SUMMARY
Some wild antelopes are fast sprinters and more resistant to fatigue than others. This study therefore investigated two wild antelope species to better understand their reported performance capability. Muscle samples collected post mortem from the vastus lateralis and longissimus lumborum of fallow deer (Dama dama) and springbok (Antidorcas marsupialis) were analysed for myosin heavy chain isoform content, citrate synthase, 3-hydroxyacyl CoA dehydrogenase, phosphofructokinase, lactate dehydrogenase and creatine kinase activities. Cross-sectional areas, fibre type and oxidative capacities of each fibre type were determined in the vastus lateralis only. The predominant fibre type in both muscle groups and species were type IIX (>50%), with springbok having more type IIX fibres than fallow deer (P<0.05). Overall cross-sectional area was not different between the two species. The metabolic pathway analyses showed high glycolytic and oxidative capacities for both species, but springbok had significantly higher CS activities than fallow deer. Large variation and overlap in oxidative capacities existed within and between the fibre types. Some type IIX fibres presented with oxidative capacities similar to those from type I and IIA fibres. The data suggest that springbok and fallow deer are able sprint at >90 and 46 km h−1, respectively, partly from having large type IIX fibre contents and high glycolytic capacities. The high oxidative capacities also suggest that these animals may be able to withstand fatigue for long periods of time.
KEY WORDS: fibre type, enzyme activity, oxidative type IIX fibre, Dama dama, Antidorcas marsupialis
INTRODUCTION
Southern Africa has a vast number of large mammalian species that present with superior exercise performance capability, either in terms of sprinting (e.g. cheetah, lion) or endurance, or both (wildebeest). However, only a few studies exist on skeletal muscle properties of selected species to explain their performance capabilities.
Skeletal muscle of particularly large mammals (including humans) generally contains three fibre types (type I, IIA and IIX fibres), with each of these fibre types differing in fuel preference and contractile properties. Varying the composition of these three fibre types gives rise to the unique functional properties of a particular muscle (e.g. soleus versus biceps). Type I (slow oxidative) fibres express the myosin heavy chain (MHC) I isoform, which gives rise to a relatively slow contraction speed (Schiaffino and Reggiani, 1996; Bottinelli, 2001). They contain large numbers of mitochondria, are efficient in using fat, glucose and glycogen aerobically [high activities of citrate synthase (CS) and 3-hydroxyacyl CoA dehydrogenase (3HAD), but low activities of lactate dehydrogenase (LDH), phosphofructokinase (PFK) and creatine kinase (CK)] to produce the required ATP and are considered highly fatigue resistant (Pette, 1985). Type IIX (fast glycolytic) fibres express the MHC IIx isoform. This isoform allows the fibre to contract fast relative to type I fibres (Bottinelli, 2001). Their primary source of ATP generation is derived from the anaerobic metabolism of glucose and glycogen via glycolysis, and hence they contain very few mitochondria (low CS and 3HAD activities), have a high glycolytic capacity (high activities of LDH, PFK and CK) and fatigue quickly (Pette, 1985). Lastly, type IIA (fast oxidative) fibres are fast contracting fibres (less so than the type IIX fibres), and they derive their contractile properties from the expression of the MHC IIa isoform. This fibre type contains large numbers of mitochondria and can produce ATP from both aerobic and anaerobic metabolism, rendering this fibre type more resistant to fatigue (Pette, 1985; Schiaffino and Reggiani, 1996). All three fibre types also differ in the amount of maximum force and power generation capability, with type I fibres being poor at both and type IIX fibres the best (Chi et al., 1983; Essén-Gustavsson and Henriksson, 1984; Bottinelli, 2001). A fourth fibre type (type IIB expressing MHC IIb), fast twitch glycolytic, is primarily abundant in limb muscles of rodents (Pette and Staron, 1993; Delp and Duan, 1996; Kohn and Myburgh, 2007). Although small quantities of this fibre type were detected in cheetah, llama and pig limb muscles, it seems that this fibre type is reserved for more specialised muscles, such as those in the eye, and is undetectable in horse, cattle, black and blue wildebeest, blesbuck, kudu, lion, caracal and brown bear (Quiroz-Rothe and Rivero, 2001; Toniolo et al., 2005; Kohn et al., 2007; Smerdu et al., 2009; Hyatt et al., 2010; Kohn et al., 2011b; Kohn et al., 2011a).
Recent investigations have shown that the vastus lateralis and longissimus lumborum muscles of feline predators (lion and caracal) exhibit a predominance of type IIX muscle fibres (>50%), with high glycolytic but relatively poor oxidative capacity (as revealed by their oxidative capacities – i.e. NADH stain, and CS and 3HAD activities) (Kohn et al., 2011b). Similar large quantities of type IIX fibres were found in tiger and cheetah muscle (Williams et al., 1997; Hyatt et al., 2010). However, the same muscle groups from black wildebeest, impala and reindeer were found to contain high proportions of type IIX fibres (30–60%), with high glycolytic and high oxidative capacities (Essén-Gustavsson and Rehbinder, 1985; Kohn et al., 2005; Kohn et al., 2011a). Thus, the muscle metabolic and fibre type profiles observed in these species closely resemble their physical activity behaviour. For example, felids are fast sprinters, reaching speeds of up to 120 km h−1 but lack endurance, whereas black wildebeest and other antelopes can maintain a relatively high running intensity for long periods of time (Skinner and Chimimba, 2005). Additionally, Kohn et al. (Kohn et al., 2011a) recently showed that black wildebeest muscle harbours type IIX muscle fibres that either contained low or high oxidative capacities in muscle sections stained for oxidative capacity. However, this is not an uncommon finding. Others have shown that type IIX fibres from rat, mouse, reindeer and horse vary significantly in oxidative capacity, having these fibres with both low and high capacities (Essén-Gustavsson and Rehbinder, 1985; Pette, 1985; Pösö et al., 1996; Linnane et al., 1999; Smerdu et al., 2009). These findings are in contrast to human muscle, as historically only type I and type IIA fibres were considered oxidative in nature (Essén-Gustavsson and Henriksson, 1984). However, the presence of high oxidative type IIX fibres in the black wildebeest was argued to sustain fast running speeds for prolonged periods of time in these animals, especially to escape predation (Kohn et al., 2011a).
Many antelope species of varying sizes roam the savannahs of the southern African content. Very few have been investigated on muscular level, yet each species presents with their own unique sprinting and endurance capabilities. The national mammal of South Africa is the springbok, Antidorcas marsupialis (Zimmermann 1780), which is indigenous to large parts of South Africa, Namibia and Botswana. They are one of the few antelope species where both males and females have horns. With its small body mass (males range between 31 and 46 kg), springbok can gallop at an unconfirmed 88–97 km h−1 and are well known for their stotting ability – periodic jump-like actions during the gallop (Garland, 1983; Skinner and Chimimba, 2005). Although not scientifically measured, the lay press have reported that they can leap more than 3 m high and reach a distance of 14 m from the point of take-off. Fallow deer, Dama dama (Linnaeus 1758), were introduced by the British in the late 19th century to South Africa, primarily for hunting. These animals adapted well to the South African climate and their population grew ever since. Where springbok (males and females) usually roam in herds, the fallow deer males are solitary animals. Males average ~67 kg and carry antlers (ranging from 50 to 70 cm), while females are smaller (44 kg on average) and carry no antlers (Dharmani, 2000). Fallow deer are not particularly fast animals (reported maximum sprinting speed of 45 km h−1) and despite their size, can only jump ~1.75 m high (Garland, 1983).
Therefore, the first aim of this study was to investigate the skeletal muscle properties of springbok and fallow deer, focusing on muscle fibre type and metabolism. Muscle groups harvested and analysed were the vastus lateralis (aids in forward propulsion and jumping) and the longissimus lumborum (aids in stabilising the back during running and jumping). Because of the ability of the springbok to run fast, and being a high jumper and migrator, it is hypothesised that their muscle contain large numbers of type IIX fibres, with high oxidative and glycolytic capacities and large fibre cross-sectional areas (CSAs), similar to that found in the black wildebeest. However, it is hypothesised that fallow deer muscle would contain large numbers of type I and IIA fibres, with high oxidative but poor glycolytic capacity, giving rise to their poor sprinting ability.
MATERIALS AND METHODS
All the methods (except the histological intensity analyses) reported below were previously described in detail by Kohn et al. (Kohn et al., 2011a). A brief summation of each analysis will follow.
Animals and tissue sampling
Adult wild animals were randomly shot during the annual cropping season by professional hunters. The cropping occurred on private game farms in the Eastern Cape province, South Africa. Muscle samples from seven female fallow deer and 12 springbok (seven males and five females) were collected post mortem from the longissimus lumborum and vastus lateralis within 4 h of death.
The muscle of interest was identified using anatomical markers. Once identified, an incision was made through the hide and the fascia, and the muscle of interest was exposed. For the vastus lateralis, the sample site was determined as half the distance between the knee and hip joint, whereas the sampling site for the longissimus lumborum was between 2 and 3 mm parallel to the spinous process between lumbar vertebrae L3 and L4 of the lumbar spine. Using a scalpel blade, a block of tissue (±1 cm3) was removed from both muscle groups and the superficial part thereof was discarded. The remaining piece was divided into smaller parts, rapidly frozen in liquid nitrogen and stored in a cryo-preservation tank (−200°C). After harvesting, samples were transported to the laboratory in liquid nitrogen and stored at −87°C until subsequent analyses.
Homogenisation of tissue
Samples were prepared for enzyme and MHC isoform analyses as described by Kohn et al. (Kohn et al., 2011a). Briefly, frozen tissue was weighed and 100 mmol l−1 potassium phosphate buffer pH 7.30 was added to a ratio of 1:19. Homogenisation was performed on ice using a Teflon tip, and sonicated twice for 10 s at 6 W (Virtis Virsonic Ultrasonic Cell Disrupter 100, Gardiner, NY, USA). The suspension was centrifuged at 1700 g for 5 min (4°C) and the protein concentration of the supernatant was then determined (Bradford, 1976). The supernatant was used for enzyme activity measurements and the pellet for MHC isoform content analysis.
Enzyme analyses
Enzyme activities were determined spectrophotometrically at 25°C as described and modified by Kohn et al. (Kohn et al., 2011b). Maximum CS (EC 4.1.3.7) and 3HAD (EC 1.1.1.35) activities represented the flux through the Kreb's cycle and β-oxidation, respectively, whereas PFK (EC 2.7.1.11) and LDH (EC 1.1.1.27) activities represented flux through the glycolytic pathway. CK (EC 2.7.3.2) activity represented rapid ATP replenishment from phosphocreatine stores. All activities are expressed as μmol min−1 g−1 protein.
MHC isoform separation, western blots and relative fibre type
All methods to identify the MHC isoforms of springbok and fallow deer have been described previously (Kohn et al., 2011a). Briefly, MHC isoforms were separated using 7% SDS-polyacrylamide gels ran for 24 h. For MHC migratory identification, a human sample from previously published research was used to serve as control (Kohn et al., 2011b). Separated isoforms were transferred to PVDF membranes and probed with antibodies specific to MHC I (BAD5), MHC I and IIa (BF35) and MHC IIx (6H1) (Lucas et al., 2000; Kohn et al., 2011b; Kohn et al., 2011a). The migration patterns were then used to identify the location of the specific MHC isoform in question. The relative MHC isoform content of the three isoforms for each muscle group was calculated as a percentage of the total from silver-stained gels using the Un-Scan-It software package (Silk Scientific Corporation, Orem, UT, USA).
Histochemical and immunohistochemistry
Histological procedures were performed as described by Kohn et al. (Kohn et al., 2011a). Briefly, 10 μm serial cross-sections were cut from the vastus lateralis muscle only in a cryostat at −25°C. Sections were stained for ATPase activity (pH 10.3) to aid in fibre boundary identification during subsequent immunohistochemical stains and analyses. Oxidative capacity of the fibres was achieved by staining the mitochondria with an NADH–nitro blue tertrazolium reaction for 30 min at 37°C (Novikoff et al., 1961). In order to ensure that the NADH reaction was in the linear phase at the end of 30 min and that the incubation time was adequate, a series of incubation times were performed for 10, 20 and 30 min. The optical densities (ODs) of the fibres were determined (see below) and the fibres were divided into dark, medium and light staining intensities, based on values obtained at the 30 min time point.
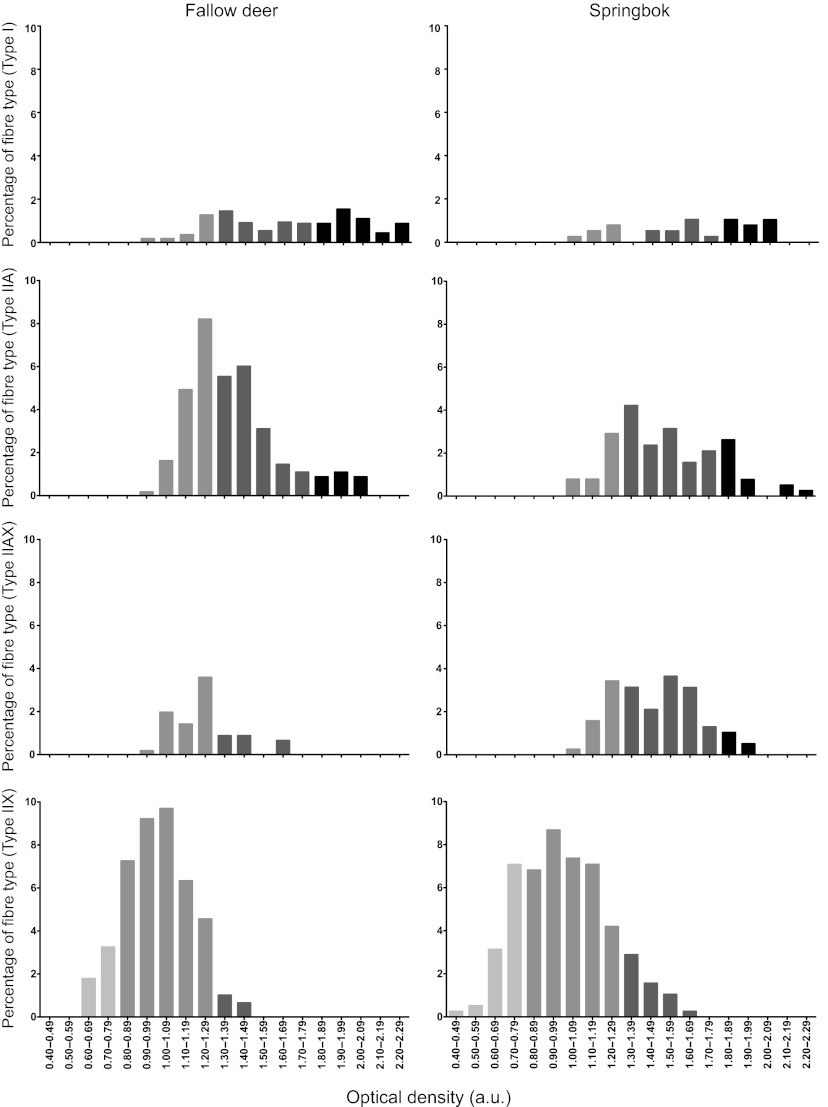
Additional sections were used for immunohistochemistry using the three antibodies used for the western blot. Immunoreactivity was visualised using a DAB staining kit (DAKO, Glostrup, Denmark). Sections were viewed under a light microscope at 10× magnification and photographed (AxioVision, Zeiss, Jena, Germany). Fibres were typed according to their reactivity to the specific antibodies and identified as type I, IIA, IIAX or IIX. Once identified, fibre CSAs were determined using the ImageJ for Mac software package (US National Institutes of Health, Bethesda, MD, USA). In order to quantify differences in oxidative capacity between fibre types, OD analysis of each fibre type was performed with pre-calibrated software (ImageJ). Frequency (percentage) distribution curves were generated for each fibre type and species. This was achieved by counting the number of fibres in a specific range of OD values (e.g. the number of fibres between 0.35 and 0.39 OD expressed as a percentage of the total number of fibres).
Statistical analyses
Values are expressed as means ± s.d. Interspecific and intraspecific differences were analysed using a one-way ANOVA with a Tukey's post hoc test. Whenever the variances differed (tested by the Bartlett's test for equal variance), the non-parametric Kruskal–Wallis ANOVA with a Dunn's post hoc test was implemented. Where only two groups were compared, an unpaired t-test (Mann–Whitney) was used. Relationships were determined using Pearson's correlation coefficient. For all statistical analyses, significance was set at P<0.05. Clarification: uppercase letters are used for fibre types derived from histology, whereas lowercase letters refer to fibre types derived from the MHC isoform analyses using SDS-PAGE.
RESULTS
Grouping of animals
Of the 12 springbok sampled, five were female. Statistical analyses of the data showed no significant difference between male and female springbok, and therefore data were pooled.
MHC isoform identification
Three MHC isoforms were separated for each species using SDS-PAGE (Fig. 1). Western blot analyses using anti-myosin I (BAD5) recognised the bottom band in all three species, confirming its location as similar to that of the human MHC I isoform (Fig. 2). BF35 (specific to MHC I and MHC IIa) was able to recognise two bands in all the species. These bands corresponded to the top (MHC IIa) and bottom (MHC I) isoforms of human. Anti-MHC IIx (6H1) recognised only the top band for human, whereas this antibody recognised the middle band in fallow deer and springbok. It also showed cross-reactivity with the bottom band (MHC I) in these antelope species. The location of the identified MHC isoforms confirms the migratory profile separated in Fig. 1.
Fig. 1.
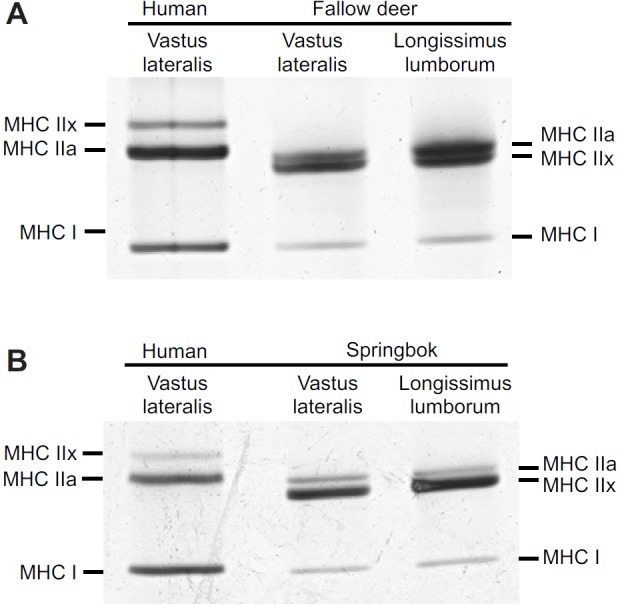
Myosin heavy chain isoforms from human, fallow deer and springbok muscles separated by SDS-PAGE. (A) Fallow deer isoforms compared with human. (B) Springbok isoforms compared with human.
Fig. 2.
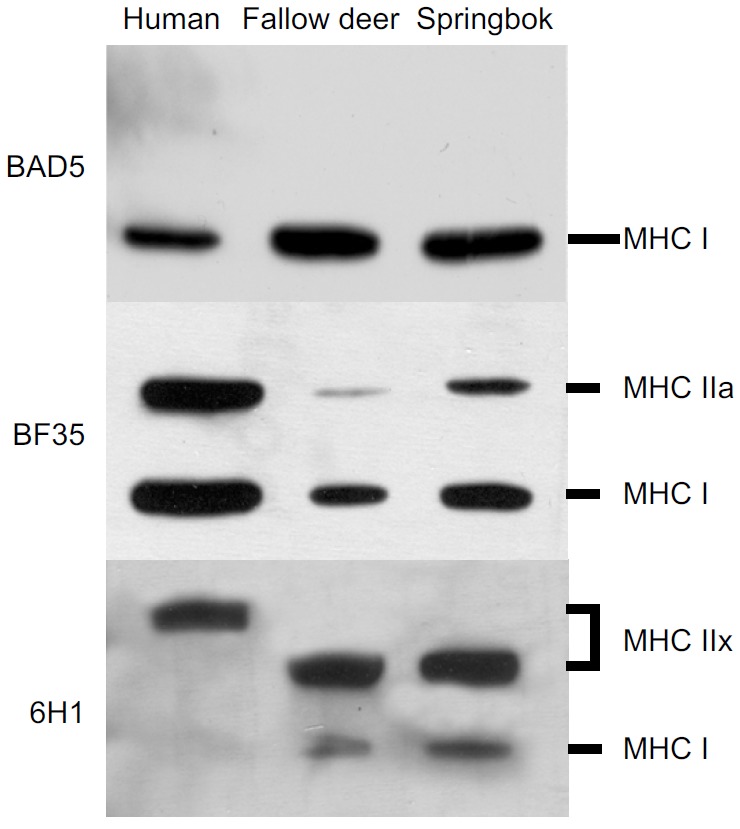
Identification of the separated MHC isoforms of human, fallow deer and springbok using antibodies specific to the three isoforms. See Results for details.
To confirm the specificity of the antibodies and hence the muscle fibre type of the antelope species, serial cross-sections from the vastus lateralis muscle pre-incubated at pH 10.3 and stained for ATPase activity (Fig. 3B), and immunohistochemistry using the above mentioned antibodies (Fig. 3C–E), were included. BAD5 only reacted with springbok MHC I fibres, whereas this antibody reacted with MHC I fibres and also showed slight cross-reactivity with MHC IIX fibres in fallow deer (Fig. 3C). BF35 reacted with only MHC I and IIA fibres (including hybrids) in both species and showed no cross-reactivity with pure MHC IIX fibres (Fig. 3D). 6H1 reacted with pure MHC IIX fibres and showed strong cross-reactivity with pure MHC I fibres in both species, but not with pure type IIA fibres (Fig. 3E).
Fig. 3.
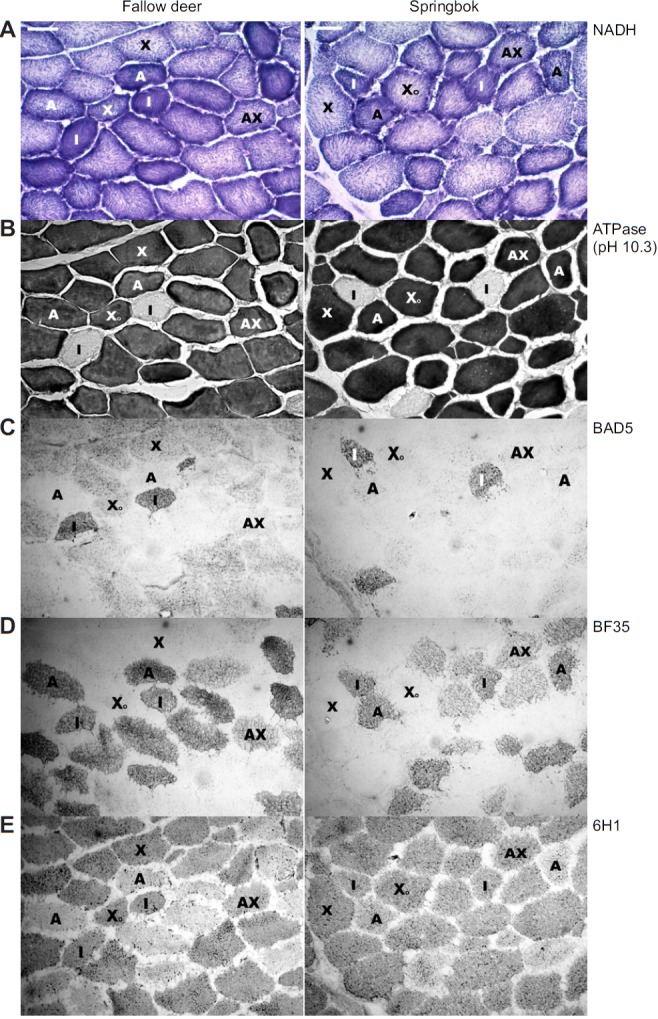
Histology of fallow deer and springbok vastus lateralis muscles. Fibres were classified (and labelled) as types I (I), IIA (A), IIAX (AX) and IIX (X). Type IIX fibres presenting with high oxidative capacity are labelled as XO. (A) NADH stain showing oxidative capacity of muscle fibres. (B) ATPase stain at pH 10.3. Type I fibres are clear. No type IC (grey) fibres are visible. All other fibres are stained dark. (C) Immunohistochemistry using an antibody specific to MHC I (BAD5). Note cross-reactivity with type IIX fibres in fallow deer. (D) Immunohistochemistry using an antibody specific to MHC I and IIa (BF35). (E) Immunohistochemistry using an antibody specific to MHC IIx (6H1). Note cross-reactivity with type I fibres in both species.
Myosin heavy chain isoform content and muscle fibre type
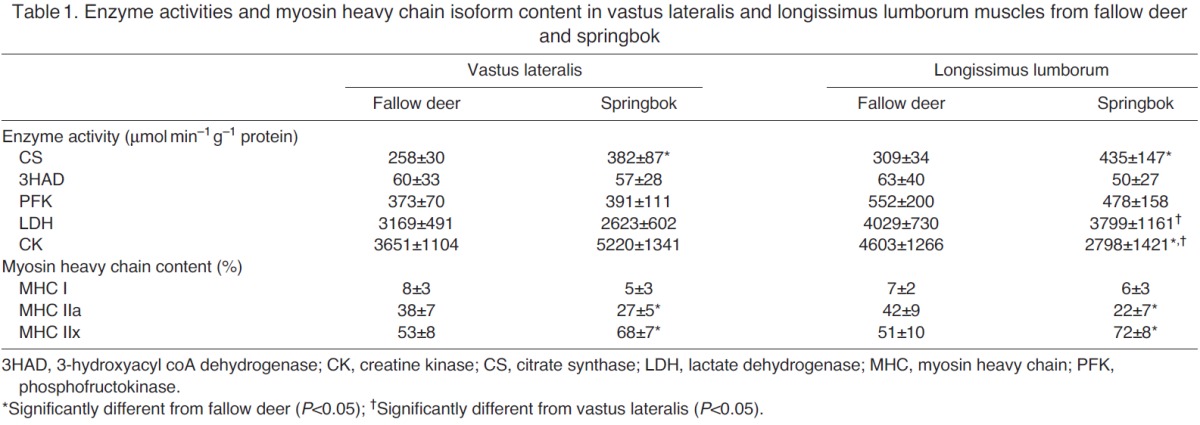
Both animals expressed an abundance of the MHC IIx isoforms in the two muscle groups, with springbok muscles containing significantly more of this isoform than fallow deer (Table 1). Fallow deer, in contrast, expressed more MHC IIa in both muscle groups. Both muscle groups from both species expressed very little MHC I.
Table 1.
Enzyme activities and myosin heavy chain isoform content in vastus lateralis and longissimus lumborum muscles from fallow deer and springbok
The isoform proportions were further confirmed using histological techniques on vastus lateralis sections only, using ATPase (pH 10.3) and immunohistochemistry (Table 2). Using these techniques, the fibres were subdivided into pure type I, IIA and IIX, and one hybrid fibre category (type IIAX). Both species had approximately equal amounts of type I fibres, with springbok having significantly less type IIA and more type IIX fibres than fallow deer (P<0.05). Although not statistically significant, springbok tended to have more hybrid type IIAX fibres than fallow deer (P=0.08).
Table 2.
Muscle fibre type and fibre cross-sectional areas of vastus lateralis muscle from fallow deer and springbok

Muscle morphology
The fibre types of vastus lateralis from both species showed a continuous increase in fibre size from type I to type IIA, IIAX and IIX (Table 2). Type IIA fibres were smaller in springbok, whereas their type IIX fibres were significantly larger than that of fallow deer. Overall, when the CSAs of the fibre types were pooled, no significant difference was found between the fibre size of fallow deer (2731±613 μm2) and that of springbok (2726±970 μm2).
Enzyme activities
The oxidative enzyme capacities (CS and 3HAD) and anaerobic metabolic pathway capacities were high in both species (Table 1). CS activities of vastus lateralis and longissimus lumborum muscles were significantly higher in springbok compared with fallow deer, whereas the capacity to utilise fat (3HAD) was similar in both muscle groups and species. With the exception of CK in the longissimus lumborum muscle (which was significantly lower in springbok), PFK, LDH and CK (vastus lateralis) activities were not different between springbok and fallow deer. In contrast, LDH and CK activities of springbok vastus lateralis were significantly lower and higher, respectively, compared with their respective longissimus lumborum activities. These differences were not apparent in the fallow deer.
Oxidative capacity quantification of muscle fibres
To ensure that the NADH reaction was in the linear phase after 30 min of incubation, three incubation time points (10, 20 and 30 min) were evaluated and OD measurements were taken (Fig. 4). As incubation time increased, the OD values increased in a linear fashion (white fibres, R2=0.76; medium fibres, R2=0.90; dark fibres, R2=0.85). The rates at which the intensity increased (slope of the lines) were faster in fibres that contained more mitochondria (dark fibres>medium fibres>light fibres). At 30 min, the reaction was still linear, but with clearer distinction between the OD of the three groups. Based on these findings, all NADH incubations were performed for 30 min.
Fig. 4.
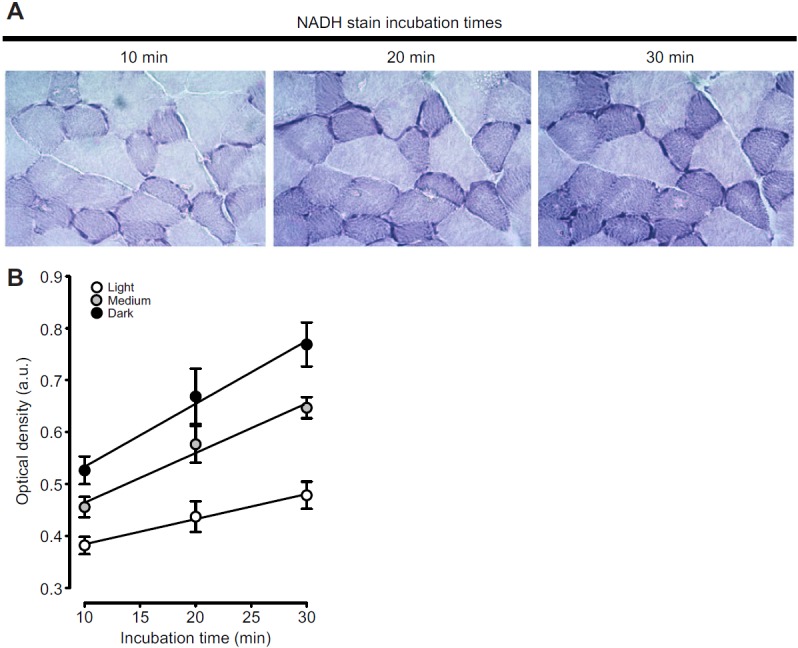
NADH stain intensities in springbok muscle as a function of incubation time. (A) Serial cross-sections incubated for 10, 20 or 30 min in NADH–nitro blue tetrazolium solution. (B) Relationship between optical density and incubation time in white (R2=0.76), medium (R2=0.90) and dark fibres (R2=0.85).
The NADH staining of springbok and fallow deer vastus lateralis muscle is shown in Fig. 3A. As expected, type I and IIA fibres had the highest oxidative capacities in both animal species, followed by type IIAX and finally type IIX. However, most notably was the presence of type IIX fibres that stained light or dark (Fig. 3A, X versus XO), indicating fibres with low or high oxidative capacities in both species.
Fig. 5 depicts fibre type frequency distributions (in percentage) of the number of fibres having the same range of OD values. The lowest and highest OD values for this analysis were 0.40 and 2.29, respectively. Most notable is the large range in oxidative capacities within a specific fibre type. Furthermore, there is large overlap in oxidative capacities between the four fibre types. Comparing the species, springbok type IIAX and IIX fibres had a greater oxidative capacity distribution than fallow deer, whereas type I and IIA seemed similar in distribution.
Fig. 5.
Frequency distribution plots (expressed as a percentage of the total number of fibres) of the oxidative capacity [optical density (OD)] from springbok and fallow deer vastus lateralis muscle. Each bar was calculated as the number of fibres that obtained OD values in a specific range (e.g. from 0.60 to 0.69 OD).
Correlations
Pooled data of the two species and their muscle groups revealed a positive relationship between CS activity and MHC IIx only (Pearson's r=0.45, P<0.01). However, this relationship was lost once the data were separated into muscle groups and species, suggesting that this relationship was an artefact of the pooling of the data sets. No other relationships were observed between any of the data sets analysed.
DISCUSSION
This is the first study to characterise skeletal muscle properties (fibre type and metabolism) of fallow deer and springbok. Both species had high oxidative and glycolytic capacities, and high type IIX (MHC IIx) fibre content. There were also large variations in oxidative capacity within a specific fibre type, but less so between fibre types.
MHC isoform identification and antibody specificity
Both species expressed three isoforms in the muscle groups analysed, which corresponded to (in order of migration) MHC IIa, MHC IIx and MHC I (Figs 1, 2). MHCs I and IIa were located on a similar migratory level as the human isoforms, whereas their MHC IIx migrated slightly below the MHC IIa isoforms. The size and pattern correspond to the MHC isoform migratory profile observed for blue and black wildebeest, blesbuck, kudu and bovines (Kohn et al., 2007; Kohn et al., 2011a). Although the migration of human, lion and caracal MHC I and IIa is similar to those above, their MHC IIx isoform shows the most diversity in size (Kohn et al., 2011b; Kohn et al., 2011a). It would therefore seem that there exists large homology between the MHC I and MHC IIa isoform structures in mammals, whereas the MHC IIx is the least conserved in structure. Further research is required, particularly for the fibres expressing MHC IIx, in order to elucidate whether the functional properties of the fibre (e.g. maximum force production, shortening velocity, etc.) are different.
The MHC I antibody BAD5 was shown to only react with the MHC I isoform in humans, lion, caracal, dog, rat and black wildebeest (Acevedo and Rivero, 2006; Kohn et al., 2011b; Kohn et al., 2011a). The present study showed similar results for the springbok on both western blot and immunohistochemistry. However, this antibody showed slight cross-reactivity with type IIX fibres from fallow deer (Fig. 3C). The explanation for this is not clear, but could be related to these animals having a different gene sequence compared with the indigenous southern African antelopes.
Antibody BF35 is specific to the MHC I and IIa isoforms. Its reactivity and specificity were confirmed in various animal species, including humans, dog, llama, felines and black wildebeest (Graziotti et al., 2001; Acevedo and Rivero, 2006; Hyatt et al., 2010; Kohn et al., 2011a). Some species that do express the MHC IIb isoform in their muscle have also shown reactivity with this antibody (e.g. llamas and rodents) (Graziotti et al., 2001). In fallow deer and springbok, this specificity was confirmed using western blots and immunohistochemistry, showing no cross-reactivity with pure type IIX (MHC IIx) fibres. To confirm the absence of the MHC IIb isoform in springbok and fallow deer, the antibody specific to the IIb isoform (10F5) was included in western blots and immunohistochemistry (data not shown). All were negative for the presence of the MHC IIb isoform or type IIB fibres, thus confirming only three fibre types for these two species. This is not an unusual find, as no MHC IIb was found in the lion, caracal or black wildebeest using the same antibody (Kohn et al., 2011b; Kohn et al., 2011a).
Muscle fibre size
On average, there was no difference between fibre CSA from fallow deer (2731±613 μm2) and that from springbok (2726±970 μm2), whereas the fibre types tended to increase in size from type I to type IIX. The average fibre size and that of the different fibre types are in accordance to that reported for black bear, blue and black wildebeest, bovines, caracal, cat, dog, horse, lion, llama and reindeer (Young, 1982; Essén-Gustavsson and Rehbinder, 1985; Pösö et al., 1996; Spurway et al., 1996; Williams et al., 1997; Serrano et al., 2000; Graziotti et al., 2001; Acevedo and Rivero, 2006; Marx et al., 2006; Toniolo et al., 2008; Smerdu et al., 2009; Kohn et al., 2011b; Kohn et al., 2011a). It is well known that, on average, human fibres tend to be larger than animals (the exception is the rhinoceros), and may increase in size as a result of training. Particularly for humans, the type of training may affect to what extent hypertrophy occurs (i.e. endurance versus resistance training). However, it seems that the small CSA of the fibres from these animals may be largely genetically determined. The main hurdle currently is the lack of understanding of what the functional significance would be for mammals to have a small fibre CSA. Kohn et al. (Kohn et al., 2011a) recently speculated that it could improve overall fibre economy (e.g. smaller O2 diffusion distance). Additionally, these authors also suggested that the smaller fibres may allow for a greater number of fibres present per muscle area, which may allow for a greater force output of the muscles. Springbok type IIX fibres were on average ~16% larger than those of fallow deer, whereas their type IIA fibres were 21% smaller. Giving the anecdotal evidence that springbok are able to sprint at speeds greater than 88 km h−1 and able to leap more than 3 m high during locomotion, it would seem that the larger CSA and shear abundance of this fibre type may significantly contribute to these physical attributes. Future studies on muscle contractile properties in the various fibre types may shed light on the role of fibre size in wild animal species.
Fibre type and metabolism of the muscles
Examination on a gross level revealed that both fallow deer and springbok muscles were dark red in colour, an observation also made in muscles from black wildebeest (Kohn et al., 2011a). This red colour is historically associated with an abundance of myoglobin present in the fibres. Furthermore, it is well known that rat muscle presenting dark in colour contains large numbers of type I and IIA fibres (Delp and Duan, 1996; Kohn and Myburgh, 2007). On the contrary, both muscle groups from the two species in this study contained more than 50% type IIX fibres, with less than 15% type I fibres. This finding seems to be a recurring observation for these types of wild animals. Previous studies on black and blue wildebeest, blesbuck, kudu, reindeer, topi, hartebeest and waterbuck have all reported high proportions of type IIX fibres, indicating a predominant genetic component affecting muscle fibre type (Essén-Gustavsson and Rehbinder, 1985; Spurway et al., 1996; Kohn et al., 2007; Kohn et al., 2011a).
Although the muscle groups serve different functions during locomotion, no difference in relative fibre type distribution (MHC isoforms) was found between the vastus lateralis and longissimus lumborum of fallow deer and springbok (Table 1). The same muscle groups analysed from lion and caracal also showed no difference in muscle fibre type composition, but differences were evident in black wildebeest (Kohn et al., 2011b; Kohn et al., 2011a). There is no doubt that large differences in muscle fibre type exist between muscle groups, but this highlights the point that not all species are the same, thus suggesting a greater genetic contribution towards fibre type distribution in muscle groups. The fibre type profile observed for the two species analysed confirms their physical performance capacity.
Glycolytic enzyme activities (PFK, LDH and CK) were all exceptionally high and similar to that found in other antelopes (black wildebeest, reindeer), horse and felines (lion and caracal) (Essén-Gustavsson and Rehbinder, 1985; Karlström et al., 1994; Kohn et al., 2011b; Kohn et al., 2011a). The findings suggest that these animals have adequate capacity to anaerobically supply sufficient ATP for activities involving sprinting and jumping.
In comparison to lion and caracal, the enzyme activities in the fallow deer and springbok responsible for aerobic (oxidative) energy production were all high (CS and 3HAD) (Kohn et al., 2011b). The activities were similar to that found for black wildebeest, reindeer, endurance-trained human athletes and the horse (Essén-Gustavsson and Rehbinder, 1985; Karlström et al., 1994; Pösö et al., 1996; Kohn et al., 2011b; Kohn et al., 2011a). CS activities in springbok muscles are significantly higher than those in fallow deer muscles, but fat metabolism is similar in both species (Table 1). It is interesting to note that fallow deer and springbok had predominantly type IIX fibres, thus refuting the evidence that a high oxidative capacity of muscle results from type I and IIA fibres (Essén-Gustavsson and Henriksson, 1984). Supporting this statement is the lack of relationship between any of the muscle fibre types and enzyme activities. This is, however, not a novel finding, as similar observations have been made in reindeer, black wildebeest, horse and black bear (Essén-Gustavsson and Rehbinder, 1985; Pösö et al., 1996; Linnane et al., 1999; Smerdu et al., 2009). The statement is further strengthened by the presence of type IIX fibres with a range of low and high oxidative capacities in both species (Fig. 5). Indeed, Kohn et al. (Kohn et al., 2011a) and Linnane et al. (Linnane et al., 1999) previously showed the presence of highly oxidative type IIX muscle fibres in black wildebeest and horses, respectively. Similarly, hartebeest and topi (both antelope species) also contained type IIX fibres with large variations in oxidative capacities (Spurway et al., 1996). However, it seems that the range of oxidative capacity within a fibre type, as well as the overlap between fibre types, may differ substantially between species and could be related to genetic factors. Unfortunately, it was not reported whether brown bear or reindeer contained type IIX fibres with high oxidative capacities. Nevertheless, the springbok muscle had more type IIX and IIAX fibres that were oxidative in nature and could explain the significantly higher CS activities in springbok.
Performance and muscle characteristics – do they concur?
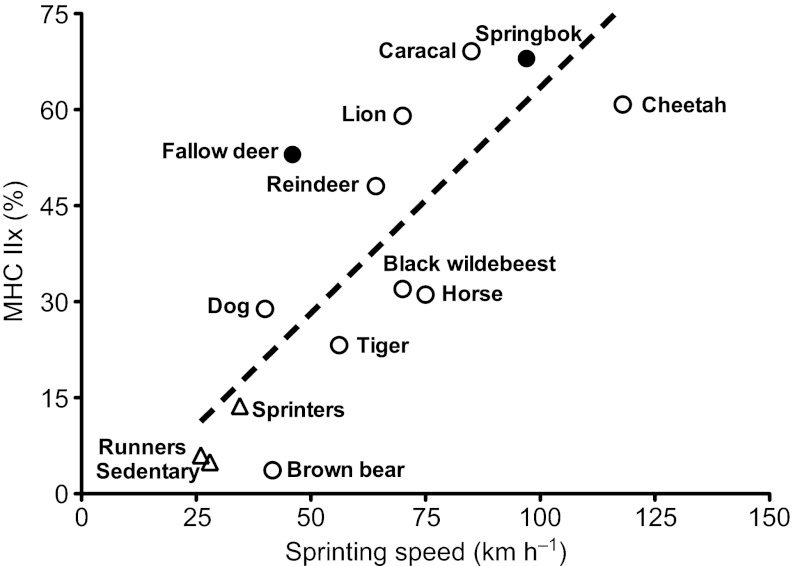
Anecdotal performance characteristics for the animals investigated here suggest that springbok are able to sprint twice as fast as fallow deer, with both species seen as possessing the ability to withstand fatigue for long periods of time, but at much lower speeds. Kohn et al. (Kohn et al., 2011a) recently showed a strong positive linear relationship between maximum sprinting speed and the MHC IIx content in various mammalian species. An adaptation of this plot to include the two species investigated (Fig. 6) strengthened this relationship, but fallow deer seemed to be the greater outlier, emphasising the other factors that could influence running speed (e.g. biomechanics, genetics, etc.). Additionally, both animals had high glycolytic capacities in their muscle, suggesting that sufficient energy could be provided to attain their respective sprinting speeds. The added high oxidative capacities (Table 1), as well as the range in oxidative type IIX fibres (Fig. 5D), would also suggest that these animals could withstand fatigue at high intensities for longer periods of time. The latter is of course purely speculative. Whether the supply of ATP via the oxidative pathways could sustain the ATP demand by the muscle at the reported high running speeds would require further investigation (e.g. lactate kinetics and oxygen and carbon dioxide kinetics during running).
Fig. 6.
The relationship between MHC IIx isoform content in muscle and reported maximal sprinting speeds. Adapted from Kohn et al. (Kohn et al., 2011a). Additional species added from the present study are indicated with a filled circle. Pearson's r=0.80, P<0.001.
Limitations of the study
On a methodological level, this study confirms the importance of using different techniques (e.g. metabolic versus antibodies versus SDS-PAGE) to classify muscle fibres into their respective types. There are many studies that have used only one such technique, resulting in the misclassification of fibres (Linnane et al., 1999). This also sheds doubt on the accuracy of assessing solely the fibre type composition of muscles in order to better understand their function, especially when comparing various species.
A final point that requires attention is the lack of studies that have accurately measured the sprinting speeds and endurance capability of wild animals. Performance data are readily available for horses, dogs and humans, as these frequently compete in races where time-keeping is considered accurate. Data for wild animals, in contrast, are scarce and to a large extent anecdotal (Garland, 1983). Therefore, the need for accurate and reliable measurements of their sprinting speeds and endurance capability is essential to enable researchers to draw concrete conclusions on the functional characteristics of wild animal skeletal muscle.
Conclusions
This is the first study to investigate skeletal muscle fibre type and metabolism of two muscle groups from fallow deer and springbok. The main findings were the presence of high proportions of type IIX fibres in the vastus lateralis and longissimus lumborum (MHC isoforms and histological types), and the presence of both high glycolytic and oxidative metabolism. Additionally, this study confirmed the presence of type IIX muscle fibres that are highly oxidative, which could aid in maintaining a high running speed for long periods of time.
ACKNOWLEDGEMENTS
All primary antibodies used in this study were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by The University of Iowa, Department of Biological Sciences. Bea Schoeman, Johan ‘Bul’ Schoeman and Neil Schoeman are thanked for their donation of the muscle samples, as well as their sincere hospitality.
LIST OF ABBREVIATIONS
- 3HAD
3-hydroxyacyl coA dehydrogenase
- CK
creatine kinase
- CS
citrate synthase
- CSA
cross-sectional area
- LDH
lactate dehydrogenase
- MHC
myosin heavy chain
- OD
optical density
- PFK
phosphofructokinase
FOOTNOTES
FUNDING
This study was funded partly by the UCT/MRC Research Unit for Exercise Science and Sports Medicine and the National Research Foundation of South Africa. T.A.K. is a recipient of the Tim and Marilyn Noakes Sports Science Postdoctoral Fellowship. Financial support for R.H. came from São Paulo State Research Financial Support.
REFERENCES
- Acevedo L. M., Rivero J. L. (2006). New insights into skeletal muscle fibre types in the dog with particular focus towards hybrid myosin phenotypes. Cell Tissue Res. 323, 283-303 [DOI] [PubMed] [Google Scholar]
- Bottinelli R. (2001). Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch. 443, 6-17 [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254 [DOI] [PubMed] [Google Scholar]
- Chi M. M., Hintz C. S., Coyle E. F., Martin W. H., 3rd, Ivy J. L., Nemeth P. M., Holloszy J. O., Lowry O. H. (1983). Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am. J. Physiol. 244, C276-C287 [DOI] [PubMed] [Google Scholar]
- Delp M. D., Duan C. (1996). Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J. Appl. Physiol. 80, 261-270 [DOI] [PubMed] [Google Scholar]
- Dharmani A. (2000). Dama dama, fallow deer. Animal Diversity Web; Available at http://animaldiversity.ummz.umich.edu/site/accounts/information/Dama_dama.html [Google Scholar]
- Essén-Gustavsson B., Henriksson J. (1984). Enzyme levels in pools of microdissected human muscle fibres of identified type. Adaptive response to exercise. Acta Physiol. Scand. 120, 505-515 [DOI] [PubMed] [Google Scholar]
- Essén-Gustavsson B., Rehbinder C. (1985). Skeletal muscle characteristics of reindeer (Rangifer tarandus L.). Comp. Biochem. Physiol. 82A, 675-679 [DOI] [PubMed] [Google Scholar]
- Garland T. (1983). The relation between maximal running speed and body mass in terrestrial mammals. J. Zool. 199, 157-170 [Google Scholar]
- Graziotti G. H., Ríos C. M., Rivero J. L. (2001). Evidence for three fast myosin heavy chain isoforms in type II skeletal muscle fibers in the adult llama (Lama glama). J. Histochem. Cytochem. 49, 1033-1044 [DOI] [PubMed] [Google Scholar]
- Hyatt J. P., Roy R. R., Rugg S., Talmadge R. J. (2010). Myosin heavy chain composition of tiger (Panthera tigris) and cheetah (Acinonyx jubatus) hindlimb muscles. J. Exp. Zool. 313A, 45-57 [DOI] [PubMed] [Google Scholar]
- Karlström K., Essén-Gustavsson B., Lindholm A. (1994). Fibre type distribution, capillarization and enzymatic profile of locomotor and nonlocomotor muscles of horses and steers. Acta Anat. 151, 97-106 [DOI] [PubMed] [Google Scholar]
- Kohn T. A., Myburgh K. H. (2007). Regional specialization of rat quadriceps myosin heavy chain isoforms occurring in distal to proximal parts of middle and deep regions is not mirrored by citrate synthase activity. J. Anat. 210, 8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn T. A., Kritzinger B., Hoffman L. C., Myburgh K. H. (2005). Characteristics of impala (Aepyceros melampus) skeletal muscles. Meat Sci. 69, 277-282 [DOI] [PubMed] [Google Scholar]
- Kohn T. A., Hoffman L. C., Myburgh K. H. (2007). Identification of myosin heavy chain isoforms in skeletal muscle of four Southern African wild ruminants. Comp. Biochem. Physiol. 148A, 399-407 [DOI] [PubMed] [Google Scholar]
- Kohn T. A., Curry J. W., Noakes T. D. (2011a). Black wildebeest skeletal muscle exhibits high oxidative capacity and a high proportion of type IIx fibres. J. Exp. Biol. 214, 4041-4047 [DOI] [PubMed] [Google Scholar]
- Kohn T. A., Burroughs R., Hartman M. J., Noakes T. D. (2011b). Fiber type and metabolic characteristics of lion (Panthera leo), caracal (Caracal caracal) and human skeletal muscle. Comp. Biochem. Physiol. 159A, 125-133 [DOI] [PubMed] [Google Scholar]
- Linnane L., Serrano A. L., Rivero J. L. (1999). Distribution of fast myosin heavy chain-based muscle fibres in the gluteus medius of untrained horses: mismatch between antigenic and ATPase determinants. J. Anat. 194, 363-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. A., Kang L. H., Hoh J. F. (2000). Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem. Biophys. Res. Commun. 272, 303-308 [DOI] [PubMed] [Google Scholar]
- Marx J. O., Olsson M. C., Larsson L. (2006). Scaling of skeletal muscle shortening velocity in mammals representing a 100,000-fold difference in body size. Pflugers Arch. 452, 222-230 [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Shin W. Y., Drucker J. (1961). Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J. Biophys. Biochem. Cytol. 9, 47-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D. (1985). Metabolic heterogeneity of muscle fibres. J. Exp. Biol. 115, 179-189 [DOI] [PubMed] [Google Scholar]
- Pette D., Staron R. S. (1993). The molecular diversity of mammalian muscle fibres. NIPS 8, 153-157 [Google Scholar]
- Pösö A. R., Nieminen M., Raulio J., Räsänen L. A., Soveri T. (1996). Skeletal muscle characteristics of racing reindeer (Rangifer tarandus). Comp. Biochem. Physiol. 114A, 277-281 [DOI] [PubMed] [Google Scholar]
- Quiroz-Rothe E., Rivero J. L. (2001). Co-ordinated expression of contractile and non-contractile features of control equine muscle fibre types characterised by immunostaining of myosin heavy chains. Histochem. Cell Biol. 116, 299-312 [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Reggiani C. (1996). Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol. Rev. 76, 371-423 [DOI] [PubMed] [Google Scholar]
- Serrano A. L., Quiroz-Rothe E., Rivero J. L. (2000). Early and long-term changes of equine skeletal muscle in response to endurance training and detraining. Pflugers Arch. 441, 263-274 [DOI] [PubMed] [Google Scholar]
- Skinner J. D., Chimimba C. T. (2005). The Mammals of the Southern African Sub Region. Cape Town, South Africa: Cambridge University Press; [Google Scholar]
- Smerdu V., Cehovin T., Strbenc M., Fazarinc G. (2009). Enzyme- and immunohistochemical aspects of skeletal muscle fibers in brown bear (Ursus arctos). J. Morphol. 270, 154-161 [DOI] [PubMed] [Google Scholar]
- Spurway N. C., Murray M. G., Gilmour W. H., Montgomery I. (1996). Quantitative skeletal muscle histochemistry of four east African ruminants. J. Anat. 188, 455-472 [PMC free article] [PubMed] [Google Scholar]
- Toniolo L., Maccatrozzo L., Patruno M., Caliaro F., Mascarello F., Reggiani C. (2005). Expression of eight distinct MHC isoforms in bovine striated muscles: evidence for MHC-2B presence only in extraocular muscles. J. Exp. Biol. 208, 4243-4253 [DOI] [PubMed] [Google Scholar]
- Toniolo L., Cancellara P., Maccatrozzo L., Patruno M., Mascarello F., Reggiani C. (2008). Masticatory myosin unveiled: first determination of contractile parameters of muscle fibers from carnivore jaw muscles. Am. J. Physiol. 295, C1535-C1542 [DOI] [PubMed] [Google Scholar]
- Williams T. M., Dobson G. P., Mathieu-Costello O., Morsbach D., Worley M. B., Phillips J. A. (1997). Skeletal muscle histology and biochemistry of an elite sprinter, the African cheetah. J. Comp. Physiol. B 167, 527-535 [DOI] [PubMed] [Google Scholar]
- Young O. A. (1982). Further studies on single fibres of bovine muscles. Biochem. J. 203, 179-184 [DOI] [PMC free article] [PubMed] [Google Scholar]