Abstract
Learning involves not only the establishment of memory per se, but also the specific details of its contents. In classical conditioning, the former concerns whether an association was learned while the latter discloses what was learned. The neural bases of associativity have been studied extensively while neural mechanisms of memory specificity have been neglected. Stimulation of the cholinergic nucleus basalis (NBs) paired with a preceding tone induces CS-specific associative memory. As different levels of acetylcholine may be released naturally during different learning situations, we asked whether the level of activation of the cholinergic neuromodulatory system can control the degree of detail that is encoded and retrieved. Adult male rats were tested pre- and post-training for behavioral responses (interruption of ongoing respiration) to tones of various frequencies (1–15 kHz, 70 dB, 2 s). Training consisted of 200 trials/day of tone (8.0 kHz, 70 dB, 2 s) either paired or unpaired with NBs (CS-NBs = 1.8 s) at moderate (65.7 ± 9.0 μA, one day) or weak (46.7 ± 12.1 μA, three training days) levels of stimulation, under conditions of controlled behavioral state (pre-trial stable respiration rate). Post-training (24 h) responses to tones revealed that moderate activation induced both associative and CS-specific behavioral memory, whereas weak activation produced associative memory lacking frequency specificity. The degree of memory specificity 24 h after training was positively correlated with the magnitude of CS-elicited increase in γ activity within the EEG during training, but only in the moderate NBs group. Thus, a low level of acetylcholine released by the nucleus basalis during learning is sufficient to induce associativity whereas a higher level of release enables the storage of greater experiential detail. γ waves, which are thought to reflect the coordinated activity of cortical cells, appear to index the encoding of CS detail. The findings demonstrate that the amount of detail in memory can be directly controlled by neural intervention.
Keywords: Auditory cortex, Encoding, Acetylcholine, Association
1. Introduction
Neural mechanisms are responsible not only for the establishment of memory per se, but also for the specific details of its contents. The issue of specificity is central to the problem of how the brain represents and stores the details of experiences and thus constitutes a core problem in the neurobiology of learning and memory. Consideration of classical (Pavlovian) conditioning can clarify the difference between the establishment and specificity of memory. The former concerns whether learning occurred while the latter concerns what was learned. For example, validation of associative memory can be provided by the use of a control group in which the conditioned (CS) and unconditioned (US) stimuli are not paired. This reveals whether an association was formed. The possibilities for specificity are much greater because the potential contents of memory are practically unlimited. For instance, even when learning occurs to a pure tone cue, subjects may have learned that a single stimulus parameter or combination thereof predicts reward, punishment or their absence, e.g., a sudden change in the stimulus environment (regardless of modality), a sound, a sound in a particular area of space, a sound with a particular timbre, a pure tone of a particular frequency range (e.g., “high” vs. “low”), a pure tone of an absolute frequency (e.g., 5.5 kHz), as well as aspects of the learning context, etc.
The specific details of what was learned ordinarily cannot be determined during training but rather can be assessed afterward by the use of appropriate tests. For example, subjects may be trained with a single stimulus (e.g., in simple conditioning) or two stimuli (e.g., in discrimination learning) but later tested with many values along a stimulus dimension (e.g., acoustic frequency), in the absence of reinforcement (i.e., extinction) (Mackintosh, 1974; Mostofsky, 1965; Pavlov, 1960). Preferential responses to the training stimulus indicate that subjects learned about the particular parameters of the training stimulus while responses to most other stimuli, as well as the training stimulus, indicate that learning was not specific to the training stimulus but rather general along the tested dimension. For example, in the case of training with a pure tone, the former outcome implies that subjects learned about the signal importance of one particular dimension of the environment (the actual frequency of the conditioned stimulus) while the latter implies that subjects did not, but may have learned that tones in general have a signal function (Mackintosh, 1974).
The neural bases of associativity have been studied extensively (Christian & Thompson, 2003; Davis, Falls, Campeau, & Kim, 1993) while those of specificity have received scant attention. Studies near the middle of the last century (1950s–1970s) revealed that learning alters the processing of signal stimuli in sensory cortices (John, 1961; Thompson, Patterson, & Teyler, 1972). More recently, hybrid experimental designs that have combined the basic protocols of the field of sensory neurophysiology with those from learning/memory have revealed that associative learning systematically modifies the processing and representation of sensory information in the auditory, somatosensory, and visual cortices (Diamond, Petersen, & Harris, 1999; Edeline, 2003; Feldman & Brecht, 2005; John, 1961; Rauschecker, 1999; Thompson et al., 1972; Weinberger, 1995). Such learning-induced plasticity can be highly specific. For example, the frequency tuning of neurons in the primary auditory cortex can be shifted toward or even to the frequency of a CS during classical and instrumental conditioning (Weinberger, 2004c); see also (Ohl & Scheich, 2004; Weinberger, 2004b).
Pairing a tone with stimulation of the cholinergic nucleus basalis (NB) induces the same type of receptive field plasticity in the primary auditory cortex that develops during standard classical and instrumental conditioning (Weinberger, 2003) and, accordingly, also expanded representation of the paired tone in the tonotopic map in the primary auditory cortex (Kilgard et al., 2001; Kilgard & Merzenich, 1998).
This report concerns an unusual approach to the neurobiology of learning and memory. Beyond the induction of cortical plasticity, it concerns the induction of actual behavioral memory by direct activation of the nucleus basalis. [We use the phrase “behavioral memory” to distinguish it from neural plasticity that develops during brain stimulation (e.g., LTP) or learning (e.g., neural correlates) because plasticity is often called “memory”; hereafter, “memory” refers to information storage that is behaviorally validated.] We chose to determine if activation of the cholinergic NB can induce memory because of the known involvement of cholinergic mechanisms in learning and memory (Deutsch, 1971; Flood, Landry, & Jarvik, 1981; Power, Vazdarjanova, & McGaugh, 2003).
Previously, we found that pairing a tone with stimulation of the nucleus basalis does induce memory, and that such memory is both associative and contains detail about the absolute frequency of the conditioned stimulus. Rats that received extensive pairing of a single tone with NB stimulation (NBs) (3000 trials over 15 days) later exhibited behavioral frequency response profiles (for both the interruption of ongoing respiration and changes in heart rate) that were maximal at the CS frequency in the absence of NBs. In contrast, rats receiving unpaired stimulation failed to develop such behavioral CS-specificity (McLin, Miasnikov, & Weinberger, 2002b, 2003). Recently, we have found that specific associative memory can be induced rapidly with a single training session of 200 trials (Miasnikov, Chen, & Weinberger, 2006).
The present study approaches the issue of the neural mechanisms of both associativity and specificity. To date, stimulation of the nucleus basalis has been shown to simultaneously induce behavioral memory that is both associative and specific. However, these two cardinal features of learning are not necessarily coupled, as evidenced by learning in which some or many of the details of an experience are not encoded or are forgotten. This long-recognized distinction is the root of studies of human recognition memory that are concerned with the cognitive and neurobiological differences between memory for detail vs. memory largely limited to a “sense of familiarity” (Rugg & Yonelinas, 2003). We asked whether the level of activation of the cholinergic system can control the degree of detail of a tonal conditioned stimulus by training different groups with different levels (“moderate” and “weak”) of NB stimulation. We further asked whether weak stimulation could produce the same effects as moderate stimulation, by tripling the number of training trials. Some findings for the group receiving moderate stimulation have been reported in another context (Miasnikov et al., 2006).
2. Materials and methods
The materials and methods were identical to those previously reported for the group that received the moderate level of NB stimulation (Miasnikov et al., 2006), except as otherwise noted, and thus will be described only briefly. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously monitored by video cameras.
2.1. Subjects and surgery
The subjects were 20 adult male Sprague–Dawley rats (115 ± 33 days of age, 429 ± 50 g weight, mean ± SD) housed individually with ad libitum food and water on a 12/12 h light–dark cycle (lights on at 7:00 am). Following several days of adaptation to the vivarium, animals were handled and learned to sit calmly during attachment of a thermistor assembly and a cable to their skull pedestal. Under general anesthesia (sodium pentobarbital, 40 mg/kg i.p., Abbott Laboratories, North Chicago, IL), an 0.8-mm diameter stainless steel recording epidural screw electrode was inserted over the right primary auditory cortex at the locus showing the largest amplitude voked potential (200–400 μV) to a contralateral click. Two screws over the frontal sinus served as reference electrodes. A stimulating electrode (concentric bipolar stainless steel, #SNEX-100x13, David Kopf Instruments, Tujunga, CA) was lowered through the contralateral hemisphere at a 45° angle in the frontal plane at AP −2.2, L 3.2 (Paxinos & Watson, 1997), entering laterally and passing medially, while stimulation was applied (200–500 μA, pairs of 0.2 ms opposite polarity pulses, 100 Hz, 200–300 ms trains; S88 stimulator, a pair of PSIU6 isolation units, Grass Instrument Co., Quincy, MA) until it reached the ipsilateral (right) NB; the final locus was determined physiologically by obtaining 1–5 s of consistent auditory cortical EEG activation. A dental acrylic pedestal was built (methyl methacrylate, Co-Oral-Ite Dental Mfg. Co., Diamond Spring, CA), two aluminum hex threaded standoffs were embedded therein, and all leads connected to a miniature socket that could be led to a commutator via a multiconductor cable. Subjects were allowed 1–2 weeks to recover from surgery.
2.2. Stimuli, recording, and data analyses
Training and testing took place while each subject was in a box (23 × 23 × 31 cm), supplied with fresh bedding and lined with acoustic-damping tile, contained in a double-walled acoustic chamber (IAC, Bronx, NY). Acoustic stimuli were pure tones [1.0–15.0 kHz, 2 s duration, cosine 10 ms rise/fall time (10% to 90%) 70 dB SPL] produced by TDT System 3 components (Tucker–Davis Technologies, Alachua, FL) and delivered to a loudspeaker (#40-1421, RadioShack, Fort Worth, TX) positioned 35 cm above the floor of the box. NBs current during training was several times weaker than that used during surgery because anesthesia greatly increases the threshold for EEG activation. The current was adjusted with respect to EEG activation threshold as described below.
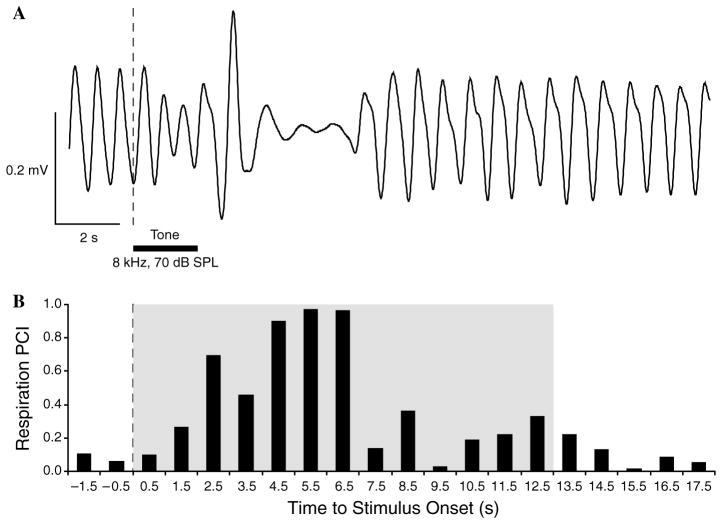
We measured the disruption of the ongoing respiration pattern to various tones following training (below) to assess the induction of memory. Respiration was detected by a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly of custom design and fabrication. The thermistor served as one of the arms of a pre-balanced resistor bridge circuit sensitive to the temperature fluctuations caused by the animal’s breathing. The output signal from the bridge was fed to the differential input of a DAM 50H amplifier (10,000×, 1–100 Hz, WPI, Sarasota, FL). The amplified signal was digitized by an ADC module (Power-1401 interface, CED, Cambridge, UK) operated under Spike-2 (CED) data acquisition/analysis software; captured data were stored on the hard drive of a Pentium-based PC. Off-line processing of respiration consisted of the calculation of Fast Fourier Transform functions (FFT, Spike-2 software, CED) for a period of 2 s preceding a trial (Pre), 2 s during a CS tone (Dur) and 24 s after the tone (Post). Major changes in respiration occurred within 13.0 s after tone onset. The respiration signal was almost completely contained within the bandwidth of 0.975–2.925 Hz. The FFT data were used to calculate a “Respiration Power Change Index” (RPCI) on a second-by-second basis. The index was sensitive to both increases and decreases of both frequency and amplitude. RPCIs were calculated as: RPCIi = (|Posti– Pre|)/(Posti + Pre). A value of 0 would indicate no change and a value of 1.0 would indicate complete cessation of respiration (Fig. 1). Statistical analyses used SPSS v.13 software (SPSS, Inc., Chicago, IL).
Fig. 1.
Respiration record and quantification. (A) An example of a regular sinusoidal baseline respiration record disrupted by onset of the CS tone presentation (vertical dotted line) following training. In this record, disruption was manifested by a decline in the depth of breathing during tone presentation followed by a strong unitary exhalation (increased amplitude “spike”) and then a ~2 s period of almost complete cessation of breathing. Horizontal black bar denotes tone presentation (2 s). (B) Quantification of the respiration record in (A). The “respiration power change index” (RPCI, see Section 2) is sensitive to both increases and decreases in signal amplitude and frequency. The shaded area indicates the 13 s quantified portion of the respiratory record.
Respiration is a sensitive behavioral measure that is interrupted both by novel and conditioned stimuli (McLin, Miasnikov, & Weinberger, 2002a). Therefore, to insure that changes in respiration due to pairing the CS tone with NB stimulation were due to associative processes, we used control groups in which these stimuli were not paired. Furthermore, we determined the degree of specificity of respiration responses to the frequency of the conditioned stimulus tone vs. non-CS tones, as non-associative processes (e.g., sensitization) would not be expected to yield CS-specific effects. Prior studies have shown that pairing a tone with stimulation of the nucleus basalis does produce associative, CS-specific changes in respiration (McLin et al., 2002a, 2002b, McLin, Miasnikov, & Weinberger, 2003; Miasnikov et al., 2006).
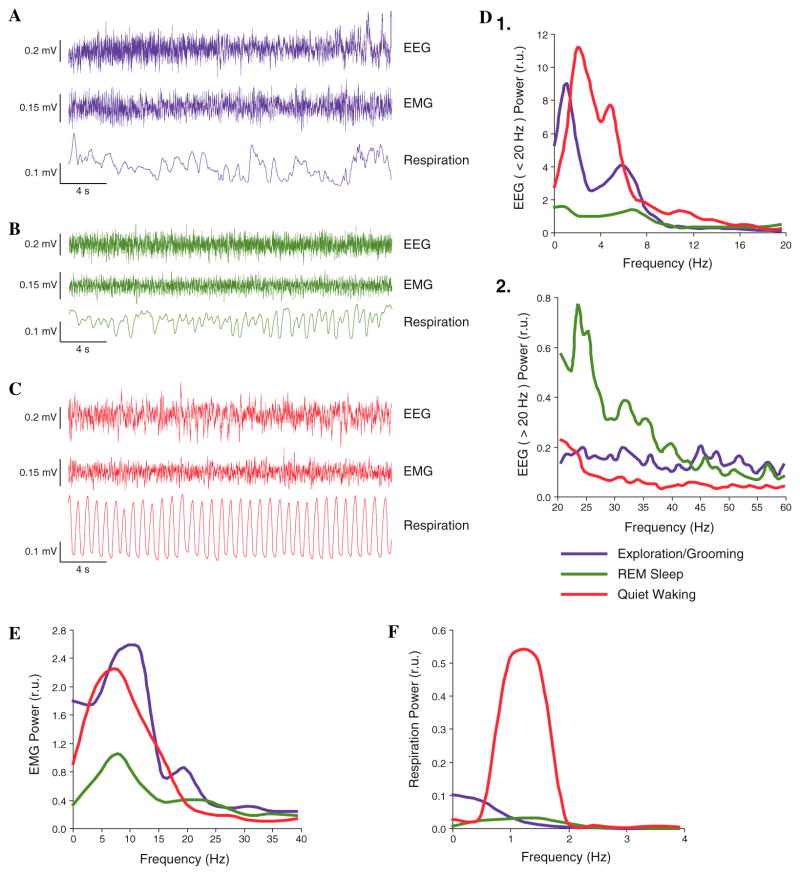
During sessions, trials were initiated for both paired and unpaired groups only when subjects were in a state of quiet waking, characterized by regular respiration (Fig. 2). This avoided the problem of stimulating the NB when cortical acetylcholine (ACh) levels are likely to be very high, as during exploration and REM sleep (Giovannini et al., 2001; Jasper & Tessier, 1971; Kametani & Kawamura, 1990; Marrosu et al., 1995), when NBs might not affect the cortex due to a ceiling effect.
Fig. 2.
State control. Examples of physiological measures for three major states. Training trials were given only when subjects were in the state of Quiet Waking (supine posture, eyes open, video monitoring). (A) Exploration/Grooming: note in particular irregular respiration. (B) REM sleep, characterized by low voltage, fast EEG, low level of EMG activity and rapid, shallow respiration. (C) Quiet Waking, distinguished by highly regular respiration. (D, E, and F) Spectral analyses of physiological indices of the examples given in (A, B, and C). REM sleep was distinctive from Exploration/Grooming and Quiet Waking in (D) EEG spectra having (1) less low frequency and (2) greater high frequency activity and (E) lower level of EMG. Quiet Waking was distinguished from Exploration/Grooming and REM sleep particularly by (F) highly regular respiration, with maximum power at ~1.3 Hz.
2.3. Experimental design
The subjects were assigned to two main groups, Weak (n = 11) and Moderate (n = 9) stimulation, each of which was subdivided into Paired and Unpaired sub-groups: Weak-Paired (WP, n = 6), Weak-Unpaired (WU, n = 5), Moderate-Paired (MP, n = 5), and Moderate-Unpaired (MU, n = 4). After recovery from surgery, NBs thresholds were determined while subjects were in a quiet waking state. NBs was delivered every few minutes at increasing levels starting at ~30 μA (100 Hz bipolar, 200 ms train) until stimulation reliably elicited a 1–5 s (WP and WU groups) or 3–8 s (MP and MU groups) epoch of cortical activation (decrease in low frequency activity often accompanied by increase in γ activity). The current levels used in subsequent training with NB stimulation did not elicit body movements. There was no significant difference in stimulation levels within subgroups (WP = 48.3 ± 10.9 μA, WU = 44.8 ± 14.5 μA, p > .65; MP = 67.6 ± 11.3 μA, MU = 63.6 ± 5.4 μA, p > .50, two-tailed t-tests). There was a significant difference between major groups (Weak = 46.7 ± 12.1 μA, Moderate = 65.7 ± 9.0 μA, p < .0015, two-tailed t-test).
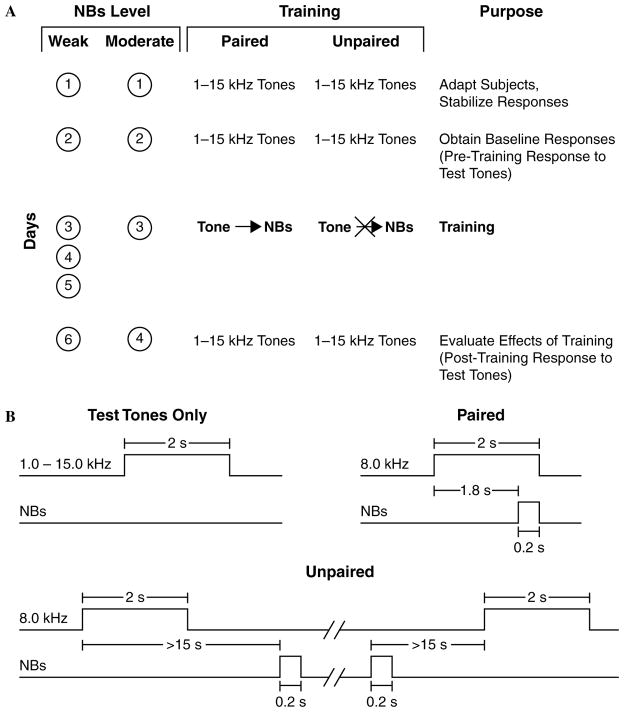
To induce and subsequently evaluate stimulus-specific memory, we used the approach of acquiring behavioral baseline responses to many frequencies, then training with one frequency and testing the training effects with many frequencies. The protocol for the Weak group required six consecutive days: Days 1–2, obtaining pre-training baseline response to test tones; Days 3–5, training (paired or unpaired CS and NBs); Day 6, obtaining post-training response to tones. The first session (Day 1) was used to acclimatize subjects to the testing environment and thus data from this session were not analyzed. The protocol for the Moderate group was the same except that training was restricted to one day (Day 3), so that the total duration of their experiment was four days (Fig. 3). Contextual transfer between training and frequency testing sessions was reduced by delivering animals to the laboratory via different circuitous routes and training them in the dark (red light) but testing them (pre-and post-training) in the light.
Fig. 3.
Experimental design. Stages of the experiment and timing of the stimuli. (A) Number of days of training and type of training for all four groups (weak and moderate NBs, paired and unpaired training). (B) Protocols for the presentation of tones and NB stimulation, for test tones and for both paired and unpaired trials. (For illustrative purposes, two types of trials are shown for unpaired training: one in which NBs follows the CS tone and one in which it precedes the CS tone. Note the minimum intervals of 15 s between tone and NBs to avoid accidental pairing.) See Section 2 for further details.
During each training session, the paired groups (WP and MP) received 200 trials of (8.0 kHz, 2 s, 70 dB SPL) followed by NBs (same level as determined post-operatively) that overlapped CS presentation and co-terminated with CS offset (i.e., the CS–US interval was 1.8 s). The unpaired groups (WU and MU) received 200 random presentations each of unpaired tone and NBs with the constraints of not more than three consecutive presentations of the same stimulus and a minimum of 15 s between tone and NBs to avoid accidental forward or backward pairing. Inter-trial intervals averaged 80 s (range ~45–150 s). On frequency test days, subjects received random presentation of tones of nine different frequencies (1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25, and 15.00 kHz, 70 dB SPL, constrained only by no more than two stimuli of the same frequency in a row) for 200 trials total. Intervals between tone presentations averaged 94 s. The frequencies were selected to avoid having simple low ratio relationships, e.g., 2:1, to prevent potential octave stimulus generalization effects. (A three-octave relationship exists between 1.00 and 8.00 kHz, but generalizations of three octaves have not been reported.) Statistical analyses of respiration responses were based on averaging the data for triplets of frequencies: 1.00–4.50, 6.25–9.75, and 11.50–15.00 kHz. The middle frequency band (6.25, 8.00, and 9.75 kHz) is referred to as the “CS band”.
2.4. Histology
Following termination of the experiments, an electrolytic lesion (4 ms pulses at 100 Hz, 500 μA for 60 s) was made with bipolar current through the stimulating electrode while the animal was under sodium pentobarbital anesthesia. It was then given an overdose of sodium pentobarbital and perfused through the heart with saline followed with 10% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The brain was removed and coordinates of the recording electrode on the skull were measured from Bregma. Following several days of post-fixation in paraformaldehyde solution with 0.8 M sucrose added for subsequent cryoprotection, the brain was sectioned at 50 μm with a freezing microtome, sections mounted onto gelatin-coated slides, dried, and stained for Nissl substance to recover the electrolytic lesion sites and determine the actual loci of stimulation. Auditory cortex recording sites, which had been determined by click-induced local field potentials, were verified by post-mortem precise measurement from the interior of the calvaria of the A–P and M–L distances from Bregma and midline, respectively, and plotted on a stereotaxic map of the auditory and surrounding areas of cortex derived from the Paxinos & Watson (1997) atlas.
2.5. Determination of effectiveness of NB stimulation on the EEG of the auditory cortex
Histological location of NB stimulation sites, while appropriate, is not sufficient for the purpose of this experiment, viz., to determine the effects of the level of NB activation on association and specificity of induced memory. Simply using weak and moderate levels of stimulating current could not guarantee different levels of physiological effectiveness based only on the location of stimulation sites within the nucleus basalis. Rather, it was essential to employ an objective measure of effectiveness, i.e., to determine that weak and moderate NBs did indeed produce two different magnitudes of effect on the cortex. Therefore, we quantified changes in the power spectra of electroencephalographic (EEG) recordings obtained from the auditory cortical recording electrode during training. The epidural ACx signal was amplified and filtered (DAM-50H amplifier, 1000×, band-pass 1.0–1000 Hz), digitized at 500 samples/s with a Power1401 hardware and Spike-2 software system and processed off-line. The FFT Power was calculated off-line at a frequency resolution of 0.975 Hz for the Power spectra up to 59.965 Hz. The FFT data were used to calculate an EEG “Power Change Index” (EEG PCI), on a second-by-second basis for each EEG frequency band separately as follows: δ: 0.98–2.92; θ: 2.93–8.78; α: 8.79–14.62; β1: 14.63–20.47; β2: 20.48–33.15; γ: 33.16–59.97. The index was sensitive to both increases and decreases in EEG Power. EEG PCIs were calculated on each trial as follows: EEG PCIi = (Posti − Pre)/(Posti + Pre), where “Pre” was the 2 s immediately preceding a trial. A negative value would indicate a decline and a positive value would indicate a rise in power within each specified frequency band relative to its baseline. The results of these analyses could be interpreted unambiguously only for the unpaired groups (WU and MU) because the paired groups (WP and MP) received the CS tone preceding NBs on each trial, which increased the EEG changes (Miasnikov et al., 2006).
3. Results
3.1. Location of electrodes
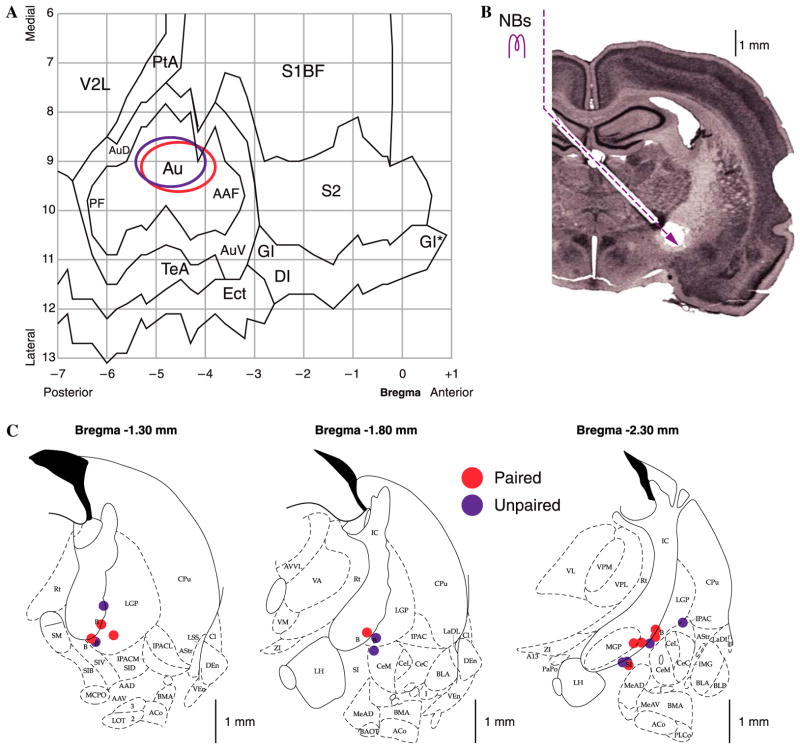
A summary of the location of electrodes is presented in Fig. 4. The cortical sites of recording are summarized in Fig. 4A. All of the electrodes were located above the primary auditory cortex. Recording sites of the paired and unpaired groups were intermingled and did not differ either in the A–P or M–L planes (t-tests, p > .20 each). Fig. 4B indicates the implantation path of the stimulating electrodes. NB stimulation sites are shown in Fig. 4C. Due to technical difficulties, stimulation placements could not be obtained for 3 of the 20 subjects, but stimulation in these, as for all subjects, produced EEG activation (see Section 2). All located tip placements were within the basal forebrain within structures containing corticopetal cholinergic cells, including those that project to the auditory cortex (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Luiten, Gaykema, Traber, & Spencer, 1987; Mesulam, Mufson, Wainer, & Levey, 1983; Rye, Wainer, Mesulam, Mufson, & Saper, 1984). Stimulation sites of the paired and unpaired groups overlapped and were not statistically different in the A–P, M–L or D–V planes (t-tests, p > .10 for each plane) (Fig. 4C).
Fig. 4.
Location of electrodes. Cortical recording sites and NB stimulation sites. (A) EEG recording loci. The ovals indicate the location of epidural recordings based on their stereotaxic coordinates using a cortical map derived from Paxinos and Watson (1997). Sites for paired and unpaired groups were over primary auditory cortex and overlapped. (B) Nissl section showing the placement of stimulating electrodes in the nucleus basalis by a contralateral approach, to avoid damage to ipsilateral structures. (C) Diagrams of three coronal sections showing the NB stimulation sites. Paired and unpaired group sites were intermingled. Abbreviations: B, basal nucleus of Meynert; CeM, amygdala central nucleus medial; CeL, amygdala central nucleus lateral; CPu, caudate–putamen; IC, internal capsule; IPAC, interstitial nucleus of posterior limb of anterior commissure; LGP, lateral globus pallidus; LH, lateral hypothalamus; SI, substantia innominata; SIB, substantia innominata, basal; SIV, substantia innominata, ventral; Au, primary auditory cortex; AAF, anterior auditory field; AuD, secondary auditory cortex, dorsal; AuV, secondary auditory cortex, ventral; PF, posterior auditory field; S1BF, primary somatosensory cortex, barrel field; S2, secondary somatosensory cortex; TeA, temporal association cortex; V2L, secondary visual cortex, lateral area.
3.2. Verification of differential effectiveness of levels of NB stimulation
As the major goal of this study is to determine the effects of different levels of NBs on induced memory, it is essential to verify that the use of weak and moderate stimuli had differential degrees of physiological effect on its major target, the cerebral cortex. We used the amount of change of the EEG to determine the effectiveness of NB stimulation. Such stimulation produces EEG “activation”, i.e., a reduction of low frequency activity (δ, θ, and α in particular) and an increase in high frequency activity, β2 and especially γ waves (McLin et al., 2002a). Moreover, such effects are mediated by cortical muscarinic receptors (Metherate & Ashe, 1993; Metherate, Cox, & Ashe, 1992; Miasnikov, McLin, & Weinberger, 2001; see also Edeline, Maho, Hars, & Hennevin, 1994b).
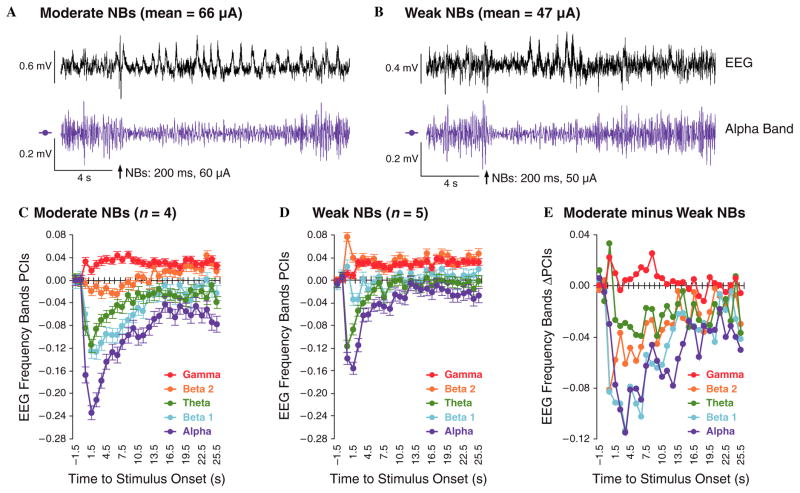
Examples of the effects of moderate and weak stimulation are provided in Fig. 5A and B, respectively. Shown are both the unfiltered EEG records and activity within the α band (8.79–14.62 Hz) (Fig. 5A and B), the latter because this band exhibited the largest change (decrease) to NBs in both groups (Fig. 5C and D). [EEG activation also produced changes in delta waves, the lowest frequencies, but the power of the δ response to NBs cannot be determined exclusively because stimulation of the nucleus basalis produces its own large cholinergic potential whose spectrum falls into the δ band (McLin, Miasnikov, & Weinberger, 2000)]. Note that the moderate level of stimulation (60 μA in this case) produced a longer duration of α reduction than did the weaker level of stimulation (50 μA). Quantification of changes in the EEG during Day 1 of training (200 trials) is provided in Fig. 5C and D for the Unpaired groups, MU and WU, respectively (see Section 2). Note that the θ, α, and β1 activity are reduced while γ activity is increased; the effect on β2 was less consistent. Note also that the magnitude of change is greater for the MU vs. the WU groups. This is seen most clearly in Fig. 5E, which shows the difference between the two groups (Moderate minus Weak). The differences were statistically significant for all EEG bands, as determined by two-tailed t-tests at the latency of maximal change within a band: θ, p < .01 (6.5 s); α, p < .00001 (3.5 s); β1, p < .00001 (3.5 s); β2, p < .00001 (0.5 s); γ, p < .02 (0.5 s). Therefore, the two levels of stimulation did have different degrees of physiological effect on the cortex.
Fig. 5.
Differential effectiveness of levels of NB stimulation. Validation that the moderate level of stimulation produced a greater effect on the auditory cortex than the weak level of stimulation. Determination of the effectiveness of NB stimulation was possible only in the unpaired groups as it was preceded by a tone in the paired groups (see also text). (A and B) Examples of the effect of NBs on the EEG for a subject in group MU (A) and group WU (B). The top line shows the entire EEG while the bottom line shows the EEG filtered to yield α waves (8.79–14.62 Hz). Note that the NBs caused a greater depression of α activity in the Moderate vs. the Weak case. (C) Group EEG spectra in response to NBs in the Moderate-Unpaired group. Note that NBs elicited a marked reduction in α, β1, and θ activity, with the largest decrease for α waves, and that it increased γ waves. (D) The same analysis for the Weak-Unpaired group. The effects were similar, but of a smaller magnitude. (E) The difference in NBs effectiveness, illustrated by subtracting changes in the Weak (WU) from the Moderate (MU) group.
3.3. Effects of NB stimulus level on associativity and specificity
For the pre-training period, a two-way ANOVA revealed a significant difference for test sound Frequencies (F(2,3947) = 8.61, p < .0002) but no difference for Groups (Paired vs. Unpaired) (F(1,3947) = 0.10, p = .76) or their interaction (F(2,3947) = 0.004, p = .99). The significant frequency effect probably reflected the fact that the audiogram of the rat is not flat in the range of 1.0–15.0 kHz (Heffner, Heffner, Contos, & Ott, 1994) (Fig. 6A).
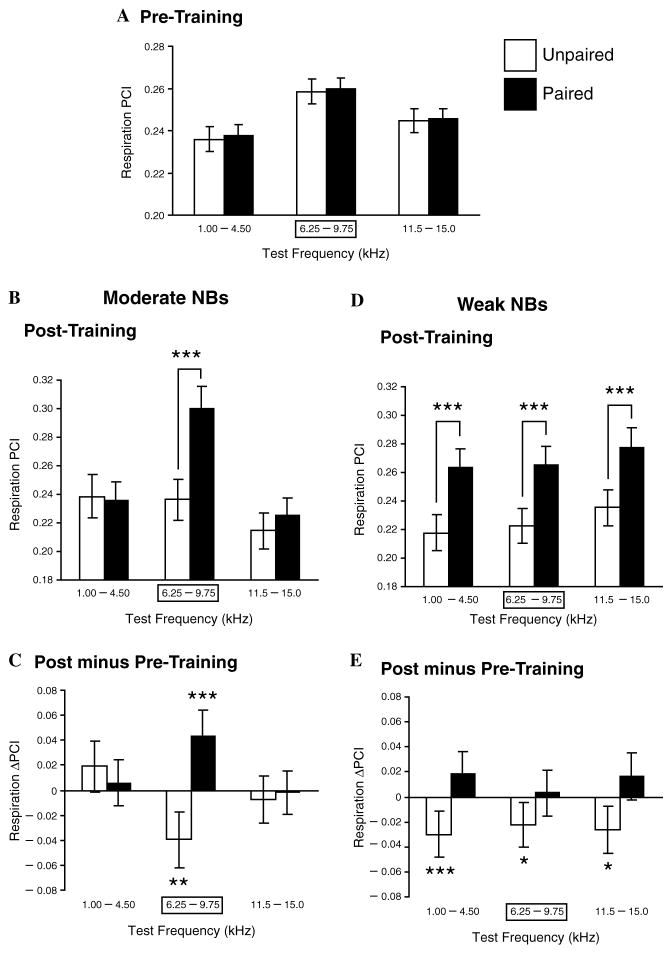
Fig. 6.
Effects of NB stimulus level on associativity and specificity. Pre-training and post-training responses to test tones in the Moderate and Weak NBs groups. (A) Pre-training responses for subjects that would be trained with either paired or unpaired CS tone and NB stimulation. There were no differences between the groups. (B) Post-training responses for the Moderate NBs groups. Note the significant difference between the paired (MP) and unpaired (MU) groups, confined to the CS-band frequencies. This indicates that training with a moderate level of NBs produced memory that was both associative and CS-specific. (C) Comparisons of changes within groups MP and MU (post minus pre-training responses to test tones). Note that the paired group (MP) had developed a significant increase to the CS-band frequencies only, while the unpaired group (MU) had developed a significant decrease, probably indicating frequency-specific habituation due to lack of pairing with NB stimulation. (D) Post-training responses for the Weak NBs groups. In contrast to the Moderate NBs group, pairing produced a significant difference in response across all test frequencies (group WP) compared to its unpaired controls (group WU). This indicates that training with weak NBs was sufficient to produce associative memory but insufficient to produce memory for frequency detail, i.e., memory that the frequency of the CS was paired with NBs. (E) Comparisons of changes within groups WP and WU showed that WP did not develop absolute increased responses but that group WU did develop significant decreases in responses across the spectrum of test frequencies. Thus, pairing the CS with weak NBs apparently prevented a habituatory decrement in group WP, which is evident in group WU. *p < .05; **p < .01; ***p < .005.
Comparison of the Moderate and Weak groups after training revealed some similarities and some differences. A two-way ANOVA within the Moderate group revealed a significant Frequencies effect (F(2,1794) = 17.55, p < .0001), as would be expected due to the rat’s audiogram, and more importantly a significant Groups effect (i.e., MP vs. MU) (F(1,1794) = 12.38, p < .0005) and a significant Frequencies × Groups Interaction (F(2,1794) = 8.82, p < .0002). Post hoc tests (Tukey) revealed that the Groups effect was due to a significantly larger response to the CS-band tones after training in the MP vs. the MU subjects (p < .000001). That is, training with a moderate level of NB stimulation induced an associative effect in the MP animals (Fig. 6B). Most importantly, the significant Interaction term indicates that the associative effect was not the same across frequencies. In fact, direct comparisons of groups MP and MU revealed that the effects of training were specific, being restricted to the CS frequency band (Fig. 6B and C). Group MP developed a significant post-training increase in response compared to the pre-training period (p < .002) while MU developed a significant decrease (p < .01). In summary, the Moderate-paired stimulation group exhibited the formation of CS-specific associative memory.
In contrast, while the Weak NBs group also had a significant post-training Group effect, i.e., WP vs. WU (F(1,2193) = 50.29, p < .0001), it had neither a significant Frequencies effect (F(2,2193) = 2.44, p = .09) nor a significant Interaction effect (F(2,2193) = 0.03, p = .97). Post hoc tests revealed that the Group effect was due to significantly different responses in the WP subjects as compared to the WU animals (1.00–4.50 kHz, p< .0003; 6.25–9.75 kHz, p< .0009; 11.50–15.00 kHz, p < .0009). The absence of Frequencies and Interaction effects indicates that, although associativity did develop, CS-specificity did not develop. Inspection of Fig. 6D and E shows that WP had larger responses than WU for each of the three frequency bands. However, none of the three frequency bands in WP had significantly increased responses. In contrast, there were significant response decreases at all three frequency bands in WU (1.00–4.50 kHz, p < .01; 6.25–9.75 kHz, p< .05; 11.50–15.00 kHz, p < .015). The significant decreases in the Unpaired groups (both MU and WU) probably reflects habituation to the repeated training tone, specific in MU and not frequency specific in WU. Thus, although there were no significant frequency-specific increased responses in group WP, pairing of tone with NB stimulation may have prevented habituatory decrements.
In summary, Weak-paired stimulation of the nucleus basalis induces associative memory, but it fails to induce memory that is specific to the CS frequency band. Accordingly, this group appears to have learned that tone (or merely sound) had gained behavioral importance whereas the Moderate NBs group seems to have learned that CS-band frequencies, rather than merely sound, had become behaviorally relevant.
3.4. EEG responses during training and specificity of memory after training
γ waves are thought to index the formation of coordinated intracortical activity of neurons, i.e., “cell assemblies” (Jefferys, Traub, & Whittington, 1996). Therefore, the encoding of greater detail (i.e., the CS frequency band vs. sound in general) might be related to the CS-elicited EEG response in γ activity during pairing. To investigate this potential relationship, we calculated the correlation between the amount of change (increase) in γ power recorded from the primary auditory cortex during CS-NBs pairing and the degree of specificity of memory.
γ response was determined for every trial for each subject by subtracting the mean of γ during the 2-s period immediately preceding CS onset from the maximal value of γ power during the CS tone (see Section 2, EEG PCI). This analysis was performed for the first day of training for group WP and, of course, the only day of training for group MP. To quantify the degree of memory specificity, we computed a “specificity index” (SI): SI = [{8.00} − ({2.75} + {13.25})/2]/{1 − 15}, where {} denotes the mean of behavioral respiration responses to the three frequencies centered on the frequency enclosed in the brackets and {1 − 15} denotes the mean of responses to all frequencies; i.e., SI is the mean of the CS band minus the mean of the average of the low and high frequency bands, all normalized by the mean response to all frequencies. This index would have a maximum value of 3.3 if all responses were elicited by frequencies in the CS band (i.e., 6.25, 8.00, and 9.75) and a minimum value of − 1.5 if no responses were elicited by frequencies in the CS band.
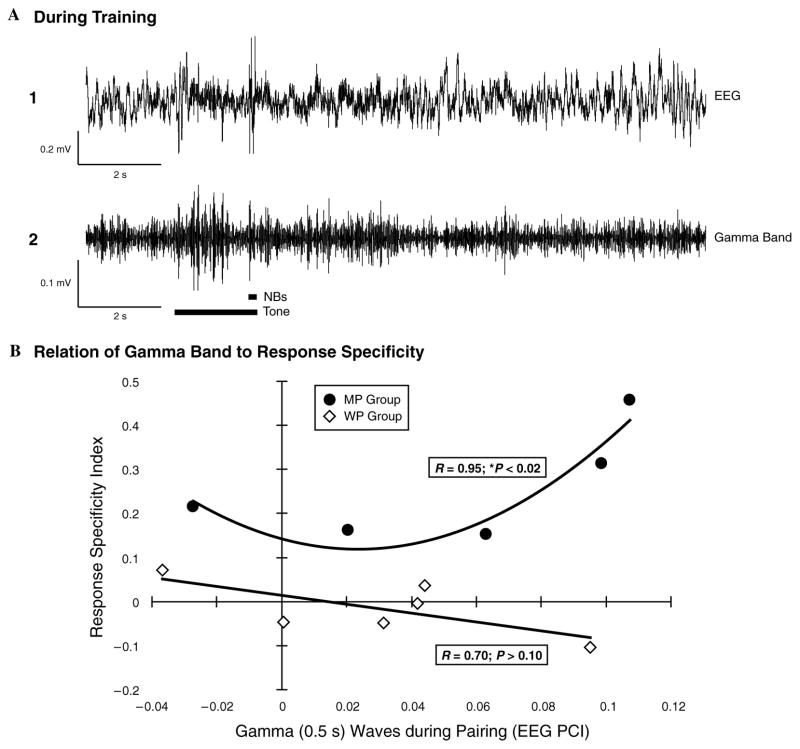
Fig. 7 shows an example of the auditory cortical response of the entire EEG spectrum and its γ band component (Fig. 7A1 and A2). Note that the maximum increase in γ occurs during the first 0.5 s after CS onset. Fig. 7B shows the scattergram of mean γ response values for the MP and WP subjects vs. the memory specificity index. Note the divergence of these two groups. The MP group shows a positive relationship between γ response during training and memory specificity as tested 24 h post-training. This relationship is best fit by a quadratic function with a correlation of 0.95, which is statistically significant (p < .02). The WP group, best fit by a linear function, shows no significant relationship (r = 0.70, p > .10). Neither linear nor quadratic correlations were significant for the two groups combined: r = 0.31, p > .10; r = 0.58, p > .05, respectively. A significant quadratic correlation suggests a threshold effect, such that CS-elicited increased γ response above some level is associated with increasing the specificity of memory in the MP group.
Fig. 7.
Relation of CS-elicited γ and specificity of behavioral auditory memory. EEG records and correlations. (A) An example of the EEG during a training trial for a subject in group MP. (1) full EEG, (2) EEG filtered to show γ activity. Note the increase in γ activity during presentation of the CS tone (long thick horizontal bar) preceding the NBs near the end of the tone (short bar). (B) Scattergram of the amount of change in γ during training [across 200 paired training trials (Day 3 for each group, see Fig. 3)] for each subject in groups MP and WP vs. the specificity of behavioral response 24 h after the termination of training. The best-fit regression for group MP is quadratic, with a correlation coefficient of 0.95, which is statistically significant (p < .02). The relationship for group WP is slightly in the opposite direction, i.e., more γ is related to less specific memory, but the best-fit regression (linear) was not significant.
We also analyzed the relationship between CS-evoked α activity during pairing and the specificity of induced memory because stimulation of the NB reduces low frequency power of the EEG and α power exhibits the greatest amount of decrease (Fig. 5). There was a tendency for increasing α suppression to be associated with higher SI values. However, in contrast to changes in γ activity, the relationship was not statistically significant (MP quadratic, r = 0.78, p > .10). Neither were other best-fit functions statistically significant: WP linear, r = 0.03, p > .10; MP and WP combined, quadratic, r = 0.37, p > .10.
4. Discussion
4.1. Validity and interpretation of the findings
Group MP manifested significantly larger post-training behavioral responses than those in group MU, and this difference was limited to the CS frequency band. Group WP also exhibited significantly different responses than its control group, WU, but this effect was not specific to the CS frequency band, being manifested also at the lower and higher frequency bands. Thus, the level of activation of the cholinergic nucleus basalis can control the level of detail in memory: moderate activation induced memory that included detail about the training frequency, whereas weak activation, while also capable of inducing associative memory, did not produce memory for detail about the training frequency.
The Moderate group exhibited associative memory by an absolute increase in response to CS-band frequencies after training compared to pre-training (Fig. 6B and C). In contrast, the Weak-Paired stimulation group did not develop an absolute difference in response magnitude after training compared to the pre-training period. Rather, association was evident in the significant decrease in response across frequency bands in the corresponding unpaired group (WU) (Fig. 6D and E). That is, although the stimulation level of group WP was insufficient to cause a frank increase in response, it was sufficient to prevent the decrement in response to tones that developed in group WU. This may be a case in which CS–US pairing prevents habituation to the CS when it is not capable of producing actual increased responses to the CS (Wagner & Brandon, 1989).
An alternative interpretation of the significant, non-specific decreased responses in the WU group is that they indicate inhibitory conditioning, i.e., that the CS tone served as a “safety signal” during training. Given that this group received strictly unpaired tone and NBs, such an account cannot be dismissed. However, inhibitory conditioning in this case would require that NBs was noxious. However, the NB is not part of any known motivational system (Pennartz, 1995) and place-preference tests have failed to detect either aversive or appetitive effects of NBs that is sufficient to induce memory (Miasnikov, Poytress, Chen, & Weinberger, 2004); see also (Wilson & Ma, 2004). The fact that group WU developed a significant decrease across all frequency bands underscores the failure for specificity to develop with weak activation of the NB. This stands in contrast to group MU which developed a significant decrease in response only for the CS frequency band (Fig. 6C). Thus, the Moderate level of NB activation appears not only to have induced a specific increase in behavioral response in group MP, but also frequency-specific habituation in group MU (Condon & Weinberger, 1991).
The current findings do reveal that weak activation of the NB is sufficient to induce associative memory. It is noteworthy that group WP received three times as many paired trials as did group MP (i.e., 600 vs. 200 trials). Nonetheless, the additional training was not sufficient to produce memory for CS-band detail. Therefore, the effectiveness of NBs in the Moderate group for the induction of CS-band detail may reflect the necessity to reach some sort of threshold, either within the NB itself or in one or more of its efferent structures.
The moderate level of NBs that produced specific associative memory might have engaged encoding for detail, consolidation of encoded information or retrieval by frequency cues presented during the post-training period. Moderate NBs could have strengthened encoding without additionally affecting consolidation or retrieval, strengthened consolidation alone, or facilitated some combination of these processes. Alternatively, the weak level of stimulation could have been insufficient to encode detail, or to induce adequate consolidation or to enable whatever combination of mnemonic neurobiological processes are necessary for the expression of specific information during post-training testing. Future studies will need to focus on the nature and extent to which the level of NB activation and the cholinergic system engages encoding, consolidation and retrieval of induced memory.
The level of specificity in memory, assessed 24 h after the completion of training, was related to the amount of CS-evoked increase in the γ band during training. However, no such relationship was found for CS-evoked changes (suppression) of α activity within the same time period (Fig. 7). [It remains possible that α might have such a relationship at a longer latency, because its suppression did not develop fully until ~3.5 s after tone onset (Fig. 5), but the presence of NBs overlapping at the end of the 2 s long CS prevents any conclusions about changes in α being caused exclusively by the tone. Future studies can employ CS-alone test trials to address this issue.] Thus, the two EEG measures, α suppression and γ facilitation, apparently do not reflect the same underlying process. Therefore, despite the tendency to regard EEG activation as a unitary phenomenon, it is important to maintain an open view and to consider that the single term “activation” may obscure multiple, possibly independent, functions.
There was a significant positive correlation between the amount of CS-elicited γ during training and the degree of memory specificity tested after training, but only for the MP group (r = 0.95, p < .02) (Fig. 7). This is intriguing because γ activity has been linked to the formation of functional neuronal ensembles that provide for object representation in learning, memory, and other cognitive functions (Başar, Başar-Eroğlu, Karakaş, & Schürmann, 2000; Kaiser & Lutzenberger, 2005; Sommer & Wennekers, 2001). Moreover, also in accord with the present findings, coherent object representations seem to be related only to induced γ, e.g., elicited by the CS, not spontaneous γ activity (Bertrand & Tallon-Baudry, 2000). Therefore, it is particularly noteworthy that group MP was the only one which developed both memory detail for CS-band frequencies and a significant relationship between memory specificity and increased CS-elicited γ. Together, these findings provide rare empirical support for the theory that the encoding of coherent detail in memory (i.e., about the CS frequency band) is the product of the formation of neuronal assemblies that store this information.
4.2. Involvement of the cholinergic system
The fact that stimulation of the nucleus basalis induced associative memory, both that which was CS-band specific, and that which was not, does not itself demonstrate that memory induction involved the cholinergic system. Direct pharmacological studies are needed to clarify this issue. However, there is good reason to infer the participation of the cholinergic system. First, cholinolytic atropine blocks EEG activation (Phillis & York, 1968; Szerb, 1964). Second, stimulation of the NB produces cortical EEG activation and release of acetylcholine in the cortex (Casamenti, Deffenu, Abbamondi, & Pepeu, 1986; Celesia & Jasper, 1966; Détári, Juhász, & Kukorelli, 1983, 1984; Détári, Juhász, & Kukorelli, 1987; Jiménez-Capdeville, Dykes, & Myasnikov, 1997; Juhász, Détári, & Kukorelli, 1985; Kukorelli, Feuer, Juhász, & Détári, 1986; Rasmusson, Clow, & Szerb, 1992, 1994; Rasmusson, Szerb, & Jordan, 1996; Szymusiak & McGinty, 1986). Third, specific neurotoxic lesions of NB cholinergic corticopetal neurons deplete the cortex of ACh and impair EEG activation, i.e., increase slow wave activity and decrease fast (e.g., γ) waves (Berntson, Shafi, & Sarter, 2002; Wenk, Stoehr, Quintana, Mobley, & Wiley, 1994). Fourth, cholinergic corticopetal neurons in the NB exhibit a strong correlation with the EEG: increased rates of discharge occur with increased activation and vice versa (Cape, Manns, Alonso, Beaudet, & Jones, 2000; Chernyshev & Weinberger, 1998; Détári, Rasmusson, & Semba, 1999; Duque, Balatoni, Détári, & Zaborszky, 2000; Szymusiak & McGinty, 1986). Fifth, GABAergic corticopetal cells in the NB, that are undoubtedly activated by NB stimulation, act synergistically with cholinergic corticopetal cells by inhibiting cortical inhibitory interneurons, thus promoting activation via disinhibition (Dykes, 1997; Freund & Meskenaite, 1992). Together, these findings demonstrate a very close relationship among activation of the NB, the cortical release of ACh and cortical activation. Indeed, they strongly support the hypothesis that the level of cortical ACh released by the nucleus basalis is a major mechanism underlying the state of the EEG (Détári et al., 1999; Metherate & Ashe, 1992). In the present study, stimulation of the NB produced the same EEG effects as documented in prior, reductionistic studies of the NB, ACh and the EEG. Therefore, it is reasonable to infer that the present findings involved engagement of the cholinergic system.
It is possible that NB stimulation additionally engaged non-cholinergic systems that were responsible for or involved in memory induction. Although this explanation cannot be discounted, recall that CS-evoked increase in γ activity was correlated with the degree of memory specificity in group MP. This significant correlation constrains the possible involvement of non-cholinergic systems. Thus, if engagement of the cholinergic system were incidental to memory induction, then the cholinergic system must have been controlled by one or more other systems that were also responsible for the formation of memory. In short, if CS-induced increased γ activity reflects in large part the actions of ACh released in the cortex, then either the cholinergic system is directly involved in memory induction or its activities reflect and index parallel control from some source common to γ regulation and memory induction.
4.3. The cholinergic nucleus basalis and the encoding and storage of experiential detail
Our previous studies found that pairing a tone with NB stimulation induced specific, associative behavioral memory (McLin et al., 2002a, 2002b). Moreover, this memory is correlated with the development of specific plasticity in the primary auditory cortex (Miasnikov et al., 2006). Thus, they directly implicate the cholinergic nucleus basalis in learning and memory of specific stimulus features.
However, these prior studies were not intended to address the issue of mechanisms whereby the cholinergic system might modulate or control the degree of detail that becomes part of memory. Some studies of cholinergic effects on sensory processing do provide a good starting point. For example, stimulation of the nucleus basalis is known to facilitate thalamocortical transmission and increase the response of the auditory cortex to sounds (Metherate & Ashe, 1991, 1992, 1993). Moreover, the duration of response facilitation is greater for higher levels of NB stimulation (Hars, Maho, Edeline, & Hennevin, 1993; Edeline, Hars, Maho, & Hennevin, 1994a). Together, these findings provide a basis for the entry and maintenance of greater sensory detail into the auditory cortex (and perhaps other auditory structures) under the auspices of the cholinergic system. These factors should promote the encoding of detail into memory.
Studies of information processing outside of the auditory system also demonstrate that the cholinergic system can enhance the encoding of experiential detail. For example, Hasselmo and colleagues have been concerned with the differential effects of high and low levels of acetylcholine on the processing of information in the piriform cortex (Barkai & Hasselmo, 1997; Hasselmo, Anderson, & Bower, 1992), entorhinal cortex (Hasselmo, Fransen, Dickson, & Alonso, 2000), and hippocampus (Giocomo & Hasselmo, 2005); see also (McGaughy, Koene, Eichenbaum, & Hasselmo, 2005). Most relevant to the present findings is the hypothesis that high levels of ACh promote the encoding of memory by facilitating sensory transmission while at the same time suppressing recurrent excitation and intracortical processing. In contrast, low levels of ACh are thought to promote retrieval from memory by facilitating intracortical processing at the expense of entry of new sensory information (Hasselmo & McGaughy, 2004). Although their formulation does not explicitly hold that high levels of ACh should increase the storage of specific detail, this outcome is consistent with their core hypothesis. Recall that while both groups MP and WP in our experiment developed associative memory, only the former, which had a higher level of NBs and a greater effect on the cortex, developed memory that was specific to acoustic frequency, i.e., that of the CS band. Therefore, these findings provide direct support for the first part of the Hasselmo et al. model, i.e., the facilitation of encoding via high levels of ACh.
4.4. Relevance for the “Thalamic–Nucleus Basalis Model” of learning-induced specific plasticity
A heuristic model of learning-induced CS-specific plasticity in the auditory cortex postulates a two-stage mechanism for the storage of information during Pavlovian fear conditioning. The first stage involves the non-lemniscal auditory thalamus, specifically the PIN/magnocellular medial geniculate (MGm) complex, which receives convergent acoustic and nociceptive input and projects to pyramidal cell apical dendrites in layer I. This was thought to initiate plasticity but be unable to induce long-term storage. The second stage involves the engagement of the nucleus basalis (via the amygdala), which in turn is able to promote consolidation and long-term retention of plasticity by the release of ACh, which engages muscarinic receptors in the cortex (Weinberger, 1998; Weinberger et al., 1990). Although the present study is not concerned with fear conditioning, the present results on γ waves, together with other findings, are relevant to this general model.
Stimulation of the PIN can evoke γ waves in the auditory cortex, although the thalamus is not essential for the generation of γ in the cortex (Brett & Barth, 1997; Brett, Krishnan, & Barth, 1996). This suggests that the non-lemniscal auditory thalamus might increase the amount of cortical γ during learning. Additionally, as mentioned previously, stimulation of the nucleus basalis and its release of ACh are known to facilitate thalamocortical transmission of acoustic information to the auditory cortex (Metherate & Ashe, 1993; Metherate & Hsieh, 2004). Moreover, thalamocortical transmission itself can promote γ waves in the auditory cortex (Metherate & Cruikshank, 1999). The present study shows that the storage of CS-related detail requires pairing a tone with NB stimulation, the latter at a “moderate” level such that the CS itself elicits increased γ activity during training. The increase in γ is itself correlated with the level of specificity in memory of CS-band frequencies. As noted above, there is support for the view that γ activity indexes the encoding of detail by the formation of neuronal ensembles. Therefore, the encoding of detail might be promoted both by the MGm/PIN facilitation of γ/ ensembles and by dual actions of the nucleus basalis—facilitation both of thalamocortical processing of the CS frequency and the γ waves that are elicited by this facilitated transmission. These processes would appear to fill in necessary detail that the original model lacks. This more detailed model is capable of being tested. For example, both the reduction of thalamocortical transmission and the prevention of γ waves should reduce the level of detail that is encoded.
4.5. Future directions
The major finding of this study is the identification of a neural mechanism that can control the level of detail that is stored in memory and recalled. We have been unable to find either prior comparable reports or systematic consideration of the neurobiological bases of memory detail. Thus, the present investigation may be seen as highlighting a neglected, but fundamental issue in the neurobiology of learning and memory. The findings have potential clinical applications, as they involve both the induction of memory by direct manipulation of a major neuromodulatory system and provide guidelines for selecting optimal conditions for effective training as well as diagnostic tools for ACh-related learning disorders. While extensive research awaits, the present report demonstrates the feasibility of addressing neural mechanisms that regulate the actual detail or “contents” of memory.
Distinctions are sometimes made between “associative memory” and “sensory specific memory”, perhaps influenced by the plethora of “types of memory” in contemporary discourse. In the present study, these terms might be applied to the Weak-Paired (WP) and Moderate-Paired (MP) groups, respectively. However, such a distinction would be misleading. Both the WP and MP groups developed associative memory, as evidenced by the difference between their behavior and those of their control groups, WU and MU, respectively. (“Perceptual learning”, i.e., improved acuity on a stimulus dimension due to extensive practice on increasingly difficult discrimination tasks, is operationally “stimulus specific learning”, but apparently does not establish detailed associative memories.) Indeed, the thrust of this paper is not on two types of memory that are implied by the terms “associative” and “sensory specific” (or “stimulus specific”), but rather on determinants of the amount of experiential detail that is encoded and retrieved within associative memory itself. It has long been recognized that subjects may learn and remember general stimulus features, e.g., that the CS was a tone, or encode and recall greater detail, e.g., that the CS was an 8.0 kHz tone (viz. Mackintosh, 1974; Mostofsky, 1965). While the goals of most studies of associative learning may be neutral with respect to the level of learned detail, subjects might have remembered a considerable amount of detail that has not been evaluated. In short, we argue not for another type of memory but rather for the benefits of determining the level of learned detail in order to achieve a more complete understanding of the neural bases of learning and memory.
As pointed out above, targeted pharmacological and other studies are needed to elucidate the role of ACh. Recent studies have found either competition between different memory systems (McIntyre, Pal, Marriott, & Gold, 2002) or cooperation between different structures (McIntyre, Marriott, & Gold, 2003) that depend on the locus and the magnitude of release of ACh. If similar studies can include control of structure-specific levels of ACh release, then it should be possible to predict the degree to which experiential detail in a given memory system will be encoded and recalled.
A major line of inquiry should concern the storage sites of the details of memory. Learning-induced plasticity in the primary auditory cortex itself has all of the major characteristics of associative memory (associativity, specificity, rapid induction, consolidation over hours and days, and very long-term retention) (reviewed in Weinberger, 2004c). However, other brain regions are undoubtedly involved. Even what may be described as a simple memory is likely to have a distributed neural substrate. Also, it is possible that information may be re-encoded and stored, following initial stimulus processing in a form not closely tied to the physical parameters of sound frequency, or more generally, any sensory dimension, complicating detection by electrophysiological or imaging approaches. Simply lesioning the auditory cortex is unlikely to provide a definitive test of this structure as a site of storage for learning-dependent changes in the representation of acoustic frequency, because auditory (and other sensory) cortex has both perceptual and mnemonic functions (Greenberg & Rubin, 2003; McGaughy et al., 2005; Palmer, Nelson, & Lindley, 1998; Rutkowski & Weinberger, 2005; Trainor, Shahin, & Roberts, 2003; Weinberger, 2004a, chap. 5). Hence, any lesion could affect either or both processes. Even this short list of necessary future research indicates that an adequate account of how the brain comes to store and access the specificity of experience constitutes a considerable, but approachable, challenge.
Acknowledgments
We thank Julia Martinson for developing the critical components of Spike-2 data acquisition and analysis software, Gabriel K. Hui for assistance with the manuscript, and Jacquie Weinberger for laboratory and administrative support. This research was funded by research grants from the National Institutes of Health, DC-02938 and DC-05592 to N.M.W.
References
- Barkai E, Hasselmo MH. Acetylcholine and associative memory in the piriform cortex. Molecular Neurobiology. 1997;15(1):17–29. doi: 10.1007/BF02740613. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Brain oscillations in perception and memory. International Journal of Psychophysiology. 2000;35(2–3):95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. European Journal of Neuroscience. 2002;16(12):2453–2461. doi: 10.1046/j.1460-9568.2002.02310.x. [DOI] [PubMed] [Google Scholar]
- Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: a possible role for object representation. International Journal of Psychophysiology. 2000;38(3):211–223. doi: 10.1016/s0167-8760(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Brett B, Barth DS. Subcortical modulation of high-frequency (gamma band) oscillating potentials in auditory cortex. Journal of Neurophysiology. 1997;78(2):573–581. doi: 10.1152/jn.1997.78.2.573. [DOI] [PubMed] [Google Scholar]
- Brett B, Krishnan G, Barth DS. The effects of subcortical lesions on evoked potentials and spontaneous high frequency (gamma-band) oscillating potentials in rat auditory cortex. Brain Research. 1996;721(1–2):155–166. doi: 10.1016/0006-8993(96)00168-0. [DOI] [PubMed] [Google Scholar]
- Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE. Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes γ and θ cortical activity together with waking and paradoxical sleep. Journal of Neuroscience. 2000;20(22):8452–8461. doi: 10.1523/JNEUROSCI.20-22-08452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamenti F, Deffenu G, Abbamondi AL, Pepeu G. Changes in cortical acetylcholine output induced by modulation of the nucleus basalis. Brain Research Bulletin. 1986;16(5):689–695. doi: 10.1016/0361-9230(86)90140-1. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Chernyshev BV, Weinberger NM. Acoustic frequency tuning of neurons in the basal forebrain of the waking guinea pig. Brain Research. 1998;793(1–2):79–94. doi: 10.1016/s0006-8993(98)00163-2. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning and Memory. 2003;10(6):427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behavioral Neuroscience. 1991;105(3):416–430. doi: 10.1037//0735-7044.105.3.416. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural Brain Research. 1993;58(1–2):175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Détári L, Juhász G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiologica Hungarica. 1983;61(3):147–154. [PubMed] [Google Scholar]
- Détári L, Juhász G, Kukorelli T. Firing properties of cat basal forebrain neurones during sleep–wakefulness cycle. Electroencephalography and Clinical Neurophysiology. 1984;58(4):362–368. doi: 10.1016/0013-4694(84)90062-2. [DOI] [PubMed] [Google Scholar]
- Détári L, Juhász G, Kukorelli T. Neuronal firing in the pallidal region: firing patterns during sleep–wakefulness cycle in cats. Electroencephalography and Clinical Neurophysiology. 1987;67(2):159–166. doi: 10.1016/0013-4694(87)90039-3. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. The role of basal fore-brain neurons in tonic and phasic activation of the cerebral cortex. Progress in Neurobiology. 1999;58(3):249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174(11):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Petersen RS, Harris JA. Learning through maps: functional significance of topographic organization in primary sensory cortex. Journal of Neurobiology. 1999;41(1):64–68. [PubMed] [Google Scholar]
- Duque A, Balatoni B, Détári L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal fore-brain. Journal of Neurophysiology. 2000;84(3):1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Mechanisms controlling neuronal plasticity in somatosensory cortex. Canadian Journal of Physiology and Pharmacology. 1997;75(5):535–545. [PubMed] [Google Scholar]
- Edeline JM. The thalamo-cortical auditory receptive fields: regulation by the states of vigilance, learning and the neuromodulatory systems. Experimental Brain Research. 2003;153(4):554–572. doi: 10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Maho C, Hennevin E. Transient and prolonged facilitation of tone-evoked responses induced by basal fore-brain stimulations in the rat auditory cortex. Experimental Brain Research. 1994a;97(3):373–386. doi: 10.1007/BF00241531. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Maho C, Hars B, Hennevin E. Non-awaking basal forebrain stimulation enhances auditory cortex responsiveness during slow-wave sleep. Brain Research. 1994b;636(2):333–337. doi: 10.1016/0006-8993(94)91033-2. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310(5749):810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Flood JF, Landry DW, Jarvik ME. Cholinergic receptor interactions and their effects on long-term memory processing. Brain Research. 1981;215(1–2):177–185. doi: 10.1016/0006-8993(81)90500-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. γ-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neo-cortex. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(2):738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Nicotinic modulation of gluta-matergic synaptic transmission in region CA3 of the hippocampus. European Journal of Neuroscience. 2005;22(6):1349–1356. doi: 10.1111/j.1460-9568.2005.04316.x. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39(4–5):687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56(1):61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. Journal of Neurophysiology. 1992;67(5):1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Fransen E, Dickson C, Alonso AA. Computational modeling of entorhinal cortex. Annals of the New York Academy of Sciences. 2000;911:418–446. doi: 10.1111/j.1749-6632.2000.tb06741.x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progress in Brain Research. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73(2):244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(3983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, Traub RD, Whittington MA. Neuronal networks for induced ‘40 Hz’ rhythms. Trends in Neurosciences. 1996;19(5):202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- Jiménez-Capdeville ME, Dykes RW, Myasnikov AA. Differential control of cortical activity by the basal forebrain in rats: a role for both cholinergic and inhibitory influences. Journal of Comparative Neurology. 1997;381(1):53–67. [PubMed] [Google Scholar]
- John ER. High nervous functions: brain functions and learning. Annual Review of Physiology. 1961;23:451–484. doi: 10.1146/annurev.ph.23.030161.002315. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhász G, Détári L, Kukorelli T. Effects of hypnogenic vagal stimulation on thalamic neuronal activity in cats. Brain Research Bulletin. 1985;15(5):437–441. doi: 10.1016/0361-9230(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Cortical oscillatory activity and the dynamics of auditory memory processing. Reviews in the Neurosciences. 2005;16(3):239–254. doi: 10.1515/revneuro.2005.16.3.239. [DOI] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep–wakefulness detected by intracerebral dialysis. Life Sciences. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. Journal of Neurophysiology. 2001;86(1):326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kukorelli T, Feuer L, Juhász G, Détári L. Effect of glutaurine on sleep–wakefulness cycle and aggressive behaviour in the cat. Acta Physiologica Hungarica. 1986;67(1):31–35. [PubMed] [Google Scholar]
- Luiten PGM, Gaykema RPA, Traber J, Spencer DG., Jr Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Research. 1987;413(2):229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Research. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME. Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatchto-sample task. Journal of Neuroscience. 2005;25(44):10273–10281. doi: 10.1523/JNEUROSCI.2386-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Cooperation between memory systems: acetylcholine release in the amygdala correlates positively with performance on a hippocampus-dependent task. Behavioral Neuroscience. 2003;117(2):320–326. doi: 10.1037/0735-7044.117.2.320. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Pal SN, Marriott LK, Gold PE. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. Journal of Neuroscience. 2002;22(3):1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, Miasnikov AA, Weinberger NM. Potentials evoked in the primary auditory cortex by stimulation of the nucleus basalis that can induce receptive field plasticity. Abs. No. 73.5. Society for Neuroscience Abstracts. 2000;26 (1):194. [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. The effects of electrical stimulation of the nucleus basalis on the electroencephalogram, heart rate, and respiration. Behavioral Neuroscience. 2002a;116(5):795–806. [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002b;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiology of Learning and Memory. 2003;79(2):152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Research. 1991;559(1):163–167. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation elicits neo-cortical activation and facilitates thalamocortical synaptic transmission: Intracellular and extracellular recordings in rat auditory cortex. Society for Neuroscience Abstracts. 1992;18(2):975. [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. Journal of Neuroscience. 1992;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma-band activity in auditory cortex in vitro. Experimental Brain Research. 1999;126(2):160–174. doi: 10.1007/s002210050726. [DOI] [PubMed] [Google Scholar]
- Metherate R, Hsieh CY. Synaptic mechanisms and cholinergic regulation in auditory cortex. Progress in Brain Research. 2004;145:143–156. doi: 10.1016/s0079-6123(03)45010-3. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006 doi: 10.1016/j.nlm.2005.12.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, McLin D, III, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. NeuroReport. 2001;12(7):1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Poytress BS, Chen JC, Weinberger NM. 2004 Abstract Viewer. Washington, DC: Society for Neuroscience; 2004. Stimulation of the nucleus basalis that induces behavioral memory in the rat is motivationally neutral. Prog. No. 207.6. [Google Scholar]
- Mostofsky DI, editor. Stimulus generalization. Palo Alto, CA: Stanford University Press; 1965. [Google Scholar]
- Ohl FW, Scheich H. Online correspondence: fallacies in behavioural interpretation of auditory cortex plasticity. Nature Reviews Neuroscience. 2004 Published online November 20, 2004 at < http://www.nature.com/nrn/journal/v5/n4/corres/nrn1366_fs.html>.
- Palmer CV, Nelson CT, Lindley GA., IV The functionally and physiologically plastic adult auditory system. Journal of the Acoustical Society of America. 1998;103(4):1705–1721. doi: 10.1121/1.421050. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, translator. New York: Dover Publications; 1960. Original work published 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA. The ascending neuromodulatory systems in learning by reinforcement: comparing computational conjectures with experimental findings. Brain Research Reviews. 1995;21(3):219–245. doi: 10.1016/0165-0173(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Phillis JW, York DH. Pharmacological studies on a cholinergic inhibition in the cerebral cortex. Brain Research. 1968;10(3):297–306. doi: 10.1016/0006-8993(68)90201-1. [DOI] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiology of Learning and Memory. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Frequency-dependent increase in cortical acetylcholine release evoked by stimulation of the nucleus basalis magnocellularis in the rat. Brain Research. 1992;594(1):150–154. doi: 10.1016/0006-8993(92)91041-c. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience. 1994;60(3):665–677. doi: 10.1016/0306-4522(94)90495-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Szerb JC, Jordan JL. Differential effects of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and N-methyl-D-aspartate receptor antagonists applied to the basal forebrain on cortical acetylcholine release and electroencephalogram desynchronization. Neuroscience. 1996;72(2):419–427. doi: 10.1016/0306-4522(95)00523-4. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends in Neurosciences. 1999;22(2):74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends in Cognitive Sciences. 2003;7(7):313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13(3):627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Sommer FT, Wennekers T. Associative memory in networks of spiking neurons. Neural Networks. 2001;14(6–7):825–834. doi: 10.1016/s0893-6080(01)00064-8. [DOI] [PubMed] [Google Scholar]
- Szerb J. The effect of tertiary and quaternary atropine on cortical acetylcholine output and on the electroencephalogram in cats. Canadian Journal of Physiology and Pharmacology. 1964;42:303–314. doi: 10.1139/y64-036. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Research. 1986;370(1):82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Patterson MM, Teyler TJ. The neurophysiology of learning. Annual Review of Psychology. 1972;23:73–104. doi: 10.1146/annurev.ps.23.020172.000445. [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Shahin A, Roberts LE. Effects of musical training on the auditory cortex in children. Annals of the New York Academy of Sciences. 2003;999:506–513. doi: 10.1196/annals.1284.061. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1989. pp. 149–189. [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annual Review of Neuroscience. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70(1–2):226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiology of Learning and Memory. 2003;80(3):268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Experience-dependent response plasticity in the auditory cortex: issues, characteristics, mechanisms, and functions. In: Parks TN, Rubel EW, Fay RR, Popper AN, editors. Springer Handbook of Auditory Research, vol 23 Plasticity of the auditory system. New York: Springer; 2004a. pp. 173–227. [Google Scholar]
- Weinberger NM. Online correspondence: correcting misconceptions of tuning shifts in auditory cortex. Nature Reviews Neuroscience. 2004b Published online November 20, 2004 at < http://www.nature.com/nrn/journal/v5/n4/corres/nrn1366_fs.html>.
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Reviews Neuroscience. 2004c;5(4):279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J. Retuning auditory cortex by learning: a preliminary model of receptive field plasticity. Concepts in Neuroscience. 1990;1(1):91–132. [Google Scholar]
- Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. Journal of Neuroscience. 1994;14(10):5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FAW, Ma YY. Reinforcement-related neurons in the primate basal forebrain respond to the learned significance of task events rather than to the hedonic attributes of reward. Cognitive Brain Research. 2004;19(1):74–81. doi: 10.1016/j.cogbrainres.2003.11.003. [DOI] [PubMed] [Google Scholar]