Abstract
Malignant gastric lymphoma, accounting only for 1% of primary gastric carcinoma, is usually a diffuse large B-cell lymphoma. Toyota et al reported that 37% of gastric perforations involved malignancy, generally gastric carcinoma. Fukuda et al found that less than 5% of malignant gastric lymphomas perforate. While it is relatively well known that perforations often take place during chemotherapy, they are rare in patients not receiving chemotherapy. To our knowledge, spontaneous perforation is rare in gastric malignant lymphoma, having been reported in the Japanese literature only 26 times, including this case, in the last 25 years.
Background
Today, the standard treatment for aggressive gastric lymphoma has shifted from surgery to chemotherapy combined with rituximab; that is, CHOP-R.1 There is as yet no established treatment strategy for gastric malignant lymphoma.2 Its merits emphasise that outcomes of malignant gastric lymphoma with spontaneous perforation was poor (less than 2 years) compared to those without perforation.3 There were two reports in 1983 and 1993 mentioning the frequency of occurrence of spontaneous perforation having been 4.0% and 4.2%, respectively.4 5 However, Law et al6 did not find any spontaneous perforations in their 61 cases of patients with malignant gastric lymphoma. Patients in the Western country reached their peak of vulnerability to spontaneous perforation in their 60s; however, the incidence seen at younger ages, mean 56 years old in Japan. It was worldwide that men were more vulnerable to it than that of women. It was only our case that patient was ready for her scheduled operation which was 2 days prior, when the perforation occurred. Therefore, the choice of treatment including surgery should be carefully deliberated.
Case presentation
An 85-year-old woman, whose medical, social and family histories were unremarkable, presented with anorexia and weight loss (a 6 kg decrease in the preceding 6 months). The patient also began to suffer from a lower back pain around on 25 March 2008. She consulted a neighbourhood doctor on 27 March.
Investigations
A subsequent endoscopic investigation identified a small number of ulcerations at the pyloric antrum of the stomach and pyloric stenosis. She was thus hospitalised, fasted and treated with fluids and proton pump inhibitors. Although her condition improved, endoscopic biopsy revealed that she had diffuse large B-cell lymphoma (DLBCL). She was discharged from the hospital in preparation of her treatment.
Status on admission in our hospital (3 June 2008): height 147 cm, weight 37 kg, temperature 36.6°C, blood pressure 124/68 mm Hg. She was able to eat a small amount of food. There was a firm, immobile, clearly bounded and egg-sized lump at the left lower abdomen.
Lab data below showed that she had anaemia, increased lactate dehydrogenase (LDH) and interleukin (IL)-2 receptor; leucocytes 3990/μl, haemoglobin (Hb) 7.5 g/dl, platelets 44.1×10/μl, C reactive protein 0.37 mg/dl, LDH 511 IU/l, IL-2 receptor 965 U/ml.
Differential diagnosis
Gastric mucosa-associated lymphoid tissue lymphoma, Burkitt lymphoma, Hodgkin's lymphoma, peptic ulcer, gastric carcinoma, small lymphocytic lymphoma, mantle cell lymphoma, follicular lymphoma, GIST and melanoma.
Treatment
She was informed of her treatment choices, namely, chemotherapy plus radiotherapy, chemotherapy plus rituximab or surgery alone. She visited the gastroenterological outpatient department of our hospital for a second opinion on 22 April. Chemotherapy plus rituximab was recommended. However, she returned to our clinic on 20 May for yet another opinion. She was subsequently admitted to our ward on 3 June, scheduled to undergo surgery on 9 June. A central venous line was placed on 5 June. She was anaemic (Hb 7.5 g/dl), so she received 2 units of concentrated red blood cells over the next 2 days.
Outcome and follow-up
On 7 June, she complained of abdominal pain and was awake until 5:00 at the nurse station owing to anxiety and sleeplessness. Her temperature rose to 38.6°C. Emergency CT showed intraperitoneal free air under the diaphragm bilaterally, under the parietal peritoneum and within the portal hepatis, and a large amount of ascites. Malignant gastric lymphoma with spontaneous perforation and pan-peritonitis was diagnosed. The following emergency operations were performed: total gastrectomy, cholecystectomy and intraperitoneal drainage (figure 1). Upon incision of the abdomen, purulent ascites was observed. The entire stomach was sclerotic, with a 30 mm perforation on the large ulcerated tumour of the anterior pyloric antrum. The ulcerated tumours measuring 65×60 mm and 90×85 mm were located at the anterior and posterior pyloric antrum, respectively (figure 2). Lymph nodes around the stomach were elastic and enlarged.
Figure 1.
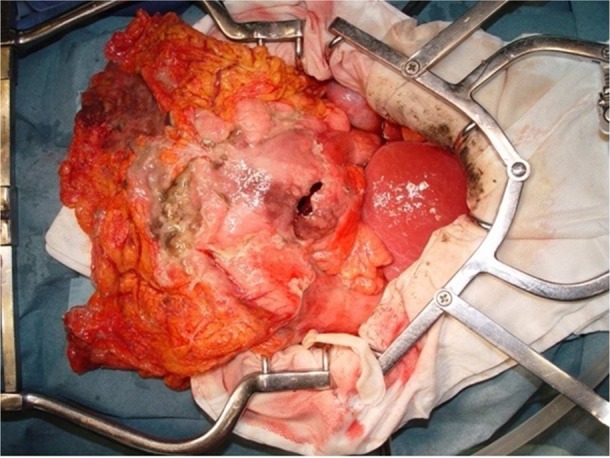
The entire stomach, with a 30 mm perforation on the large ulcerated tumour of the anterior pyloric antrum, is shown.
Figure 2.
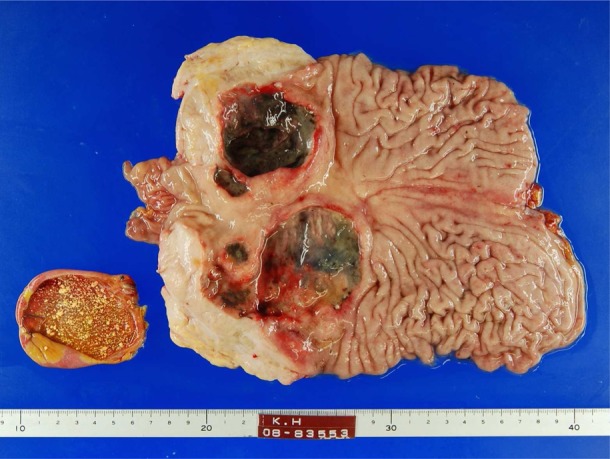
The gallbladder, ulcerated tumours measuring 65×60 mm and 90×85 mm located at the anterior and posterior of pyloric antrum, respectively, are shown.
Pathological results reported ulcerative type with focal perforations, infiltrating the serosa. H&E staining showed diffuse, B-cell type and large-cell lymphoma. Immunohistochemical staining showed the tumour to be CD20 and CD79a positive, but CD3 and cyclin D1 negative (figure 3). The number of metastatic lymph nodes was 31/54. Dissemination to the major omentum was confirmed. The Lugano classification was considered to be Stage II1.
Figure 3.
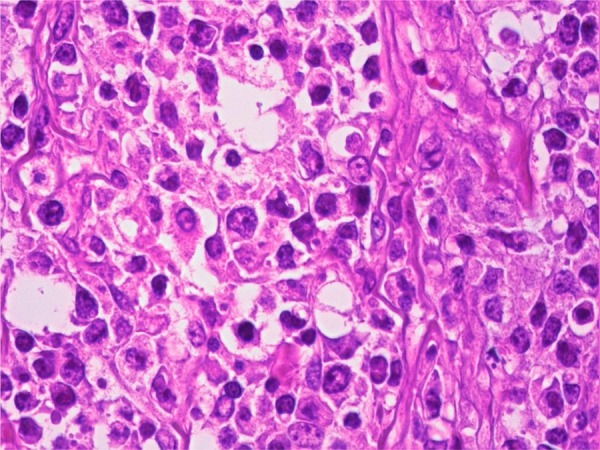
Pathological investigation: H&E staining showed diffuse, B-cell type, large-cell lymphoma. Magnification: ×40.
She recovered from the operation uneventfully and was discharged from the hospital 25 days postoperatively. She refused to undergo adjuvant chemotherapy and ascites recurred 4.5 months after surgery. She again refused any treatment then. She only began to take an opioid as a palliative care 6 months after surgery. The patient died 6.5 months after surgery.
Discussion
Tanaka et al reported on 24 cases of malignant lymphoma with spontaneous perforation. Their 24 cases plus our present case and an additional case reported in the meantime are presented in table 1. Perforation occurred in the body of the stomach in 15 cases and in the pylorus in 13 cases. Tumour sizes ranged from 15 to 250 mm, mean 67.6 mm. There were eight cases of excavated type and six of ulcerative type according to Sano's classification,7 suggesting tumours with ulceration to be common in perforation cases. Pathologically, diffuse large cell lymphoma was seen in 12 cases. According to Mitsunaga, pathology of malignant lymphoma often exists in conjunction with other tumour types. Large, ulcerative-type tumours are reportedly more likely to perforate.8
Table 1.
Twenty-six reported cases of malignant gastric lymphoma with spontaneous perforation 11–28
| Age | Tumour size | Size of perforation | Outcome | Sex | Location | Sano's class | LSG class | Operation | Adjuvant chemotherapy | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1985 Kanzaki | 42 | 3.2×3.2 cm | 0.5 cm | >18 | M | M | Burkitt | VEMP | ||
| 1987 Oda | 43 | 6 mm | > 2 | M | L | Non-Hodgkin's | Subtotal | |||
| 1989 Takeda | 85 | DLBC | ||||||||
| 1990 Yamaguchi | 57 | Subtotal | Chemotherapy | |||||||
| 1992 Ando | 22 | 1.5×1.4 cm | >30 | M | M | DMBC | Subtotal | CHO(P) | ||
| 1992 Yanagi | 65 | 18.5×10 cm | 2×2.5 cm, 4.5×2.5 cm | >10 | M | L | DLBC | CHOP | ||
| 1994 Shinjyo | 14 | 9×4 mm | F | L | Subtotal | Chemotherapy | ||||
| 1996 Uni | 45 | 5 mm | F | M | DMBC | Subtotal | VEPA | |||
| 1996 Mori | 87 | F | L | DMBC | Subtotal | |||||
| 1997 Ono | 43 | 25 cm | 2 cm | >3 | F | L | DLBC | Closure | CHOP | |
| 1997 Shiomi | 71 | 15×13 cm | 2 cm | 7 | M | M | Excarvated | DLBC | Total | THP-COP |
| 1997 Banya | 43 | 25 cm | 2 cm | M | L | DLBC | Closure | CHOP | ||
| 1997 Fukuda | 45 | 8×9 cm | 7 mm | >25 | M | M | DMBC | Subtotal | VEPA | |
| 1998 Yanagisawa | 72 | 7.5×6.0 cm | >15 | M | M | DLBC | Subtotal | CHOP | ||
| 1998 Yanagisawa | 26 | 6.5×4.5 | >14 | M | M | DLBC | Subtotal | CHOP | ||
| 1998 Housui | 58 | 8 mm | F | DLBC | Total | Chemotherapy | ||||
| 1999 Miyamoto | 46 | 3.0×2.8 cm | 6 mm | >62 | M | L | MALT | |||
| 2000 Yabuki | 53 | 8×6 cm | 1.0×0.5 cm | >28 | M | M | DMBC | Subtotal | CHOP | |
| 2001 Hoshino | 59 | Nk | 3 mm | F | M~L | DMBC | Total | Chemotherapy | ||
| 2002 Yokoyama | 64 | 10 mm | M | DLBC | Subtotal | CHOP | ||||
| 2005 Mori | 65 | 3×3 cm | 5 mm | 2 | F | M | DMBC | Total | CHOP | |
| 2006 Tanaka | 65 | 10 cm | Total | |||||||
| 2007 Tanaka | 84 | 9×8 cm | 1 cm | >13 | F | M | Excarvated | DLBC | Subtotal | Chemotherapy |
| 2007 Hirai | 70 | >3 | L~M | DLBC | Total | Chemotherapy | ||||
| 2008 Matsunaga | 73 | 4.5×2.0 cm | 3 cm | >3 | M | M | DLBC | Total | R-CHOP | |
| 2008 Our case | 85 | 6.5×6 cm, 9×8.5 cm | 30 mm | 6 | F | L | Excarvated | DLBC | Total |
CHOP, cyclophosphamide, doxorubicin, vincristine, prednison; DLBC, diffuse large B-cell; DMBC, diffuse medium B-cell; LSG, lymphoma study group classification; MALT, mucosa associated lymphoid tissue; THP-COP, pirarubicin, cyclophosphamide, vincristine, prednisone; VEMP, etoposide, cyclophosphamide, mitoxantrone, prednisone; VEPA, vincristine, cyclophosphamide, prednisolone, adriamycin.
Tanaka et al also noted that pretreatment diagnosis is rare, occurring in only 6 of 24 cases. In the usual course of events, as soon as the diagnosis is made, treatment starts. It is widely known that perforation occasionally occurs in patients receiving chemotherapy. In contrast, spontaneous perforation of malignant gastric lymphoma is rare. The present patient was 85 years old and refused chemotherapy. However, the main tumour was 90 mm in size, raising the possibility of perforation regardless of whether chemotherapy was being administered.
Today, the standard treatment for aggressive gastric lymphoma has shifted from surgery to chemotherapy combined with rituximab; that is, CHOP-R. Aviles et al reported the 10-year survival rate of 589 patients with early stage primary gastric DLBCL, according to choice of treatment. The patients were divided into four treatment groups: surgery, surgery plus radiation, surgery plus chemotherapy and CHOP. The 10-year survival rates were 28%, 23%, 82% and 92%, respectively.9 It is somewhat surprising that patients who chose chemotherapy alone achieved the best result. Nevertheless, a simple comparison of results between Japan and Western nations is problematic. This is partly due to differences in the standard of management of gastric carcinoma. Gastric carcinoma is common in Japan, where the surgical death rate is less than 2%, whereas in Western nations, the rate is substantially higher partly because of D2 gastrectomy not having become standard yet. Nakamura et al compared the survival rate of patients choosing surgery to those selecting chemotherapy or radiotherapy, and found differences did not reach statistical significance. There is as yet no established treatment strategy for gastric malignant lymphoma. Its merits emphasis that outcomes of malignant gastric lymphoma with spontaneous perforation was poor (less than 2 years) compared to those without perforation.3 It seems that number of metastases to lymph nodes at the emergency surgery may predict the outcomes.2 3 10 The patient had spontaneous perforation 2 days prior to her scheduled operation in our case; the choice of treatment including surgery should be carefully deliberated.
Learning points.
Tumours with ulceration to be common in perforation cases.
Large, ulcerative-type tumours are reportedly more likely to perforate.
Outcomes of malignant gastric lymphoma with spontaneous perforation were poor (less than 2 years) compared to those without perforation.
The choice of treatment including surgery should be carefully deliberated.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Toyota T, Nichi M. Gastric perforation (in Japanese). Shyujyutsu (Surgery) 1989;43:623–30 [Google Scholar]
- 2.Fukuda N, Watanabe J, Yamakawa T, et al. A case of perforation of huge malignant gastric lymphoma. Jpn Clin Surg 1998;59:698–701 [Google Scholar]
- 3.Kanzaki M, Yokoyama T, Saito Y, et al. A case of perforated gastric malignant lymphoma (in Japanese). J Tokyo Womens Med Coll 1985;55:1069–73 [Google Scholar]
- 4.Mittal B, Wasserman T, Griffith R. Non-Hodgkin's lymphoma of the stomach. Am J Gastroenterol 1983;78:780–7 [PubMed] [Google Scholar]
- 5.Taal BG, Burgers JMV, Heerde P, et al. The clinical spectrum and treatment of primary non-Hodgkin's lymphoma of the stomach. Ann Oncol 1993;4:839–46 [DOI] [PubMed] [Google Scholar]
- 6.Law MM, Williams SB, Wong JH. Role of surgery in the management of primary lymphoma of the gastrointestinal tract. J Surg Oncol 1996;61:199–204 [DOI] [PubMed] [Google Scholar]
- 7.Sano R. Classification of malignant gastric lymphoma (in Japanese). Igakusyoin, Tokyo 1987;257–75 [Google Scholar]
- 8.Tanaka T, Iwasa M. Haneda H. A case of malignant gastric lymphoma with spontaneous perforation (in Japanese). Nippon Shoukaki Geka(Jpn Gastroenterol Surg) 2007;40:26–9 [Google Scholar]
- 9.Mitsunaga A. Malignant lymphoma (in Japanese).In: Takemoto T, Nagasako K, eds. Diagnostic endoscopy textbook\xE2\x91, esopha·stomach·duodenal. 2nd edn Bunkodo, Tokyo, 2000:208 [Google Scholar]
- 10.Aviles A, Nambo MJ, Neri N, et al. The role of surgery in primary gastric lymphoma: results of a controlled clinical trial. Ann Surg 2004;240:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura S, Matsumoto T, Nakamura Set al. Treatment and prognosis of primary gastric B-cell lymphoma (in Japanese). Stomach Bowles 2004;39:261–75 [Google Scholar]
- 12.Brooks JJ, Enterline HT. Primary gastric lymphomas—a clinicopathologic study of 58 cases with long-term follow up and literature review. Cancer 1983;51:701–11 [DOI] [PubMed] [Google Scholar]
- 13.Dworkin B, Lightdale CJ, Weingrad DN, et al. Primary gastric lymphoma—a review of 50 cases. Dig Dis Sci 1982;27:986–92 [DOI] [PubMed] [Google Scholar]
- 14.Ando O, Sato T, Umeda Aet al. A rare case of gastric malignant lymphoma which was diagnosed due to perforation. J Nihon Univ Med Assoc 1992;51:1021–4 [Google Scholar]
- 15.Oda G, Mishima Y, Yoshinaga K, et al. A case of malignant gastric lymphoma with spontaneous perforation (in Japanese). Jap Clin Surg Med 1987:48:1379 [Google Scholar]
- 16.Takeda T, Hasumi T, Akiyama T, et al. A case of malignant gastric lymphoma with perforation (in Japanese). Nippon Shoukakibyou(Jpn Gastroenterol disease) 1989;86:148 [Google Scholar]
- 17.Yamaguchi Y, Hitamura H, Iwase M, et al. A case of perforated malignant gastric lymphoma (in Japanese). Jap Clin Surg Med 1999;51:2558 [Google Scholar]
- 18.Yanagi S, Kouya K, Kudo Y, et al. A case of perforated malignant gastric lymphoma (in Japanese). Donan Med 1992;27: 9–12 [Google Scholar]
- 19.Shinjyo H, Tokiwa K, Kure N, et al. A case of paediatric malignant gastric lymphoma with perforation (in Japanese). Paediatric Cancer 1994;31:542 [Google Scholar]
- 20.Mori H, Shirai Y, Kamiya T, et al. A case of perforated malignant gastric lymphoma (in Japanese). Jap Abd Emerg Med 1996;16:255 [Google Scholar]
- 21.Banya K, Ono S, Ishi S, et al. A study of perforated malignant lymphoma (in Japanese). Chiba Med 1997;49:1186 [Google Scholar]
- 22.Ono S, Tanaka T, Yoshino H, et al. A case of perforated malignant gastric lymphoma (in Japanese). Jap Abd Emerg Med 1997;17:710 [Google Scholar]
- 23.Yanagisawa S, Tanaka T, Tsuchiya T, et al. Two cases of malignant gastric lymphoma with spontaneous perforation (in Japanese). Jap Clin Surg 1998;59:586 [Google Scholar]
- 24.Hosui S, Senoo T, Mukouyama H, et al. A case of perforated malignant gastric lymphoma (in Japanese). Jap Clin Surg 1998;59:2701 [Google Scholar]
- 25.Hosino Y, Sato H, Iwagaki T, et al. A case of malignant gastric lymphoma diagnosed by gastric perforation (in Japanese). Jap Clin Surg 2001;62:1593 [Google Scholar]
- 26.Mori R, Miura M, Takahashi T, et al. A case of malignant gastric lymphoma with gastric perforation and intestinal obstruction (in Japanese). Jap Clin Surg 2005;66:1903–7 [Google Scholar]
- 27.Hirai E, Ide M, Suzuki S, et al. A case of malignant gastric lymphoma with gastric perforation managed by total gastremtomy (in Japanese). Jap Clin Surg 2007;68:935 [Google Scholar]
- 28.Yokoyama T, Kawamura T, Matsuda T, et al. A case of rapidly progressed malignant gastric lymphoma with perforation (in Japanese). Jap Clin Surg 2002;63:2830 [Google Scholar]


