestin-GFP+/ DsRed+ and Nestin-GFP−/DsRed+ together, all red cells in the SVZ were Nestin-GFP+ 2 weeks later, suggesting that only the Nestin-GFP+ neural progenitor cells migrate toward the SVZ. Left panels are brightfield images, while the second and third columns show GFP and DsRed fluorescence, respectively. Nuclei were stained with Hoechst. Merged images are shown in the far right column. (C) and (D) Scale bars = 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Nestin-GFP+ progenitor cells do not form tumors
A previous report indicates that NG2-glia is a source of tumors [86]. To examine their tumorigenicity, we subcutaneously injected 1 × 106 Nestin-GFP+ cells in 100 μl of PBS into the flanks of immunodeficient mice (n = 4) and monitored them every other day by visual inspection and palpation. Six weeks after cell injection, we did not detect tumor formation in any of the animals. Similarly, intracranial xenografting of these cells in the striatum did not form tumors 2 months after injection (data not shown), supporting the concept that Nestin-GFP+ cells can be used in regenerative therapy.
Discussion
Our previous work shows that skeletal muscle interstitial Nestin-GFP+ cells form neural progenitors and their differentiation depends on soluble factors from the in vitro niche created by skeletal muscle derived cells. After isolation, neural progenitors proliferate [17]. Here, we studied more in depth the characteristics of these progenitor cells.
Nestin-GFP+ cells share NG2-glia cell characteristics
Nestin-GFP+ neural progenitor cells express NG2 proteoglycan, a known pericyte marker [69], but, unlike pericytes, they are committed to neural lineage and do not differentiate into muscle as do pericytes purified from skeletal muscle [78–80] or aorta [66]. NG2 has also been identified in resident glial progenitors [87], which have neural progenitor activity after brain injury [88,89].
We report here that Nestin-GFP+ progenitor cells express other NG2-glia markers, such as O4 [72], PDGFRα [73], A2B5 [74], NGF receptor (p75) [76], and glutamate receptor 1 (AMPA) [75]. The expression of glutamate receptor 1(AMPA) agrees with our previous data [17], showing that Nestin-GFP+ progenitor cells respond to repeated glutamate challenges and, like AMPA receptors in the CNS, are desensitized during prolonged glutamate application [90](Table 3). All these surface markers are useful in sorting Nestin-GFP+ progenitor cells from a mixed cell population (Fig. 8).
Table 3.
Comparative properties of NG2-glia and skeletal muscle-derived Nestin-GFP+ progenitor cells.
| Characteristics | NG2-glia cells | Nestin-GFP+ cells |
|---|---|---|
| Nestin expression | Positive [97] | Positive* |
| NG2 expression | Positive [32] | Positive* |
| O4 expression | Positive [72] | Positive* |
| NGF receptor (p75) expression | Positive [76] | Positive* |
| Glutamate receptor 1 (AMPA) expression | Positive [75] | Positive* |
| A2B5 expression | Positive [74] | Positive* |
| Kir6.1 expression | Positive [74] | Positive* |
| Significant proliferation | Yes | Yes [17] |
| potential (replicative capacity) | [26,70,93,102] | |
| Response to neurotransmitters | Yes [103] | Yes [17] |
| Neurosphere-forming cells | Yes [82] | Yes [17] |
| Migrate long distance in the brain | Yes [32,104,105] | Yes * |
Present paper.
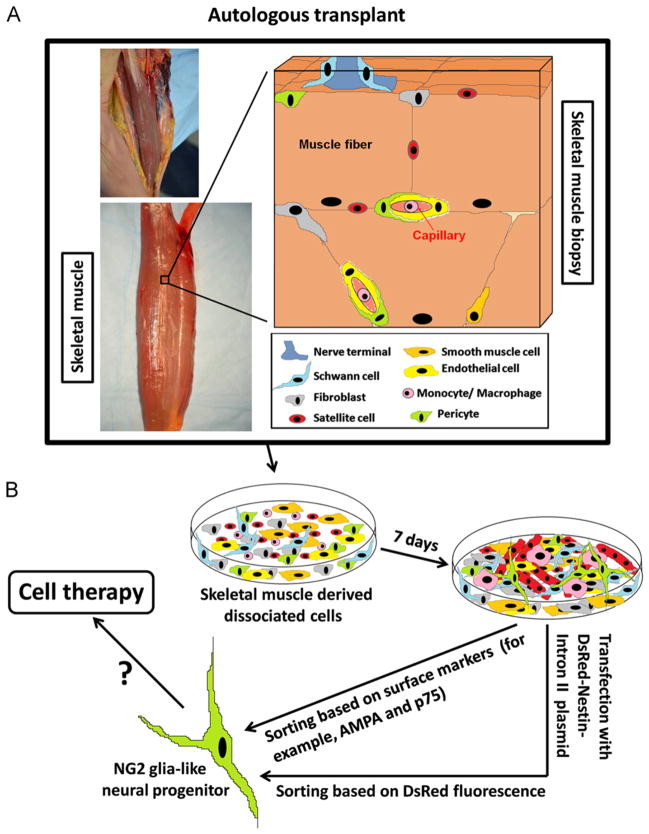
Fig. 8.
Diagram representing the origin of Nestin-GFP+ progenitor cells derived from a skeletal muscle biopsy. (A) The skeletal muscle consists of bundles of multinucleated myofibers, each carrying a population of satellite cells. Various mesenchymal cells are present in the muscle interstitium: fibroblasts (gray), smooth muscle cells (orange), endothelial cells (yellow), and pericytes (green), which emit processes along and around capillaries. The communication between the motor axon terminal (dark blue) and muscle fiber (neuromuscular junction) is represented. Schwann cells associated with the nerve terminal are shown (light blue). (B) Various cell types are represented in enzymatically dissociated muscles cultured for 7 days: fibroblasts (gray), smooth muscle cells (orange), endothelial cells (yellow), macrophages (pink) derived from circulating monocytes, Schwann cells (light blue), pericytes (green), multinucleated myotubes (red) and myoblasts (red) derived from satellite cells (red), and a small population of neural progenitor cells (NG2-glia like cells) (green) derived from GFP+ cells (green). Surface markers or use of DsRed-Nestin-Intron II plasmid (Fig. 2) allow neural progenitor cell sorting and eventual application for cell therapy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We recorded inward rectifying potassium but not sodium currents in muscle-derived neural cells, as reported for some brain NG2-glia cells [91]. The potassium currents correspond with Kir6.1 immunodetection. This channel expression is limited to brain pericytes [77], astrocytes [92], and NG2-glia cells [74] but absent in pericytes located outside the CNS [77]. NG2-glia cells are thought to be present in the postnatal SVZ [93], a neurogenic area in adult animals [94]. When we injected Nestin-GFP+ progenitor cells into the undamaged brains of adult mice, they survived and migrated through the CNS, like NG2-glia cells [84], ending at the SVZ. We also found that Nestin-GFP+ progenitor cells proliferated and formed neurospheres in vitro [17](Table 3). Taken together, these data suggest that Nestin-GFP+ cells may be multipotent neural progenitors, as suggested for NG2-glia cells in the brain [27].
Previous studies have proposed that immature neural cells derived from tissue stem cells may express markers characteristic of both neurons and glia [83]. We found cells co-expressing markers of immature neurons (Tuj1) [17] and NG2-glia (such as O4, PDGFRα, A2B5, glutamate receptor 1-AMPA, and NGF receptor (p75)) derived from skeletal muscle interstitial cells (Fig. 8).
Skeletal muscle is the most enriched source of Nestin-GFP+ progenitor cells
Despite the fact that Nestin-GFP+ progenitor cells can be isolated from different sources, they are more abundant in skeletal muscle than adipose tissue and bone marrow. This finding agrees with previous data showing that muscle-derived cells have a higher rate of positive NGF receptors than any other peripheral tissue [95].
A major risk factor for most neurodegenerative disorders [96] is the continuous reduction in the number of brain neural progenitors [97]. As the number of neural progenitors from skeletal muscle does not decline significantly with age, they can be used to regenerate the nervous system throughout life.
Given their therapeutic potential, we wanted to find a tool that would enable identification of neural progenitor cells derived from skeletal muscle in nontransgenic animals. We developed a DsRed-Nestin-Intron II plasmid to identify them in skeletal muscle cultures from nontransgenic mice or any other species in vitro. While autologous skeletal muscle could be an important source for cell therapy for neurological diseases (Fig. 8), the potential transplantation capacity, engraftment, and differentiation potential in specific neuronal subtypes of Nestin-GFP+ progenitor cells must be better defined.
Are Nestin-GFP+/NG2+ cells neural progenitors?
Their expression of specific markers indicates that Nestin-GFP+/ NG2+ cells are predifferentiated into neural lineage [17]; our results also show that they differ from pericytes, as they cannot differentiate into other lineages. However, we cannot rule out the possibility that they can revert to a multipotent state under certain conditions. Previous studies suggest that NG2-glia generate mature neurons, astrocytes, and oligodendrocytes and are a resource for remediating degenerative diseases involving both neurons and glia [71,93,98–101]. Further research is needed to understand the molecular and cellular mechanisms that regulate their lineage restriction and plasticity.
Conclusion
This study establishes that skeletal muscle-derived Nestin-GFP+ neural progenitor cells share some properties with NG2-glia cells, are committed to neural lineage, and can be efficiently isolated from the skeletal muscle of various species, including nonhuman primates and aging mice, suggesting that autologous transplantation may be a potential application for central nervous system therapy.
Supplementary Material
Acknowledgments
The present study was supported by a PUSH grant from the Wake Forest Comprehensive Cancer Center to Drs. Akiva Mintz and Osvaldo Delbono, grants from the National Institutes of Health/ National Institute on Aging (AG13934 and AG15820) to Dr. Osvaldo Delbono, the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the National Institute of Aging (R01AG040209), National Institute of Mental Health (R01MH092928) and NYSTEM to Dr. Grigori N. Enikolopov. We thank Dr. James Wood of the Comprehensive Cancer Center of Wake Forest University School of Medicine (WFUSM) for his expert support on flow cytometry, and Dr. Xin Feng of the WFUSM Department of Otolaryngology for providing monkey muscle samples, and Dr. W. Stallcup (Sanford-Burnham Medical Research Institute, CA), for kindly sharing the PDGFRα antibody with us.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.yexcr.2012.09.008.
Footnotes
Disclaimer: Authors declare no conflict of interest exists.
References
- 1.Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6:252–273. [PubMed] [Google Scholar]
- 2.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004;364:1872–1883. doi: 10.1016/S0140-6736(04)17443-6. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. doi: 10.1016/j.expneurol.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Silva AT, Wardhaugh T, Dolatshad NF, Jones S, Saffrey MJ. Neural progenitors from isolated postnatal rat myenteric ganglia: expansion as neurospheres and differentiation in vitro. Brain Res. 2008;1218:47–53. doi: 10.1016/j.brainres.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 6.Yap EC. Myofascial pain—an overview. Ann Acad Med Singapore. 2007;36:43–48. [PubMed] [Google Scholar]
- 7.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zammit PS. All muscle satellite cells are equal, but are some more equal than others? J Cell Sci. 2008;121:2975–2982. doi: 10.1242/jcs.019661. [DOI] [PubMed] [Google Scholar]
- 9.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, Robbins P, Fu FH, Huard J. Effect of bone morpho-genetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A:1032–1039. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Goldring MB. Are bone morphogenetic proteins effective inducers of cartilage repair? Ex vivo transduction of muscle-derived stem cells. Arthritis Rheum. 2006;54:387–389. doi: 10.1002/art.21756. [DOI] [PubMed] [Google Scholar]
- 12.Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22–26. [PubMed] [Google Scholar]
- 13.Kondo T, Case J, Srour EF, Hashino E. Skeletal muscle-derived progenitor cells exhibit neural competence. Neuroreport. 2006;17:1–4. doi: 10.1097/01.wnr.0000192732.00535.ff. [DOI] [PubMed] [Google Scholar]
- 14.Arsic N, Mamaeva D, Lamb NJ, Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266–1280. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Schultz SS, Lucas PA. Human stem cells isolated from adult skeletal muscle differentiate into neural phenotypes. J Neurosci Methods. 2006;152:144–155. doi: 10.1016/j.jneumeth.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Ramos M, Vourc’h P, Young HE, Lucas PA, Wu Y, Chivatakarn O, Zaman R, Dunkelman N, el-Kalay MA, Chesselet MF. Neuronal differentiation of stem cells isolated from adult muscle. J Neurosci Res. 2002;69:894–907. doi: 10.1002/jnr.10374. [DOI] [PubMed] [Google Scholar]
- 17.Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One. 2011;6:e16816. doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 22.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Nat Acad Sci USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama A, Yang L, Itoh S, Mori K, Tanaka J. Microglia, a potential source of neurons, astrocytes, and oligodendrocytes. Glia. 2004;45:96–104. doi: 10.1002/glia.10306. [DOI] [PubMed] [Google Scholar]
- 24.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 27.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 29.Renganathan M, Messi ML, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem. 1998;273:28845–28851. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- 30.Payne AM, Zheng Z, Gonzalez E, Wang ZM, Messi ML, Delbono O. External Ca(2+)-dependent excitation–contraction coupling in a population of ageing mouse skeletal muscle fibres. J Physiol. 2004;560:137–155. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor JR, Zheng Z, Wang ZM, Payne AM, Messi ML, Delbono O. Increased CaVbeta1A expression with aging contributes to skeletal muscle weakness. Aging Cell. 2009;8:584–594. doi: 10.1111/j.1474-9726.2009.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 33.Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, Nagy A. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 34.Zammit PS, Carvajal JJ, Golding JP, Morgan JE, Summerbell D, Zolnerciks J, Partridge TA, Rigby PW, Beauchamp JR. Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol. 2004;273:454–465. doi: 10.1016/j.ydbio.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 35.Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell) Dev Biol. 2002;251:221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- 36.Zhang T, Birbrair A, Wang ZM, Taylor J, Messi ML, Delbono O. Troponin T nuclear localization and its role in aging skeletal muscle. Age (Dordr) 2011 doi: 10.1007/s11357-011-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong H, Jin Z, Chen Y, Zhang T, Bian W, Cui X, Jing N. First intron of nestin gene regulates its expression during C2C12 myoblast differentiation. Acta Biochim Biophys Sin (Shanghai) 2008;40:526–532. doi: 10.1111/j.1745-7270.2008.00428.x. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 39.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 40.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 42.Erceg S, Lainez S, Ronaghi M, Stojkovic P, Perez-Arago MA, Moreno-Manzano V, Moreno-Palanques R, Planells-Cases R, Stojkovic M. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One. 2008;3:e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 44.Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106(Pt 4):1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- 45.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kachinsky AM, Dominov JA, Miller JB. Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. J Histochem Cytochem. 1995;43:843–847. doi: 10.1177/43.8.7542682. [DOI] [PubMed] [Google Scholar]
- 47.Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111(Pt 14):1951–1961. doi: 10.1242/jcs.111.14.1951. [DOI] [PubMed] [Google Scholar]
- 48.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–249. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- 49.Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947–956. [PubMed] [Google Scholar]
- 50.Mokry J, Nemecek S. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biol (Praha) 1998;44:155–161. [PubMed] [Google Scholar]
- 51.Lardon J, Rooman I, Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol. 2002;117:535–540. doi: 10.1007/s00418-002-0412-4. [DOI] [PubMed] [Google Scholar]
- 52.Treutelaar MK, Skidmore JM, Dias-Leme CL, Hara M, Zhang L, Simeone D, Martin DM, Burant CF. Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes. 2003;52:2503–2512. doi: 10.2337/diabetes.52.10.2503. [DOI] [PubMed] [Google Scholar]
- 53.Delacour A, Nepote V, Trumpp A, Herrera PL. Nestin expression in pancreatic exocrine cell lineages. Mech Dev. 2004;121:3–14. doi: 10.1016/j.mod.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15–25. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Mokry J, Nemecek S. Immunohistochemical detection of intermediate filament nestin. Acta Med (Hradec Kralove) 1998;41:73–80. [PubMed] [Google Scholar]
- 56.Walcott JC, Provis JM. Muller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Exp Ophthalmol. 2003;31:246–249. doi: 10.1046/j.1442-9071.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 57.Sun XY, An J. Expression of nestin, an intermediate filament protein, in human fetal hepatic stem cells. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:207–209. [PubMed] [Google Scholar]
- 58.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 59.Hunziker E, Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem Biophys Res Commun. 2000;271:116–119. doi: 10.1006/bbrc.2000.2611. [DOI] [PubMed] [Google Scholar]
- 60.Friedman B, Zaremba S, Hockfield S. Monoclonal antibody rat 401 recognizes Schwann cells in mature and developing peripheral nerve. J Comp Neurol. 1990;295:43–51. doi: 10.1002/cne.902950105. [DOI] [PubMed] [Google Scholar]
- 61.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 62.Gallo V, Armstrong RC. Developmental and growth factor-induced regulation of nestin in oligodendrocyte lineage cells. J Neurosci. 1995;15:394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- 65.Yamaguchi M. Analysis of neurogenesis using transgenic mice expressing GFP with nestin gene regulatory regions. Chem Senses. 2005;30(Suppl 1):i117–118. doi: 10.1093/chemse/bjh142. [DOI] [PubMed] [Google Scholar]
- 66.Ugarte G, Cappellari O, Perani L, Pistocchi A, Cossu G. Noggin recruits mesoderm progenitors from the dorsal aorta to a skeletal myogenic fate. Dev Biol. 2012;365:91–100. doi: 10.1016/j.ydbio.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, Yamada KA. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 70.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- 71.Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 73.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 74.Fogal B, McClaskey C, Yan S, Yan H, Rivkees SA. Diazoxide promotes oligodendrocyte precursor cell proliferation and myelination. PLoS One. 2010;5:e10906. doi: 10.1371/journal.pone.0010906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 78.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 79.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 81.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 82.Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 84.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 85.Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23:461–472. doi: 10.1016/s0896-6273(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 86.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754–768. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- 90.Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, Veh RW. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol Cell Neurosci. 2001;18:671–690. doi: 10.1006/mcne.2001.1048. [DOI] [PubMed] [Google Scholar]
- 93.Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garzon-Muvdi T, Quinones-Hinojosa A. Neural stem cell niches and homing: recruitment and integration into functional tissues. ILAR J. 2009;51:3–23. doi: 10.1093/ilar.51.1.3. [DOI] [PubMed] [Google Scholar]
- 95.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 96.Lizio R, Vecchio F, Frisoni GB, Ferri R, Rodriguez G, Babiloni C. Electroencephalographic rhythms in Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:927573. doi: 10.4061/2011/927573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004;18:2963–2972. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Omlin FX, Waldmeyer J. Differentiation of neuron-like cells in cultured rat optic nerves: a neuron or common neuron-glia progenitor? Dev Biol. 1989;133:247–253. doi: 10.1016/0012-1606(89)90315-1. [DOI] [PubMed] [Google Scholar]
- 101.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 102.Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Nat Acad Sci USA. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamilton N, Hubbard PS, Butt AM. Effects of glutamate receptor activation on NG2-glia in the rat optic nerve. J Anat. 2009;214:208–218. doi: 10.1111/j.1469-7580.2008.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- 105.Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination n corpus callosum. Nat Neurosci. 2010;13:287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.