Abstract
The purpose of this study was to determine the relationship between methylation status of the insulin-like growth factor 2 (IGF-2) gene and methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphisms in bladder transitional cell carcinoma tissues in a Chinese population. The polymorphisms of the folate metabolism enzyme gene MTHFR were studied by restrictive fragment length polymorphism (RFLP). PCR-based methods of DNA methylation analysis were used to detect the CpG island methylation status of the IGF-2 gene. The association between the methylation status of the IGF-2 gene and clinical characteristics, as well as MTHFR C677T polymorphisms, was analyzed. Aberrant hypomethylation of the IGF-2 gene was found in 68.3% bladder cancer tissues and 12.4% normal bladder tissues, respectively, while hypomethylation was not detected in almost all normal bladder tissues. The hypomethylation rate of the IGF-2 gene in cancer tissues was significantly higher in patients with lymph node metastasis than in those without lymph node metastasis (46.3% vs 17.2%, P = 0.018). No association was found between aberrant DNA methylation and selected factors including sex, age, tobacco smoking, alcohol consumption and green tea consumption. After adjusting for potential confounding variables the variant allele of MTHFR C677T was found to be associated with hypomethylation of the IGF-2 gene. Compared with wildtype CC, the odds ratio was 4.33 (95% CI=1.06-10.59) for CT and 4.95 (95% CI=1.18-12.74) for TT. MTHFR 677 CC and CT genotypes might be one of the reasons that cause abnormal hypomethylation of the IGF-2 gene, and the aberrant CpG island hypomethylation of the IGF-2 gene may contribute to the genesis and progression of bladder transitional cell carcinoma.
Keywords: methylenetetrahydrofolate reductase, folate, epidemiology, methylation, bladder transitional cell carcinoma, insulin-like growth factor 2 (IGF-2)
INTRODUCTION
Approximately 500,000 individuals in the U.S have been diagnosed with bladder cancer[1], i.e., transitional cell carcinoma transitional cell carcinoma of the bladder. It is one of the most common malignancies affecting the genitourinary tract and is characterized by multifocality and a high incidence of recurrence[2]. Although its carcinogenesis is unclear so far, the consensus is that the accumulation of multiple genetic and epigenetic alternations lead to the activation of proto-oncogenes and/or inactivation of tumor suppressor genes[3]-[5].
Aberrant DNA methylation is now recognized as an important epigenetic alteration which occurs early in human cancers[6], including bladder cancer[7]. In general, DNA methylation alterations, which play a strong role in tumorigenesis[8], are probably the most widely studied epigenetic alterations in cancer[9] and are increasingly becoming a hot subject of research. Carcinogenesis is associated with changes in this epigenetic phenomenon, including two distinct and seemingly opposing trends: global decrease in cytosine methylation (hypomethylation or unmethylation) and methylation of cytosine in CpG islands (hypermethylation)[10]. One study, performed in Spain, demonstrated that neoplasia is correlated with overall genomic hypomethylation[11]. It reported that failure to repress genes appropriately by abnormal demethylation of tissue-restricted genes or by hypomethylation of proto-oncogenes could result in the loss of tissue specificity and could promote cancer formation.
Thus, the exploration of its modulating mechanisms is expected to play important roles in the early diagnosis, treatment and prognosis of tumors. As the monocarbon unit is required for DNA, synthesis of methylation is provided by the folate metabolism pathway. Methylenetetrahydrofolate reductase (MTHFR) plays a critical role in folate metabolism, which is an important pathway for DNA methylation. Folate metabolism impaired by the genetic variants (C667T and A1298C) of MTHFR could change DNA methylation pattern, including promoter hypomethylation, which has been frequently observed in cancer[12]. However, little research has been done regarding this topic in transitional cell carcinoma. Therefore, his study was conducted to investigate the correlation between the methylation pattern of the proto-oncogene IGF-2 in transitional cell carcinoma and the gene polymorphism of the folate metabolism enzyme, as well as their clinical characteristics.
MATERIALS AND METHODS
Sample information
Frozen bladder tissue and blood samples, including 125 carcinoma samples and 125 normal tissue samples, from 125 subjects were obtained from the First Affiliated Hospital of Nanjing Medical University, China. The tumor type was classified as transitional cell carcinoma by two experienced pathologists following the World Health Organization (WHO) standard. All patients had well-documented clinical histories and follow-up information. The study was approved by our institute's Human Research Ethics Committee.
Isolation of genomic DNA from tissues
Genomic DNA was isolated from all tissues by using Promega's wizard DNA isolation kit according to the manufacturer's instructions. Bladder cancer tissue and normal bladder tissue were obtained after surgical resection and stored at -70°C. The tissues were then incubated at 55°C in homogenization buffer containing 50 mmol/L Tris (pH 8.0), 1 mmol/L EDTA, 0.5% Tween-20, and 5 mg/mL proteinase K for 3 h, and genomic DNA was isolated using Promega's DNA isolation kit.
PCR-based methods of DNA methylation analysis
Following the manufacturer's recommendations, approximately 500 ng of the obtained DNA was digested at 37°C for 14 h with 10 units of the methylation-specific restriction endonuclease Hpa II (Roche Molecular Biochemicals, Mannheim, Germany), which recognizes the methylated sequence 5′-C↓CGG-3′. The digested DNA was subjected to PCR amplification with the designed primers using Primer 3 plus Software (SourceForge, Inc., Mountain View, CA,USA) encompassing the CpG clusters in exon 9 of the IGF2 gene. The forward primer: 5′-GAAGATGCTGCTGTGCTTCC-3′, and the reverse primer: 5′-AGTGAGCAAAACTGCCGC-3′ were synthesized commercially by TIANGEN Biotechnologies (Beijing, China). Genomic PCR without Hpa II digestion for each sample was used as internal control. Dilutions of DNA from the digestion reaction were then used for each PCR. PCR conditions were 2 min at 94°C, followed by 27 cycles of 94°C for 30 sec, 53°C for 30 sec, and 68°C for 1 min for each primer set. The PCR products were analyzed by 2% agarose gel electrophoresis, and the amplified bands were analyzed in UV I Tech Gel Documentation system (UVI-Tech Ltd., Cambridge, United Kingdom). Undigested DNA of each sample was selected as an internal control. All normal bladder controls were set up with each batch of transitional cell carcinoma samples processed.
Genotyping of the MTHFR gene by PCR-RFLP
Genomic DNA was extracted from freshly frozen blood using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The extracted DNA was stored at 4°C for subsequent analysis. Genotyping of the MTHFR C677T was performed by PCR-RFLP. PCR amplification was performed using 5′-CAAAGGCCACCCCGAAGC-3′ and 5′-AGGACGGTGCGGTGAGAGTG-3′ (Sangon, Shanghai, China) as the forward and reverse primer pairs, respectively. Each amplification reaction was performed in a total volume of 25 µL, containing 10×PCR buffer (1.8 mmol/L MgCl2), 1 U Taq polymerase, 2.5 mmol/L of each dNTP (Tiangen, Beijing, China), 5 pmol/L of each primer and 2 µL of genomic DNA. PCR was run at 94°C for 5 min and 34 cycles at 94°C for 45 sec, 61.5°C for 40 sec and 72°C for 50 sec. This was followed by a final extension at 72°C for 7 min. Then, 5 units of Hinf I enzyme was added directly to the PCR products and digested at 37°C overnight. After restriction enzyme digestion of the amplified DNA, genotypes were identified by electrophoresis on 2% agarose gels and visualized with ethidium-bromide staining under ultraviolet illumination. Genotypes were scored by an experienced reader blinded to the epidemiological data and serum lipid levels. The PCR products were purified by low melting point gel electrophoresis and phenol extraction, and then the DNA sequences were analyzed in Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China).
Statistical analysis
All statistical analyses were conducted with SPSS 11.0 (Chicago, Illinois, USA) and STATA 9.2 (Stata Corp, College Station, TX, USA) statistical software. Folate intake was calculated by monitoring the subjects' daily food intake, and then the sum of all folate taken from various foods was calculated as the total folate intake with reference to the nutrition values specified in the “food compositions”. Folate intake levels were divided into four groups according to the percentile interval P25, P50 and P75, i.e. Q1, Q2, Q3 and Q4 groups from low to high, respectively. The correlation between IGF-2 gene hypomethylation frequency and age, sex, smoking history, history of alcohol consumption, history of tea consumption, sites of pathological changes and TNM staging, was done by Pearson χ2 test. In the case of a sample size unsuitable for Pearson χ2 test, Fisher exact test would be used instead. Unconditional logistic regression model was carried out to analyze the correlation between IGF-2 gene methylation and MTHFR C677T genetic polymorphism with age, gender and folate intake as potential confounding variables.
RESULTS
Demographic and clinical characteristics of the study participants
We obtained bladder cancer tissues and normal tissue samples from 125 patients with transitional cell carcinoma. The subjects had a median age of 62 years; 81 (64.8%) were male and 44 (35.2%) were female. Twenty-three (54.4%) of the cases had pathological bladder changes located in the bladder side wall; 68 (27.2%) cases had pathological changes in the lateral bladder wall; 34 (18.4%) cases had pathological changes at the bladder trigone area. According to the TNM staging criteria, T1, T2, T3 and T4 accounted for 16.8%, 36.8%, 40.0%, and 6.4%, respectively. Fifty-nine (47.2%) of the cases had regional lymph node metastases, while remote metastasis only occurred in one case (0.8%).
Methylation status of the IGF-2 gene and its correlation with clinical characteristics
The correlation between IGF-2 gene hypomethylation frequency and age, gender, smoking, alcohol drinking, tea intake etc., was not statistically significant (Table 1). According to the methylation reaction electrophoresis results (Fig. 1), the hypomethylation frequency of the IGF-2 gene in transitional cell carcinoma tissues was about 68.3% while 12.4% was detected in normal bladder tissues. The N stage in TNM staging, i.e., the occurrence of lymph node metastasis, was associated with IGF-2 gene methylation frequency. Specifically, the IGF-2 gene in cancer patients with lymph node metastasis had a methylation frequency of 46.3%, which was significantly lower than that in patients without lymph node metastasis (17.2%, P < 0.05, Table 2).
Table 1. Association between the IGF2 gene methylation and sex, age and selected factors in transitional cell carcinoma (TCC) patients.
| Variables | Cases(n) | Frequency of hypomethylation[n (%)] |
|
| TCC tissues | Normal bladder tissues | ||
| Sex | |||
| Male | 81 | 21(25.9) | 8(9.9) |
| Female | 44 | 13(29.5) | 6(13.6) |
| P value | 0.664 | 0.561 | |
| Age (years) | |||
| <60 | 49 | 12(24.5) | 8(16.3) |
| ≥60 | 76 | 22(28.9) | 6(7.9) |
| P value | 0.585 | 0.144 | |
| Tobacco smoking | |||
| Never | 68 | 20(29.4) | 8(11.8) |
| Ever | 57 | 14(24.6) | 6(10.5) |
| P value | 0.544 | 0.827 | |
| Alcohol drinking | |||
| Never | 71 | 21(29.6) | 8(11.3) |
| Ever | 54 | 13(24.1) | 6(11.1) |
| P value | 0.493 | 0.978 | |
| Green tea drinking | |||
| Never | 77 | 23(29.9) | 10(13.0) |
| Ever | 48 | 11(22.9) | 4(8.3) |
| P value | 0.395 | 0.422 | |
Smoking is the inhalation of the smoke of burning tobacco that is used mostly in three forms: cigarettes, pipes, and cigars. Alcohol is consumed largely for their physiological and psychological effects. The two items were divided into never and ever according to their histories. P values for Fisher's exact test.
Fig. 1. Analysis of the methylation of the IGF2 gene by PCR-based methods.
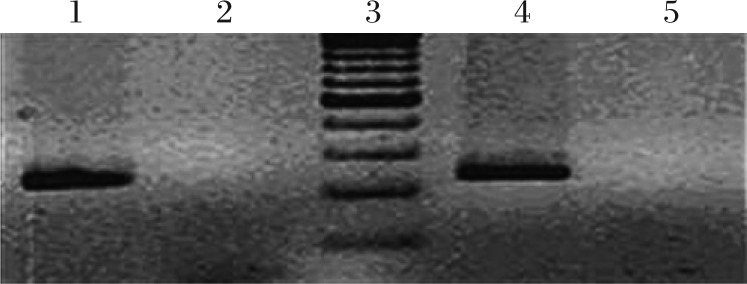
DNA samples of transitional cell carcinoma patients were studied for methylation of the IFG2 gene by PCR-based methods as detailed in “MATERIALS AND METHODS”. Agarose gel electrophoresis showed the presence of a 214 bp DNA band in lane 1 and 4, which indicated that the CpG cluster at exon 9 of the IGF2 gene was methylated. No such band was present at lane 2 and 5, which indicated that there was no methylation of the IGF2 gene at exon 9 CpG cluster. lane 3: DNA 100 bp molecular weight marker.
Table 2. Association between hypomethylation of the IGF2 and clinical characteristics in transitional cell carcinoma (TCC) patients.
| Variables | Cases(n) | Frequency of hypomethylation[n (%)] |
|
| TCC tissues | Normal bladder tissues | ||
| Site | |||
| Side wall | 23 | 8(34.8) | 3(13.0) |
| Lateral wall | 68 | 14(20.6) | 7(10.3) |
| Trigone | 34 | 12(35.3) | 4(11.8) |
| P value | 0.193 | 0.929 | |
| T stage | |||
| Tl/2 | 67 | 18(26.9) | 8(11.9) |
| T3/4 | 58 | 16(27.6) | 6(10.3) |
| P value | 0.928 | 0.778 | |
| N stage | |||
| N0 | 66 | 11(17.2) | 7(10.6) |
| N1 | 59 | 27(46.3) | 7(11.9) |
| P value | 0.018 | 0.824 | |
| M stage | |||
| M0 | 124 | 33(26.6) | 14(11.3) |
| M1 | 1 | 1 (100) | 0 |
| P value | 0.272 | 1.000 | |
P values for Fisher's exact test.
Relationship between IGF-2 gene methylation status and polymorphism of MTHFR C677T
We compared the correlation between the IGF-2 gene methylation status and MTHFR genotype CC, CT and TT in transitional cell carcinoma tissues and normal bladder tissue. After adjusting other potential confounding variables such as age, gender, folate intake, we found that the IGF-2 gene hypomethylation frequency in the transitional cell carcinoma tissues from carriers of MTHFR variant CT and TT genotypes was significantly increased compared with the MTHFR wildtype genotype CC, with the OR values of 4.33 (95% CI=1.06-10.59) and 4.95 (95% CI=1.18-12.74), respectively (Figs. 2, 3 and Table 3).
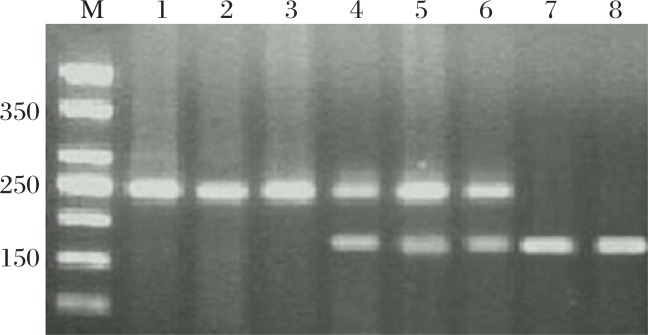
Fig. 2. MTHFR C 677T gene fragment length polymorphism distribution by Hinf I restriction enzyme (2.5% agarose gel electrophoresis).
M: 100 bp ladder Marker; Lanes 1-3: CC genotype; Lanes 4-6: CT genotype; Lanes 7 and 8: TT genotype.
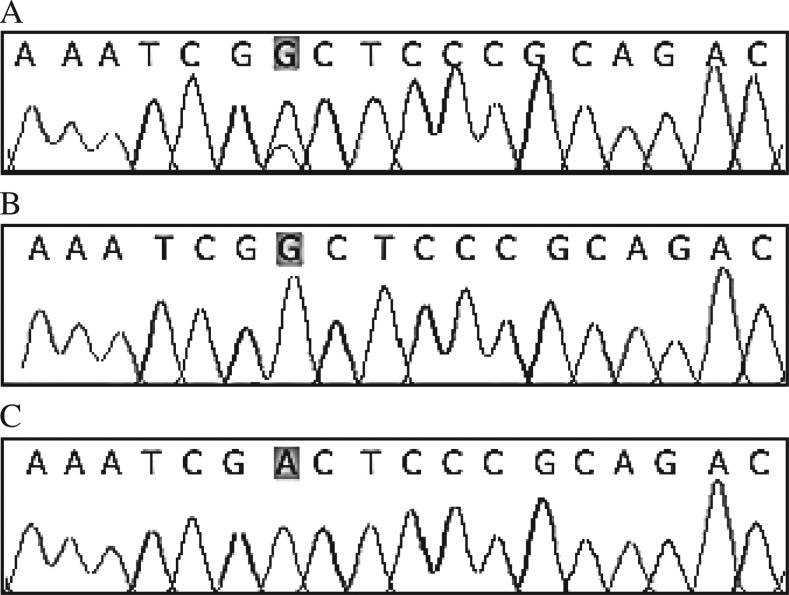
Fig. 3. Partial nucleotide sequence of the MTHFR C677T locus.
A: CT genotype; B: CC genotype; C: TT genotype.
Table 3. Association between hypomethylation of IGF-2 and MTHFR C677T polymorphisms in transitional cell carcinoma (TCC) patients.
| Variables | OR(95% CI) * |
|
| TCC tissues | Normal bladder tissues | |
| Age(years) | 1.01(0.10-1.08) | 0.91(0.83-1.00) |
| Sex | ||
| Male | 1.00 | 1.00 |
| Female | 1.32(0.53-3.24) | 2.37(0.65-8.65) |
| Folate intake(µg/d) | ||
| Q1 | 1.00 | 1.00 |
| Q2 | 2.53(0.89-7.14) | 2.65(0.58-11.99) |
| Q3 | 0.58(0.15-2.21) | 0.34(0.03-3.78) |
| Q4 | 5.09(1.02-25.36) | 4.23(0.51-34.79) |
| MTHFR C677T | ||
| CC | 1.00 | 1.00 |
| CT | 4.33 (1.06-10.59) | 2.90(0.63-13.33) |
| TT | 4.95 (1.18-12.74) | 0.80(0.11-5.72) |
*Adjusted by age, sex, folate intake and MTHFR C677T genotypes. The cut point of folate intake was quartile (µg/d), Ql: 0-26.2; Q2: 26.3-99.4; Q3: 99.5-311.0; Q4: ≥ 311.1.
DISCUSSION
Genomic DNA methylation is the best-studied epigenetic modification currently discovered. The process is promoted by DNA methyltransferases which use S-adenosyl-methionine as the methyl donor[13]. 5-methylcytosine is formed through the addition of a methyl group to the fifth carbon position of the cytosinepyrimidine ring by DNA methyltransferases[14]. DNA methylation is an epigenetic mechanism, which plays an important role in regulating gene expression at the transcription level[15]. Through methylation of cytosine of the CpG island in the gene promoter and other CpG-riched regions, some redundant genes in certain tissues or cells can be silenced, while demethylation can function as an activator in some tissueor stage-specific genes, which contribute to the temporal and spatial regulation of gene expression[16],[17]. Previous studies have described global genomic hypomehtylation in several malignant cancers, including bladder carcinoma[18] and other organic carcinomas, by evaluating genomic repetitive sequences. Therefore, these epigenetic mechanisms in the transcriptional regulation of genes play critical roles in cancer progression[19].
IGF-2 is a member of the IGF family, which is a circulating peptide hormone and locally acting growth factor with both paracrine and autocrine functions[20],[21]. IGF-2 could encourage cell division in a variety of tissues. The IGF-2 gene is located at chromosome 11p15.5 and is flanked by the insulin and H19 genes in a region that is known to have differential parental methylation. The IGF-2 gene is associated with CpG regions that have allele-specific DNA methylation known as differentially methylated regions (DMRs). IGF-2 consists of nine exons in humans; a few are non-coding leader exons, while exons 6-9 encode prepro-IGF-2 polypeptide. There are four CpG islands within the IGF2 gene. The first CpG island maps between the first two untranslated exons and is found to be fully methylated on both alleles in all tissues. The second and third CpG islands (DMR0) map to promoter 2, 4 (P2-P4) and the IGF2 antisense (IGF2-AS) transcripts, and this region is fully unmethylated in all tissues. However, a subsequent study showed that paternal DMR0 was fully methylated, contradicting the previous studies[22],[23]. The final CpG island maps to exon 9, which has previously been defined as DMR in both human and mouse, with the paternal allele being more methylated than the maternal allele[24]. Although the IGF-2 is the first autosomal gene identified to exhibit imprinting, a study indicated that 2/9 (22.2%) of cases displaying loss of imprinting (LOI) of the IGF-2 and 2/16 (12.5%) had LOI of H19 in bladder cancer[25], However, the precise regulatory mechanism of the IGF-2 in transitional cell carcinoma is not clear. The present study is the first to assess the methylation status of the exon 9 CpG cluster in samples from transitional cell carcinoma and neighboring normal bladder tissue by PCR-based methylation assay. In our present study, the IGF-2 gene hypomethylation rate in transitional cell carcinoma was 68.3% compared with the normal healthy controls. The presence of regional lymph node metastasis was also associated with decreased IGF-2 gene methylation frequency, i.e., in the cancer tissues from patients with lymph node metastasis, the IGF-2 gene hypomethylation frequency (46.3%) was significantly higher than that of patients without lymph node metastasis (17.2%), suggesting that abnormal IGF-2 gene hypomethylation might serve as a valuable biomarker in transitional cell carcinoma diagnosis and a potential indicator of transitional cell carcinoma prognosis.
Unlike genetic changes, epigenetic alterations are more dynamic and often reversible, depending on the presence or removal of the inducing factors[26]. Further exploration of the impact factors for epigenetic alterations was of great value for the early prevention and intervention of diseases, but knowledge in this field is still relatively lacking[27]. It is found in this study that environmental factors (for example, dietary nutrients) are associated with genomic DNA methylation, especially deficiency in methyl group donors such as vitamin A and methionine. Zhang et al.[28] showed that a dietary pattern characterized by a high intake of vegetables and fruits might protect against global DNA hypomethylation. Folate is an important dietary nutrient, which accepts one-carbon units from one-carbon unit donors. DNA methylation status was determined as a functional endpoint, suggesting that abnormal folate metabolism may affect the genomic methylation state negatively. MTHFR is a key enzyme regulating folate metabolism, which affects DNA methylation and synthesis[29]. MTHFR converts 5, 10-methylentetrahydrofolate to 5-methyltetrahydrofolate, which is required for homocysteine methylation to methionine. Methionine is then activated to S-adenosylmethionine, a universal methyl donor in numerous transmethylation reactions, including methylation of DNA, RNA, proteins, and other molecules[30]. Moreover, the enzyme activities of MTHFR 677TT are only 30% of those of the wildtype 677CC genotypes, while the enzyme activities of the MTHFR 677CT are 60% of the wildtype enzyme[31]. Changing activity of MTHFR enzyme is associated with polymorphism in the MTHFR gene, which would affect its abilities involved in DNA synthesis and the supply of methyl group. Therefore, the association between MTHFR gene polymorphism and DNA methylation is given high priority all over the world. Friso et al.[29] reported that in human lymphocytes, the gene-nutrient interaction affecting DNA methylation in 1298AA was mainly due to the coexistence of the 677TT genotype. Axume et al.[32] suggested that the MTHFR 677TT genotype and folate interacted to lower global leukocyte DNA methylation patterns in young Mexican American women. Moreover, Supic et al.[33] found a significant association between TT genotype and methylation status of the RASSF1A gene in oral squamous cell carcinoma patients.
In this study, we found that carriers of MTHFR CC and CT mutation genotypes increased the IGF-2 gene hypomethylation frequency in their transitional cell carcinoma tissues, which was associated with the level of folate intake. Source of dietary folate, amount of folate intake, as well as body metabolism will affect the supply of methyl group required for DNA methylation. Ma et al.[34] also provided support for an important role of folate metabolism in colon carcinogenesis. These results suggest that the 677TT mutation in MTHFR reduces colon cancer risk, perhaps by increasing 5,10-methylenetetrahydrofolate levels for DNA synthesis, but that low folate intake or high alcohol consumption may negate some of the protective effect. It is generally believed that with sufficient folate intake, the risk of cancer in MTHFR 677CT or TT genotype carriers would be reduced, since low activity of folate metabolic enzyme will facilitate DNA synthesis, while adequate folate can provide sufficient methyl groups for DNA methylation. Conversely, if folate intake is deficient, DNA synthesis, repair and methylation would all be affected. A deficiency in 5, 10-methylene THF supply would result in difficulty executing the protective function of MTHFR mutation genotype, therefore disturbing the DNA methylation process[35],[36]. However, during carcinogenesis, the whole genome is in a hypomethylated state, accompanied by hypermethylation of specific genes. Therefore, further studies to investigate how individual folate intake level and folate metabolism pathway disorder affect this co-existence of hyper- and hypo-methylation state seem justified.
In summary, abnormal methylation in exon 9 of the IGF-2 gene might contribute to the initiation and development of transitional cell carcinoma. Individuals with MTHFR 677CT or TT genotype may be a positive factor in carcinogenesis by demethylating exon 9 of the IGF-2 gene in vivo. Therefore, exploration of abnormal DNA methylation distribution is expected to play an important role in early individual diagnosis and in monitoring the prognosis of transitional cell carcinoma.
Acknowledgments
We thank Yunfei Wei, Xin Yu, and Haibing He (Department of Urology, the First Affiliated Hospital of Nanjing Medical University) for critical comments and scientific editing.
Footnotes
The authors reported no conflicts of interests
References
- 1.Marsit CJ, Houseman EA, Christensen BC, Gagne L, Wrensch MR, Nelson HH, et al. Identification of methylated genes associated with aggressive bladder cancer. PLoS One. 2010;5:e12334. doi: 10.1371/journal.pone.0012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vikram R, Sandler CM, Ng CS. Imaging and staging of transitional cell carcinoma: part 1, lower urinary tract. AJR Am J Roentgenol. 2009;192:1481–7. doi: 10.2214/AJR.08.1318. [DOI] [PubMed] [Google Scholar]
- 3.Hansen MF, Cavenee WK. Tumor suppressors: recessive mutations that lead to cancer. Cell. 1988;53:173–4. doi: 10.1016/0092-8674(88)90376-5. [DOI] [PubMed] [Google Scholar]
- 4.Hunter T. Oncoprotein networks. Cell. 1997;88:333–46. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 5.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang JY, Lu R, Mikovits JA, Cheng ZH, Zhu HY, Chen YX. Regulation of hMSH2 and hMLH1 expression in the human colon cancer cell line SW1116 by DNA methyltransferase 1. Cancer Lett. 2006;233:124–30. doi: 10.1016/j.canlet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Tada Y, Yokomizo A, Shiota M, Tsunoda T, Plass C, Naito S. Aberrant DNA methylation of T-cell leukemia, homeobox 3 modulates cisplatin sensitivity in bladder cancer. Int J Oncol. 2011;39:727–33. doi: 10.3892/ijo.2011.1049. [DOI] [PubMed] [Google Scholar]
- 8.Brait M, Sidransky D. Cancer epigenetics: above and beyond. Toxicol Mech Methods. 2011;21:275–88. doi: 10.3109/15376516.2011.562671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eze OP, Starker LF, Carling T. The role of epigenetic alterations in papillary thyroid carcinogenesis. J Thyroid Res. 2011;2011:895470. doi: 10.4061/2011/895470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi IS, Estecio MR, Nagano Y, Kim do H, White JA, Yao JC, et al. Hypomethylation of LINE-1 and Alu in well-differentiated neuroendocrine tumors (pancreatic endocrine tumors and carcinoid tumors) Mod Pathol. 2007;20:802–10. doi: 10.1038/modpathol.3800825. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo V, Castellvi-Bel S, Balaguer F, Pellise M, Ocana T, Castells A. Epigenetics of cancer. Gastroenterol Hepatol. 2008;31:37–45. doi: 10.1157/13114573. [DOI] [PubMed] [Google Scholar]
- 12.Oyama K, Kawakami K, Maeda K, Ishiguro K, Watanabe G. The association between methylenetetrahydrofolate reductase polymorphism and promoter methylation in proximal colon cancer. Anticancer Res. 2004;24:649–54. [PubMed] [Google Scholar]
- 13.Batra V, Mishra KP. Modulation of DNA methyltransferase profile by methyl donor starvation followed by gamma irradiation. Mol Cell Biochem. 2007;294:181–7. doi: 10.1007/s11010-006-9258-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhu D, Hunter SB, Vertino PM, Van Meir EG. Overexpression of MBD2 in Glioblastoma Maintains Epigenetic Silencing and Inhibits the Antiangiogenic Function of the Tumor Suppressor Gene BAI1. Cancer Res. 2011;71:5859–70. doi: 10.1158/0008-5472.CAN-11-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Vieira A. DNA methylation, riboswitches, and transcription factor activity: fundamental mechanisms of gene-nutrient interactions involving vitamins. Mol Biol Rep. 2006;33:253–6. doi: 10.1007/s11033-006-9005-y. [DOI] [PubMed] [Google Scholar]
- 16.Milutinovic S, D'Alessio AC, Detich N, Szyf M. Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis. 2007;28:560–71. doi: 10.1093/carcin/bgl167. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson L, Singh GK, Osterwalder T, Roman GW, Davis RL, Keshishian H. Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics. 2008;178:215–34. doi: 10.1534/genetics.107.081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalitchagorn K, Shuangshoti S, Hourpai N, Kongruttanachok N, Tangkijvanich P, Thong-ngam D, Voravud N, et al. Distinctive pattern of LINE-1 methylation level in normal tissues and the association with carcinogenesis. Oncogene. 2004;23:8841–6. doi: 10.1038/sj.onc.1208137. [DOI] [PubMed] [Google Scholar]
- 19.Fraga MF, Agrelo R, Esteller M. Cross-talk between aging and cancer: the epigenetic language. Ann N Y Acad Sci. 2007;1100:60–74. doi: 10.1196/annals.1395.005. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Katsaros D, Wiley A, Rigault de la Longrais IA, Risch HA, Puopolo M, et al. The relationship of insulin-like growth factor-II, insulin-like growth factor binding protein-3, and estrogen receptor-alpha expression to disease progression in epithelial ovarian cancer. Clin Cancer Res. 2006;12:1208–14. doi: 10.1158/1078-0432.CCR-05-1801. [DOI] [PubMed] [Google Scholar]
- 21.Elzagheid A, Kuopio T, Pyrhonen S, Collan Y. Lymph node status as a guide to selection of available prognostic markers in breast cancer: the clinical practice of the future? Diagn Pathol. 2006;1:41. doi: 10.1186/1746-1596-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murrell A, Ito Y, Verde G, Huddleston J, Woodfine K, Silengo MC, et al. Distinct methylation changes at the IGF2-H19 locus in congenital growth disorders and cancer. PLoS One. 2008;3:e1849. doi: 10.1371/journal.pone.0001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–5. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 24.Feil R, Charlton J, Bird AP, Walter J, Reik W. Methylation analysis on individual chromosomes: improved protocol for bisulphite genomic sequencing. Nucleic Acids Res. 1994;22:695–6. doi: 10.1093/nar/22.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byun HM, Wong HL, Birnstein EA, Wolff EM, Liang G, Yang AS. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67:10753–8. doi: 10.1158/0008-5472.CAN-07-0329. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Li Y, Tollefsbol TO. Gene-environment interactions and epigenetic basis of human diseases. Curr Issues Mol Biol. 2008;10:25–36. [PMC free article] [PubMed] [Google Scholar]
- 27.Feinberg AP. The epigenetics of cancer etiology. Semin Cancer Biol. 2004;14:427–32. doi: 10.1016/j.semcancer.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang FF, Morabia A, Carroll J, Gonzalez K, Fulda K, Kaur M, et al. Dietary patterns are associated with levels of global genomic DNA methylation in a cancer-free population. J Nutr. 2011;141:1165–71. doi: 10.3945/jn.110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friso S, Girelli D, Trabetti E, Olivieri O, Guarini P, Pignatti PF, et al. The MTHFR 1298A>C polymorphism and genomic DNA methylation in human lymphocytes. Cancer Epidemiol Biomarkers Prev. 2005;14:938–43. doi: 10.1158/1055-9965.EPI-04-0601. [DOI] [PubMed] [Google Scholar]
- 30.Cellarier E, Durando X, Vasson MP, Farges MC, Demiden A, Maurizis JC, et al. Methionine dependency and cancer treatment. Cancer Treat Rev. 2003;29:489–99. doi: 10.1016/s0305-7372(03)00118-x. [DOI] [PubMed] [Google Scholar]
- 31.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 32.Axume J, Smith SS, Pogribny IP, Moriarty DJ, Caudill MA. The MTHFR 677TT genotype and folate intake interact to lower global leukocyte DNA methylation in young Mexican American women. Nutr Res. 2007;27:1365–17. doi: 10.1016/j.nutres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Supic G, Jovic N, Kozomara R, Zeljic K, Magic Z. Interaction between the MTHFR C677T polymorphism and alcohol--impact on oral cancer risk and multiple DNA methylation of tumor-related genes. J Dent Res. 2011;90:65–70. doi: 10.1177/0022034510385243. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Stampfer MJ, Giovannucci E, Artigas C, Hunter DJ, Fuchs C, et al. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997;57:1098–102. [PubMed] [Google Scholar]
- 35.Yang CX, Matsuo K, Ito H, Shinoda M, Hatooka S, Hirose K, et al. Gene-environment interactions between alcohol drinking and the MTHFR C677T polymorphism impact on esophageal cancer risk: results of a case-control study in Japan. Carcinogenesis. 2005;26:1285–90. doi: 10.1093/carcin/bgi076. [DOI] [PubMed] [Google Scholar]
- 36.Wang LD, Guo RF, Fan ZM, He X, Gao SS, Guo HQ, et al. Association of methylenetetrahydrofolate reductase and thymidylate synthase promoter polymorphisms with genetic susceptibility to esophageal and cardia cancer in a Chinese high-risk population. Dis Esophagus. 2005;18:177–84. doi: 10.1111/j.1442-2050.2005.00492.x. [DOI] [PubMed] [Google Scholar]