Abstract
The goal of this paper is to describe the simultaneous influence of social and genetic risk factors on declines in cognitive functioning among older American adults. We use detailed information about the social characteristics of older adults' neighborhoods from the Chicago Health and Aging Project (n = 1655; ages 65+) in conjunction with information about respondent's APOE genotype to predict changes in cognitive function over time. Results indicate that the presence of the ɛ4 allele is associated with a significantly lower cognitive function score at baseline and greater declines in cognitive function compared to those without this risk allele. Importantly, we also show significant variation in the effect of the ɛ4 allele across neighborhoods and our results indicate that this genotype is more strongly associated with cognitive function for residents of neighborhoods with the lowest levels of social disorder. Our findings support the non-causal social push gene–environment interaction model.
Keywords: ApoE4, Cognitive decline, Neighborhoods, Gene-environment interaction, USA
Introduction
The ɛ4 allele is a polymorphism in the apolipoprotein E (APOE) gene that has been associated with the early onset of cognitive decline and is more prevalent among those with Alzheimer's disease compared to the rest of the population (Corderet al., 1993; Small, Rosnick, Fratiglioni, & Backman, 2004). Importantly, there is a great deal of variability in the magnitude of the effects of the ɛ4 allele across studies (Small et al., 2004), which has produced a fairly small average effect size (Williams, Plassman, Burke, Holsinger, & Benjamin, 2010). Although differences in effect size may reflect random variation, they could also allude to the influence of variable environments on the potency of APOE-E4 in relation to cognitivefunction; a gene–environment (G × E) interaction (Shanahan & Hofer, 2005; Raine, 2002). In this paper, we use data from a longitudinal study of older American adults from 20 census tracts in Chicago to examine differences in the effects of the allele for residents of neighborhoods that differ markedly from one another with respect to social disorder.
Gene–environment interaction and aging
There is an emerging interest in more precisely defining the role of APOE-E4 in cognitive function and cognitive decline in older populations. Specifically, researchers point to differences in the effect of this risk allele as a function of different behavioral and environmental factors (Lee, Glass, James, Bandeen-Roche, & Schwartz, 2011; Peavey et al., 2007). In one of the earliest studies in this area, Haan, Shemanski, Jagust, Manolio, and Kuller (1999) found that several biological risk factors for cardio-vascular disease were more strongly linked to cognitive decline for those with at least one ɛ4 allele compared to those without this risk allele. Similar results are shown in more recent studies in which the association between cognitive performance and biomarkers including beta-carotene (Hu et al., 2006), vitamin B-12 (Feng, Li, Yap, Kua, & Ng, 2009), estrogen (Yaffe, Haan, Byers, Tangen, & Kuller, 2000), and cortisol (Lee et al., 2008) are systematically different for carriers of the ɛ4 allele compared to others.
These studies focus on proximal biological risk factors but similar results have started to emerge from studies that have focused on psychological and social characteristics that, from an etiological perspective, reflect a more distal relationship to the underlying disease process (Lee et al., 2011; Link & Phelan, 1995; Peavy et al., 2007). For example, using data from the Health and Retirement Study, a large study of older adults in the United States, McCardle and Prescott (2010) find no main effect of APOE-E4 on decline in episodic memory but they show steeper declines in memory for the ɛ4 carriers compared to the non-ɛ4 carriers among those with less than 8 years of education. This finding is in line with the “social trigger” G × E model in which particular environmental contexts trigger genetic risk factors (Shanahan & Hofer, 2005). In a thorough review of the literature, Reiss and Leve (2007) argue that there is wide support for the triggering perspective and state that “[i]n virtually all publications reporting positive results for this phenomenon, a substantial association between allele and behavior is observed under adverse environmental circumstances but not under favorable circumstances” (pp. 1006–7).
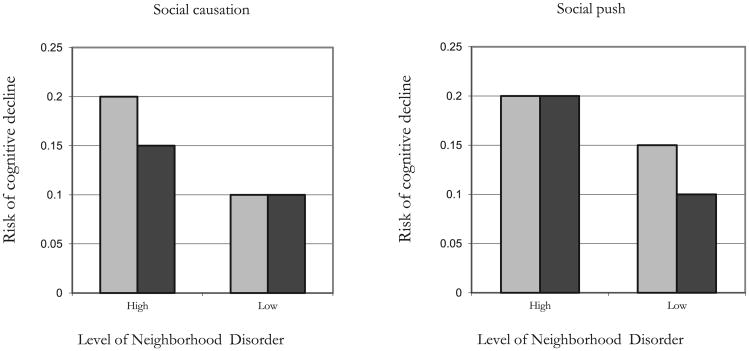
The social trigger approach assumes that the interactive relationships between genes and environments are causal; that is, G × E interactions are interpreted to mean that specific environmental conditions are required for a polymorphism to become expressed, leading to its differential associations with behavioral phenotypes or disease risk (Meaney, 2010). Although this model makes intuitive and biological sense, it is worth noting that statistical interactions between a measured E and a measured G may also be observed in the absence of a causal (interactive) relationship. For example, social contexts characterized by high levels of disadvantage may have such a dominant effect on the occurrence of specific behaviors or diseases that they may “overwhelm” the typically more subtle genetic effects on outcomes. Raine (2002) refers to this situation as a social push model, arguing that social environments may push certain phenotypes forward irrespective of the distribution of genetic risk factors; only when these adverse social conditions are minimized will the genetic influences become apparent, allowing “biology to shine through” (Raine, 2002: 13). Scarr (1993: 5) provides a similar perspective in which she elaborates on Hartmann's (1958) notion of the “average expectable environment.” This general evolutionary perspective emphasizes “normal organisms in normal environments” and Scarr argues that “[e]nvironments that fall outside of the species-normal range will not promote normal developmental patterns” (Scarr, 1993:5). According to her perspective, forces related to genetic inheritance are not likely to cause individual differences in phenotype for organisms within environments that are atypical. This “social push” perspective is illustrated in Fig. 1.
Fig. 1.
Causal and non-causal gene-environment interaction models. Note: Models above denote conceptual examples of different G × E models. The light shaded bar indicates the risk of cognitive decline for those with a risky genotype and the dark shaded bar is the risk of cognitive decline for those without the risky genotype.
In this paper, we turn our focus to psychosocial stress as an important environmental condition that may modify the relationship between genotype and cognitive outcomes in late-life to test causal and non-causal G × E models. Previous research has shown that markers of stressful life experiences may interact with specific polymorphisms in relation to behavioral and disease-related outcomes (Caspi et al., 2003) and more recent research is providing clues about the physiological mechanisms behind these complex interactions (Su et al., 2009). Such work has also begun to emerge for cognitive aging. In a small volunteer sample, Peavy and colleagues found that APOE-E4 positive older adults reporting high levels of stress had a substantially poorer performance on memory tasks than low stress persons, whose performance was similar to either high stress or low stress APOE-E4 negative persons (Peavy et al., 2007).
Much of the present work on psychosocial stress by gene interaction has emphasized individual-level stress exposures, often defined as experiences or perceptions of stressful life conditions. Very little is known about the degree to which stressful conditions in the actual environment such as work places or neighborhoods interact with genetic risk factors in producing specific behavioral or disease outcomes. A notable exception is a recent study that suggests that living in more hazardous neighborhoods is associated with worse executive functioning and processing speed among persons with an ɛ4 allele, but not in those without this allele (Lee et al., 2011). Another important limitation of present work on G × E interactions in relation to late-life cognitive function is that it has relied on cross-sectional cognitive function data. Such data do not permit solid inferences regarding the role of G × E interactions in aging-related disease processes, as they do not differentiate between early-life and life-course influences on cognition and aging-related disease effects on cognitive decline. Serial cognitive performance data are better suited to the establishment of aging-related declines in cognition associated with dementia, Alzheimer's disease and their pre-clinical, early stage manifestations.
The purpose of this study is to explore the G × E mechanism that may structure the relationship between APOE-E4, neighborhood social environment, and change in cognitive function in older age. The social trigger model represents a causal mechanism in which the association between APOE-E4 and cognitive decline is triggered by specific social circumstances, in our study represented by adverse neighborhood conditions (Fig. 1, model 1). In this case, we would expect that the ɛ4 allele would have a more deleterious effect on cognitive function for those in the most disorganized residential environments. On the other hand, the social push model represents the non-causal mechanism, which rests on the assumption that in highly adverse environments, social (and physical) conditions overwhelm any small genetic effects on cognitive decline, and genotype may therefore not discriminate between patterns of cognitive decline under these circumstances; the social environment is ‘pushing’ the phenotype rather than genetic factors (Raine, 2002). In this model, non-adverse social environments free of social disorder may create a context in which genetic factors become more salient in complex phenotypes such as cognitive decline (Fig. 1, model 2). Thus, we would expect the ɛ4 allele to have a more deleterious effect on cognitive function for residents of neighborhoods with the least amount of social disorder. This perspective may be particularly relevant to mixed evidence regarding the links between the and ɛ4 allele and Alzheimer's disease across racial and ethnic groups. For example, Tang and colleagues show a two and a half fold increase in the risk of Alzheimers for ɛ4 carriers but only among non-Hispanic white adults and not among African Americans or Hispanics (Tang et al., 1998). Given the large differences in the typical neighborhoods for white and minority adults in the US, the Tang and colleagues' results are in line with the social push perspective where social factors are “pushing” Alzheimer's onset more so for racial and ethnic minorities than for non-Hispanic whites.
We contribute to this literature by taking advantage of a multilevel and longitudinal data source derived from the Chicago Health and Aging Project (CHAP) to examine the nature of the G × E interaction of neighborhood conditions and APOE-E4 in relation to cognitive decline in older age. The multilevel framework allows us to nest individual respondents within particular neighborhoods and to assess aspects of the social environment that are otherwise difficult to measure and the repeated measures of cognitive functioning build on the work of Lee et al. (2011) and others because it allows us to measure change in cognitive functioning as related to genetic and social influences.
Methods
Design and population
Data for this analysis come from CHAP, an ongoing, longitudinal study of risk factors for Alzheimer's disease and other aging-related outcomes. Details of the CHAP study design and population have been described previously (Bienias, Beckett, Bennett, Wilson, & Evans, 2003), but are summarized briefly below. CHAP is designed as a geographically-defined population-based study that includes all persons aged 65 years and older living in three adjacent community areas on the south-side of Chicago. Recruitment of the initial cohort (Original Cohort) was based on a complete census of the study population, which began in 1993 and was completed in about three years. Other than age, there were no inclusion or exclusion criteria. Recruitment and baseline assessments were done in conjunction with the census, and yielded a sample size of 6158 participants (78.9% participation rate). All surviving participants are invited for repeat assessments conducted at approximately three-year intervals, which have generally achieved a participation rate of 80–85%. These assessments (referred to as Population Interview) include standardized questions on medical history, lifestyle factors, demographic and psychosocial characteristics, as well as standardized tests of physical and cognitive function. CHAP is currently in its sixth cycle of interviews for the surviving members of this cohort. In addition, random samples are drawn from participants who have completed the Population Interview at each cycle of data collection, stratified by age, race, sex, and change in cognitive function; participants of this sample receive a standardized, detailed Clinical Evaluation in order to identify (new) cases of Alzheimer's disease. Participants of the Clinical Evaluation also provide a blood sample (or cheek swab) from which DNA is isolated. As of the third cycle of interviews (in 2000), CHAP has started to recruit residents from the same study area who had turned 65 since the beginning of the study, and it has continued to do so in all subsequent cycles, thus establishing Successive Cohorts of newly-aged community residents. Assessments methods for these participants are identical to and fully integrated with those performed in the Original Cohort, including the selection of a stratified random sample for clinical evaluation.
All CHAP procedures are approved by the IRB at Rush University Medical Center, and all participants have provided signed, informed consent. The analysis sample of the present study is based on participants who completed a Clinical Evaluation during one of the first four assessment cycles, conducted between 1993 and 2006, and who had at least one follow-up assessment of cognitive function and APOE data (N = 1655). Data for individual-level demographic characteristics (date of birth, sex, race, education) are derived from the first CHAP Population Interview each participant completed; data on neighborhood conditions comes from interviews with CHAP participants who were not included in this analysis sample (see below); data on initial cognitive function and APOE-E4 come from the Clinical Evaluation from which participants were selected for this sample; and data on follow-up cognitive function come from subsequent Population Interviews.
Measures
Assessment of cognitive function is based on four tests including the East Boston Memory Test of immediate and delayed memory (Albert et al., 1991), the symbol digit modalities test as measure of perceptual speed (Smith, 1984), and the Mini Mental State Examination (MMSE) as a measure of general cognitive function (Folstein, Anthony, Parhad, Duffy, & Gruenberg, 1985). As described previously, scores on each test are first standardized by converting raw scores to z-scores based on the baseline distribution in CHAP, and then averaged across tests to produce a summary score of global cognitive function (Wilson et al., 2002). This procedure minimizes floor and ceiling effects within each test, and produces a variable that has an approximately normal distribution. Data from the last (most recent) available Population Interview for each participant are used as follow-up assessment to compute change in cognitive function. For comparative purposes, the levels of impairment based on the MMSE were as follows: severe (0–10): 1.8%, moderate (11–20) 4.9%, and mild (21–26) 20.8%. These rates increased to 5.8%, 12.7%, and 29.3%, respectively, in the follow-up period. Table 1 provides descriptive statistics for all variables used in the analysis.
Table 1.
Descriptive statistics for all variables used in the analysis (n = 1655).
| APOE-E4 genotype | |||
|---|---|---|---|
|
|
|||
| No | YES | P. | |
| Baseline cognitive function | .39 | .33 | <.138 |
| Follow-up cognitive function | −.01 | −.24 | <.0001 |
| Change in cognitive function | −.40 | −.58 | <.0001 |
| Age (baseline) | 74.71 | 73.38 | <.0001 |
| Sex (male) | .38 | .40 | <.419 |
| Black | .48 | .58 | <.001 |
| Education (baseline) | 12.74 | 12.72 | <.899 |
| Duration of residence | 32.61 | 32.14 | <.633 |
| Follow-up time | 9.11 | 9.01 | <.623 |
| Neighborhood disorder | −.02 | .02 | <.001 |
| Neighborhood disadvantage | −.07 | .12 | <.001 |
Note: Data come from the Chicago Health and Aging Project. Cell entries represent unweighted descriptive statistics (means for continuous variables and proportions for categorical variables). Neighborhood-level means and standard deviations are based on observations nested within 20 census tracts. There was anaverage of 103.2 respondents per neighborhood (SD = 44.9) with a minimum of 22 and a maximum of 206.
We used 8 other variables in our analyses, including 6 individual-level and 2 neighborhood-level, measures. The primary variable of interest is the presence of at least one APOE-E4 allele. In total 534 (32.3%) 1655 respondents in our sample had this risk allele which is somewhat higher than expected given the known distribution of this allele (Eisenberg, Kuzawa, & Hayes, 2010) and the age of the study population (Smith, 2002). This may be due in part to the fact that the presence is higher among African Americans (40%), who comprise more than one-half (51.2%) of our sample, than non-Hispanic whites (27%). All models include race as a control variable to account for the potential influence of population stratification on our findings. The other individual-level variables include age (measured at the time of Clinical Evaluation that serves as baseline for the analysis), sex, race, (black and non- black) and education (years of formal schooling completed). In addition, we use terms for duration (in years) between initial and follow-up assessment of cognitive function, and for years living in the neighborhood as additional control variables.
Our primary neighborhood-level variable is a measure of social disorder obtained in the 1993 study. We developed this measure in CHAP based on a factor analysis of a series of standardized questions on perceptions of the neighborhood environment that were added to the CHAP interview (Cagney et al., 2009). The social disorder measure is based on 7 items that focus on conditions that may represent neglect or threat in the neighborhood environment (e.g., presence of trash and litter, vandalism, unsafe conditions to walk, and broken curbs and sidewalks). Raw scores for individual items are converted to z-scores, and then averaged across items to create an individual-level measure of (perceptions of) social disorder at the census tract level and this measures has a reliability coefficient of .85 (Cagney et al., 2009). To minimize same source bias, we only use responses from CHAP participants who are not included in the present sample but come from the same neighborhood areas. To more precisely define the role of social disorder in its interaction APOE-E4 with respect to cognitive decline, we also include a compositional neighborhood-level variable for the general socioeconomic characteristics of the neighborhood population, based on the following four items derived from the 2000 Census: 1) proportion of households with incomes less than $25,000/year; 2) proportion receiving public assistance; 3) proportion of adult population with a college degree; and 4) proportion of dwelling with a value of > $200,000 (alpha = .94) (Cagney at al., 2009).
Statistical analysis
We use the lmer package (Bates & Maechler, 2010) updated for R 2.12.0 (R Development Core Team, 2010) to fit all multilevel models used in this paper. The lmer package is used to fit generalized linear models for nested data structures like the CHAP data described above and it is particularly useful for our purposes because it allows for the use of sampling weights.
| (1) |
The multilevel model, described in Equation (1), is comparable to a traditional linear model but it contains two additional estimates. The ζ1j term characterizes the average cognition score for residents of neighborhood j (random intercept) and the ζ2jχij term provides an offset to the average effect of APOE-E4 (β2) for residents of neighborhood j (random slope). Variance of this estimate ( ) provides evidence for neighborhood differences in genetic effects on cognition. We also allow for correlation between the random intercept and the random slope. This is a particularly important component of these models because it purges the G × E estimates of any influence of gene–environmental correlation. In addition to these models, we also estimate separate regression models for residents of neighborhoods with low and high levels of neighborhood disorder that are conceptually in line with Fig. 1. To adjust for the non-independence of observations within each neighborhood, we include a random intercept for these models.
Results
As shown in Table 1, APOE-E4 genotype is not associated with baseline differences in cognitive function (p < .138) but E4 carriers declined an average of .58 points compared to .40 points among those without this allele. This difference is statistically significant (p < .0001) and corresponds with a relatively small effect size (Cohen's d = .22). APOE-E4 genotype is also associated with age, race, and our two neighborhood-level indicators. This is most likely due to the increased prevalence of the E4 allele among African Americans compared to white respondents. As such, it is important to adjust for individual-level factors that are associated with both E4 genotype and cognitive decline that may also be associated with neighborhood composition. The adjusted multivariate estimates are presented in Table 2.
Table 2.
eighborhood variation in the effect of APOE-E4 genotype on change in cognitive functioning: multilevel regression estimates (standard error).
| Model 1 | Model 2 | |
|---|---|---|
| Intercept | −290*** (.021) | −290*** (.021) |
| Baseline cognitive function | −.170*** (.016) | −.171*** (.016) |
| APOE-E4 genotype | −.123*** (.024) | −.125*** (.023) |
| Neighborhood disorder | −.415*** (.106) | −.468*** (.107) |
| Neighborhood disadvantage | −.004 (.019) | −.004 (.019) |
| Respondent race [Black = 1] | .040 (.031) | .038 (.031) |
| Respondent education (std.) | .019* (.009) | .018* (.009) |
| Respondent age (std.) | −.256*** (.011) | −.257*** (.011) |
| Respondent sex [Male= 1] | .004 (.016) | .005 (.016) |
| Duration of residence | .051*** (.009) | .050*** (.009) |
| Follow-up time | −.057*** (.009) | −.057*** (.009) |
| APOE-E4 * Disorder | .371** (.139) | |
|
| ||
| SD (slope) | .071 | .066 |
| −2LL | 5934.774 | 5933.274 |
| Likelihood ratio | 8.542 | 1.500 |
| df | 1 | 1 |
| p < | .003 | .220 |
Note: all data have been weighted to correct for sampling design of the Chicago Health and Aging Project. The full sample models include a random effect for APOE-E4 genotype at the neighborhood-level. The likelihood ratios compare the -2LogLikelihood estimates of the current model to the prior baseline model. Corresponding degrees of freedom and probability of overall model improvement are provided.
p <.001,
p <.01,
p <.05.
Table 2 presents estimates from 2 multilevel regression models in which the dependent variable, change in cognitive function, is nested within 20 neighborhoods. The random slope also allows the effect of APOE-E4 genotype to vary across neighborhoods. This variation is described by the standard deviation of the slope (SD = .071). After adjusting for individual and neighborhood-level risk factors, we continue to see a negative and significant influence of the APOE-E4 genotype on change in cognitive function (b = –.123, p < .001), which is only slightly smaller compared to the unadjusted difference in Table 1 (b = –.18, p < .001). We also show a strong and negative effect of social disorder (b = –.415, p < .001) on change in cognitive function over time. Importantly, the inclusion of the random slope for APOE-E4 genotype compared to a null model without this additional term yields a significant improvement in the overall model fit (LR = 8.542, p < .003) providing support for the notion that the effect of APOE-E4 varies significantly across neighborhoods in the CHAP study area.
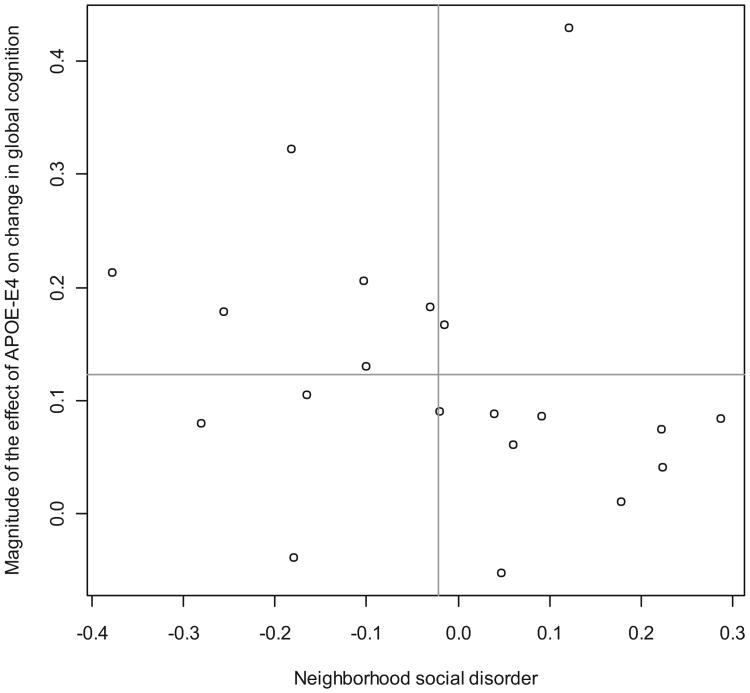
To visualize the meaning of the variation in the effect of APOE-E4 across neighborhoods, Fig. 2 plots the empirical Bayes estimates from Model 1 of Table 2 against levels of neighborhood social disorder. These estimates describe a neighborhood-specific effect of APOE-E4 and to improve the interpretation of these results, the vertical axis presents the absolute value of these estimates so that they are in line with our conceptual model in Fig. 1. That is, higher levels have a stronger deleterious influence on cognitive decline. Two important findings emerge from this figure. First, based on these estimates, the value of .12 (Model 1 of Table 2) is simply the average effect of APOE-E4 genotype. In some neighborhoods, there is little to no effect of this genotype on cognitive decline but in some neighborhoods the effect is 2–3 times higher. This dispersion is summarized with the standard deviation of the random slope and highlights the significance of the contextual G × E framework for interpreting genetic influences. Second, as anticipated by the social push perspective, the effect of APOE-E4 genotype on cognitive decline appears to be the strongest in neighborhoods with the lowest level of social disorder. In most cases, neighborhoods that are above the mean for social disorder also have effects of the APOE-E4 allele that are below the mean. Similarly, six neighborhoods that have relatively low levels of social disorder have average effects of APOE-E4 genotype that are above the mean.
Fig. 2.
Neighborhood-level effects of ApoE4 on change in cognitive function as a function of neighborhood social disorder. Note: Values on the horizontal axis describe the level of social disorder that is present across the 20 neighborhoods in the CHAP study. The values on the vertical axis denote the absolute value of the effect of the ApoE4 allele on changes in cognitive function from the baseline to the follow-up for each of the 20 neighborhoods. The neighborhood-level slopes are derived from the first model in Table 2. The lines correspond to the mean values for the vertical and horizontal axes.
This relationship is moderately sized (bivariate correlation between random slope and disorder r = - .22) but as indicated by the interaction term presented in Model 2 of Table 2, this G × E effect is statistically significant (p < .01). Table 3 examines this same association using a slightly different modeling strategy. Here, we differentiate between neighborhoods with high disorder (those above the mean for disorder) and neighborhoods with low disorder (those below the mean for disorder). As shown in this table, the effect of APOE-E4 genotype is 2.2 times higher (b = −.186 compared to b = −.083) in neighborhoods with low disorder compared to those with high disorder. Again, these findings are in line with the social push G × E perspective. Because this modeling strategy also allows the effects of all covariates to differ by the level of neighborhood disorder it provides further evidence for the interactive results presented in Table 2.
Table 3.
Association between APOE-E4 genotype and cognitive decline by neighborhood disorder.
| Level of neighborhood social disorder | ||
|---|---|---|
|
|
||
| Low | High | |
| Intercept | −.308*** (.039) | −.187*** (.049) |
| Baseline cognitive function | −.174*** (.030) | −.169*** (.019) |
| APOE-E4 Genotype | −.186*** (.027) | −.083*** (.021) |
| Neighborhood disadvantage | −.087** (.030) | −.080 (.045) |
| Respondent race [Black = 1] | .086 (.046) | −.038 (.042) |
| Respondent education (std.) | −.027 (.015) | .033** (.012) |
| Respondent age (std.) | −.293*** (.015) | −.245*** (.015) |
| Respondent sex [Male = 1] | .063* (.025) | −.027 (.022) |
| Duration of residence | .045*** (.011) | .064** *(.013) |
| Follow-up time | −.057*** (.013) | −.053*** (.012) |
|
| ||
| −2LL | 2532.806 | 3362.590 |
| Likelihood ratio | 12.230 | 2.872 |
| df | 1 | 1 |
| p < | .000 | .090 |
Note: all data have been weighted to correct for sampling design of the Chicago Health and Aging Project. Cell entries denote random intercepts models that capture differences in the mean level of cognitive function across neighborhoods. A random slope for APOE-E4 genotype is not estimated in these models. The likelihood ratios compare the -2LogLikelihood estimates of the current model to the prior baseline model. Corresponding degrees of freedom and probability of overall model improvement are provided.
p < .001,
p < .01,
p < .05.
Discussion
Previous research on the interaction between APOE-E4 genotype and environmental factors has clearly demonstrated the plasticity of the APOE-E4-cognitition association (Hunter, 2005). However, there are limitations of the current G × E work related to APOE-E4 genotype and cognition among older adults. For example, the bulk of the research emphasizes psychological, behavioral, or physiological aspects of individuals when considering the environment (Feng et al., 2009; Lee et al., 2008) and only one existing paper (Lee et al., 2011) has focused on broad social factors as the environmental component. This perspective is particularly relevant to social scientists who are primarily interested in the social and geographic boundaries of individuals' daily lives (Sampson, Morenoff, & Gannon-Rowley, 2002). It is within these social spaces that persons can either access resources during times of need or alternatively, where they are exposed to social risk factors (Sampson & Raudenbush, 2004). This perspective has influenced social scientific research on the genetic and environmental influences on complex behaviors such as problem behavior (Cleveland, 2003) and smoking (Boardman, Saint Onge, Haberstick, Timberlake, & Hewitt, 2008; Boardman, 2009) and only one paper has specifically explored contextual moderation of genetic factors related to cognitive function (Lee et al., 2011).
The emphasis on neighborhood environments is also important because a key limitation of G × E studies is the possibility of gene–environmental correlation (rGE). If the specific genotype and the measured environmental factor are correlated, this association can mask true causal pathways; researchers remain uncertain as to whether the measured polymorphism affects response to the environmental factor or simply increases the risk of exposure to the environmental factor. This is particularly problematic for environmental factors that may be influenced by the same genetic factors that are causing the phenotype of interest. In the presence of rGE, many believe that G × E parameter estimates can be biased (Jaffee & Price, 2007). This issue has been discussed extensively and the new consensus is that G × E research should use exogenous environmental factors when available (Conley, 2009). We argue that selection of friends and even workplaces may have a genetic component, but selection into a residential neighborhood is further removed from this risk, especially selection contingent on cognitive decline. A neighborhood-indicator of E may therefore provide a more robust measure of the environment in G × E studies because it reduces the likelihood of rGE.
Population stratification also denotes a passive form of rGE because black respondents are a) more likely to have the ɛ4 allele compared to whites (pb = .397, pw = .274, p < .007) b) more likely to live in neighborhoods with higher levels of social disorder (χ̄w = .13, χ̄b = .27, p < .0001), and c) more likely to have lower cognitive function at baseline (χ̄w = .14, χ̄b = −.51, p < .0001). This raises the possibility that the observed G ×E interactions are a function of the combination of allele frequency and neighborhood differences between black and white adults. To explore this possibility, we re-estimated the second model in Table 2 after adding race × APOE-E4 and race × social disorder interaction terms. These additional adjustments did not change our key findings; we still show a strong (in fact even stronger) interaction between the APOE-E4 and social disorder (b = .45, se = .20; t = 2.26).
Limitations and considerations
There are two aspects of our study that warrant additional discussion. First, it is important to consider differences between our results and those reported by Lee et al. (2011). The key difference is the longitudinal nature of our data, which allows us to interpret our findings more directly in the context of age-associated changes in cognitive function that are likely the result of progressive neuro- degenerative conditions such as Alzheimer's disease. In fact, this is one of the first studies of which we are aware that has reported a prospective G × E interaction effect in relation to an important health outcome. In contrast, their study was limited to cross- sectional cognitive function data, which tend to be much more reflective of early-life factors such as educational experiences than late-life neurodegenerative disease processes (Wilson et al., 2009). In addition, the Lee at al. study used a sample of older adults and examined 7 domains of cognitive function and while neighborhood psychosocial hazards are strongly linked to each measure of cognitive function, they find no main effects of APOE-E4 on any of these 7 domains. However, when considered in combination with elevated levels of psychosocial hazards (a 12- item scale based on socioeconomic census measures, crime reports, the presence of liquor outlets, and vacant housing statistics), those with the ɛ4 allele demonstrated significantly lower cognitive performance scores compared to those without this allele. This is the “strong” variant of the social causation model and although our findings also show moderation in the genetic effects as a function of neighborhood characteristics, we find the opposite direction of the interaction term in our support of the social push model. Differences in the results may be due to the age composition (our sample is considerably older (mean ∼ 72) compared to theirs (mean ∼59)) or the fact that we have smaller number of neighborhoods in our study (n = 20) compared to theirs (n = 65). It is also important to note that we identify a main effect of both social disorder and ApoE4 genotype while this is not the case for Lee et al. (2011). They show this association for the highest quartile of neighborhood psychosocial hazards but we show an effect in neighborhoods with both high and low levels of social disorder. Given the differences in the study designs and the divergent results, we encourage researchers with comparable data to provide results across a range of metropolitan contexts to better elucidate the links between APOE-E4 and cognition.
An additional issue for readers to consider is that we use a composite indicator of global cognition, which is comprised of different components of cognitive health including memory, spatial and temporal orientation, and general knowledge. As stress is consistently linked to declines in memory (Kuhlmann, Piel, & Wolf, 2005) and processing speed (Richardson & VanderKaay Tomasulo, 2011) and less so with general knowledge including spatial and temporal orientation (which are important indictors in the MMSE), it is possible that our results are specific to just one of our indicators. In ancillary analyses (results available upon request), we examined statistical models comparable to model 2 in Table 2 for each indicator separately. According to these preliminary results, the APOE-E4 is consistently related to change in all four measures of cognitive functioning and we observed significant interactions with APOE-E4 and neighborhood disorder for symbol digit, immediate recall, and delayed recall that are in the same direction as the interaction for overall global cognition. However, this same interaction is not evident for MMSE. While this may have to do with the notorious ceiling effects and other measurement issues of the MMSE (Nieuwenhuis-Mark, 2010), it is also possible that the neighborhood disorder * APOE-E4 interaction is more relevant to these memory related tasks because of acute and ambient stressors in highly disordered neighborhoods. While this goes beyond the scope of this paper, we encourage future researchers to investigate this possibility.
Conclusion
In sum, results provide another example of non-causal G × E effects. As described elsewhere, genetic factors may become more relevant within relatively stable and integrated social environments (Raine, 2002). This social push perspective anticipates that within highly disorganized social contexts, the ambient, acute, and chronic stresses will be far more influential on various phenotypes compared to fairly small effects due to specific alleles. If levels of neighborhood social disorder fall outside of the “average expectable environment” (Scarr, 1993) then highly selected alleles may not be linked to phenotype because of the absence of the requisite “normal” environmental conditions. If the social environment pushes the phenotype rather than genetic factors (Raine, 2002) then, as we show here, APOE-E4 has a significantly stronger influence on cognition for persons within environments with fewer environmental stressors and strains. This is important because the bulk of the G × E research focuses on causal models. For example, Rutter, Moffitt, and Caspi (2006) summarize the G × E framework when they state: The question is not “Is there any environmental risk?” or “How big is the average effect of an environmental pathogen across all people exposed to it?” but rather “Who is at the greatest risk from an environmental pathogen?” This question is in line with our inquiry because it assumes a main effect of an established environmental pathogen (which we demonstrate), but it departs from our perspective because it assumes that genetic risk will exacerbate known environmental risks in a causal manner. Instead, we believe that the social and built environments can actually mask subtle genetic risk factors. The use of data sources such as CHAP that contain rich measures of the environment help to clarify contexts in which we are observing change in cognition and as such provide a more focused assessment of the various risk factors.
Acknowledgments
This research was funded in part by the following grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): R01 HD060726 and R24HD066613-01. National Institute on Aging (AG11101) and the National Institute of Environmental Health Sciences (ES10902).
References
- Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. International Journal of Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-36. 2010 http://CRAN.R-project.org/package=lme4.
- Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) Journal of Alzheimers Disorders. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- Boardman JD. State-level moderation of genetic tendencies to smoke. American Journal of Public Health. 2009;99:480–486. doi: 10.2105/AJPH.2008.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Saint Onge JM, Haberstick BC, Timberlake DS, Hewitt JK. Do schools moderate the genetic determinants of smoking? Behavior Genetics. 2008;38:234–246. doi: 10.1007/s10519-008-9197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney KA, Glass TA, Skarupski KA, Barnes LL, Schwartz BS, Mendes de Leon CF. Neighborhood-level cohesion and disorder: measurement and validation in two older adult urban populations. Journals of Gerontology B: Psychological Sciences. 2009;64:415–424. doi: 10.1093/geronb/gbn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cleveland HH. Disadvantaged neighborhoods and adolescent aggression: behavioral genetic evidence of contextual effects. Journal of Research on Adolescence. 2003;13:211–238. [Google Scholar]
- Conley D. The promise and challenges of incorporating genetic data into longitudinal social science surveys and research. Biodemography and Social Biology. 2009;55:238–251. doi: 10.1080/19485560903415807. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apoliprotein E (APOE) gene: climate, local adaptations and evolutionary history. American Journal of Physical Anthropology. 2010;143(1):100–111. doi: 10.1002/ajpa.21298. [DOI] [PubMed] [Google Scholar]
- Feng L, Li J, Yap KB, Kua EH, Ng TP. Vitamin B-12, apolipoprotein E genotype, and cognitive performance in community-living older adults: evidence of a gene-micronutrient interaction. American Journal of Clinical Nutrition. 2009;89:1263–1268. doi: 10.3945/ajcn.2008.26969. [DOI] [PubMed] [Google Scholar]
- Folstein M, Anthony JC, Parhad I, Duffy B, Gruenberg EM. The meaning of cognitive impairment in the elderly. Journal of American Geriatric Society. 1985;33:228–235. doi: 10.1111/j.1532-5415.1985.tb07109.x. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Journal of the American Medical Association. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Hartmann H. Ego psychology and the problem of adaptation. New York: International Universities Press; 1958. [Google Scholar]
- Hu P, Bretsky P, Crimmins EM, Guralnik JM, Reuben DB, Seeman TE. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E4 alleles: MacArthur studies of successful aging. Journal of Gerontology A: Biological Sciences and Medical Sciences. 2006;61:616–620. doi: 10.1093/gerona/61.6.616. [DOI] [PubMed] [Google Scholar]
- Hunter DJ. Gene–environment interactions in human diseases. Nature Reviews: Genetics. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. The Journal of Neuroscience. 2005;25(11):2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, James BD, Bandeen-Roche K, Schwartz BS. Neighborhood psychosocial environment, apolipoprotein E genotype, and cognitive function in older adults. Archives of General Psychiatry. 2011;68:314–321. doi: 10.1001/archgenpsychiatry.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, et al. Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. American Journal of Psychiatry. 2008;165:1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, Phelan JC. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995;35:80–94. [PubMed] [Google Scholar]
- McCardle JJ, Prescott CA. Contemporary modeling of gene × environment effects in randomized multivariate longitudinal studies. Perspectives in Psychological Science. 2010;5:606–621. doi: 10.1177/1745691610383510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis-Mark RE. The death knoll for the MMSE: has it outlived its purpose? Journal of Geriatric Psychiatry. 2010;23(3):151–157. doi: 10.1177/0891988710363714. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, et al. The effects of prolonged stress and ApoE genotype on memory and cortisol in older adults. Biological Psychiatry. 2007;62:472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. ISBN 3-900051-07-0. URL. http://www.R-project.org/ [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Reiss D, Leve LD. Genetic expression outside the skin: clues to mechanisms of genotype × environment interaction. Development and Psychopathology. 2007;19:1005–1027. doi: 10.1017/S0954579407000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AE, VanderKaay Tomasulo MM. Influence of acute stress on spatial tasks in humans. Physiology & Behavior. 2011;103(5):459–466. doi: 10.1016/j.physbeh.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing “Neighborhood Effects”: social processes and new directions in research. Annual Review of Sociology. 2002;28:443–478. [Google Scholar]
- Sampson RJ, Raudenbush SW. Seeing disorder: neighborhood stigma and the social construction of “Broken windows”. Social Psychology Quarterly. 2004;67:319–342. [Google Scholar]
- Scarr S. Developmental theories for the 1990s: developmental and individual differences. Child Development. 1993;63:1–19. [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. Journals of Gerontology: Series B. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol digit modalities test manual-revised. Los Angeles: Western Psychological; 1984. [Google Scholar]
- Smith JD. Apolipoproteins and aging: emerging mechanisms. Ageing Research Review. 2002;1:345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Su S, Zhao J, Bremner JD, Miller AH, Tang W, Bouzyk M, et al. Serotonin transporter gene, depressive symptoms, and interleukin-6. Circulation: Cardiovascular Genetics. 2009;2:614–620. doi: 10.1161/CIRCGENETICS.109.870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The APOE-ɛ 4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Journal of the American Medical Association. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Preventing Alzheimer's disease and cognitive decline. AHRQ Evidence Reports/Technology Assessments. 2010;No. 193 [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72(5):460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]