Abstract
Increasing life expectancies paired with age-related comorbidities have resulted in the continued growth of the elderly surgical population. In this group, age-associated changes and decreased physiological reserve impede the body’s ability to maintain homeostasis during times of physiological stress, with a subsequent decrease in physiological reserve. This can lead to age-related physiological and cognitive dysfunction resulting in perioperative complications. Changes in the cardiovascular, pulmonary, nervous, hepatorenal, endocrine, skin, and soft tissue systems are discussed as they are connected to the perioperative experience. Alterations affect both the pharmacodynamics and pharmacokinetics of administered drugs. Elderly patients with coexisting diseases are at a greater risk for polypharmacy that can further complicate anesthetic management. Consequently, the importance of conducting a focused preoperative evaluation and identifying potential risk factors is strongly emphasized. Efforts to maintain intraoperative normothermia have been shown to be of great importance. Procedures to maintain stable body temperature throughout the perioperative period are presented. The choice of anesthetic technique, in regard to a regional versus general anesthetic approach, is debated widely in the literature. The type of anesthesia to be administered should be assessed on a case-by-case basis, with special consideration given to the health status of the patient, the type of operation being conducted, and the expertise of the anesthesiologist. Specifically addressed in this article are age-related cognitive issues such as postoperative cognitive dysfunction and postoperative delirium. Strategies are suggested for avoiding these pitfalls.
Keywords: geriatric anesthesiology, elderly physiological changes, operation considerations, geriatric surgery
Introduction
In 2008, the United States (US) Census Bureau reported that persons over the age of 65 comprise approximately 13% of the total US population. Improving social conditions, combined with medical science advancements, have resulted in marked reductions in morbidity and death from diseases that had once been fatal. Consequently, the average life expectancy for this group has steadily increased and is now estimated to be 78 years.1,2
Health issue awareness has resulted in a decrease in the prevalence of chronic illness in elderly individuals. Advances in surgical, anesthetic, and intensive care techniques have therefore led to a larger proportion of this expanding elderly population presenting to undergo surgical procedures.3,4 Studies have estimated that approximately 53% of all surgical procedures are performed on patients over the age of 65. Projections estimate that approximately half of the population over the age of 65 will require surgery once in their lives.4
The principles of physiology of the aged are not merely a linear extension when compared to the middle-aged adult. This geriatric surgical population represents a unique clinical group; moreover, such elderly individuals typically suffer from a number of chronic conditions such as, but not limited to, cardiovascular disease, arthritis, thyroid disease, and emphysema.5 However, increasing age should not be viewed as a pathologic condition; rather, it represents the variation within senescent biological processes. It does appear, though, that advancing age is an independent predictor of poorer perioperative outcomes.6 The aging process impedes the body’s ability to maintain homeostasis during times of physiologic stress7; as a result, elderly individuals undergo a number of physiologic changes that impact their response to anesthesia. Age-related organ system changes are reviewed below; a discussion of the most salient pre-, peri-, and postoperative concerns for physicians caring for the elderly patient follows.
Cardiovascular
Age-related cardiovascular changes are leading factors impacting perioperative outcomes among elderly patients.8 Cardiovascular reserve is significantly reduced with aging: notably, cardiac output declines by almost 50% between the ages of 20 and 80.9 The number of cardiac myocytes decreases as one ages, and remaining myocytes regularly undergo hypertrophy. There is an increased density in the supporting structures, including an increase in the number of fibroblasts.10,11 The resulting interstitial fibrosis is believed to be the underlying etiology of decreased myocardial compliance. A less-compliant ventricle affects both diastolic relaxation and systolic contraction. Additionally, there is increased arterial stiffness resulting in increased afterload, increased systolic blood pressure, and, thus, left ventricular hypertrophy.10,11
Such senescence-induced cardiovascular changes also affect the cells of the conduction system. There is a decrease in the number of sinoatrial nodal cells; consequently, elderly patients are subject to an increased incidence of dysrhythmias.12 Vagal tone is increased resulting in a decreased basal heart rate. Although the exact etiology remains unknown, aged individuals also experience decreased sensitivity to beta-adrenergic transmission.13–15
Pulmonary
Structural changes to the respiratory system also occur with aging. These alterations involve both the lung parenchyma and the chest wall.16–22 A decline in the lung elastic recoil leads to an increase in lung compliance (Figure 1 ),21 a pathologic process similar to that of emphysema.16,22 Increased parenchymal compliance is accompanied by a decrease in chest wall compliance. This reduction in compliance is secondary to changes in the bony thorax, loss of respiratory muscle mass, and additional effects associated with osteoporosis. Decreases in arterial oxygen tension and alveolar gas exchange, paired with alterations in compliance, lead to increases in ventilation perfusion mismatch.19
Figure 1.
Pressure volume curves in a 20-year-old (upper panel) and 60-year-old (lower panel). As age increases, there is an increase in lung compliance, residual volume, and functional residual capacity with a decrease in chest wall compliance. TLC indicates total lung capacity; FRC, functional residual capacity; RV, residual volume.
When compared to the younger adult, the elderly individual breathes with both a smaller tidal volume and more rapid respiratory rate.18 Increased residual volume and functional residual capacity are increased, while vital capacity, forced expiratory volume (FEV1), forced vital capacity (FVC), and peak expiratory flow rate are all reduced (Figures 2 and 3). Moreover, there is a decreased central nervous system response to hypoxia, hypercapnia, as well as the ability to sense respiratory challenges such as bronchoconstriction.20
Figure 2.

Forced vital capacity (FVC) and forced expiratory volume (FEV1) among males (M) and females (F). The aged individual has reductions in FVC and FEV1.
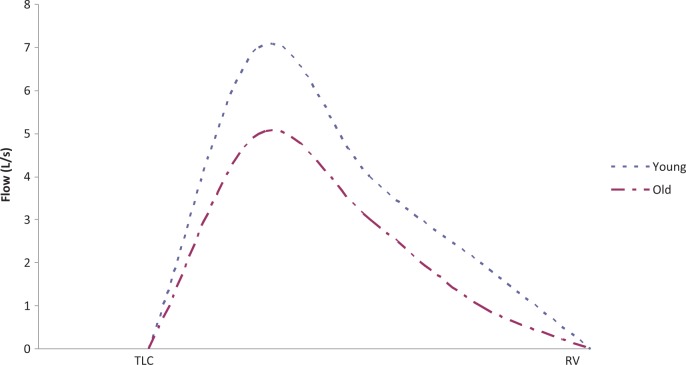
Figure 3.
Expiratory flow rate curve in younger and older subjects. TLC indicates total lung capacity; RV, residual volume. Young is between the ages 20 and 49 and old is between the ages 50 and 84.
Respiratory protective mechanisms are impaired in elderly individuals. Ciliary function in the respiratory epithelium is decreased. As a result of a fewer number of glandular epithelial cells, mucus production is decreased. Additionally, upper airway muscular support deteriorates causing declines in pharyngeal sensitivity and motor function. Decreased mucus production, in addition to a reduced ability to cough, impairs the efficient clearance of bacteria from the respiratory tract leading to an increased risk of pneumonia.23
Physiologic changes in combination with the loss of protective mechanisms places the elderly patient at greater risk of developing respiratory failure, and causes higher incidences of mechanical ventilation dependence, and ventilation-associated pneumonia.17
Hepatorenal
Even in the absence of pathology, the aged individual undergoes reductions in both hepatic and splanchnic blood flow with consequent liver involution. Decreased blood flow through the liver also results in lower levels of microsomal enzyme function, thereby potentially impairing the body’s ability to metabolize drugs.24 Drug metabolism grossly depends on a number of cytochrome P450 (CYP) phase I and/or phase II reactions in the liver. However, phase II pathways, including glucuronidation, acetylation, or sulfation, are essentially unchanged with age.25–27 Thus, it is suspected that the 30% decrease in hepatic clearance of drugs among elderly individuals is due to age-related impairments in CYP-mediated phase I reactions.28 Further, there is a decline in plasma proteins produced by the liver, such as albumin, and protein drug binding is decreased.29 Among elderly men, there is a decreased production of plasma cholinesterase.29 This enzyme metabolizes the depolarizing agent succinylcholine and may result in a prolongation of its action.
A significant loss of renal mass is also seen in the elderly patient. Due to decreases in cardiac output, causing a decrease in renal blood flow, a decline in glomerular filtration rate takes place.30,31 In fact, nearly 50% of all functioning glomeruli are lost by the age of 80.29 The remaining functional renal tubules become less sensitive to hormones including aldosterone, vasopressin, and atrial natriuretic peptide. The elderly patient’s ability to either dilute or concentrate their urine is, thereby, effectively decreased.
Endocrine
In those patients with unrelated diabetes diagnoses, the ability to handle increased glucose loads is compromised due to increased insulin resistance. Elderly individuals produce less thyroid-stimulating hormone, free T3, growth hormone, aldosterone, and androgens; these alterations may lead to global changes in body composition,32,33 such as decreased muscle mass and greater fat composition, and may be accompanied by a reduced responsiveness to thyroxin, renin, and aldosterone.32,33
Thermoregulation
Body temperature regulation occurs through a negative feedback loop mechanism. The hypothalamus increases vasomotor reflexes due to hypothermia.34 Such reflexes are blunted under anesthesia.35 Furthermore, the elderly patient is more prone to increased heat loss and hypothermia as a result of changes in their body composition resulting in decreases in muscle mass and increases in surface area to body mass ratio.36–39 Decreased muscle mass attenuates the basal and resting heat production by nearly 20% when compared to individuals in their 30s.40 When subjected to cold challenges, such as in the operating room, the aged individual has a lessened protective vasoconstrictor response.41–43
Central Nervous System
Aging is associated with a reduction in the number of peripheral neurons and these nerves have slower conduction velocity.44,45 The efficiency in which nerves and muscles couple is decreased and results in atrophy and denervation of both the sensory and motor systems.29,46
In comparison to adults in their 20s, 80-year-olds have lost approximately 30% of their total brain mass.47 Decreased cerebral blood flow and cerebral oxygen consumption metabolic rates are seen in these patients. Such changes are associated with a decrease in the number of neurotransmitters and declines in the number of available neuroreceptors.29
Cognition can be described as higher-level functions of the brain, involving language, imagination, perception, and planning. It can be viewed in 2 components: fluid and crystallized.48 Fluid cognition encompasses processing speeds, ability to solve problems, and spatial manipulation. Crystallized cognition encompasses tasks such as word vocabulary and knowledge, general knowledge, and occupational proficiency.48 Fluid cognition peaks among individuals in their 20s and declines thereafter. In contrast, crystallized cognition does not decline with time; however, its rate of rise is not as rapid once reaching adulthood in the mid 20s.49 It is suspected that crystallized intelligence may even experience slight declines once individuals reach the ninth decade of life.49
The aged individual suffers greater variability in their behavioral responses to cognitive tests in comparison to the younger individual. Furthermore, elderly individuals may have difficulties filtering extraneous information and stimuli.50 Although the degree that individuals are affected varies, there is an overall decline in cognition among elderly persons, and normal cognitive decline has been described as a drop in IQ by 1 to 2 standard deviations from the age of 20 to 70.46,51 These impairments affect fluid cognition more than crystallized cognition and decrease efficiency in processing speed, working memory, and long-term memory.52
Approximately 80% of all individuals over the age of 60 demonstrate preclinical stages of Alzheimer’s disease, Parkinson’s disease dementia with Lewy bodies, or cerebrovascular disease.53 Furthermore, as life expectancy continues to increase, a greater proportion of elderly individuals will begin to demonstrate clinical signs of neurodegenerative disease.53 Unfortunately, when clinical signs are recognizable, disease in the brain is severe, widespread, and likely irreversible.
Skin and Soft Tissue
Atrophy of back muscles, and the decreased distance among the intervertebral disks, leads to a 5- to 7-cm reduction in height by the age of 70.45,54 There is a also a reduction in overall muscle mass and a decline in general strength; lost muscle mass is replaced by increases in stores of body fat.44,55 Structural weakness is compounded due to degenerative joint diseases including arthritis and osteoporosis. Elderly skin has not only been subjected to the consequences of time but also a prolonged exposure to the environment. Deterioration of both the vasculature and the supporting structures of the dermis, such as collagen and elastin, have deteriorated.56 Thus, elderly skin becomes particularly fragile and readily prone to trauma. Particular care must be taken when positioning the older patient in the operating room.
Preoperative Considerations
The elderly surgical population characteristically suffers from a greater number of comorbidities often requiring multiple medications, thus placing patients at risk for polypharmacy. It has been estimated that approximately 40% of people over the age of 65 are taking 5 or more drugs.5 The most common drug-drug interactions occur with nonprescription medications.57 As physicians involved in the preoperative management of such patients, it is important to thoroughly review not only prescription medications but also over-the-counter medications including integrative therapies such as herbs and alternative medicine remedies. The American Society of Anesthesiologists (ASA) recommends that all such supplements be discontinued 2 weeks prior to surgery.58,59 These substances can interfere with anesthesia and potentially produce problems during surgery; for example, Ginkgo Biloba may increase surgical bleeding.
Physical examination of the elderly surgical candidate should be used as a guide to indicate which preoperative studies may direct further testing, if necessary. For example, the role of routine preoperative spirometry is of limited value; however, spirometry should be performed when there is a clinical suspicion of chronic obstructive pulmonary disease (COPD). Although preoperative chest x-rays are regularly ordered by clinicians, the information that they provide is indeed limited. Preoperative chest x-rays should be reserved for otherwise healthy patients who suffer cardiopulmonary disease and are undergoing either abdominal or thoracic surgery.60 There have been conflicting reports with regard to the prevention of pulmonary complications and the timing of smoking cessation prior to surgery.61–64 Therefore, in counseling patients preoperatively, the timing of smoking cessation is often a point of controversy. In elective surgeries, the greatest benefit in smoking cessation may occur when it is initiated 2 months prior to the procedure.65
As previously mentioned, a number of age-related cardiovascular changes occur among elderly persons, and thus the gross majority of individuals over the age of 70 will demonstrate an abnormal electrocardiogram (EKG).66 The value of EKGs in the geriatric population must be tailored toward the cardiac risk factors of the patient, the urgency of surgery to be performed, and the type of surgery being performed.67 Cardiac risk factors may include preexisting cardiac disease, age, functional capacity, and comorbid conditions such as peripheral vascular disease and diabetes mellitus.67 Furthermore, risk stratifications of surgeries can be identified by the practice guidelines set by the American College of Cardiologists and the American Heart Association.67 Recommendations for provocative testing such as exercise or pharmacologic stress testing and coronary angiography should be also directed by these practice guidelines.67
Intraoperative Considerations
The older surgical candidate often presents with decreased cardiac reserve and is less well equipped to respond to both hypovolemia and hypotension. These patients can also be observed to have an exaggerated blood pressure response during induction of anesthesia. Despite these changes, there is little evidence to support routine placement of pulmonary artery (PA) catheters in high-risk individuals undergoing major abdominal, thoracic, vascular, or orthopedic surgeries.68
Such reductions in physiological reserve are not limited to cardiac function. A similar paradigm is seen with body temperature maintenance. As discussed, age-related changes in body composition and metabolism place these patients at a particularly high risk of hypothermia. The deleterious effects of such hypothermia may include prolonged bleeding, poor immune function, and increased wound infections.69
Efforts to maintain normothermia should be made throughout the perioperative period. Prior to arrival in the surgical suite, the elderly patient should be kept warm; active warming measures should be taken if the patient’s oral temperature is below 36°C. In the operating room, prior to the induction of anesthesia, skin exposure should be limited. Patients should be actively warmed if the anticipated anesthesia time is longer than 60 minutes. Warm fluids should be administered and the patient’s temperature should be continuously measured and maintained at or above 36°C. During the postoperative phase, active warming should be continued if body temperature is less than 36°C; shivering can be treated with meperidine.35
The choice of an optimal anesthetic technique in elderly individuals is fiercely debated in the anesthesia literature. It was hypothesized that the benefits of regional anesthesia (RA) versus general anesthesia (GA) were 2-fold: to create a stress-free anesthetic and to provide preemptive analgesia.70 Despite diminished sedation requirements, there appears to be no difference in the incidence of postoperative cognitive dysfunction (POCD) between elderly individuals receiving RA versus GA.71,72
If neuraxial anesthesia is considered, its use must be tailored toward the unique patient and his/her preexisting conditions. Recently, there has been an increase in the use of thrombolytic therapies. To date, there is no hard evidence recommending timing of placement and removal of neuraxial blockade with relationship to discontinuation of thrombolytic therapy.73 With respect to subcutaneous prophylactic unfractionated heparin, there are no contraindications for neuraxial blockade among patients who have received subcutaneous prophylactic doses. However, patients who have received 4 or more days of subcutaneous prophylactic unfractionated heparin should undergo evaluation of platelet counts to exclude the possibility of heparin-induced thrombocytopenia.73 Clearly, neuraxial anesthesia should be avoided in all patients who are coagulopathic.
Recently, the use of low-molecular weight heparin (LMWH) has become conventional, thus raising many questions regarding its use in the face of neuraxial techniques.74 If LMWH is administered in the preoperative period, a time interval of 10 to 12 hours should pass between the last administered dose of LMWH and placement of neuraxial blockade. With doses exceeding, prophylactic LMWH (Enoxaparin 40 mg once daily) require a 24-hour waiting period from the last LMWH dose to time of needle insertion for neuraxial anesthesia. In the postoperative period, single LMWH dosing should be initiated at a minimum of 6 hours postoperatively. If an indwelling neuraxial catheter remains present, the catheter should be removed at a minimum of 10 hours following the last dose of LMWH. Future dosing of LMWH after catheter removal should only occur at a minimum of 2 hours following removal of neuraxial catheter. If twice daily LMWH is required, indwelling catheters should be removed prior to starting LMWH administration.73
If a neuraxial technique is being considered, and the patient is receiving chronic oral anticoagulant therapy, these medications must be ceased a minimum of 4 days prior to the planned procedure. Such neuraxial techniques should only be performed after normalization of coagulation studies. If initiation of oral anticoagulants occurs during the period 24 hours prior to planned neuraxial blockade, or a second dose is given within 24 hours of the planned neuraxial blockade, prothrombin time (PT) and international normalized ratio (INR) levels should be measured. In such patients, in order to prevent the occurrence of epidural hematoma, the indwelling catheter removal should occur at INR levels less than 1.5. Moreover, neurologic testing should occur at regular intervals and continue 24 hours after catheter removal.73,75
Antiplatelet medication use is ubiquitous. Currently, there is no evidence to suggest that nonsteroidal anti-inflammatory drugs (NSAIDs), in sole use, increase the risk of developing a spinal hematoma. In contrast, the evidence with respect to ticlopidine and clopidogrel is not based on experience with neuraxial blockade but rather surgical and interventional outcomes. The literature suggests that prior to insertion of a neuraxial blockade, patients should discontinue ticlopidine and clopidogrel, 14 days and 7 days, respectively. However, due to its novelty, the data regarding fondaparinux and neuraxial techniques are lacking. As such, general recommendations cannot be made until further clinical trials exist. The use of neuraxial techniques and the anticoagulated patient is a complex relationship and few generalizations can be made. As always, patients must be considered on an individual basis, with careful attention to use of multiple anticoagulation agents, and the risks and benefits must be assiduously weighed.73
In a systematic review of regional anesthesia in total hip arthroplasty (THA), patients who received RA versus GA were noted to have decreased blood loss and less postoperative pain, nausea, and vomiting. However, no differences were noted between RA and GA with regard to length of stay, mortality, duration of surgery, or incidence of deep-vein thrombosis.76 Regional anesthesia is not without complications or failures. The estimated failure rate of RA, among the elderly patient population, ranges from 5% to 10%. Major complications including cardiac arrest, respiratory failure, seizure, and peripheral nerve toxicity have been reported.70,77 Furthermore, RA, through redistribution of blood volume, can lead to significant changes in hemodynamics.70 In the carefully selected elderly individual, RA is an excellent choice, but the risks and benefits must be weighed. As such, a gross generalization regarding choice of anesthetic technique cannot be made.
Once an anesthetic technique has been chosen, alterations in drug administration must be planned for the elderly patient. The aged patients' requirement for inhaled anesthetic agents is generally decreased. An 80-year-old individual has a 20% reduction in minimal alveolar concentration (MAC) value for inhaled agents as compared to individuals in their 40s.78–80 However, recovery from inhaled agents among elderly patients may be delayed due to decreases in minute ventilation and increases in volume of distribution.24 Increased body fat contents of elderly individuals serve to increase the volume of distribution and also increase the elimination half-time of lipid-soluble drugs. This increased volume of distribution, in conjunction with decreased hepatic clearance, can prolong the effects of such drugs as opioids and benzodiazepines. Furthermore, alterations in receptors and neurotransmitters can make the elderly patient particularly sensitive to the effects of benzodiazepines and opioids.81 The action of neuromuscular blockers (NMB) is not affected by increasing age; however, those NMBs that require elimination by either hepatic or renal functions have prolonged duration of action.
Decreases in neuronal sensitivity will make the elderly patient more sensitive to epidural and spinal anesthesia. Thus, when considering neuraxial anesthesia in this group, the dose of local anesthetic administered must be adjusted accordingly.82,83
Postoperative Considerations
Criteria promulgated by Beers represented an early attempt to identify drugs that may pose a risk of adverse events among elderly individuals.84 Newer modifications have been made to these criteria, and it is suggested to avoid drugs with significant anticholinergic activity among elderly patients such as diphenhydramine, hydroxine, and promethazine. Beers also recommends avoiding the use of meperidine, avoiding longer-acting benzodiazepines like diazepam, and limiting intermediate-acting benzodiazepines like lorazepam when possible. The avoidance of anticholinergic drugs in the perioperative period may lessen the likelihood of developing postoperative delirium (POD).84
Postoperative delirium and POCD are cognitive impairments that can occur following surgery. It is important to discern these 2 entities. Postoperative delirium is a transient phenomenon in which patients can experience fluctuating disturbances in consciousness.85 Postoperative cognitive dysfunction can be defined as a decline in higher-level cognitive functions of the brain encompassing language, imagination, perception, and planning.86 Postoperative delirium, in contrast to POCD, can usually be seen within the first 3 days during the postoperative period, whereas POCD can be diagnosed days to weeks following surgery. Perioperative factors have been associated with the development of POD including intraoperative blood loss, electrolyte imbalances, and sepsis, and treatment of POD is targeted at the underlying etiology.87 Preventative measures such as good sleep hygiene, early ambulation, and cognitive stimulation can reduce the incidence of POD.88 Although some risk factors for developing POCD have been identified, such as increasing age and decreased levels of education, the underlying etiology of POCD is unknown.86 Thus, without an identified cause, there are no known preventative measures or treatments for POCD.
Both pain and pain medication may contribute to POD.89 Elderly patients have an increased threshold for pain.90–92 It seems that older patients may complain of pain less frequently. However, these patients should not be placed on scheduled doses of pain medication. As mentioned previously, opioids must be used with caution due to changes in the elderly individual’s volume of distribution, clearance, and protein binding. The elimination half-time of morphine is prolonged to 4.5 hours compared to the 1.5 hours in younger patients.93,94 A balanced approach to analgesia in the elderly patient is necessary in managing the postoperative period, titrating medications carefully to effect. Such a methodology may include morphine, patient-controlled analgesia, weak opioids, nonopioid drugs, and regional analgesia when appropriate.
Other predictors of perioperative outcomes among elderly individuals include nutritional and postoperative pulmonary status. Malnutrition, specifically a serum albumin level below 35 g/L, has been associated with higher levels of morbidity and mortality.95,96 Strategies to hasten the return to preoperative nutritional status in the postoperative period are employed despite no proven benefit in decreasing postoperative complications by providing patients with supplemental, nutrition, such as parental or enteral nutrition.97,98 Despite this detail, strategies to hasten return preoperative nutritional status in the postoperative period should be employed.
Aged patients should also be encouraged to actively prevent pulmonary complications. A number of lung expansion techniques are available including but not limited to deep breathing exercises, incentive spirometry, and chest physical therapy. No technique has been shown to be superior; however, incentive spirometry is frequently employed due to its simplicity.60
Conclusion
With the rapid growth of the elderly population, along with increased comorbidities and greater life expectancy, geriatric surgery has become more frequent and requires careful tailoring of anesthesia technique. The aging process produces physiological, anatomical, and cognitive changes within the major organ systems of the body. Such changes have a significant impact on perioperative outcomes. It is not aging alone, per se, that alters perioperative risk, but rather its effect in association with age-related comorbidities. It is essential that the anesthesiologist be aware of the alterations associated with aging, coexisting diseases, and patient medications in order to provide the most effective perioperative treatment for this group of aged patients.
References
- 1. United States Census Bureau State and County Quickfacts Accessed March 28, 2010. from http://quickfacts.census.gov/qfd/states/00000.html
- 2. Life Expectancy Accessed March 28, 2010. from http://www.cdc.gov/nchs/fastats/lifexpec.htm
- 3. Owens WD. Overview of anesthesia for the geriatric patient. Int Anesthesiol Clin. 1988;26(2):96–97 [DOI] [PubMed] [Google Scholar]
- 4. Veering BT. Management of anaesthesia in elderly patients. Curr Opin Anaesthesiol. 1999;12(3):333–336 [DOI] [PubMed] [Google Scholar]
- 5. Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kazmers A, Perkins AJ, Jacobs LA. Outcomes after abdominal aortic aneurysm repair in those > or =80 years of age: recent Veterans Affairs experience. Ann Vasc Surg. 1998;12(2):106–112 [DOI] [PubMed] [Google Scholar]
- 7. Tasch MD. The autonomic nervous system and geriatric anesthesia. Int Anesthesiol Clin. 1988;26(2):143–151 [DOI] [PubMed] [Google Scholar]
- 8. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153–184 [DOI] [PubMed] [Google Scholar]
- 9. Musunuru V. The geriatric patient. In: Stoelting R, Dierdorf S. eds. Anesthesia and Co-existing Disease. New York: Churchill Livingstone; 1983 [Google Scholar]
- 10. Groban L. Diastolic dysfunction in the older heart. J Cardiothorac Vasc Anesth. 2005;19(2):228–236 [DOI] [PubMed] [Google Scholar]
- 11. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146 [DOI] [PubMed] [Google Scholar]
- 12. Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85(6):2176–2184 [DOI] [PubMed] [Google Scholar]
- 13. Colangelo PM, Blouin RA, Steinmetz JE, McNamara PJ, DeMaria AN, Wedlund PJ. Age and beta-adrenergic receptor sensitivity to S(−)- and R,S(+/−)-propranolol in humans. Clin Pharmacol Ther. 1992;51(5):549–554 [DOI] [PubMed] [Google Scholar]
- 14. Fitzgerald D, Doyle V, Kelly JG, O'Malley K. Cardiac sensitivity to isoprenaline, lymphocyte beta-adrenoceptors and age. Clin Sci (Lond). 1984;66(6):697–699 [DOI] [PubMed] [Google Scholar]
- 15. Vestal RE, Wood AJ, Shand DG. Reduced beta-adrenoceptor sensitivity in the elderly. Clin Pharmacol Ther. 1979;26(2):181–186 [DOI] [PubMed] [Google Scholar]
- 16. Campbell EJ, Lefrak SS. How aging affects the structure and function of the respiratory system. Geriatrics. 1978;33(6):68–74 [PubMed] [Google Scholar]
- 17. Chalfin DB. Outcome assessment in elderly patients with critical illness and respiratory failure. Clin Chest Med. 1993;14(3):583–589 [PubMed] [Google Scholar]
- 18. Krumpe PE, Knudson RJ, Parsons G, Reiser K. The aging respiratory system. Clin Geriatr Med. 1985;1(1):143–175 [PubMed] [Google Scholar]
- 19. Nickalls RW, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003;91(2):170–174 [DOI] [PubMed] [Google Scholar]
- 20. Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124(4):387–391 [DOI] [PubMed] [Google Scholar]
- 21. Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol. 1968;25(6):664–671 [DOI] [PubMed] [Google Scholar]
- 22. Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest. 1992;101(3):800–809 [DOI] [PubMed] [Google Scholar]
- 23. Zaugg M, Lucchinetti E. Respiratory function in the elderly. Anesthesiol Clin North Am. 2000;18(1):47–58, vi [DOI] [PubMed] [Google Scholar]
- 24. Conzen P, Peter K. Inhalation anaesthesia at the extremes of age: geriatric anaesthesia. Anaesthesia. 1995;50(suppl):29–33 [DOI] [PubMed] [Google Scholar]
- 25. Greenblatt DJ, Shader RI, Harmatz JS. Implications of altered drug disposition in the elderly: studies of benzodiazepines. J Clin Pharmacol. 1989;29(10):866–872 [DOI] [PubMed] [Google Scholar]
- 26. Advenier C, Saint-Aubin A, Gobert C, Houin G, Albengres E, Tillement JP. Pharmacokinetics of isoniazid in the elderly. Br J Clin Pharmacol. 1980;10(2):167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villesen HH, Banning AM, Petersen RH, et al. Pharmacokinetics of morphine and oxycodone following intravenous administration in elderly patients. Ther Clin Risk Manag. 2007;3(5):961–967 [PMC free article] [PubMed] [Google Scholar]
- 28. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76 [DOI] [PubMed] [Google Scholar]
- 29. Sophie S. Anaesthesia for the elderly patient. J Pak Med Assoc. 2007;57(4):196–201 [PubMed] [Google Scholar]
- 30. Buemi M, Nostro L, Aloisi C, Cosentini V, Criseo M, Frisina N. Kidney aging: from phenotype to genetics. Rejuvenation Res. 2005;8(2):101–109 [DOI] [PubMed] [Google Scholar]
- 31. Muhlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology. 1999;45(5):243–253 [DOI] [PubMed] [Google Scholar]
- 32. Mooradian AD. Normal age-related changes in thyroid hormone economy. Clin Geriatr Med. 1995;11(2):159–169 [PubMed] [Google Scholar]
- 33. Peeters RP. Thyroid hormones and aging. Hormones (Athens). 2008;7(1):28–35 [DOI] [PubMed] [Google Scholar]
- 34. Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201(4350):16–22 [DOI] [PubMed] [Google Scholar]
- 35. Torossian A. Thermal management during anaesthesia and thermoregulation standards for the prevention of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22(4):659–668 [DOI] [PubMed] [Google Scholar]
- 36. Fox RH, Solman AJ, Isaacs R, Fry AJ, MacDonald IC. A new method for monitoring deep body temperature from the skin surface. Clin Sci. 1973;44(1):81–86 [DOI] [PubMed] [Google Scholar]
- 37. Howell TH. Normal temperatures in old age. Lancet. 1948;1(6501):517. [DOI] [PubMed] [Google Scholar]
- 38. Salvosa CB, Payne PR, Wheeler EF. Environmental conditions and body temperatures of elderly women living alone or in local authority home. Br Med J. 1971;4(5788):656–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morrison RC. Hypothermia in the elderly. Int Anesthesiol Clin. 1988;26(2):124–133 [DOI] [PubMed] [Google Scholar]
- 40. Poehlman E. Endurance exercise in aging humans: effects on energy metabolism. In: Holloszy H. ed. Exercise and Sports Science Reviews. Baltimore, MD: Williams and Wilkins; 1994:251–284 [PubMed] [Google Scholar]
- 41. Falk B, Bar-Or O, Smolander J, Frost G. Response to rest and exercise in the cold: effects of age and aerobic fitness. J Appl Physiol. 1994;76(1):72–78 [DOI] [PubMed] [Google Scholar]
- 42. Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol. 1996;80(2):512–515 [DOI] [PubMed] [Google Scholar]
- 43. Khan F, Spence VA, Belch JJ. Cutaneous vascular responses and thermoregulation in relation to age. Clin Sci (Lond). 1992;82(5):521–528 [DOI] [PubMed] [Google Scholar]
- 44. Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol. 1997;82(1):93–101 [DOI] [PubMed] [Google Scholar]
- 45. Tonner PH, Kampen J, Scholz J. Pathophysiological changes in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17(2):163–177 [DOI] [PubMed] [Google Scholar]
- 46. Ramaiah R, Lam AM. Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin. 2009;27(3):485–496 [DOI] [PubMed] [Google Scholar]
- 47. Brody H. The aging brain. Acta Neurol Scand Suppl. 1992;137:40–44 [DOI] [PubMed] [Google Scholar]
- 48. Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician. 2004;33(10):783–787 [PubMed] [Google Scholar]
- 49. Christensen H. What cognitive changes can be expected with normal ageing?. Aust N Z J Psychiatry. 2001;35(6):768–775 [DOI] [PubMed] [Google Scholar]
- 50. Salthouse TA. Memory aging from 18 to 80. Alzheimer Dis Assoc Disord. 2003;17(3):162–167 [DOI] [PubMed] [Google Scholar]
- 51. Meguro K, Shimada M, Yamaguchi S, et al. Cognitive function and frontal lobe atrophy in normal elderly adults: implications for dementia not as aging-related disorders and the reserve hypothesis. Psychiatry Clin Neurosci. 2001;55(6):565–572 [DOI] [PubMed] [Google Scholar]
- 52. Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004;2004(23):pe26 [DOI] [PubMed] [Google Scholar]
- 54. Tessler MJ, Kardash K, Wahba RM, Kleiman SJ, Trihas ST, Rossignol M. The performance of spinal anesthesia is marginally more difficult in the elderly. Reg Anesth Pain Med. 1999;24(2):126–130 [DOI] [PubMed] [Google Scholar]
- 55. Faulkner JA, Brooks SV, Zerba E. Skeletal muscle weakness and fatigue in old age: underlying mechanisms. Annu Rev Gerontol Geriatr. 1990;10:147–166 [DOI] [PubMed] [Google Scholar]
- 56. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73–86 [DOI] [PubMed] [Google Scholar]
- 57. Barnett SR. Polypharmacy and perioperative medications in the elderly. Anesthesiol Clin. 2009;27(3):377–389 [DOI] [PubMed] [Google Scholar]
- 58. Anesthesiologists warn: if you’re taking herbal products, tell your doctor before surgery. http://www.asahq.org/patientEducation/herbPatient.pdf Accessed May 25, 2010.
- 59. Leak JA. Perioperative considerations in the management of the patient taking herbal medicines. Curr Opin Anaesthesiol. 2000;13(3):321–325 [DOI] [PubMed] [Google Scholar]
- 60. Neragi-Miandoab S, Wayne M, Cioroiu M, Zank LM, Mills C. Preoperative evaluation and a risk assessment in patients undergoing abdominal surgery. Surg Today. 2010;40(2):108–13 [DOI] [PubMed] [Google Scholar]
- 61. Moore S, Mills BB, Moore RD, Miklos JR, Mattox TF. Perisurgical smoking cessation and reduction of postoperative complications. Am J Obstet Gynecol. 2005;192(5):1718–1721 [DOI] [PubMed] [Google Scholar]
- 62. Myles PS, Iacono GA, Hunt JO, et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology. 2002;97(4):842–847 [DOI] [PubMed] [Google Scholar]
- 63. Smetana GW. A 68-year-old man with COPD contemplating colon cancer surgery. JAMA. 2007;297(19):2121–2130 [DOI] [PubMed] [Google Scholar]
- 64. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98(8):760–766 [DOI] [PubMed] [Google Scholar]
- 65. Smetana GW, Conde MV. Preoperative pulmonary update. Clin Geriatr Med. 2008;24(4):607–624, vii [DOI] [PubMed] [Google Scholar]
- 66. Loran DB, Hyde BR, Zwischenberger JB. Perioperative management of special populations: the geriatric patient. Surg Clin North Am. 2005;85(6):1259–1266, xi [DOI] [PubMed] [Google Scholar]
- 67. Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007;50(17):e159–e241 [DOI] [PubMed] [Google Scholar]
- 68. Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14 [DOI] [PubMed] [Google Scholar]
- 69. Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22(4):645–657 [DOI] [PubMed] [Google Scholar]
- 70. Roy RC. Choosing general versus regional anesthesia for the elderly. Anesthesiol Clin North Am. 2000;18(1):91–104, vii [DOI] [PubMed] [Google Scholar]
- 71. Haan J, van Kleef JW, Bloem BR, et al. Cognitive function after spinal or general anesthesia for transurethral prostatectomy in elderly men. J Am Geriatr Soc. 1991;39(6):596–600 [DOI] [PubMed] [Google Scholar]
- 72. Rasmussen LS, Johnson T, Kuipers HM, et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47(3):260–266 [DOI] [PubMed] [Google Scholar]
- 73. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35(1):64–101 [DOI] [PubMed] [Google Scholar]
- 74. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1 suppl):64S–94S [DOI] [PubMed] [Google Scholar]
- 75. Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28(3):172–197 [DOI] [PubMed] [Google Scholar]
- 76. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anaesthesia improve outcome after total hip arthroplasty? A systematic review. Br J Anaesth. 2009;103(3):335–345 [DOI] [PubMed] [Google Scholar]
- 77. Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97(5):1274–1280 [DOI] [PubMed] [Google Scholar]
- 78. Gold MI, Abello D, Herrington C. Minimum alveolar concentration of desflurane in patients older than 65 yr. Anesthesiology. 1993;79(4):710–714 [DOI] [PubMed] [Google Scholar]
- 79. Munson ES, Hoffman JC, Eger EI 2nd. Use of cyclopropane to test generality of anesthetic requirement in the elderly. Anesth Analg. 1984;63(11):998–1000 [PubMed] [Google Scholar]
- 80. Rampil IJ, Lockhart SH, Zwass MS, et al. Clinical characteristics of desflurane in surgical patients: minimum alveolar concentration. Anesthesiology. 1991;74(3):429–433 [DOI] [PubMed] [Google Scholar]
- 81. Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990;6(2):269–283 [PubMed] [Google Scholar]
- 82. Sadean MR, Glass PS. Pharmacokinetics in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17(2):191–205 [DOI] [PubMed] [Google Scholar]
- 83. Vuyk J. Pharmacodynamics in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17(2):207–218 [DOI] [PubMed] [Google Scholar]
- 84. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157(14):1531–1536 [PubMed] [Google Scholar]
- 85. Parikh SS, Chung F. Postoperative delirium in the elderly. Anesth Analg. 1995;80(6):1223–1232 [DOI] [PubMed] [Google Scholar]
- 86. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861 [DOI] [PubMed] [Google Scholar]
- 87. Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105(5):380–384 [DOI] [PubMed] [Google Scholar]
- 88. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857 [PubMed] [Google Scholar]
- 89. Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86(4):781–785 [DOI] [PubMed] [Google Scholar]
- 90. French R, Van Vliet H, Cowan F, et al. Hormonally impregnated intrauterine systems (IUSs) versus other forms of reversible contraceptives as effective methods of preventing pregnancy. Cochrane Database Syst Rev. 2004(3):CD001776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gagliese L, Katz J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103(1-2):11–20 [DOI] [PubMed] [Google Scholar]
- 92. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20(4):227–239 [DOI] [PubMed] [Google Scholar]
- 93. Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21(1):109–127 [DOI] [PubMed] [Google Scholar]
- 94. Owen JA, Sitar DS, Berger L, Brownell L, Duke PC, Mitenko PA. Age-related morphine kinetics. Clin Pharmacol Ther. 1983;34(3):364–368 [DOI] [PubMed] [Google Scholar]
- 95. Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134(1):36–42 [DOI] [PubMed] [Google Scholar]
- 96. Weber RS, Hankins P, Rosenbaum B, Raad I. Nonwound infections following head and neck oncologic surgery. Laryngoscope. 1993;103(1 Pt 1):22–27 [DOI] [PubMed] [Google Scholar]
- 97. Perioperative total parenteral nutrition in surgical patients The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325(8):525–532 [DOI] [PubMed] [Google Scholar]
- 98. Detsky AS, Baker JP, O’Rourke K, Goel V. Perioperative parenteral nutrition: a meta-analysis. Ann Intern Med. 1987;107(2):195–203 [DOI] [PubMed] [Google Scholar]