Abstract
In this issue, Singer et al. (2012) reveal that the nucleoporin Nup98 supports adaptation to genotoxic stress by protecting specific p53-induced mRNAs from exosome-dependent degradation, suggesting that wild-type Nup98 may possess tumor suppressor function.
The nuclear pore complex (NPC) is the gateway that allows trafficking of molecules between the nucleus and the cytoplasm. However, over the past decade, the role of constituents of the NPC, termed nucleoporins (Nups), has extended beyond nucleocytoplasmic trafficking. Some nucleoporins, such as Nup98, are found at the NPC as well as the nuclear interior (Enninga et al., 2002; Griffis et al., 2002). This nucleoplasmic pool has been shown to regulate transcription of genes involved in cell-cycle control, stress, and development (Egecioglu and Brickner, 2011). During mitosis, Nup98 is required for preservation of the mitotic checkpoint and proper spindle assembly (Cross and Powers, 2011; Jeganathan et al., 2005). Additionally, Nup98 is involved in pathological conditions including viral infection (Enninga et al., 2002) and leukemogenesis (Xu and Powers, 2009). In leukemias, chromosomal translocations result in fusions of Nup98 with transcriptional factors, which lead to abnormal transcriptional activity that drive leukemogenesis (Wang et al., 2009; Xu and Powers, 2009). However, a role for wild-type Nup98 in tumor development has not been previously described. In this issue, Singer et al. (2012) uncover another function for Nup98 as a posttranscriptional regulator that stabilizes selected p53 target mRNAs, which may support the tumor-suppressor activity of p53.
From a focused siRNA screen against nuclear transport factors, Singer et al. (2012) revealed that a subset of Nups, including Nup98, were required for upregulation of p21 mRNA upon p53 induction by genotoxic stress (Figure 1). However, levels of several other p53 target genes were not affected, suggesting that Nup98 is involved in the induction of specific p53 targets. Further analysis showed that basal levels of p21 mRNA were not affected by depletion of Nup98 and that downregulation of p21 mRNA levels by Nup98 depletion, upon p53 induction, was independent of transcription or splicing. These results indicated that modulation of p21 mRNA levels by Nup98 occurred posttranscriptionally and raised the possibility of p21 mRNA degradation. Indeed, depletion of Nup98 decreased p21 mRNA levels in both the nucleus and cytoplasm in an exosome-dependent mechanism. Since Nup98 has a role in mRNA export (Enninga et al., 2002; Powers et al., 1997), it is plausible that, as in yeast, depletion of Nup98 leads to nuclear accumulation of p21 mRNA, which is followed by rapid mRNA degradation through the exosome. The authors found that Nup98 bound p21 mRNA at the 3′UTR and this interaction was required for p21 mRNA stabilization upon induction of p53 (Figure 1). A key Nup98 region involved in the interaction with the p21 mRNA was mapped to the FG-repeat region, where the mRNA export receptor NXF1/TAP binds Nup98, suggesting that mRNA export may play a role in the regulation of p21 mRNA degradation by the exosome.
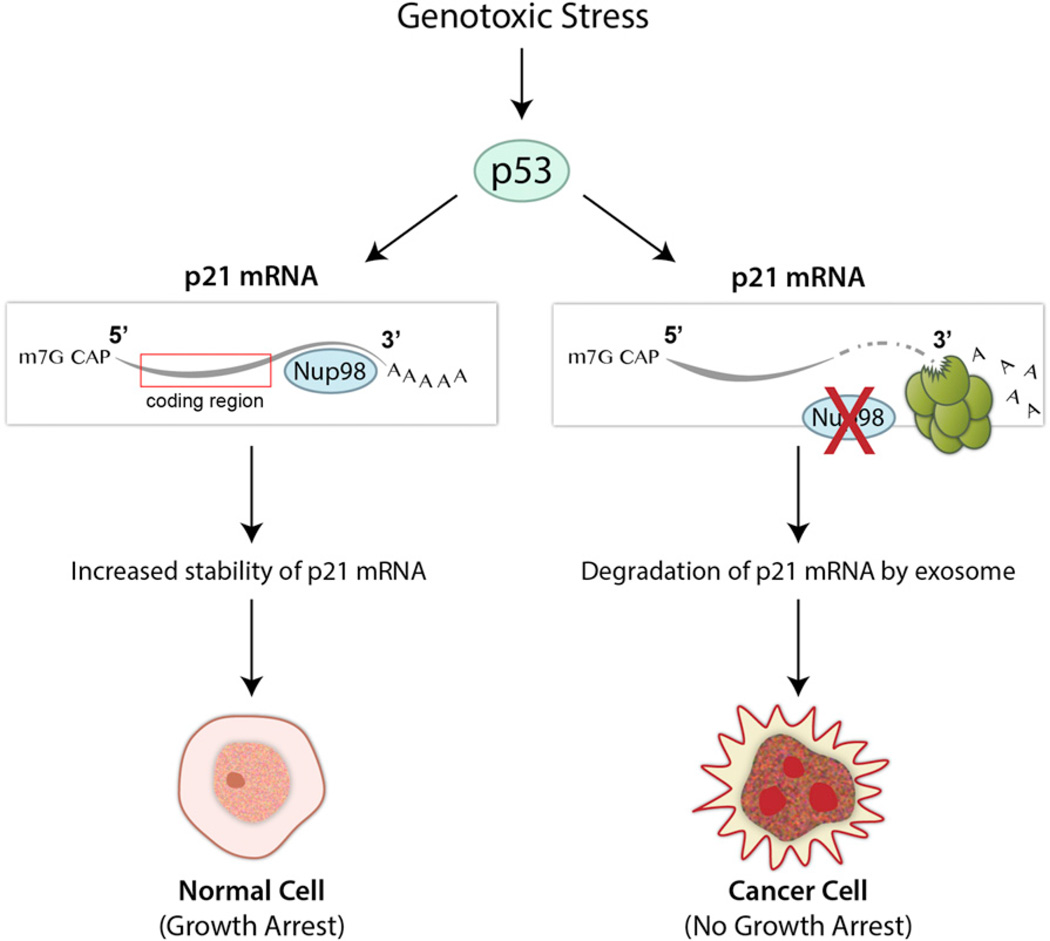
Figure 1. Nup98 Regulates the Stability of Selected p53-Induced mRNAs and Possibly Suppresses Tumorigenesis.
In response to genotoxic stimuli, the tumor suppressor protein p53 upregulates target genes such as p21 to regulate the cell cycle and DNA repair. In normal cells, the interaction of Nup98 with the 3′UTR of p21 mRNA protects p21 from degradation. In the absence of Nup98, the exposed p21 mRNA is targeted for exosome-mediated degradation. Singer et al. (2012) showed that downregulation of Nup98 expression occurs in some types of cancer, which may lead to destabilization of selected p53 target mRNAs, contributing to tumorigenesis.
It is unknown whether Nup98 follows the p21 mRNA to the cytoplasm or if Nup98 recruits additional factors to p21 mRNA inside the nucleus or at the NPC, which would then protect p21 mRNA from degradation in the cytoplasm. There is evidence that a pool of Nup98 shuttles between the nucleus and the cytoplasm (Griffis et al., 2002). Future studies should clarify whether a cytoplasmic pool of Nup98 prevents p21 mRNA degradation prior to translation, as transport factors have been shown to follow mRNAs to the translation machinery (Bolger et al., 2008). Since basal levels of p21 mRNA are not regulated by Nup98, one possibility suggested by the authors is that Nup98 may be posttranslationally modified upon p53 induction, favoring its interaction with the 3′UTR of p21 mRNA.
To identify additional potential mRNA targets of Nup98, the authors used a search algorithm to find RNA motifs based on predictive differences in gene expression. They uncovered a C-rich motif whose presence is highly correlated with mRNA stabilization upon p53 activation. The p21 mRNA was sixth in a list of bona fide p53 targets that were ranked by total motif score, with 14-3-3σ at the top of the list. Similar to p21, Nup98 was required for induction of the cell-cycle inhibitor 14-3-3σ, and the 3′UTR of the 14-3-3σ mRNA coimmunoprecipitated with Nup98. These results suggested that the identified C-rich motif represents a regulatory region in mRNAs induced by p53 and that the interaction of this region with Nup98 protects these mRNAs from degradation. A systematic search of mRNAs that interact with Nup98 upon p53 induction should give further insights into the biological signatures that could reveal additional functions of Nup98 as regulator of p53 diverse activities.
Some of the functions of p21 include cell-cycle regulation and growth arrest, resulting in inhibition of cell death in response to stress. Singer et al. (2012) demonstrated that reduced levels of p21, as a result of Nup98 depletion, did not affect cell-cycle progression but did increase cell death upon DNA damage. Additionally, Nup98 knockdown in cells treated with a p53 activator resulted in reduced senescence, a cellular process also regulated by p21. These results strongly suggest that Nup98 supports the p53 stress-response checkpoint (Figure 1). Elaboration of the significance of this relationship in normal development and/or tissue homeostasis remains to be determined and will likely reveal important aspects of the p53 response network that are currently unappreciated.
Throughout their study, the authors noted that Nup98 had the strongest effect on p21 levels in cell lines derived from liver cancers. Indeed, quantification of Nup98 expression in a mouse model of hepatocellular carcinoma (HCC) showed downregulation in tumor versus normal tissue. Furthermore, an analysis of samples from patients with HCC revealed that Nup98 expression was decreased in 25% of the tumors and that p21 expression was positively correlated with Nup98. These results suggest that Nup98 regulation of p21 levels may occur in a significant portion of patients with HCC. Since Nup98 regulation requires p21 activation by p53, it is assumed that this subset of HCC expresses wild-type p53. However, a large number of cancers express mutant p53 that does not activate downstream targets. Therefore, it will be interesting to determine if Nup98 can regulate p21 induction via a p53-independent mechanism. Furthermore, revisiting the mouse model in which Nup98 and Rae1 haploinsufficiency led to aneuploidy (Jeganathan et al., 2005) may reveal whether this phenotype is also related to the function of Nup98 in the stabilization of mRNAs such as p21. In sum, the study by Singer et al. (2012) defines a potential role for wild-type Nup98 as a tumor suppressor and indicates that loss-of-function lesions at the Nup98 locus could contribute to oncogenesis while bypassing p53 mutation.
ACKNOWLEDGMENTS
The authors are supported by grants from the NIH and CPRIT.
REFERENCES
- Bolger TA, Folkmann AW, Tran EJ, Wente SR. Cell. 2008;134:624–633. doi: 10.1016/j.cell.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MK, Powers MA. Mol. Biol. Cell. 2011;22:661–672. doi: 10.1091/mbc.E10-06-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu D, Brickner JH. Curr. Opin. Cell Biol. 2011;23:338–345. doi: 10.1016/j.ceb.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J, Levy DE, Blobel G, Fontoura BM. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Mol. Biol. Cell. 2002;13:1282–1297. doi: 10.1091/mbc.01-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan KB, Malureanu L, van Deursen JM. Nature. 2005;438:1036–1039. doi: 10.1038/nature04221. [DOI] [PubMed] [Google Scholar]
- Powers MA, Forbes DJ, Dahlberg JE, Lund E. J. Cell Biol. 1997;136:241–250. doi: 10.1083/jcb.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S, Zhao R, Barsotti AM, Ouwehand A, Fazollahi M, Coutavas E, Breuhahn K, Neumann O, Longerich T, Pusterla T, et al. Mol. Cell. 2012;48:799–810. doi: 10.1016/j.molcel.2012.09.020. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, Luo JL, Patel DJ, Allis CD. Nature. 2009;459:847–851. doi: 10.1038/nature08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Powers MA. Semin. Cell Dev. Biol. 2009;20:620–630. doi: 10.1016/j.semcdb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]