Abstract
The brain is in many ways an immunologically and pharmacologically privileged site. The blood–brain barrier (BBB) of the cerebrovascular endothelium and its participation in the complex structure of the neurovascular unit (NVU) restrict access of immune cells and immune mediators to the central nervous system (CNS). In pathologic conditions, very well-organized immunologic responses can develop within the CNS, raising important questions about the real nature and the intrinsic and extrinsic regulation of this immune privilege. We assess the interactions of immune cells and immune mediators with the BBB and NVU in neurologic disease, cerebrovascular disease, and intracerebral tumors. The goals of this review are to outline key scientific advances and the status of the science central to both the neuroinflammation and CNS barriers fields, and highlight the opportunities and priorities in advancing brain barriers research in the context of the larger immunology and neuroscience disciplines. This review article was developed from reports presented at the 2011 Annual Blood-Brain Barrier Consortium Meeting.
Keywords: blood–brain barrier, multiple sclerosis, neuroimaging, neuroinflammation, neurovascular unit
Introduction
The blood–brain barrier (BBB) limits the entry of immune cells and immune mediators into the central nervous system (CNS). The anatomical basis of this barrier lies in the tight junctions formed between endothelial cells (ECs) and their low pinocytotic activity. The surrounding microenvironment also has a fundamental role in promoting the distinctive features of the BBB. The neurovascular unit (NVU) is a conceptual construct that consists of horizontal elements comprising microvessels (endothelium, basal lamina, and astrocyte endfeet), and vertical elements including intervening astrocytes, the neurons and their axons, and other supporting cells such as pericytes. The NVU construct emphasizes the influence of CNS and vascular cellular and acellular elements and secreted factors on the unique physiology of cerebrovascular endothelium.1, 2, 3, 4, 5, 6 Interactions among the horizontal and vertical components of the NVU are critical to understand CNS immune responses.
The concept of CNS immune privilege arose from the apparent segregation of immune responses occurring in the brain from those in the peripheral immune system.4, 7, 8 Its features include (1) limited penetration of the BBB by antibodies, immune mediators, and immune cells from the systemic circulation; (2) a lack of lymphatic vessels in the parenchyma to drain antigens and immune cells from the CNS to peripheral lymph nodes; (3) inability of microglial and astroglial cells to sustain immune responses, (4) paucity of dendritic cells (DCs) in the parenchyma; and (5) low levels of major histocompatibility complex expression and delayed, reduced, or absent responses in the brain.
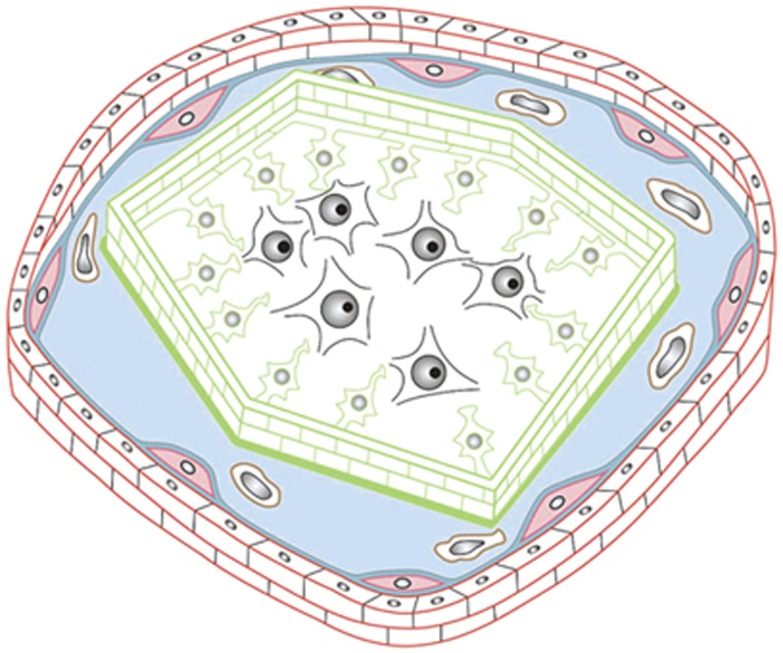
In fact, there is a relative and specialized immune privilege of the CNS. Leukocyte penetration into the CNS proceeds through multiple routes,9, 10 including (1) blood-to-subarachnoid space via leptomeningeal vessels, (2) blood-to-parenchymal perivascular space through the BBB, (3) blood-to-cerebrospinal fluid (CSF) via the choroid plexus, and (4) blood to CSF via meningeal spaces and through the ependyma lining ventricles. Under homeostatic conditions in nondisease states leukocyte trafficking is relatively low and the cells rarely enter the neuropil.8, 11 Rather, leukocytes accumulate on the abluminal side of CNS microvessels12 where they encounter perivascular antigen-presenting cells, collect CNS antigens, and patrol the CNS barriers.13 Leukocyte trafficking can increase considerably in inflammation and disease.11 Memory T cells are found in the CSF under steady-state conditions8 and within the parenchyma long after brain antigen challenge.14 Microglia are essential components of CNS innate immunity, immune defense mechanisms independent of antigens,15, 16 and can respond to environmental or endogenous insults by changing the balance between proinflammatory and antiinflammatory macrophages.17 Thus, the different barriers and routes used by immune cells to access the CNS establish a unique architecture for CNS immune privilege resembling that of a medieval castle (Figure 1).11
Figure 1.
The anatomical basis of the immune privilege of the central nervous system (CNS) resembles the architecture of a medieval castle. The CSF drained space (blue) resembles the castle moat, which is bordered at the outside by the BBB—the outer castle wall—and toward the inside by the glia limitans—the inner castle wall. In the cartoon, the outer castle wall is established by endothelial cells (ECs) (red bricks) protected toward the castle moat by the endothelial basement membrane (blue line), in which a high number of pericytes is embedded. The inner castle wall is mounted by astrocytic endfeet (green) protected toward the castle moat by the parenchymal basement membrane laid down by astrocytes (green line). Inside the castle, that is the CNS parenchyma, the royal family of sensitive neurons (black) resides with their servants, the glial cells. Immunosurveillance is restricted to the castle moat, where perivascular macrophages (brown cells) serve as guards that continuously collect information from within the castle, and present this information to the messengers, the immunosurveilling T cells that can cross the outer wall. If in their communication with the castle moat guards, T cells recognize their antigen they will get activated, clonally expand and open gates in the outer castle wall allowing the entry of more immune cells from the periphery. Within the castle moat, the immune cells then mount an invasion of the castle, breaching the inner castle wall and entering the castle with the aim to eliminate any intruders. If for reasons unknown, T cells turn their attack to the inhabitants of the castle; chronic immune cell invasion of the castle will eventually kill many of the castle inhabitants leading to neuronal deficits as observed in chronic neuroinflammatory diseases of the CNS.11
In pathologic conditions, such as neurodegenerative diseases, cerebrovascular disease or injury, and intracerebral tumors, organized immunologic cascades develop within the NVU and the CNS that produce distinct disease entities and contribute to disease pathogenesis.6, 18 Multiple interactions within the NVU and molecular pathways/soluble factors contribute to changes in the relative state of CNS immune privilege before, during, and years after immunologic response and disease.
The 2011 Annual Blood-Brain Barrier Consortium Meeting was directed toward ‘The brain as an immunologically and pharmacologically privileged site.' This review is the summary compiled from six individual reports presented at the meeting. Participants in each subcommittee report are listed in Table 1.
Table 1. Co-Chairs and members of the five working groups.
| Multiple sclerosis and demyelination |
|---|
| Co-Chairs: Britta Engelhardt and Alexandre Prat |
| Contributors: Ingo Bechman, Nicole Schaeren-Wiemers, and Jack Antel |
| Alzheimer's and Parkinson's disease |
| Co-Chairs: Richard Smeyne and Ruben Boado |
| Contributors: Howard E Gendelman, Malu Tansey, and William Bowers, Eain Cornford |
| Central nervous system genetic disease |
| Co-Chairs: David Begley and Maurizio Scarpa |
| Contributors: Brian Eliceiri, Philip Elsinga, Alon Friedman, Andrea Kassner, Paul Lockman, Timothy Murphy, Chris B Schaffer, Quentin Smith, and Ursula Tuor |
| Cerebrovascular disease |
| Co-Chairs: John Hallenbeck and Gregory del Zoppo |
| Contributors: Kyra Becker, Ulrich Dirnagl, Richard Milner, Joseph LaManna, Eng Lo, Pablo Villoslada, Roland Veltkamp, and Lester Drewes |
| Malignant brain tumors |
| Co-Chairs: John Ohlfest and Russell Lonser |
| Contributors: Paul R Walker, Pedro R Lowenstein, Maria G Castro, Christopher A Hunter, Aaron Johnson, William Elmquist, Krystof Bankiewicz, and Zhengping Zhuang |
Immune privilege in neurologic disease
Multiple Sclerosis
Multiple sclerosis (MS) is the prototypical human neuroinflammatory CNS disease, with extensive focal and disseminated infiltration of mononuclear cells in the white and gray matter. The unique architecture that provides relative CNS immune privilege (Figure 1)11 leads to the hypothesis that lesion distribution in MS should reflect the original sites of entry for immune cells. While MS was long considered to affect only the white matter, recent neuroimaging and histopathologic analysis show that the cerebral cortex is extensively involved during early MS. Intravital two-photon imaging in a rat experimental autoimmune encephalomyelitis (EAE) model of MS shows effector T cell binding and diapedesis through leptomeningeal vessels as well as the BBB.19 Thus, meningeal vessels might provide cues for immune cells to reach the leptomeningeal space and migrate from there to the parenchyma. In clinical specimens, subpial demyelinating lesions are topographically associated with meningeal infiltrates.20, 21 The presence of MS lesions around the optic nerves and in periventricular white matter and spinal cord further strengthens the concept of immune cell invasion not only via the BBB, but also via the leptomeningeal spaces and the blood–CSF barrier. The restrictive nature of the permeability barrier portion of the NVU in leptomeningeal vessels is not uniform and ‘gradients' of restriction or protection can be found in distinct CNS areas. There is limited information on the regional variability of BBB function within CNS regions and its relation to lesion localization.
The differential transmigration of distinct immune cells under homeostatic and inflammatory conditions suggests that changes in the NVU impact CNS immune privilege. There is circumstantial evidence suggesting that distinct leukocyte subsets have different capacity to enter, survive, or reside within the healthy CNS. Studies investigating the molecular mechanisms of immune cell entry into the CNS in EAE have focused on CD4+ T lymphocyte transmigration.22 Extrapolation to other immune cell subpopulations may be incorrect. Expression of matrix proteins in the vascular basal lamina and expression of integrins and other adhesion molecules by cells of the NVU can be altered by both acute and chronic MS and EAE.1, 23 Live-cell imaging in EAE has shown that although DCs use α4 integrins for adhesion to the BBB they are much more efficient in their interaction with the inflamed BBB than are T cells.24 In vitro studies and EAE models of MS have shown that monocytes, macrophages, and DCs use adhesion molecules such as ICAM-1, ALCAM, and ninjurin-1 to interact with the endothelium and migrate to the CNS.25, 26 CD4+ T and B lymphocyte extravasation appears to rely on the expression of counter ligands for ICAM-1 and VCAM, while CD8+ T lymphocyte migration appears dependent on the VLA-4-VCAM pathway.26, 27 The molecular mechanisms used by other immune cells such as B cells, granulocytes, and natural killer cells to migrate into the CNS in MS remain largely unexplored. These findings show that distinct immune cell populations might require a particular set of molecules to access the CNS. Such requirements could prove useful in terms of therapeutics.
Mechanisms of antigen presentation from the CNS to the periphery in MS neuroinflammation remain unclear. The CSF communicates with brain interstitial fluid and carries brain antigens to arachnoid granulations and the cribriform plate where they can egress to blood.10 The choroid plexus transports, processes, and presents antigens in MS,28 and serves as a major portal for immune surveillance.10, 29 The CNS lacks a classical lymphatic system, but animal models show that CNS antigens30 and even nanoparticles31 are drained from the CNS and localize in deep cervical lymph nodes, through a drainage system of the cribriform plate. It is unknown if antigen-loaded immune cells can exit the CNS and reach the draining lymph nodes or if antigens diffuse from the CNS to the lymph nodes. Dendritic cells charged with myelin peptides and colocalized with myelin antibody-reactive T cells were found in the cervical lymph nodes of EAE monkeys and possibly in MS,30 but it is unclear whether these peptides were phagocytosed in the CNS by DCs and transported to lymph nodes, or captured in the periphery by DCs that then migrated to the lymph nodes. It is also not clear whether other cellular and noncellular NVU components are involved in myelin peptide transport and how presentation of brain antigens in draining lymph nodes orchestrates immune and regulatory responses targeted to the CNS.
Alzheimer's Disease and Parkinson's Disease
Cerebral vascular EC dysfunction and leukocyte transmigration across the BBB are early events in the development of Alzheimer's disease (AD), Parkinson's disease (PD), and other neurodegenerative diseases,18, 32, 33 but it is difficult to delineate whether they represent a cause or a consequence of the disease. The phenotypes of AD and PD are linked to advanced age, and pathology often starts years before symptoms.34 The BBB multidrug resistance function decreases in a brain region-specific manner with age35 resulting in decreased clearance of neurotoxic compounds and increased oxidative stress in the brain, elevating the risk of neurodegenerative pathology.33
Aberrant protein secretion or configuration can be actively immunogenic. In AD, soluble amyloid β stimulates the transmigration of monocytes, enhances tau pathology, and induces secretion of proinflammatory cytokines and chemokines.32, 36, 37 Microglia and macrophages are activated by amyloid β.16, 17 In PD, α-synuclein activates microglial cells in vitro,38 and may be proinflammatory in vivo.39 It remains to be determined what component(s) of protein misfolding (synuclein, tau, etc.) affect inflammatory responses, as well as what secreted factors elicit specific neuronal damage.
Gene-wide association studies implicate immune components in the etiology of PD40 and AD.41 Further elucidation of the association of genetic loci that contain genes related to immunocompetence and pathogen defense may increase options for therapeutic intervention. There is potential for neuroimmune modulation as a therapeutic strategy to prevent, attenuate, or at least delay onset of neurodegenerative diseases. In addition, disease-specific immune processes must be compared with neurologically normal age-matched individuals, including regional response to immune stimulation,42 mapping of CNS cell types,43 and mechanisms for long-lived glial cell activation response to insult.
Central Nervous System Genetic Disease
A number of inherited diseases directly affect the CNS. Good examples are lysosomal storage disorders where mutation of a single gene encoding a lysosomal enzyme or transporter can result in the production of a defective protein and lead to a specific disorder. Depending on the residual amount of enzyme function, CNS deterioration in metabolic disorders may be chronic and progressive or severe and rapid, while others show little or no neuronopathy.44, 45 Many of the inherited neurodegenerative diseases and lysosomal disorders have an inflammatory component characterized by microglial activation and secretion of inflammatory cytokines.46, 47 Inflammation may secondarily produce changes in BBB function or BBB pathology may be primary in the disease natural history.48, 49 In some lysosomal storage disorders, the BBB is clearly compromised with extravasation of plasma proteins or large molecular weight tracers.50 Inflammatory mediators such as chemokines and tumor necrosis factor α are significantly elevated in Gaucher's disease and are associated with both BBB permeability changes and neuronal cell death.51 The role of adhesion molecules and the various components of the NVU have not been systematically assessed in these rare diseases. The variety of CNS genetic disease models thus provide tools for assessing mechanisms to modulate different classes of inflammatory mediators and determine their individual roles in NVU function and pathology.
Treatment of neuronopathic lysosomal storage disorders requires effective CNS delivery of small molecular weight therapeutics and macromolecules across the BBB for enzyme replacement therapy. Early bone marrow transplantation can provide a source of enzyme secreting cells in the CNS by exploiting the normal trafficking of mononuclear cells across the BBB.52 So far bone marrow transplantation has only proven effective in the general and CNS treatment of Mucopolysaccharidosis Type I (Hurler's disease) and adrenoleukodystrophy, and some improvement of CNS symptoms has been reported with metachromatic leukodystrophy and Nieman–Pick disease.53 In other disorders, the number of migrating cells or the quantity of enzyme secreted may be too little to be effective in preventing neurodegeneration. Animal models of CNS genetic diseases could be used to assess general mechanisms of peripheral leukocyte migration and long-term residence in the brain.47
Immune privilege in cerebrovascular disease
Focal cerebral ischemia causes direct injury to the cellular and noncellular elements of the NVU and induces inflammatory processes that alter the relationships of ECs, extracellular matrix (ECM), and astroglial cells, resulting in acute and profound changes in the microvascular permeability barrier and loss of the vertical organization of the permeability barrier of the NVU.2, 54, 55 Patchy increased permeability to molecules as large as fibrinogen, IgG, or nanoparticles is seen within 2 to 4 hours after middle cerebral artery occlusion.56, 57 Conversely, hypoxic conditions stimulate expression of BBB tight junction protein ZO-1 in vitro,56 and claudin-5 and occludin expression in vivo.55, 58 Endothelial cell integrins α1β1, α3β1, and α6β1 decrease by 2 hours after middle cerebral artery occlusion.2, 23, 54 Inhibition of the adhesion function β1 integrins on microvessel ECs (‘outside-in' signaling) in vitro significantly reduces claudin-5 expression, and increases permeability to small molecules by confluent endothelium.55 The ECM contains inactive matrix metalloproteinases, growth factors, and other agents that may be activated on ischemic insult59, 60, 61 while cellular elements of the NVU generate matrix proteases that could potentially lead to changes in ECM constituents over the course of hours after focal cerebral ischemia.61 Integrin α6β4 and αβ dystroglycan on astrocyte endfeet disappear after middle cerebral artery occlusion,2, 23, 54, 62 which is associated with detachment of astrocyte endfeet and the appearance of perivascular edema.63 These studies suggest that adhesive interactions between the endothelium and the ECM contribute to the pharmacologic and immunologic barrier, and that physiologic remodeling of cerebral vessels is a carefully synchronized and orchestrated process.62
Leukocyte adhesion during focal ischemia is mediated by the postcapillary venule endothelium, which expresses receptors necessary for leukocyte transmigration.64, 65 Recent in vitro assays suggest that leukocytes enter the brain via breaks in the basal lamina ECM, but evidence of active matrix metalloproteinases that cause ECM degradation in vivo is lacking. Since leukocytes usually remain in the Virchow-Robin space of large vessels of normal brain without reaching the neuropil,10 matrix metalloproteinases could function as processing enzymes, for example, by modulating the function of chemokines.66 The situation in the postcapillary venule is likely different.
The role of the blood–CSF barrier in the pathobiology of cerebrovascular disease is underappreciated. The choroid plexus immunologic ‘barrier' is selectively vulnerable to ischemic damage, whereby reduction in blood flow of ∼40% produces energy failure and epithelial cell damage compared with a reduction of ∼80% required for cortical injury.67 The damaged blood–CSF barrier may expose adjacent hippocampus and subcortical white matter to blood solutes, immune responses, and inflammatory mediators that provoke local cytotoxicity,67 and may also deprive them of cytoprotective growth factors. Choroid epithelial cells subject to transient forebrain ischemia undergo patchy degeneration by 30 minutes reperfusion, suggesting that adjacent areas within the choroid epithelial cell layer have different cellular contexts at ischemia onset. Transplantation of cultured choroid epithelial cells68 or microencapsulated choroid plexus grafts69 into the CSF is apparently cytoprotective in models of ischemia because of growth factor release and suppression of inflammation locally.
Immune privilege in malignant brain tumors
Macrophage/microglial infiltration is a hallmark of glioblastoma, comprising upwards of a third of the tumor mass.70 Adhesion proteins and integrin signaling are frequently altered in glioblastoma71 and brain metastases,72 and CNS tumors disrupt the integrity of the BBB and change the composition of the ECM and astrocytes in the NVU.73 Nevertheless, the importance of interactions of immune cells and immune mediators with the NVU in CNS tumors, and their general role in either tumor development or therapy remains unclear.
Brain tumors are relatively abundant in mutated proteins that should constitute immunogenic tumor-specific neoantigens.74 Nonetheless, the immune system rarely successfully attacks CNS cancers, and brain tumor patients often show immune defects.75 In animal models, tumors implanted in the mammary fat pad,76 or skin77 were rejected by the immune response, while brain xenografts of the same tumors were not rejected. The brain xenografts showed higher expression of TGFβ, more tumor infiltrating regulatory T cells, and functional impairment of tumor infiltrating DCs.76, 77 In one clinical study, 18% of immunosuppressed patients receiving organ transplantation from a donor with a primary brain tumor developed cancer of brain origin in the transplanted organ.78 This finding indicates that immune function in glioma patients is competent to suppress metastatic tumors but not the primary brain tumor.
The brain microenvironment is important in dampening cell-mediated immune responses. The paraneoplastic neurologic disorders (PNDs) provide important insights into how tolerance to cell-associated brain antigens is regulated. They are caused by immune responses to brain-specific antigens that are ectopically expressed in tumors (hereafter referred to as onconeuronal antigens). For example, a fraction of patients with small cell lung cancer develop neurologic symptoms after immune responses against the HuD protein that is normally expressed in neurons even though all the primary tumors express HuD.79, 80 Most PND patients present with neurologic symptoms associated with autoimmune attack of the brain rather than symptoms of tumor burden and indeed symptomatic tumor progression is typically delayed.81 T cells reactive to onconeuronal antigens have been documented in the CSF of PND patients.80 The PNDs reveal that there is normally strict peripheral tolerance to brain-specific antigens, and even when tolerance to onconeuronal antigens is broken, only under certain circumstances does that immune response cause brain pathology. Cause-and-effect relationships have not been established that can unambiguously explain how tolerance to cell-associated brain antigens is regulated in brain tumor patients.
Anticancer vaccines have shown encouraging safety in glioma patients, but efficacy has not been shown in randomized controlled clinical trials.82 Neuroinflammatory side effects are seldom observed after brain tumor immunotherapy. In one study, 56 patients with recurrent malignant glioma were vaccinated with DCs pulsed with autologous tumor lysate and only one patient exhibited vaccine-associated edema and stupor,83 while others report no vaccine-related symptomatic neurologic side effects.84, 85 Considering neuroinflammatory PNDs, the relative ease of inducing EAE in animals, and the neuroinflammatory toxicities observed in AD patients who were vaccinated with an amyloid β peptide fragment,86 a paradox exists regarding brain tumors. Why is induction of symptomatic autoimmunity so rare in glioma-bearing hosts treated by, for instance, tumor cell lysate vaccines containing numerous self-antigens?85 These observations raise important questions regarding how mechanisms of immunologic tolerance may be unique to specific brain diseases. Until the mechanisms that license effector T cells to enter the brain are uncovered in primary brain tumors, it will be difficult to realize the goal of effective brain tumor immunotherapy.
Our knowledge about how standard brain tumor therapies such as radiation, chemotherapy, and antiangiogenics affect immune responses is incomplete and contradictory. Radiation induces DNA repair pathways, which result in the upregulation of costimulatory ligands recognized by receptors expressed in T cells and natural killer cells.87 Despite this, immunotherapy in the form of vaccines or adoptively transferred lymphocytes has almost universally been administered after radiation therapy is complete. Another case in point is chemotherapy-induced lymphopenia. The prevailing hypothesis is that lymphopenia increases the concentrations of homeostatic cytokines necessary for T-cell survival, thereby opening a ‘window of opportunity' to expand tumor-reactive T cells in vivo.88 Conversely, the diversity and competence of T cells can be diminished for years after chemotherapy-induced lymphopenia.89 Indeed, adoptive immunotherapy with CD8 T cells is more effective in lymphoreplete hosts relative to lymphopenic hosts, especially when regulatory T cells are deleted concurrent with IL-7 administration.90 Comparative studies combining standard therapies with immune-based approaches are immediately needed.
Recommendations/priorities for advancing knowledge
Alterations in Barrier Properties In Vitro
The field would benefit by better understanding the roles of adhesion molecules for maintaining barrier integrity via EC–ECM–astrocyte interactions, and signaling effects by these receptors and by specific ECM components. The mechanisms of barrier opening (e.g., roles of matrix proteases/matrix metalloproteinases) and the signals that regulate localization and/or activation of tight junction proteins including soluble stimuli and molecular cross-talk (e.g., integrins/αβ dystroglycan) are of interest. New BBB models using multiple cell types and physiologic flow conditions will provide better systems for studying immune cell interaction with brain vasculature components.91 Tools that would be helpful include the identification of a promoter specific for brain ECs, ECM-, and adhesion receptor-based inhibitors, and viral vectors that specifically target CNS ECs. Recent laboratory work developing major new formulations of the nature of the permeability barrier underwrite the need for a concerted effort to focus research to define the normative relationships of the barrier components and their function. Additionally, we need to know how NVU properties change under conditions of injury, hypoxia, and disease, and in response to discrete stimuli (Table 2). The ultimate goal is to develop an understanding that would permit targeting network dynamics in a beneficial way instead of trying to manipulate single molecular mechanisms to protect the brain.
Table 2. Schema for a multidisciplinary program to define the neurovascular unit.
| Conceptual models | Neurovascular unit Stress responses Preservation of homeostasis |
|---|---|
| Experimental models | Cell culture systems (including human brain cells) Slice culture Small and large animal models |
| Outcomes | Hypothesis-driven experiments for animal models, slice culture, and cell culture systems Brain cell transcriptomes and proteomes at multiple time points, with mapping of the interactomes Iterative models of network dynamics |
| Resources required | Multidisciplinary team of neuroscientists, stroke experimentalists and clinicians, vascular biologists, computational biologists, statisticians, computer scientists, bioengineers, and bioinformatics experts |
Alterations in Immune Cell Trafficking In Vivo
Special attention must be paid to understanding how tolerance to brain antigens is regulated. An important aspect of immune surveillance, particularly as related to the choroid plexus, is to further understand its role in priming regulatory T cells to brain antigens, the trafficking of natural or adaptive T cells, and the local antigen presentation to regulatory T cells.92 More effort is needed to define the molecular determinants of priming and trafficking that result in high numbers of tumoricidal T cells entering brain parenchyma. It is vital that future studies do not focus only on T cells, but on checkpoints controlled by the innate immune system as well as in discerning the mechanisms involved in the migration of distinct immune cells types across the NVU. Fundamental differences in immune cell trafficking across species should be defined form anatomical and physiologic perspectives.
Mechanisms to Improve Therapy Delivery Across the Blood–Brain Barrier
Treatment for many CNS diseases will require safe and effective delivery of large molecular weight proteins, viral vectors, or cells across the BBB.93 Liposome encapsulated agents and drug containing nanoparticles may be delivered to brain tumors by convection-enhanced delivery.94 Infusion with convection bypasses the BBB to allow semihomogeneous perfusion through large regions of brain and may increase therapeutic efficacy by optimizing drug delivery kinetics and minimizing systemic toxicity.95 Despite the potential of convection-enhanced delivery in lesions such as brain tumors,95 clinical trials of convection in PD were negative and discontinued because of lack of efficacy.
Bifunctional fusion proteins and immunoliposomes that target endogenous BBB transport systems, i.e., transferrin and insulin receptors,96, 97 have been successfully used to treat experimental models of AD98 and PD.99, 100 Pending further development into pharmacological/toxicological studies and clinical trials, these molecules may become a new generation of neuropharmaceuticals. Most gene vector systems do not appear to cross the BBB, but the viral vector AAV9 may be neurotropic and may show promise for brain delivery.101, 102 AAV9-mediated delivery of erythropoietin to the CNS protects against PD neurodegeneration.103 However, safety concerns about the use of AAV vectors in humans have been increased,104 and long-term safety studies are needed to draw final conclusions on this issue.
Animal Models
Improved animal models are needed that better recapitulate the phenotype of CNS disorders and have improved clinical predictive value. Single gene mutations provide a number of good animal models for lysosomal storage disorders that mimic the human disease,46, 47, 48, 49, 53 and should be more fully used to evaluate immune components of neurologic disease. In contrast, in genetic models of PD (synuclein, LRRK2, PINK1, etc.), animals do not fully develop a phenotype that is seen in human disease.105 The same is true for animal models of MS, whether genetic or immunization based. Further studies are needed to understand the mechanisms by which animal models overcome the disease phenotype, and to develop alternatives that do show neuroinflammation and neurodegeneration. Differences in immunologic responses across species should be pointed out in animal models of CNS inflammatory and neurologic disease.
For tumor models, cell culture conditions affect expression of genes involved in immune suppression so studies may reflect culture artifacts rather than reveal insights into the immunology of tumors.106 Preclinical trials in spontaneous mouse models107, 108 or naturally occurring dog models109 may be more predictive of human responses.
Imaging
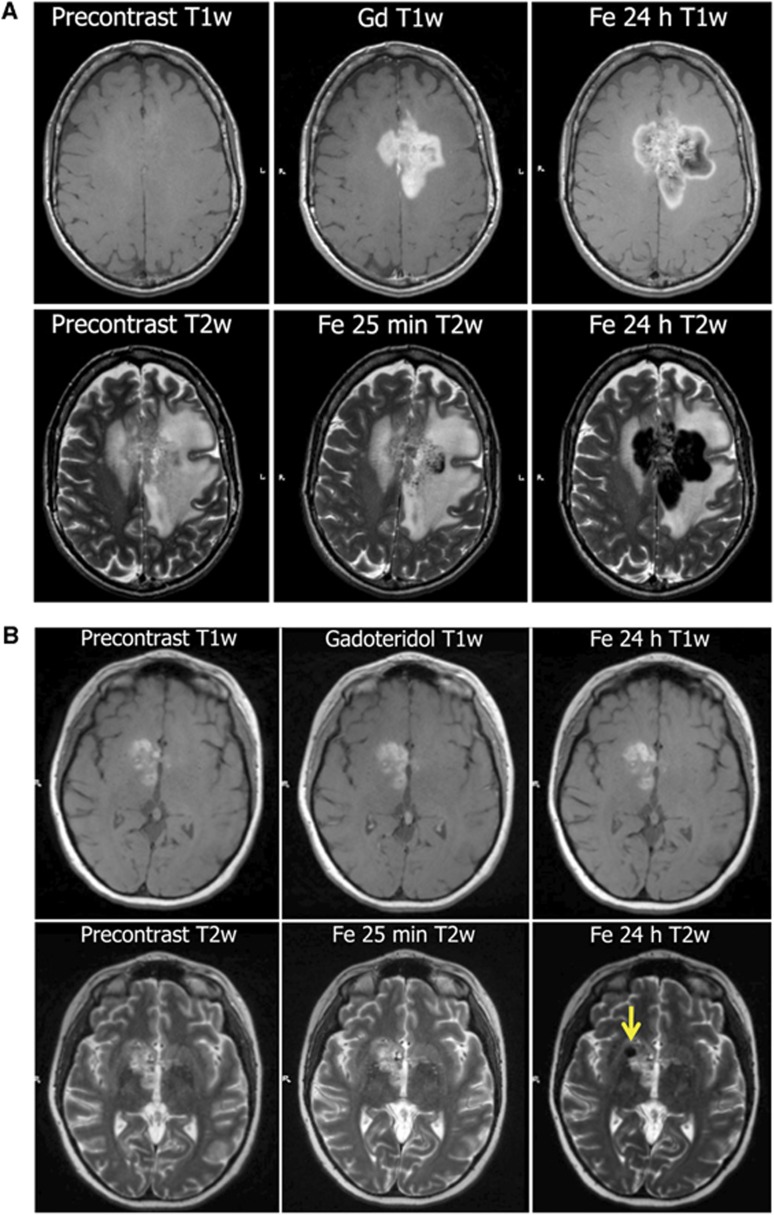
It would be valuable to visualize lymphocyte trafficking, changes in intracerebral lesions, and biomarkers of response to immunotherapy. Magnetic resonance imaging (MRI) using superparamagnetic nanoparticle conjugated antibodies may show specific immune cell subsets in vivo.110 Ultrasmall superparamagnetic iron oxide nanoparticles such as FDA-approved ferumoxytol can be taken up by immune cells, and should find wider use in evaluating the immune component of intracerebral lesions.111 Figure 2 shows examples of MRI after uptake of nanoparticles in brain tumor and an MS lesion.
Figure 2.
Imaging inflammation. Ferumoxytol iron oxide nanoparticles are taken up by microglia in central nervous system (CNS) lesions and can be visualized on magnetic resonance imaging (MRI).113 (A) Delayed detection of inflammation in glioblastoma. MRI of 57-year-old woman with confusion, cognitive difficulties, and diagnosis of glioblastoma multiforme 9 months after chemoradiotherapy and on adjuvant temozolomide chemotherapy. Top: T1-weighted (T1w) MRI before contrast, after gadolinium contrast (Gd) or 24 hours after ferumoxytol (Fe). Ferumoxytol signal enhancement shows a larger tumor volume than gadoteridol because of uptake in inflammatory cells in the infiltrating edge of the tumor. Bottom: T2-weighted (T2w) MRI before contrast, 25 minutes after ferumoxytol, and 24 hours after ferumoxytol. Signal dropout is because of ferumoxytol uptake by inflammatory cells.113 (B) Imaging an inflammatory component of multiple sclerosis (MS) with ferumoxytol. MRI of 31-year-old woman with fatigue, confusion, and nondiagnostic biopsy. Presumptive diagnosis of primary CNS lymphoma was disproven at biopsy after ferumoxytol imaging and showed demyelination. Top: T1-weighted (T1w) MRI before contrast, after gadolinium contrast (Gd) or 24 hours after ferumoxytol (Fe) in a patient with MS. Bottom: T2-weighted (T2w) MRI before contrast, 25 minutes after ferumoxytol, and 24 hours after ferumoxytol. Arrow indicates signal dropout because of ferumoxytol uptake in inflammatory cells.
Clinical Studies
Special emphasis should be placed on human studies. A standardized tissue procurement protocol and imaging plan should be implemented to obtain information on immune status, metabolism, genetics, and gene expression in neurologic disease and intracerebral tumors. Brain tumor patients with PND, where naturally occurring antitumor immunity is coupled to brain autoimmunity, will be useful to study immune cell trafficking and immunotherapy. Phase II clinical trials should be double blinded and placebo controlled, so that therapy failure can be determined early, rather than investing in phase III studies powered to detect incremental improvements. Understanding the systems biology that orchestrates the pharmacological and immunologic barrier characteristics should be a priority.112
Summary
The cell components, the ECM, and the vertical organization of the microvessel permeability barrier of the NVU are dynamic, with changes depending on type of injury and time frame of events. It is clear that in animals and patients, only under certain ill-defined circumstances do cytotoxic lymphocytes enter the brain parenchyma. Immune cell migration into the brain in acute and chronic brain diseases and related signaling deserve a reassessment using state-of-the-art tools. Key questions regarding the role of the immune system in disease development involve understanding the interactions between the humoral and the innate nervous immune system, the timeframe of changes, their longevity, and relationship of immune components with final CNS phenotype. Therapeutic approaches to neurologic disease must take into account both the BBB and an array of immunologic processes in the CNS.
Acknowledgments
We would like to thank all of the people who attended the Brain as an Immunologically and Pharmacologically Privileged Site meeting (March 24–26, 2011), Stevenson, Washington, USA. Special thanks to Aliana Culp who was instrumental in the development of this report.
The authors declare no conflict of interest.
Footnotes
Funding was provided by an NIH R13 Meeting grant (9R13NS076353) and by the Walter S and Lucienne Driskill Foundation to EAN.
References
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann NY Acad Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis TP, et al. Strategies for advancing brain-barriers translational research. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane RW, Hickey WF. Immunology of the nervous system. Oxford University Press: New York; 1997. [Google Scholar]
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JI, Teale JM. Evidence for differential changes of junctional complex proteins in murine neurocysticercosis dependent upon CNS vasculature. Brain Res. 2007;1169:98–111. doi: 10.1016/j.brainres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67:1113–1121. doi: 10.1097/NEN.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Exp Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci. 2005;233:55–59. doi: 10.1016/j.jns.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Leukocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Hinojoza JR, Maeda A, Chen M. Endothelial cell integrin laminin receptor expression in multiple sclerosis lesions. Am J Pathol. 1998;153:405–415. doi: 10.1016/S0002-9440(10)65584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol. 2010;184:7196–7206. doi: 10.4049/jimmunol.0901404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Kebir H, Terouz S, Alvarez JI, Lécuyer MA, Gendron S, et al. Role of Ninjurin-1 in the migration of myeloid cells to CNS inflammatory lesions. Ann Neurol. 2011;70:751–763. doi: 10.1002/ana.22519. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Kebir H, Alvarez JI, Marceau G, Bernard M, Bourbonnière L, et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on α4 integrin. Brain Behav Immun. 2011;134:3560–3577. doi: 10.1093/brain/awr268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellino M, Votta B, Condello C, Piacentino C, Romagnolo A, Merola A, et al. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199:133–141. doi: 10.1016/j.jneuroim.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119:75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- de Vos AF, van Meurs M, Brok HP, Boven LA, Hintzen RQ, van der Valk P, et al. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol. 2002;169:5415–5423. doi: 10.4049/jimmunol.169.10.5415. [DOI] [PubMed] [Google Scholar]
- Muldoon LL, Varallyay P, Kraemer DF, Kiwic G, Pinkston K, Walker-Rosenfeld SL, et al. Trafficking of superparamagnetic iron oxide particles (Combidex) from brain to lymph nodes in the rat. Neuropathol Appl Neurobiol. 2004;30:70–79. doi: 10.1046/j.0305-1846.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. Cell Transplant. 2007;16:285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, et al. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Kortekaas R, Bart J, Willemsen AT, de Klerk OL, de Vries JJ, et al. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30:1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm. 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2010;69:337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson's disease. J Neuroimmune Pharmacol. 2009;4:419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PLoS ONE. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Jiao Y, Shepherd KR, Smeyne RJ. Glia cell number modulates sensitivity to MPTP in mice. Glia. 2005;52:144–152. doi: 10.1002/glia.20233. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Pontikis CC, Scarpa M. Lysosomal storage diseases and the blood-brain barrier. Curr Pharm Des. 2008;14:1566–1580. doi: 10.2174/138161208784705504. [DOI] [PubMed] [Google Scholar]
- Bellettato CM, Scarpa M. Pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis. 2010;33:347–362. doi: 10.1007/s10545-010-9075-9. [DOI] [PubMed] [Google Scholar]
- Arfi A, Richard M, Gandolphe C, Bonnefont-Rousselot D, Therond P, Scherman D. Neuroinflammatory and oxidative stress phenomena in MPS IIIA mouse model: the positive effect of long-term aspirin treatment. Mol Genet Metab. 2011;103:18–25. doi: 10.1016/j.ymgme.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, Hopwood JJ. Lessons learnt from animal models: pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis. 2010;33:363–371. doi: 10.1007/s10545-010-9078-6. [DOI] [PubMed] [Google Scholar]
- Farfel-Becker T, Vitner EB, Pressey SN, Eilam R, Cooper JD, Futerman AH. Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum Mol Genet. 2011;20:1375–1386. doi: 10.1093/hmg/ddr019. [DOI] [PubMed] [Google Scholar]
- Killedar S, Dirosario J, Divers E, Popovich PG, McCarty DM, Fu H. Mucopolysaccharidosis IIIB, a lysosomal storage disease, triggers a pathogenic CNS autoimmune response. J Neuroinflammation. 2010;7:39. doi: 10.1186/1742-2094-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem. 2010;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner EB, Farfel-Becker T, Eilam R, Biton I, Futerman AH. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher's disease. Brain. 2012;135:1724–1735. doi: 10.1093/brain/aws095. [DOI] [PubMed] [Google Scholar]
- Dobrenis C.Cell-mediated delivery systemsIn: Platt FM, Walkley SU, (eds). Lysosomal disorders of the brain Oxford University Press: Oxford, UK; 2004339–380. [Google Scholar]
- Stroobants S, Gerlach D, Matthes F, Hartmann D, Fogh J, Gieselmann V, et al. Intracerebroventricular enzyme infusion corrects central nervous system pathology and dysfunction in a mouse model of metachromatic leukodystrophy. Hum Mol Genet. 2011;20:2760–2769. doi: 10.1093/hmg/ddr175. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab. 2011;31:1972–1985. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ.Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion Stroke 1994251847–1853.discussion 1853–1854. [DOI] [PubMed] [Google Scholar]
- Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–1859. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–1348. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- Li L, Welser JV, Dore-Duffy P, Del Zoppo GJ, LaManna JC, Milner R. In the hypoxic central nervous system, endothelial cell proliferation is followed by astrocyte activation, proliferation, and increased expression of the α6β4 integrin and dystroglycan. Glia. 2010;58:1157–1167. doi: 10.1002/glia.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Kim EH, del Zoppo GJ, Heo JH. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res. 2009;87:668–676. doi: 10.1002/jnr.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Keep RF. The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab. 2006;26:675–683. doi: 10.1038/sj.jcbfm.9600224. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Taguchi A, Kitayama H, Watanabe Y, Ohta M, Yoshihara T, et al. Transplantation of cultured choroid plexus epithelial cells via cerebrospinal fluid shows prominent neuroprotective effects against acute ischemic brain injury in the rat. Neurosci Lett. 2010;469:283–288. doi: 10.1016/j.neulet.2009.09.060. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport. 2004;15:1543–1547. doi: 10.1097/01.wnr.0000133298.84901.cf. [DOI] [PubMed] [Google Scholar]
- Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseley A, Boone S, Wojton J, Yu L, Yoo JY, Yu J, et al. Extracellular matrix protein CCN1 limits oncolytic efficacy in glioma. Cancer Res. 2012;72:1353–1362. doi: 10.1158/0008-5472.CAN-11-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Mason MD, Jiang WG. Tight junctions in cancer metastasis. Front Biosci. 2011;16:898–936. doi: 10.2741/3726. [DOI] [PubMed] [Google Scholar]
- Lee J, Lund-Smith C, Borboa A, Gonzalez AM, Baird A, Eliceiri BP. Glioma-induced remodeling of the neurovascular unit. Brain Res. 2009;1288:125–134. doi: 10.1016/j.brainres.2009.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Gharagozlou S, Vengco I, Chen W, Ohlfest JR. Effective CpG immunotherapy of breast carcinoma prevents but fails to eradicate established brain metastasis. Clin Cancer Res. 2008;14:5484–5493. doi: 10.1158/1078-0432.CCR-07-4139. [DOI] [PubMed] [Google Scholar]
- Biollaz G, Bernasconi L, Cretton C, Puntener U, Frei K, Fontana A, et al. Site-specific anti-tumor immunity: differences in DC function, TGF-beta production and numbers of intratumoral Foxp3+ Treg. Eur J Immunol. 2009;39:1323–1333. doi: 10.1002/eji.200838921. [DOI] [PubMed] [Google Scholar]
- Collignon FP, Holland EC, Feng S. Organ donors with malignant gliomas: an update. Am J Transplant. 2004;4:15–21. doi: 10.1046/j.1600-6143.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- DeLuca I, Blachere NE, Santomasso B, Darnell RB. Tolerance to the neuron-specific paraneoplastic HuD antigen. PLoS ONE. 2009;4:e5739. doi: 10.1371/journal.pone.0005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WK, Deluca IJ, Thomas A, Fak J, Williams T, Buckley N, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest. 2009;119:2042–2051. doi: 10.1172/JCI36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Darnell RB. Paraneoplastic neurological degenerations: keys to tumour immunity. Nat Rev Cancer. 2004;4:36–44. doi: 10.1038/nrc1255. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Walker AI, Ward SJ. Cancer vaccines: will we ever learn. Expert Rev Anticancer Ther. 2009;9:67–74. doi: 10.1586/14737140.9.1.67. [DOI] [PubMed] [Google Scholar]
- De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T cell infiltration and survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi AR, Clarke AM, Wooldridge T, Waldmann H, Hale G, Symmons D, et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: twelve-year outcomes. Arthritis Rheum. 2008;58:370–375. doi: 10.1002/art.23122. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang H, Meadors J, Poon R, Guimond M, Mackall CL. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 2009;114:3831–3840. doi: 10.1182/blood-2009-03-212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31:767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Pardridge WM.Blood-brain barrier drug targeting: the future of brain drug development Mol Interv 2003390–105.51. [DOI] [PubMed] [Google Scholar]
- Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble CO, Krauze MT, Drummond DC, Yamashita Y, Saito R, Berger MS, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- Boado RJ. Blood-brain barrier transport of non-viral gene and RNAi therapeutics. Pharm Res. 2007;24:1772–1787. doi: 10.1007/s11095-007-9321-5. [DOI] [PubMed] [Google Scholar]
- Boado RJ. A new generation of neurobiological drugs engineered to overcome the challenges of brain drug delivery. Drug News Perspect. 2008;21:489–503. doi: 10.1358/dnp.2008.21.9.1290820. [DOI] [PubMed] [Google Scholar]
- Zhou QH, Fu A, Boado RJ, Hui EK, Lu JZ, Pardridge WM. Receptor-mediated abeta amyloid antibody targeting to Alzheimer's disease mouse brain. Mol Pharm. 2011;8:280–285. doi: 10.1021/mp1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Intravenous treatment of experimental Parkinson's disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood-brain barrier. Brain Res. 2010;1352:208–213. doi: 10.1016/j.brainres.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Brain penetrating IgG-erythropoietin fusion protein is neuroprotective following intravenous treatment in Parkinson's disease in the mouse. Brain Res. 2011;1382:315–320. doi: 10.1016/j.brainres.2011.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Dirosario J, Killedar S, Zaraspe K, McCarty DM. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther. 2011;19:1025–1033. doi: 10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YQ, Ma BF, Zhao LR, Tatom JB, Li B, Jiang LX, et al. AAV9-mediated erythropoietin gene delivery into the brain protects nigral dopaminergic neurons in a rat model of Parkinson's disease. Gene Ther. 2010;17:83–94. doi: 10.1038/gt.2009.113. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LY, Wu AS, Doucette T, Wei J, Priebe W, Fuller GN, et al. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immunotherapeutic responses. Clin Cancer Res. 2010;16:5722–5733. doi: 10.1158/1078-0432.CCR-10-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Thang NN, Derouazi M, Philippin G, Arcidiaco S, Di Berardino-Besson W, Masson F, et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010;70:4829–4839. doi: 10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- Pluhar GE, Grogan PT, Seiler C, Goulart M, Santacruz KS, Carlson C, et al. Anti-tumor immune response correlates with neurological symptoms in a dog with spontaneous astrocytoma treated by gene and vaccine therapy. Vaccine. 2010;28:3371–3378. doi: 10.1016/j.vaccine.2010.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirko I, Johnson A, Ciric B, Gamez J, Macura SI, Pease LR, et al. In vivo magnetic resonance imaging of immune cells in the central nervous system with superparamagnetic antibodies. FASEB J. 2004;18:179–182. doi: 10.1096/fj.02-1124fje. [DOI] [PubMed] [Google Scholar]
- Weinstein J, Varallyay C, Dosa E, Gharmanov S, Hamilton B, Rooney W, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and therapeutics in CNS pathology, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villoslada P, Steinman L, Baranzini SE. Systems biology and its application to the understanding of neurological diseases. Ann Neurol. 2009;65:124–139. doi: 10.1002/ana.21634. [DOI] [PubMed] [Google Scholar]
- Dosa E, Guillaume DJ, Haluska M, Lacy CA, Hamilton BE, Njus JM, et al. Magnetic resonance imaging of intracranial tumors: intra-patient comparison of gadoteridol and ferumoxytol. Neuro Oncol. 2011;13:251–260. doi: 10.1093/neuonc/noq172. [DOI] [PMC free article] [PubMed] [Google Scholar]