Abstract
The myogenic response has a critical role in regulation of blood flow to the brain. Increased intraluminal pressure elicits vasoconstriction, whereas decreased intraluminal pressure induces vasodilatation, thereby maintaining flow constant over the normal physiologic blood pressure range. Improved understanding of the molecular mechanisms underlying the myogenic response is crucial to identify deficiencies with pathologic consequences, such as cerebral vasospasm, hypertension, and stroke, and to identify potential therapeutic targets. Three mechanisms have been suggested to be involved in the myogenic response: (1) membrane depolarization, which induces Ca2+ entry, activation of myosin light chain kinase, phosphorylation of the myosin regulatory light chains (LC20), increased actomyosin MgATPase activity, cross-bridge cycling, and vasoconstriction; (2) activation of the RhoA/Rho-associated kinase (ROCK) pathway, leading to inhibition of myosin light chain phosphatase by phosphorylation of MYPT1, the myosin targeting regulatory subunit of the phosphatase, and increased LC20 phosphorylation; and (3) activation of the ROCK and protein kinase C pathways, leading to actin polymerization and the formation of enhanced connections between the actin cytoskeleton, plasma membrane, and extracellular matrix to augment force transmission. This review describes these three mechanisms, emphasizing recent developments regarding the importance of dynamic actin polymerization in the myogenic response of the cerebral vasculature.
Keywords: actin polymerization, calcium sensitization, myogenic response, myosin light chain phosphorylation, Rho-associated kinase
Introduction—The myogenic response
Cognition and brain integrity are critically dependent on appropriate control of blood flow within the cerebral circulation, with insufficient flow leading to ischemia and excessive flow provoking small-vessel rupture and blood–brain barrier disruption. Cerebral blood flow is controlled through the interplay of several physiologic factors that regulate the contractile state of vascular smooth muscle cells within the walls of cerebral arteries and arterioles. These factors include intravascular pressure and circulating or locally released vasoactive molecules.1, 2
Bayliss3 made the seminal observation that small arteries are sensitive to intravascular pressure, with vasoconstriction occurring in response to an increase in pressure and vasodilatation in response to a decrease in pressure. This mechanism, known as the myogenic response,2 maintains blood flow constant during variations in systemic pressure, i.e., flow autoregulation.4 The sensitivity of resistance vessels (< ∼200 μm internal diameter) to pressure can be traced to cellular mechanisms inherent to the smooth muscle cells, i.e., intrinsic myogenic mechanisms.2 The modulatory actions of extrinsic vasoactive molecules are superimposed on these intrinsic mechanisms to match blood flow to metabolic demand under dynamic physiologic conditions,2 such as regional neuronal activity.1
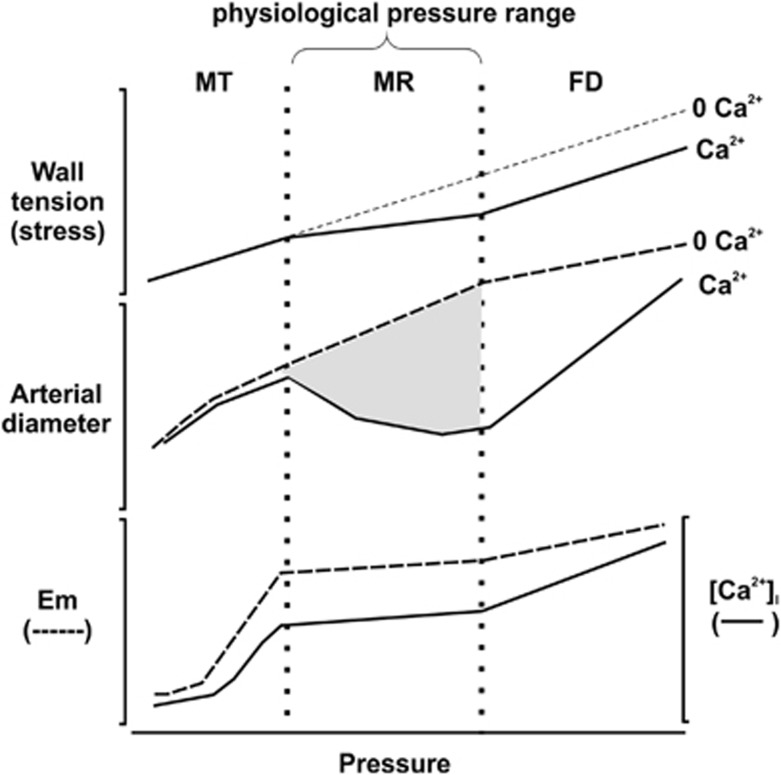
Vascular smooth muscle cells of cerebral resistance vessels, therefore, respond to an increase in intravascular pressure and increased wall tension by contracting, with diameter progressively decreasing with pressure elevation beyond a threshold level (Figure 1).5, 6 The intrinsic development of myogenic tone permits flow autoregulation by reducing flow with pressure elevation, and permitting dilatation and increased flow with pressure reduction. Each resistance vessel has a different operating range of pressure,2, 7 e.g., rat middle cerebral arteries exhibit myogenic constriction between ∼60 and 130 mm Hg,6, 8, 9 but penetrating arterioles are active between ∼20 and 140 mm Hg.10 Outside the autoregulatory range, diameter (Figure 1) and flow (Figure 2) increase proportionally with pressure in the presence of physiologic extracellular-free Ca2+ concentrations. High pressure overwhelms the capacity of the smooth muscle cells to generate increased force, leading to forced dilatation and unregulated flow augmentation with further pressure increase (Figures 1 and 2).2, 11
Figure 1.
The relationship between wall tension, arterial diameter, membrane potential or [Ca2+]i, and intraluminal pressure in rat cerebral arteries. Three phases of the diameter response to increased intraluminal pressure have been identified. As pressure is increased toward the normal physiologic range, myogenic tone (MT) begins to develop. Myogenic reactivity (MR) is observed within the physiologic pressure range, beyond which forced dilatation (FD) occurs as myogenic vasoconstriction fails to counteract the high intraluminal pressure. The myogenic response within the physiologic pressure range is shown in gray shading and is the difference between the diameter versus pressure curves in the absence and presence of extracellular Ca2+. Circumferential wall tension or stress is thought to be the stimulus for the myogenic response. Importantly, membrane potential (Em) and [Ca2+]i increase as pressure is increased to 40 to 60 mm Hg, but exhibit modest changes over the physiologic pressure range. Redrawn from Figure 8 of Osol et al (2002).11
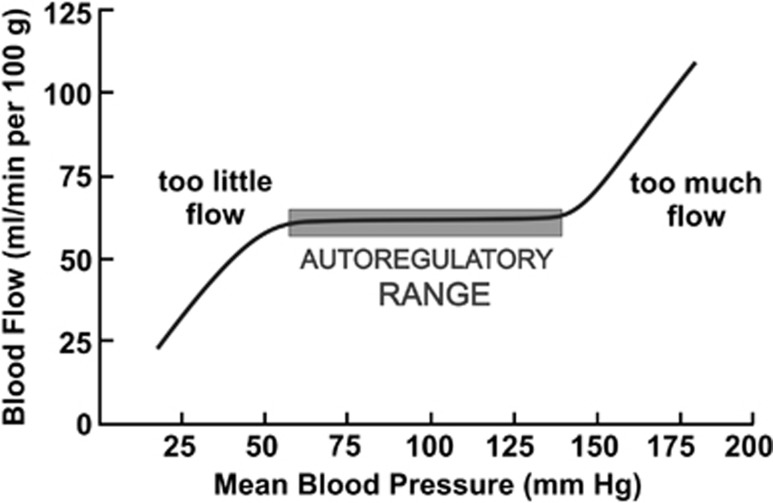
Figure 2.
The relationship between blood flow and perfusion pressure in rat middle and posterior cerebral arteries. The autoregulatory region extends from ∼60 to 130 mm Hg and is indicated by the shading. The presence of a myogenic response maintains flow constant over a wide range of pressure. Outside the autoregulatory range, there is too little flow at <60 mm Hg and, at high pressure, force generation by vascular smooth muscle cells is insufficient to counteract the distending force of intracellular pressure, resulting in forced dilatation and too much flow.
The cerebral myogenic response is independent of, but modulated by extrinsic factors from the endothelium (including nitric oxide, prostacyclin, electrical coupling, endothelin-1, and thromboxane A2), perivascular nerves (e.g., nitric oxide, calcitonin gene-related peptide, and serotonin), the blood (e.g., angiotensin II), or released locally from astrocytes (e.g., prostaglandins and K+ ions) surrounding smaller arterioles (i.e., neurovascular coupling).2, 7 In vivo measurement of the myogenic response is complicated by these extrinsic factors, but the experimental approach of in vitro pressure myography facilitates analysis of the molecular basis of the myogenic response and its modulation by individual factors in isolation.2, 7
Inappropriate myogenic control of cerebral blood flow is observed in animal models of hypertension, stroke, and diabetes, and is prognostic of neurologic deterioration and poor outcome in patients with these conditions.12, 13, 14, 15, 16, 17 Current strategies to optimize cerebral perfusion pressure and improve flow autoregulation are crude (e.g., volume expansion, dopamine, or epinephrine to increase systemic pressure) and not appropriate in some clinical situations.16 Progress in the treatment of dysfunctional cerebral autoregulation requires elucidation of the molecular basis of the myogenic response and the defects responsible for abnormal myogenic control of cerebral blood flow.
Much of our knowledge of the cellular mechanisms of excitation–contraction coupling (the process whereby a stimulus, e.g., a contractile agonist or an increase in intraluminal pressure, induces contraction) and its regulation in vascular smooth muscle has come from studies of large conduit arteries. This is due principally to the size of these vessels, which provide adequate amounts of tissue for biochemical analysis. Since resistance arteries of <200 μm internal diameter are primarily responsible for blood flow regulation,18 it is essential that the molecular mechanisms that regulate contraction and relaxation of such vessels will be studied directly. Recent advances, particularly improvements in the sensitivity of detection and quantification of protein phosphorylation levels,8, 9, 19 have enabled direct analysis of signal transduction pathways involved in the control of contraction and relaxation of smaller vessels, including the myogenic response of cerebral and other resistance arteries.
The mechanosensor
The mechanosensor responds to an increase in intraluminal pressure and transduces this stimulus to vasoconstriction. Candidate mechanosensors include the integrins and G protein-coupled receptors. Integrins are transmembrane glycoproteins that link the extracellular matrix, via interaction with proteins such as collagen, laminin, and fibronectin,20, 21 to the cytoskeleton, via interaction with focal adhesion proteins, and thereby transduce mechanical force across the plasma membrane. Evidence has been provided in support of a role for integrins in the myogenic response,22 which was found to be suppressed by integrin-specific peptides and blocking antibodies.20, 21, 23 Smooth muscle integrins are located in membrane-associated dense plaques, structural analogs of focal adhesions of cultured cells.24 Integrin stimulation (clustering) induces tyrosine phosphorylation and activation of focal adhesion kinase (FAK) and Src family tyrosine kinases, and increased tyrosine phosphorylation is associated with the myogenic response.25, 26 Furthermore, the myogenic response of cerebral arteries is sensitive to tyrosine kinase inhibition.27 There are likely several important substrates of these activated tyrosine kinases, but only some have been identified. Candidates, based on agonist- and depolarization-induced tyrosine phosphorylation in airway and vascular smooth muscles, include the cytoskeletal proteins paxillin28, 29, 30 and talin,31 the adapter protein p130 Crk-associated substrate (CAS)32 and p42/44 mitogen-activated protein kinases (MAPKs).33 Stimulation of α5β1 integrin has been implicated in activation of the L-type Ca2+ channel in vascular myocytes via a tyrosine phosphorylation cascade involving FAK, Src, and various focal adhesion proteins, such as paxillin and vinculin.34
Gq/11-coupled receptors (specifically AT1 receptors) have also been proposed as mechanosensors in the myogenic response, with mechanically activated receptors adopting an active conformation that enables G protein coupling, activation of phospholipase C, opening of TRPC channels, and membrane depolarization.35
The role of Ca2+ in the myogenic response
The primary mechanism for the activation of vascular smooth muscle contraction involves an increase in cytosolic-free Ca2+ concentration ([Ca2+]i) in response to a variety of contractile stimuli, including smooth muscle cell membrane depolarization elicited by neurotransmitters released from nerve terminals, occupancy of a range of smooth muscle plasma membrane G protein-coupled receptors by ligands transported in the bloodstream or released from neighboring cells (e.g., endothelial cells, parenchymal cells, fibroblasts, and adipocytes), and an increase in intraluminal pressure.2, 36, 37 This increase in [Ca2+]i results from Ca2+ entry from the extracellular space and Ca2+ release from intracellular stores, principally the sarcoplasmic reticulum.
Increased arterial wall tension because of an increase in intraluminal pressure is detected by the mechanosensor, which evokes membrane potential depolarization via activation of nonselective cation channels, leading to opening of voltage-gated Ca2+ channels in the sarcolemma.2, 6 Ca2+ then enters the cell and decreases its electrochemical gradient (Figure 3). Ca2+ binds to calmodulin and the (Ca2+)4-calmodulin complex activates myosin light chain kinase (MLCK).38, 39 MLCK phosphorylates a unique substrate, the two 20-kDa regulatory light chain subunits (LC20) of myosin II.40 This phosphotransferase reaction, whereby a phosphoryl group is covalently attached to Ser-19 of LC20, is necessary and sufficient for activation of the MgATPase activity of actomyosin, cross-bridge cycling and contraction of demembranated smooth muscle.41
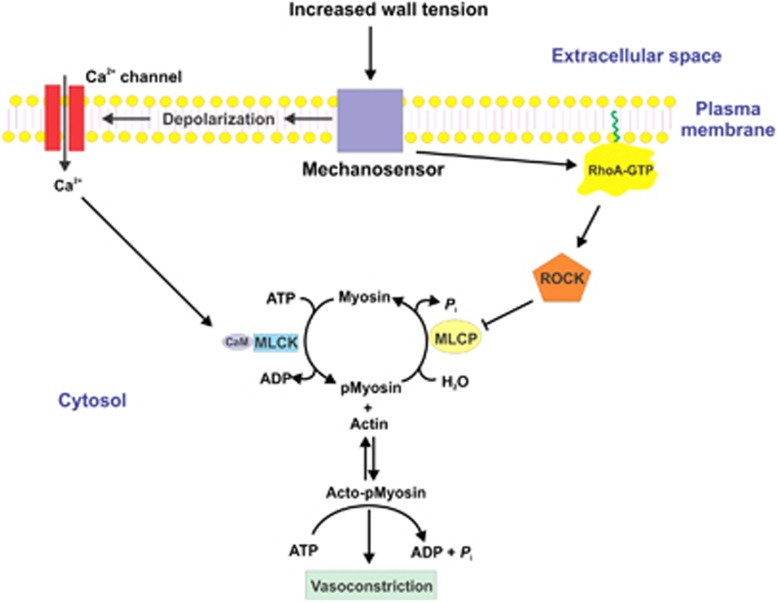
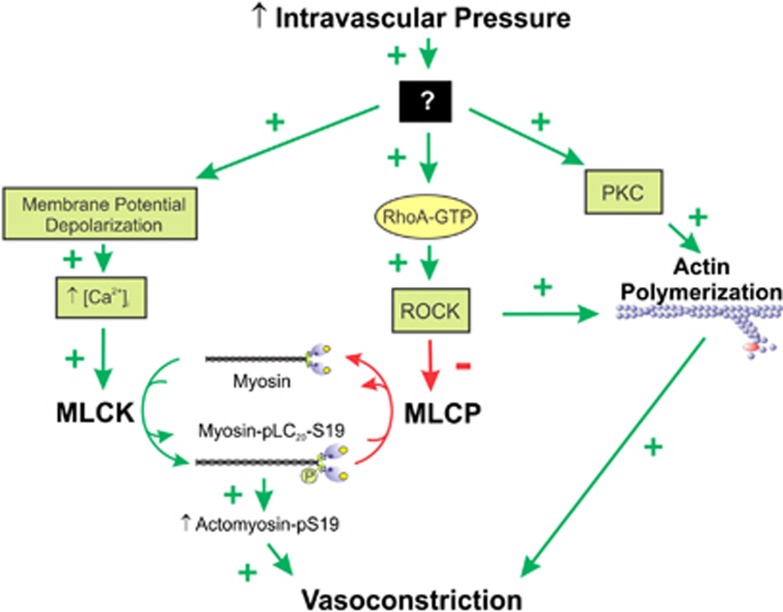
Figure 3.
Pressure-induced vasoconstriction via increased [Ca2+]i and via Ca2+ sensitization mediated by inhibition of myosin light chain phosphatase (MLCP). The mechanosensor detects an increase in arterial wall tension, which leads to membrane depolarization, opening of voltage-gated Ca2+ channels, and influx of Ca2+ from the extracellular space. Extracellular-free [Ca2+] is ∼2.5 mM and cytoplasmic-free [Ca2+] at rest is ∼120 to 150 nmol/L. After stimulation, the free [Ca2+] in the cytoplasm rises to ∼300 to 500 nmol/L. This increase in [Ca2+]i saturates the four Ca2+-binding sites of calmodulin (CaM), leading to activation of Ca2+/CaM-dependent myosin light chain kinase (MLCK). Activated MLCK phosphorylates the two 20-kDa light chain subunits of myosin II at Ser-19, which activates cross-bridge cycling and vasoconstriction, driven by the energy derived from the hydrolysis of ATP within the head regions of phosphorylated myosin bound to actin filaments. Detection of an increase in wall tension by the mechanosensor also leads to activation of the small GTPase RhoA. The mechanism of activation of RhoA likely involves activation of a guanine nucleotide exchange factor, which catalyzes GDP-GTP exchange on RhoA. This induces dissociation of a guanine nucleotide dissociation inhibitor and a conformational change involving exposure of a hydrophobic geranylgeranyl moiety on RhoA. RhoA-GTP then translocates from the cytosol to the plasma membrane where it attaches to the membrane via the geranylgeranyl moiety (indicated in green) and activates Rho-associated kinase (ROCK). Activated ROCK phosphorylates the myosin targeting subunit of MLCP, MYPT1, at Thr-855 (and perhaps Thr-697), resulting in inhibition of phosphatase activity, increased phosphorylation of LC20 at Ser-19 by MLCK, and enhanced contraction, without an additional change in [Ca2+]i. ROCK can also phosphorylate CPI-17 at Thr-18, which thereby becomes a potent inhibitor of MLCP; however, this does not occur during the myogenic response of rat cerebral arteries. Protein kinase C (PKC) can also phosphorylate CPI-17 at Thr-38 but, since this does not occur during the myogenic response of rat cerebral arteries, this mechanism cannot explain the role of PKC in the myogenic response.
It was initially considered that the myogenic response is due exclusively to increased MLCK activity.6 However, pressure elevation also evokes asynchronous Ca2+ waves within individual vascular smooth muscle cells because of release of Ca2+ from the sarcoplasmic reticulum,42, 43 similarly to the Ca2+ waves observed during agonist-induced contraction.44 Thus, myogenic constriction is suppressed by membrane hyperpolarization, block of voltage-gated Ca2+ channels, removal of extracellular Ca2+, or application of ML-7 (which inhibits MLCK activity).2, 6, 43, 45, 46 The extent of myogenic depolarization must be precisely controlled to obtain an appropriate increase in [Ca2+]i. This control is provided by negative-feedback activation of large-conductance Ca2+-activated K+ channels2, 47, 48 and voltage-gated K+ channels.46, 48, 49, 50, 51, 52
Ca2+ sensitization via inhibition of myosin light chain phosphatase
Knot and Nelson6 showed that an increase in intravascular pressure in rat cerebral arteries from 60 to 100 mm Hg depolarized the membrane potential by 8 mV and increased [Ca2+]i by ∼55 nmol/L. Pressure-induced depolarization, leading to activation of voltage-gated Ca2+ channels, entry of extracellular Ca2+ and Ca2+-dependent activation of MLCK, is clearly necessary for myogenic vasoconstriction.6, 42 However, these changes are not sufficient to explain the phenomenon.2, 7, 11, 53, 54, 55, 56 On the basis of the three phases described in Figure 1, which are characterized by distinct pressure-dependent changes in [Ca2+]i that do not parallel arterial diameter, Osol et al11 suggested the existence of additional regulatory mechanisms in the myogenic response. One such mechanism involves the inhibition of myosin light chain phosphatase (MLCP) via Rho-associated kinase (ROCK) or protein kinase C (PKC), leading to an increase in LC20 phosphorylation and vasoconstriction without a change in [Ca2+]i. This process is generally referred to as Ca2+ sensitization (Figure 3) and was first described for agonist-induced smooth muscle contraction.57, 58, 59 The ability of inhibitors of ROCK and PKC, or suppression of RhoA or ROCK with dominant-negative mutants, to block myogenic constriction increased the possibility that Ca2+ sensitization may also be involved in the myogenic response.2, 11, 14, 54, 55, 56, 60, 61, 62
Myosin light chain phosphatase is a type 1 protein serine/threonine phosphatase. The native enzyme is a trimer composed of a catalytic subunit (PP1cδ), a regulatory MYPT1 subunit (myosin phosphatase targeting subunit)63 and a 20- to 21-kDa subunit that is not required for activity or regulation.64 ROCK phosphorylates two sites in MYPT1 (Thr-697 and Thr-855 in rat sequence numbering), both of which result in reduced phosphatase activity.65, 66 ROCK67 and PKC68 phosphorylate CPI-17, a 17-kDa cytosolic protein that becomes a potent inhibitor of MLCP when phosphorylated at Thr-38.69 In its phosphoryated state, CPI-17 binds to the catalytic subunit of MLCP and inhibits the activity of the holoenzyme. The ROCK-catalyzed phosphorylation of MYPT1 and CPI-17 thereby shifts the MLCK: MLCP activity ratio in favor of the kinase, resulting in an increase in LC20 phosphorylation at Ser-19 and contraction without a change in [Ca2+]i.
While MLCP exhibits constitutive activity, it is subject to complex regulation, both inhibition and activation.64 We will focus on mechanisms of inhibition of MLCP activity since they are largely responsible for Ca2+ sensitization, the mechanism whereby contraction occurs without a change in [Ca2+]i.57 A variety of ligands interact with G protein-coupled receptors that are linked to members of the G12/13 family of heterotrimeric G proteins. Ligand occupancy of the receptor activates guanine nucleotide exchange factors such as LARG, PDZ-RhoGEF, and p115RhoGEF,57, 70 which in turn activate the small GTPase RhoA by catalyzing GDP-GTP exchange.57, 71 RhoA-GTP activates ROCK, which phosphorylates the regulatory subunit of MLCP, leading to inhibition of phosphatase activity.72 Studies with PKC inhibitors have suggested a role for this kinase in the myogenic response of resistance arteries, including cerebral arteries,14, 54, 73, 74 but the underlying mechanism remains unclear. For example, the PKC inhibitors GF109203X (a general PKC inhibitor) and Gö6976 (a selective inhibitor of the Ca2+-dependent PKC isoforms) suppressed the myogenic response of rat middle cerebral arteries.8 This could have been explained by inhibition of pressure-induced phosphorylation of CPI-17 at Thr-38, the PKC site;69 however, CPI-17 was not phosphorylated at any pressure examined (10, 60, or 100 mm Hg). Furthermore, while GF109203X induced vasodilatation at 100 mm Hg, there was no change in phosphorylation of MYPT1 at either of the two phosphatase inhibitory sites (Thr-697 and Thr-855) or in phosphorylation of LC20. We concluded that PKC-dependent Ca2+ sensitization via phosphorylation of CPI-17 and MYPT1, which would lead to MLCP inhibition, does not contribute to the myogenic response. We can also conclude from these experiments that the PKC inhibitors do not block the myogenic response by inhibiting ROCK.
Integrins have been widely implicated in activation of the Rho family of small GTPases.75 Since ROCK is a prominent downstream target of RhoA, Ca2+ sensitization via the RhoA/ROCK pathway attracted a great deal of interest in the context of the myogenic response. Attenuation of this pathway by inhibitors of ROCK, or dominant-negative mutants of RhoA or ROCK, suppressed the myogenic response.14, 54, 55, 61, 62, 76 Direct demonstration that increased intraluminal pressure induced phosphorylation of MYPT1 supported an important role for Ca2+ sensitization in the myogenic response of cerebral resistance arteries.8 Myogenic vasoconstriction, which was markedly inhibited by pretreatment with two structurally distinct ROCK inhibitors, Y27632 and H1152, correlated with an increase in phosphorylation of MYPT1 at Thr-855 and of LC20 at Ser-19. Importantly, these increases in phosphorylation were observed when intraluminal pressure was increased from 60 to 100 mm Hg, i.e., within the physiologic range. Interestingly, no changes in phosphorylation of MYPT1 at Thr-697, or of CPI-17 at Thr-38, were detected. MYPT1 exhibited basal phosphorylation (at 10 mm Hg intraluminal pressure) at both Thr-697 and Thr-855, suggesting that MLCP is partially inhibited under these conditions, whereas no phosphorylation of CPI-17 was detected at any pressure.8
Ca2+ sensitization is also involved in the contractile response of cerebral resistance arteries to serotonin in the presence of physiologic levels of intraluminal pressurization. Vasoconstriction induced by serotonin at 10 mm Hg was observed to correlate with an increase in phosphorylation of LC20(Ser-19), but with no evidence of a change in MYPT1 phosphorylation.9 In contrast, a combination of increased intraluminal pressure (to 60 mm Hg) and serotonin elicited further increases in vasoconstriction, MYPT1(Thr-855) and LC20(Ser-19) phosphorylation, and an increase in MYPT1(Thr-697) phosphorylation, all of which were attenuated by ROCK inhibition.9 Similar effects were observed in response to a supra-physiologic pressure increase (to 140 mm Hg). Once again, no phosphorylation of CPI-17 was detected under any conditions.9 These data imply that the cellular mechanism(s) used by extrinsic vasoconstrictor factors to evoke contraction are influenced by the presence of myogenic activation. In the example cited above, serotonin-evoked contraction was due exclusively to MLCK activation in the absence of intraluminal pressurization. However, in the presence of myogenic depolarization and a preexisting activation of MLCK at 60 mm Hg, subsequent vasoconstriction in response to serotonin required that an alternative mechanism of MLCP inhibition be used to increase the level of LC20(Ser-19) phosphorylation and force generation. A major goal for future research must be to understand the molecular basis of the integration of extrinsic vasoconstrictor and dilator signaling with the cellular mechanisms that are responsible for the myogenic response.
Actin cytoskeletal dynamics
Accumulating evidence indicates that dynamic cytoskeletal reorganization influences force generation in smooth muscle.24 Actin filaments of the contractile apparatus are anchored to the cytoplasmic tails of integrins by a complex of adhesion proteins, and to each other at cytosolic dense bodies. Integrins and focal adhesion complexes are connected to, and reinforced by the cortical actin cytoskeleton. Together, these elements distribute and transmit force generated by cross-bridge cycling at the level of the contractile apparatus (myofilaments) over the cell membrane and to the extracellular matrix. Dynamic reorganization of the actin cytoskeleton is postulated to strengthen these interactions and enhance force transmission. The importance of actin polymerization in smooth muscle contraction was originally suggested by the effects of inhibitors of actin polymerization on the contractile responses of a variety of smooth muscle tissues. Thus, treatment of airway,77, 78 vascular,28, 29, 79, 80, 81, 82, 83 gastrointestinal,84 and uterine81 smooth muscles with latrunculins (which sequester G-actin monomers), or cytochalasins (which cap the fast-growing, barbed end of actin filaments), markedly attenuate contractility. Furthermore, blockade of specific steps in the activation of actin polymerization inhibits contraction in response to a variety of stimuli. For example, dynamic reorganization in agonist-evoked smooth muscle contraction,24 and possibly the myogenic response,11, 80, 85, 86 have been attributed to ROCK, PKC, and/or mechanostimulation of FAK.87, 88, 89 The requirement of an intact cytoskeleton for myogenic constriction is suggested by the use of cytochalasins (which induce depolymerization) and jasplakinolide (which enhances polymerization).80, 85, 86 Alternatively, latrunculin B is thought to disrupt dynamic reorganization of the actin cytoskeleton in the myogenic response by binding G-actin.80, 85, 90 The effects on smooth muscle contractility described above were not attributable to disruption of the contractile apparatus itself (i.e., the myofilaments), suggesting that formation of new actin filaments is essential for normal contractile function.
A large body of evidence supports the conclusion that smooth muscle contraction is accompanied by an increase in F-actin and a decrease in G-actin content.78, 80, 86, 91, 92, 93 Quantification of F- and G-actin levels at rest and after stimulation with acetylcholine (canine trachea) or norepinephrine (carotid artery) indicated that a small proportion of cellular actin (∼10% to 12%) undergoes polymerization, the F-actin content increasing from ∼70% to 80% at rest to ∼80% to 92% after stimulation.32, 89, 92, 94 This is consistent with the existence of two pools of cellular actin: one (‘contractile actin') involved in the contractile machinery, associated with tropomyosin and stabilized in the filamentous form, and the other (‘cytoskeletal actin') localized to the cell cortex, not associated with tropomyosin and undergoing reversible polymerization–depolymerization.95 Contractile and cytoskeletal actins may involve distinct isoforms,96, 97 but this is controversial.92, 98
Actin Dynamics in the Myogenic Response
As discussed earlier, the myogenic response requires MLCK activation via increased [Ca2+]i and MLCP inhibition via MYPT1 phosphorylation at Thr-855, leading to an increase in LC20 phosphorylation.37 Several lines of evidence suggest, however, that the myogenic response of rat cerebral arteries cannot be fully explained by increased MLCK and decreased MLCP activities: (1) depletion of RhoA in rat cerebral arteries in organ culture abolished the contractile response to an increase in intraluminal pressure from 15 to 80 mm Hg without affecting LC20 phosphorylation, i.e., without a change in the MLCK:MLCP activity ratio;88 (2) microcystin treatment (MLCP inhibition) induced robust LC20 phosphorylation in RhoA-depleted arteries, but failed to elicit a contractile response;88 (3) PKC inhibition abolished the myogenic response without a change in phosphorylation of LC20, CPI-17, or MYPT1;8 (4) serotonin-induced vasoconstriction at 10 and 60 mm Hg was almost completely abolished by inhibition of ROCK or PKC, but the level of LC20 phosphorylation declined only to the level observed in control tissues at 60 mm Hg, at which pressure a significant myogenic response was observed;8, 9 (5) serotonin increased LC20 phosphorylation by ∼25% at 10 mm Hg and ∼10% at 60 mm Hg, but the vasoconstriction at 60 mm Hg was significantly greater;9 and (6) under three different experimental conditions (an increase in intraluminal pressure from 80 to 120 mm Hg, application of serotonin at 80 mm Hg, or pressurization to 80 mm Hg in the presence of serotonin), pressure- or agonist-induced vasoconstriction was observed without an increase in LC20 phosphorylation above the preexisting level of ∼0.5 mol Pi/mol LC20 (El-Yazbi et al, submitted). These observations suggested that the myogenic response of cerebral vessels involves a third mechanism, in addition to MLCK activation and MLCP inhibition. Based on the evidence provided above, a likely candidate for this third mechanism involves dynamic regulation of the actin cytoskeleton.
In summary: (1) de novo formation of actin filaments is required for force development; (2) actin polymerization and myosin phosphorylation are independent events (but see Chen et al99 for evidence of actin polymerization in response to LC20 phosphorylation); and (3) both actin filament formation and myosin phosphorylation are required for smooth muscle contraction.
We recently investigated pressure-induced actin polymerization as the potential third mechanism involved in the myogenic response of rat middle cerebral arteries (El-Yazbi et al, submitted). Latrunculin B treatment had no effect on resting diameter (at 10 mm Hg intraluminal pressure) or on the magnitude of serotonin-induced constriction at 10 mm Hg, but induced dilatation to the passive diameter observed in the absence of extracellular Ca2+ in vessels pressurized to 80 mm Hg. This vasodilatation occurred without a change in LC20 phosphorylation. Latrunculin B also completely dilated vessels preconstricted with serotonin at 80 mm Hg, and vessels constricted in response to an increase in pressure from 10 to 80 mm Hg after serotonin pretreatment. These results are consistent with force generation during the myogenic response, and in response to serotonin in the presence of myogenic tone, being sensitive to sequestration of G-actin. Quantification of G-actin levels under different conditions supported a role for actin polymerization in the myogenic response. G-actin content progressively decreased as intraluminal pressure was increased from 10 to 80 to 120 mm Hg. However, serotonin treatment at 10 mm Hg was not associated with a significant change in G-actin content. Latrunculin B treatment at 80 mm Hg, however, was accompanied by a fourfold increase in G-actin content. Finally, G-actin levels were significantly lower in the presence than in the absence of serotonin at 80 mm Hg, and at 80 mm Hg after pretreatment with serotonin, compared with 10 mm Hg in the presence of serotonin. Therefore, in every case in which vasoconstriction occurred in the absence of a change in LC20 phosphorylation, G-actin content was reduced.
Our observation that cerebral arteries treated with latrunculin B at 120 mm Hg, or at 80 mm Hg in the presence of serotonin, dilated to the passive diameter rather than to the preexisting diameter at 80 mm Hg or in the presence of serotonin at 10 mm Hg, suggests that increased actin polymerization is not the only process involved in the reorganization of the cytoskeleton. Since LC20 phosphorylation did not change, we can conclude that sequestration of G-actin by latrunculin B does not affect membrane potential, Ca2+ influx, MLCK or ROCK activation, or MLCP inhibition. Complete loss of force would be predicted, however, if pressurization evoked a remodeling process that involves breaking of existing connections between the contractile machinery, the plasma membrane, and the extracellular matrix that are required for force distribution and transmission. Severing of existing connections within the cytoskeleton and between the actin cytoskeleton and the contractile machinery would prevent transmission of force generated by cross-bridge cycling to the sarcolemma and extracellular matrix, resulting in complete dilatation. α-Actinin may have a key role in this regard: recruitment of α-actinin to membrane-associated dense bodies in tracheal smooth muscle is required for force development but not for actin polymerization or LC20 phosphorylation.100 α-Actinin crosslinks actin filaments and also binds to integrins,101 which would strengthen the connections between the cytoskeleton and extracellular matrix, thereby enhancing the efficiency of the transmission of force generated by cross-bridge cycling.
Based on accumulating evidence of a change in F-actin: G-actin ratio with pressure elevation in resistance arteries, it is worthwhile to consider the potential physiologic significance of cytoskeletal reorganization in the myogenic response. A well-recognized, fundamental feature of smooth muscle contraction is that force is maintained during sustained contraction, although the level of LC20(Ser-19) phosphorylation and the rate of ATP hydrolysis by actomyosin ATPase (i.e., energy consumption) are substantially lower than during the phase of force generation after agonist or mechanical stimulation.102, 103 The molecular basis of this phenomenon of force maintenance at reduced levels of LC20 phosphorylation and energy consumption, referred to as the ‘latch state,' is not known. It has been attributed to: (1) dephosphorylated, slowly cycling cross-bridges,104 (2) slower kinetics of myosin head interaction with actin when phosphorylated at a single versus both heads,105 and more recently, (3) stimulated actin polymerization.30 Consistent with the latter view, Jones et al106 showed that F-actin stabilization with phalloidin before Ca2+-induced activation of Triton X-100-permeabilized airway smooth muscle was associated with an increased rate of ATP hydrolysis compared with untreated tissues during sustained contractions. The lower level of energy consumption associated with sustained contraction may arise because of a reduction in load on the contractile apparatus as a result of the dynamic reorganization of actin filaments. In the context of the myogenic response, reorganization and strengthening of the cortical cytoskeleton, as well as connections between the contractile apparatus and cytoskeleton at dense bodies may, therefore, optimize the efficiency of energy utilization during sustained contractions that are required to hold arteriolar diameter constant at maintained levels of physiologic intraluminal pressurization. Direct measurements of ATP hydrolysis in the presence and absence of cytoskeletal reorganization after changes in intraluminal pressure are required to substantiate this view.
Cellular Signaling Mechanisms and Adapter/Effector Proteins Involved in Actin Cytoskeletal Dynamics
As yet, we know very little about the signal transduction pathways that mediate pressure-induced actin cytoskeletal rearrangement during the myogenic response in the cerebral vasculature. Work in this area is being directed by previous studies with larger, agonist-activated smooth muscle tissues that lack myogenic tone. Based largely on the work of Gunst et al24 with airway smooth muscle, newly formed actin filaments in the subplasmalemmal region are thought to connect to membrane-associated dense plaques, specifically the cytoplasmic domain of β integrins, via linker proteins such as talin and vinculin. β Integrins thereby form a physical continuum that connects the extracellular matrix to the contractile machinery to mediate mechanical force transmission. Several proteins are recruited to the dense plaques on stimulation, e.g., FAK, talin, paxillin, vinculin,93, 107 α-actinin,100 and the Ca2+-dependent nonreceptor tyrosine kinase PYK2 (proline-rich tyrosine kinase 2).28 An early event in the response of tracheal smooth muscle to acetylcholine is the recruitment to the membrane of a stable cytosolic trimeric complex108 composed of the protein kinase ILK (integrin-linked kinase), which binds to the cytoplasmic domain of β integrins and also serves a scaffolding role,109 PINCH (particularly interesting new cysteine-histidine-rich protein, an LIM (Lin 11, Isl-1, Mec-3) domain-only adapter protein)110 and α-parvin (an actin-binding protein).111 Association of the complex with paxillin and vinculin initiates actin polymerization via the actin nucleation initiating factor N-WASP (neuronal Wiskott-Aldrich syndrome protein) pathway.108
The Neuronal Wiskott-Aldrich Syndrome Protein Pathway
Various pathways leading to N-WASP activation have been identified. For example, FAK is activated by autophosphorylation at Tyr-397 in response to integrin activation.112, 113 Paxillin tyrosine phosphorylation, which is catalyzed by activated FAK and other tyrosine kinases, has been observed in tracheal107, 114 and vascular smooth muscles,28, 29, 30 and phosphorylated paxillin activates N-WASP via the adapter protein CrkII and the small GTPase Cdc42 (Figure 4).91, 94
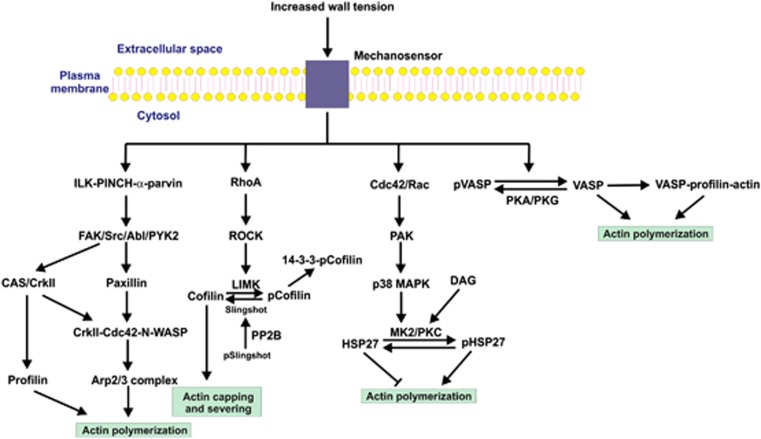
Figure 4.
Potential pathways leading to actin polymerization in the myogenic response. Several signal transduction pathways have been implicated in the regulation of actin polymerization, based largely on studies of agonist stimulation of airway and vascular smooth muscles, but the possibility that one or more of them may have a role in the myogenic response deserves investigation. An early event after stimulation is the recruitment to the plasma membrane of a trimeric complex composed of integrin-linked kinase (ILK), PINCH (particularly interesting new cysteine-histidine-rich protein), and α-parvin. A variety of tyrosine kinases are capable of phosphorylating paxillin, leading to the sequential activation of N-WASP (neuronal Wiskott-Aldrich syndrome protein) and the actin-related protein (Arp)2/3 complex, which activates actin polymerization. N-WASP can also be activated via p130 CAS (Crk-associated substrate) and the adapter protein, CrkII. In addition, the actin-binding protein profilin lies downstream of CAS and induces actin polymerization. In addition to their role in Ca2+ sensitization via myosin light chain phosphatase (MLCP) inhibition, RhoA and Rho-associated kinase (ROCK) may be involved in regulation of actin polymerization through the actin capping and severing protein cofilin. Lin 11, Isl-1, Mec-3 kinase (LIMK) is a substrate of ROCK which, when phosphorylated within its activation loop, phosphorylates cofilin at Ser-3. Phosphocofilin (pCofilin) loses the ability to cap and sever actin filaments, so activation of this pathway would stabilize preformed actin filaments in the subplasmalemmal domain. HSP27 is an actin capping protein that inhibits actin polymerization. This effect is alleviated by phosphorylation (pHSP27) by MK2, which lies downstream of p38 MAPK (mitogen-activated protein kinase) and the small GTPase-activated PAK (p21-activated protein kinase). Protein kinase C (PKC), activated by diacylglycerol (DAG), phosphorylates the same sites in HSP27 as does MK2. The vasodilator-stimulated phosphoprotein (VASP) is a PKA and PKG substrate, with phosphorylation (pVASP) preventing its interaction with actin and the profilin–actin complex. Dephosphorylation favors binding of VASP to G-actin and profilin–actin, which enhance actin polymerization. While the role of VASP has not been studied in relation to the myogenic response, the possibility that increased wall tension leads to dephosphorylation of VASP deserves attention.
An alternative pathway to activation of N-WASP involves the focal adhesion protein CAS,115, 116 which is phosphorylated by the tyrosine kinases Src or Abl in response to contractile stimulation.32 Norepinephrine induced an increase in force, LC20 phosphorylation, and F-actin: G-actin ratio in canine carotid artery.116 Downregulation of CAS by antisense oligonucleotide treatment inhibited this norepinephrine-induced force response and the increase in F-actin: G-actin ratio, without affecting LC20 phosphorylation.32 Phosphorylation of CAS by Abl increased its binding to CrkII117 and contractile stimulation increased the association of CrkII with N-WASP,91 thereby exposing a binding site for the actin-related protein (Arp)2/3 complex to initiate actin polymerization and branching.91, 92, 94, 117 Silencing of Abl by short-hairpin RNA reduced CAS phosphorylation in resistance arteries on contractile stimulation.117 Abl itself is activated by phosphorylation at Tyr-412 and this has been shown to occur in vascular smooth muscle cells in response to angiotensin II.118 PYK2 may also phosphorylate CAS and paxillin.119, 120
p130 Crk-associated substrate also lies upstream of the actin regulatory protein, profilin, which binds to actin monomers and can promote actin polymerization:121 binding of the profilin–actin complex to N-WASP can deliver actin monomers to the Arp2/3 complex nucleation site or the barbed end of a growing actin filament.122 Profilin-mediated actin polymerization has been implicated in the contractile response of canine carotid arteries to norepinephrine, referred to above, with the demonstration that latrunculin A pretreatment or downregulation of profilin expression by antisense oligonucleotide treatment inhibits norepinephrine-induced force development and the increase in F-actin: G-actin ratio, without affecting the increase in LC20 phosphorylation. Finally, downregulation of CAS prevented the association of profilin with G-actin.32 Future studies are needed to elucidate the importance and specific components of the N-WASP pathway(s) in the myogenic response of cerebral resistance arteries.
The ROCK-LIMK-Cofilin Pathway
In addition to regulating Ca2+ sensitization via inhibition of MLCP, the small GTPase RhoA affects actin polymerization (Figure 4).87, 123, 124 Support for a role for RhoA in the myogenic response came from the observation that RhoA translocates from the cytosol to the plasma membrane during the myogenic response of mesenteric arteries, and the myogenic response is blocked by treatment with C3 exoenzyme, which ADP ribosylates and inactivates RhoA,125 or by overexpression of dominant-negative RhoA.62 Several studies suggest that ROCK is the important downstream target of activated RhoA leading to actin polymerization126, 127 and that ROCK has an important role in the myogenic response of cerebral, renal, and mesenteric vascular beds, as discussed earlier. Inhibition of ROCK activity also abolished UTP-induced actin polymerization and induced dilatation of vessels that were preconstricted with UTP.90
The actin depolymerizing factor/cofilin family of proteins has been implicated in canine tracheal smooth muscle contraction.128 Cofilin binds to and severs actin filaments, providing G-actin monomers and short, capped actin filaments with free barbed ends that provide nucleation sites for the formation of new actin filaments by the Arp2/3 complex. Cofilin is phosphorylated at Ser-3 by LIM kinase (LIMK), which is activated by phosphorylation within its activation loop by ROCK, among others.129 Phosphorylation of cofilin abolished its actin binding and severing function.130, 131 Phosphocofilin binds to the intracellular scaffolding protein, 14-3-3, which sequesters cofilin and protects it from dephosphorylation.132 The phosphatase(s) responsible for cofilin dephosphorylation in smooth muscle is unknown, but in other cell types two specific cofilin phosphatases have been identified: slingshot and chronophin.133, 134 Slingshot can be activated by dephosphorylation, and studies with cultured cells have implicated calcineurin (the Ca2+/calmodulin-dependent type 2B protein serine/threonine phosphatase, PP2B) as the responsible phosphatase.135 Slingshot has also been shown to dephosphorylate and thereby inactivate LIMK.136 Acetylcholine-induced contraction of canine tracheal smooth muscle correlates with dephosphorylation (activation) of cofilin, and expression of the dominant-negative phosphomimetic S3E mutant of cofilin in canine tracheal smooth muscle inhibited acetylcholine-induced cofilin dephosphorylation, actin polymerization and contraction.128 Cofilin function appears to be restricted to a subcellular pool of actin that is distinct from the stable actin filaments involved in actomyosin complex formation and cross-bridge cycling. The latter pool is associated with tropomyosin, which inhibits cofilin binding, whereas the cortical actin pool involved in dynamic polymerization–depolymerization is free of tropomyosin and, therefore, accessible to cofilin.137, 138
At first glance, it would appear that activation of the ROCK-LIMK-cofilin pathway has the opposite effect to what would be predicted, i.e., alleviation of cofilin's ability to cap and sever actin filaments would prevent the formation of new actin filaments that have been implicated in force development. However, the time course of events is likely to be critical here. Cofilin in resting vascular smooth muscle of the swine carotid artery is phosphorylated at Ser-3 and becomes dephosphorylated in response to K+-induced depolarization, which activates voltage-gated Ca2+ channels to allow the entry of extracellular Ca2+ and activation of contraction via LC20 phosphorylation.30 However, cofilin dephosphorylation is a slow process compared with force development: significant dephosphorylation of cofilin was not detected until steady-state force had already been developed in response to K+. Removal of the depolarizing stimulus resulted in phosphorylation of cofilin. We suggest, therefore, that the ROCK-LIMK-cofilin pathway is constitutively active and cofilin remains phosphorylated and, therefore, may be inactive during the force development phase of the myogenic response. Actin polymerization during this rapid phase is then likely because of activation of the N-WASP pathway. Subsequently, when cofilin becomes dephosphorylated, its actin capping and severing activity is expressed to sustain dynamic actin polymerization during the force maintenance phase of the contractile response. Dephosphorylation of cofilin, therefore, may have a key role in cortical actin polymerization required for force maintenance rather than force development. However, stretch of the rat portal vein in organ culture increased cofilin phosphorylation and the F-actin: G-actin ratio.139 Furthermore, different contractile agonists have been shown to either increase or have no effect on cofilin phosphorylation in pulmonary arterial ring preparations.140
The HSP27 Pathway
HSP27 is a well-known chaperone protein that provides protection against physical and chemical stresses. It has also been implicated, however, in regulation of the actin cytoskeleton.141 The unphosphorylated protein inhibits actin polymerization by capping actin filaments, a property that is lost on phosphorylation.142 HSP27 is phosphorylated143 by MK2 (MAPK-activated protein kinase 2),144 which itself is activated by phosphorylation by p38 MAPK that lies downstream of PAK (p21-activated kinase) and other kinases (Figure 4).145 Agonist-induced translocation of HSP27 to membranes and the contractile apparatus has been shown in smooth muscle.93, 146 Norepinephrine stimulation of rat mesenteric resistance vessels results in the activation of p38 MAPK (leading to phosphorylation of HSP27) and Src (leading to phosphorylation of paxillin). HSP27 phosphorylation induces the translocation of HSP27 from the cytoskeleton to the cytosol, where it interacts with phosphorylated paxillin, thereby alleviating HSP27-mediated inhibition of actin polymerization and leading to increased F-actin content.93 To date, there is no evidence implicating HSP27 phosphorylation in the myogenic response, but this pathway is worth investigating in this context. It is also of potentially great importance that PKCδ and, less effectively, PKCα, have been shown to phosphorylate the same sites in HSP27 as MK2,144 given that PKC has been implicated in the myogenic response.8, 9, 14, 54, 73, 74 Direct evidence of PKC-mediated actin polymerization during the myogenic response has come from measurements of G-actin content in rat gracilis arteries. The myogenic response was inhibited by pretreatment with the PKC inhibitor GF109203X, without an effect on CPI-17 phosphorylation, and increased intraluminal pressure decreased G-actin content in a GF109203X-dependent manner (Moreno-Dominguez et al, submitted).
Another heat-shock protein, HSP20, has been implicated in cyclic nucleotide-dependent relaxation of vascular smooth muscle: HSP20 phosphorylated at Ser-16 by cAMP-dependent protein kinase (PKA) competes with phosphocofilin for binding to 14-3-3, leading to an increase in the amount of activated (dephosphorylated) cofilin.147
The Vasodilator-stimulated Phosphoprotein Pathway
Vasodilator-stimulated phosphoprotein (VASP) is a member of the Ena/VASP family of proteins that are involved in regulation of actin dynamics through actin filament elongation, and have been implicated in cancer cell invasion and metastasis, morphogenesis, axon guidance, endothelial barrier function148 and, more recently, airway smooth muscle contraction.149 It binds directly to G-actin to induce actin polymerization150 and is localized to focal adhesions through interactions with proteins such as zyxin151 and vinculin.152 It also recruits profilin–actin heterodimers via its central proline-rich domain.153 It contains two principal cyclic nucleotide-dependent protein kinase phosphorylation sites: Ser-153 (predominant PKA site) and Ser-235 (predominant PKG (cGMP-dependent protein kinase) site) with minor phosphorylation of Thr-274 by both kinases.154 Ser-153 is located within the proline-rich domain and has been implicated in targeting of VASP, whereas Ser-235 and Thr-274 lie within the actin-binding domain and are involved in the regulation of actin polymerization.155 Phosphorylation inhibits VASP/G-actin interaction and prevents VASP-induced actin filament formation (Figure 4).156
Vasodilator-stimulated phosphoprotein partially colocalizes with α-actinin and vinculin in isolated aortic smooth muscle cells, consistent with its localization to adhesion plaques and dense bodies.83 Phosphorylation of VASP decreases during phenylephrine stimulation, and treatment with cytochalasin D inhibits phenylephrine-induced actin polymerization in freshly isolated ferret aortic smooth muscle cells and force development in aortic rings.83 However, no effect on the phenylephrine-induced increase in LC20 phosphorylation was observed in the aortic ring preparations treated with cytochalasin D. Knockdown of VASP significantly reduces phenylephrine-induced force generation. Introduction into freshly isolated aortic smooth muscle cells of the expressed EVH1 domain of human EVL (another Ena/VASP family member), fused to the HIV-1 TAT sequence to achieve membrane permeability, inhibits phenylephrine-induced actin polymerization, whereas the corresponding F78S mutant (which should prevent interaction with vinculin and other target proteins)157 has no effect. Finally, phenylephrine treatment induces modest, transient decreases in phosphorylation of VASP at all three PKA/PKG sites. The potential role of VASP and its dephosphorylation in dynamic actin cytoskeletal regulation in the cerebral circulation will be an important avenue for future exploration.
The relative importance of the pathways regulating actin polymerization is likely to vary according to cell type and mode of activation. It is, therefore, crucially important to identify the signal transduction pathways in diverse vascular beds that respond to specific stimuli. The myogenic response in rat cerebral arteries represents one important example.
Concluding remarks
Evidence has been presented indicative of the involvement of three signaling pathways in the myogenic response (Figure 5): (1) Ca2+-induced activation of contraction via MLCK-catalyzed phosphorylation of LC20; (2) Ca2+ sensitization of contraction elicited by Rho-associated kinase-mediated phosphorylation and inhibition of MLCP; and (3) Ca2+ sensitization of contraction elicited by dynamic regulation of the actin cytoskeleton. It is unclear whether a single or multiple mechanosensors are involved in activation of these three pathways. It is important to emphasize that Ca2+ sensitization pathways are ineffective in the absence of Ca2+-dependent activation of MLCK. Ca2+ channel blockers and removal of extracellular Ca2+ are well known to abolish the myogenic response.43, 48 This does not mean, however, that MLCP inhibition and actin polymerization are not involved in the myogenic response since cross-bridge cycling absolutely requires LC20 phosphorylation, which generally requires an increase in [Ca2+]i. It is important to note, therefore, that any treatment or manipulation that directly or indirectly affects the activity of voltage-gated Ca2+ channels or Ca2+ influx may confound interpretation of the involvement of MLCP inhibition and dynamic regulation of the actin cytoskeleton.
Figure 5.
Three signaling pathways are implicated in the myogenic response: (1) Ca2+-induced activation of contraction via myosin light chain kinase (MLCK)-catalyzed phosphorylation of LC20 in response to membrane depolarization; (2) Ca2+ sensitization of contraction elicited by Rho-associated kinase (ROCK)-mediated phosphorylation of MYPT1 and inhibition of myosin light chain phosphatase (MLCP); and (3) Ca2+ sensitization of contraction elicited by dynamic regulation of the actin cytoskeleton mediated by protein kinase C (PKC) and ROCK. The mechanisms whereby an increase in wall tension and intravascular pressure activate these signaling pathways remain to be identified, but likely involve integrins and G protein-coupled receptors as mechanosensors.
The cerebral myogenic response is key to three fundamental mechanisms: (1) it maintains cerebral blood flow constant during variations in systemic pressure (i.e., flow autoregulation);11 (2) it establishes a regional blood flow reserve so that local flow may be matched to metabolic demand by allowing vasodilatation from a partially constricted state;2 and (3) it provides a critical shield that protects brain capillaries and the blood–brain barrier from damage by elevated intravascular pressure.2, 158, 159 Inappropriate myogenic behavior impacts each of these. Enhanced myogenic constriction at low pressure and reduced force generation with increasing pressure are observed in several conditions that have an impact on neurologic function in humans, including hypertension, cerebral vasospasm, hemorrhagic and ischemic stroke, diabetes, and head trauma.1, 12, 13, 14, 15, 16, 17, 158, 160, 161 Impaired dilatation at low perfusion pressure in hypertension compromises blood flow reserve and increases the risk of ischemia. Conversely, impaired myogenic constriction leading to uncontrolled elevated levels of blood flow (i.e., ‘breakthrough') in malignant hypertension, hemorrhagic stroke, type 2 diabetes, and trauma is associated with secondary damage owing to blood–brain barrier disruption, small vessel rupture (microbleeds), edema, and increased intracranial pressure. Notably, loss of the myogenic response may be a critical prelude to irreversible, ‘true' vascular remodeling within the arterial wall involving hypertrophy and hyperplasia.161, 162, 163 Only eutrophic remodeling of existing wall material around a smaller lumen is observed in small arteries that possess a myogenic response (conduit vessels lacking myogenic tone exhibit ‘true' remodeling).163 However, the loss of myogenic behavior and an inability of vessels to withstand the stress of increased pressure-evoked wall tension stimulate an adaptive, structural response to control blood flow.161, 162, 163 Understanding the molecular defects that result in the loss of myogenic responsiveness will provide crucial information concerning the fundamental changes that contribute to this key event in the progression of vascular dysfunction.
There remain numerous gaps in our understanding of the signaling pathways involved in the myogenic response, their applicability to smooth muscle tissues in general, and whether they are involved in acute contractile responses and slower effects such as cell migration and proliferation. Essentially, actin polymerization is regulated by proteins that control the availability of monomeric G-actin and of free barbed (plus) ends of short, nucleating actin filaments.24 While relatively little is known regarding the identity of actin regulatory proteins in the context of smooth muscle contraction and the myogenic response, a number of candidate proteins have emerged from extensive knowledge of the regulation of actin polymerization–depolymerization in cell migration.164, 165 In the context of the cerebral circulation, we are embarking on a period of identification of the proteins involved in dynamic regulation of the actin cytoskeleton as an essential component of the myogenic response. This will lead to elucidation of defects responsible for pathologies of the cerebral circulation and identification of novel drug targets.
Finally, it is worth closing with a note about the concept of Ca2+ sensitization of smooth muscle contraction. Ca2+ sensitization can be defined as an increase in force without a change in [Ca2+]i. This can be achieved by activation of signaling pathways that lead to inhibition of MLCP activity and an increase in actin polymerization. It must be emphasized that activation of either of these mechanisms without a sufficiently high [Ca2+]i will not induce contraction since there must be a threshold level of LC20 phosphorylation to activate cross-bridge cycling.
Acknowledgments
The authors are very grateful to current and former laboratory personnel who have made important contributions to our work on the molecular basis of the myogenic response in resistance arteries: Tim Chen, Olaia Colinas, Ahmed F El-Yazbi, Rosalyn P Johnson, Alejandro Moreno-Dominguez, Kosuke Takeya, Emma J Walsh, Xi Zoë Zhong, and Hai-Lei Zhu.
The authors declare no conflicts of interests.
Footnotes
This study was supported by grants from the Canadian Institutes of Health Research (MOP-111262 to MPW and MOP-13505 to WCC and MPW). MPW is an Alberta Innovates—Health Solutions Scientist and Canada Research Chair (Tier 1) in Vascular Smooth Muscle Research. WCC is the Andrew Family Professor in Cardiovascular Research.
References
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol. 1985;249:H914–H921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 1999;96:313–326. [PubMed] [Google Scholar]
- Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009;587:2537–2553. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Yazbi AF, Johnson RP, Walsh EJ, Takeya K, Walsh MP, Cole WC. Pressure-dependent contribution of Rho kinase-mediated calcium sensitization in serotonin-evoked vasoconstriction of rat cerebral arteries. J Physiol. 2010;588:1747–1762. doi: 10.1113/jphysiol.2010.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation. 2008;15:495–501. doi: 10.1080/10739680801986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behaviour. Am J Physiol Heart Circ Physiol. 2002;283:H2260–H2267. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- Immink RV, van den Born BJ, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation. 2004;110:2241–2245. doi: 10.1161/01.CIR.0000144472.08647.40. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H1917–H1922. doi: 10.1152/ajpheart.01012.2004. [DOI] [PubMed] [Google Scholar]
- Kim YS, Immink RV, Stok WJ, Karemaker JM, Secher NH, van Lieshout JJ. Dynamic cerebral autoregulatory capacity is affected early in Type 2 diabetes. Clin Sci (Lond) 2008;115:255–262. doi: 10.1042/CS20070458. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990;70:921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- Takeya K, Loutzenhiser K, Shiraishi M, Loutzenhiser RD, Walsh MP. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: The first quantification in renal arterioles. Am J Physiol Renal Physiol. 2008;294:F1487–F1492. doi: 10.1152/ajprenal.00060.2008. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, et al. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. αvβ3- and α5β1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, et al. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol. 2001;280:H1427–H1433. doi: 10.1152/ajpheart.2001.280.4.H1427. [DOI] [PubMed] [Google Scholar]
- Mogford JE, Platts SH, Davis GE, Meininger GA. Vascular smooth muscle αvβ3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res. 1996;79:821–826. doi: 10.1161/01.res.79.4.821. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Zhang W. Actin cytoskeleton dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TV, Spurrell BE, Hill MA. Tyrosine phosphorylation following alterations in arteriolar intraluminal pressure and wall tension. Am J Physiol Heart Circ Physiol. 2001;281:H1047–H1056. doi: 10.1152/ajpheart.2001.281.3.H1047. [DOI] [PubMed] [Google Scholar]
- Murphy TV, Spurrell BE, Hill MA. Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways. Clin Exp Pharmacol Physiol. 2002;29:612–619. doi: 10.1046/j.1440-1681.2002.03698.x. [DOI] [PubMed] [Google Scholar]
- Masumoto N, Nakayama K, Oyabe A, Uchino M, Ishii K, Obara K, et al. Specific attenuation of the pressure-induced contraction of rat cerebral artery by herbimycin A. Eur J Pharmacol. 1997;330:55–63. doi: 10.1016/s0014-2999(97)00166-0. [DOI] [PubMed] [Google Scholar]
- Ohanian V, Gatfield K, Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension. 2005;46:93–99. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol. 2007;293:C993–C1002. doi: 10.1152/ajpcell.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani AD, Walsh MP, Rembold CM. Tissue length modulates ‘stimulated actin polymerization', force augmentation, and the rate of swine carotid artery contraction. Am J Physiol Cell Physiol. 2011;301:C1470–C1478. doi: 10.1152/ajpcell.00149.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Adam LP, Wu MF, Walker TL, Gunst SJ. Phosphorylation of dense-plaque proteins talin and paxillin during tracheal smooth muscle contraction. Am J Physiol Cell Physiol. 1995;268:C563–C571. doi: 10.1152/ajpcell.1995.268.3.C563. [DOI] [PubMed] [Google Scholar]
- Tang DD, Tan J. Role of Crk-associated substrate in the regulation of vascular smooth muscle contraction. Hypertension. 2003;42:858–863. doi: 10.1161/01.HYP.0000085333.76141.33. [DOI] [PubMed] [Google Scholar]
- Spurrell BE, Murphy TV, Hill MA. Intraluminal pressure stimulates MAPK phosphorylation in arterioles: temporal dissociation from myogenic contractile response. Am J Physiol Heart Circ Physiol. 2003;285:H1764–H1773. doi: 10.1152/ajpheart.00468.2003. [DOI] [PubMed] [Google Scholar]
- Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by α5β1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276:30285–30292. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- Mederos Y, Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys. 2011;510:160–173. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Allen BG, Walsh MP. The biochemical basis of the regulation of smooth-muscle contraction. Trends Biochem Sci. 1994;19:362–368. doi: 10.1016/0968-0004(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Wilson DP, Sutherland C, Walsh MP. Ca2+ activation of smooth muscle contraction: evidence for the involvement of calmodulin that is bound to the Triton-insoluble fraction even in the absence of Ca2+ J Biol Chem. 2002;277:2186–2192. doi: 10.1074/jbc.M110056200. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- Walsh MP, Bridenbaugh R, Hartshorne DJ, Kerrick WGL. Phosphorylation-dependent activated tension in skinned gizzard muscle fibres in the absence of Ca2+ J Biol Chem. 1982;257:5987–5990. [PubMed] [Google Scholar]
- Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, et al. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol. 2010;588:3983–4005. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharia J, Zhang J, Wier WG. Ca2+ signaling in mouse mesenteric small arteries: myogenic tone and adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol. 2007;292:H1523–H1532. doi: 10.1152/ajpheart.00670.2006. [DOI] [PubMed] [Google Scholar]
- Zou H, Ratz PH, Hill MA. Role of myosin phosphorylation and [Ca2+]i in myogenic reactivity and arteriolar tone. Am J Physiol Heart Circ Physiol. 1995;269:H1590–H1596. doi: 10.1152/ajpheart.1995.269.5.H1590. [DOI] [PubMed] [Google Scholar]
- Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol. 2006;291:C348–C356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol. 2009;587:625–640. doi: 10.1113/jphysiol.2008.165217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, et al. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588:3277–3293. doi: 10.1113/jphysiol.2010.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, et al. Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol. 2010;588:4519–4537. doi: 10.1113/jphysiol.2010.196618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokina NI, Knot HJ, Nelson MT, Osol G. Increased Ca2+ sensitivity as a key mechanism of PKC-induced constriction in pressurized cerebral arteries. Am J Physiol Heart Circ Physiol. 1999;277:H1178–H1188. doi: 10.1152/ajpheart.1999.277.3.H1178. [DOI] [PubMed] [Google Scholar]
- Lagaud G, Gaudreault N, Moore ED, Van Breemen C, Laher I. Pressure-dependent myogenic constriction of cerebral arteries occurs independently of voltage-dependent activation. Am J Physiol Heart Circ Physiol. 2002;283:H2187–H2195. doi: 10.1152/ajpheart.00554.2002. [DOI] [PubMed] [Google Scholar]
- Gokina NI, Park KM, Elroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol. 2005;98:1940–1948. doi: 10.1152/japplphysiol.01104.2004. [DOI] [PubMed] [Google Scholar]
- Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104:109–115. doi: 10.1254/jphs.cp0070027. [DOI] [PubMed] [Google Scholar]
- Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- VanBavel E, van der Meulen ET, Spaan JA. Role of Rho-associated protein kinase in tone and calcium sensitivity of cannulated rat mesenteric small arteries. Exp Physiol. 2001;86:585–592. doi: 10.1113/eph8602217. [DOI] [PubMed] [Google Scholar]
- Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, et al. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic activity of protein phosphatase type 1δ. Arch Biochem Biophys. 2011;510:147–159. doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdődi F. Role of protein phosphatase type 1 in contractile functions: Myosin phosphatase. J Biol Chem. 2004;279:37211–37214. doi: 10.1074/jbc.R400018200. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa H, Isaka N, Nishikawa M, Hartshorne DJ, et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Murányi A, Derkach D, Erdődi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: Inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 2005;579:6611–6615. doi: 10.1016/j.febslet.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, et al. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000;475:197–200. doi: 10.1016/s0014-5793(00)01654-9. [DOI] [PubMed] [Google Scholar]
- Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aortic media and characterization. J Biochem (Tokyo) 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009;284:35273–35277. doi: 10.1074/jbc.R109.059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Cierpicki T, Momotani K, Artamonov MV, Derewenda U, Bushweller JH, et al. On the mechanism of autoinhibition of the RhoA specific nucleotide exchange factor PDZRhoGEF. BMC Struct Biol. 2009;9:36. doi: 10.1186/1472-6807-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swärd K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertension Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- Karibe A, Watanabe J, Horiguchi S, Takeuchi M, Suzuki S, Funakoshi S, et al. Role of cytosolic Ca2+ and protein kinase C in developing myogenic contraction in isolated rat small arteries. Am J Physiol Heart Circ Physiol. 1997;272:H1165–H1172. doi: 10.1152/ajpheart.1997.272.3.H1165. [DOI] [PubMed] [Google Scholar]
- Wesselman JP, Spaan JA, van der Meulen ET, VanBavel E. Role of protein kinase C in myogenic calcium-contraction coupling of rat cannulated mesenteric small arteries. Clin Exp Pharmacol Physiol. 2001;28:848–855. doi: 10.1046/j.1440-1681.2001.03534.x. [DOI] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Dubroca C, You D, Lévy BI, Loufrani L, Henrion D. Involvement of RhoA/Rho kinase pathway in myogenic tone in the rabbit facial vein. Hypertension. 2005;45:974–979. doi: 10.1161/01.HYP.0000164582.63421.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn T, Kim SA, Hai CM. Length-dependent modulation of smooth muscle activation: effects of agonist, cytochalasin, and temperature. Am J Physiol Cell Physiol. 1998;274:C1601–C1607. doi: 10.1152/ajpcell.1998.274.6.C1601. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol. 1999;519:829–840. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler KB, Krill J, Alberghini TV, Evans JN. Effect of cytochalasin D on smooth muscle contraction. Cell Motil. 1983;3:545–551. doi: 10.1002/cm.970030521. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behaviour. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- Shaw L, Ahmed S, Austin C, Taggart MJ. Inhibitors of actin filament polymerization attenuate force but not global intracellular calcium in isolated pressurized resistance arteries. J Vasc Res. 2003;40:1–10. doi: 10.1159/000068940. [DOI] [PubMed] [Google Scholar]
- Chen X, Pavlish K, Zhang HY, Benoit JN. Effects of chronic portal hypertension on agonist-induced actin polymerization in small mesenteric arteries. Am J Physiol Heart Circ Physiol. 2006;290:H1915–H1921. doi: 10.1152/ajpheart.00643.2005. [DOI] [PubMed] [Google Scholar]
- Kim HR, Graceffa P, Ferron F, Gallant C, Boczkowska M, Dominguez R, et al. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator-stimulated phosphoprotein. Am J Physiol Cell Physiol. 2010;298:C559–C571. doi: 10.1152/ajpcell.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss S, Koch G, Kreye VA, Aktories K. Inhibition of the contraction of the isolated longitudinal muscle of the guinea-pig ileum by botulinum C2 toxin: evidence for a role of G/F-actin transition in smooth muscle contraction. Naunyn Schmiederbergs Arch Pharmacol. 1989;340:345–351. doi: 10.1007/BF00168521. [DOI] [PubMed] [Google Scholar]
- Gokina OsolG. Actin cytoskeletal modulation of pressure-induced depolarization and Ca2+ influx in cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;282:H1410–H1420. doi: 10.1152/ajpheart.00441.2001. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Bailey SR, Flavahan WA, Mitra S, Flavahan S. Imaging remodelling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol. 2005;288:H660–H669. doi: 10.1152/ajpheart.00608.2004. [DOI] [PubMed] [Google Scholar]
- Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol Lung Cell Mol Physiol. 1999;277:L653–L661. doi: 10.1152/ajplung.1999.277.3.L653. [DOI] [PubMed] [Google Scholar]
- Corteling RL, Brett SE, Yin H, Zheng XL, Walsh MP, Welsh DG. The functional consequence of RhoA knockdown by RNA interference in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;293:H440–H447. doi: 10.1152/ajpheart.01374.2006. [DOI] [PubMed] [Google Scholar]
- Zhang W, Du L, Gunst SJ. The effects of the small GTPase RhoA on the muscarinic contraction of airway smooth muscle result from its role in regulating actin polymerization. Am J Physiol Cell Physiol. 2010;299:C298–C306. doi: 10.1152/ajpcell.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luykenaar KD, Abd El-Rahman R, Walsh MP, Welsh DG. Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am J Physiol Heart Circ Physiol. 2009;296:H917–H926. doi: 10.1152/ajpheart.01206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem. 2005;280:23380–23389. doi: 10.1074/jbc.M413390200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1145–C1160. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Forman S, Quinlan RA, Ohanian J, Ohanian V. Regulation of contractility by Hsp27 and Hic-5 in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2008;294:H961–H969. doi: 10.1152/ajpheart.00939.2007. [DOI] [PubMed] [Google Scholar]
- Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem. 2004;279:51722–51728. doi: 10.1074/jbc.M408351200. [DOI] [PubMed] [Google Scholar]
- Small JV. Structure-function relationships in smooth muscle: the missing links. Bioessays. 1995;17:785–792. doi: 10.1002/bies.950170908. [DOI] [PubMed] [Google Scholar]
- Parker CA, Takahashi K, Tao T, Morgan KG. Cytoskeletal targeting of calponin in differentiated, contractile smooth muscle cells of the ferret. J Physiol. 1998;508:187–198. doi: 10.1111/j.1469-7793.1998.187br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew JS, Moos C, Murphy RA. Localization of isoactins in isolated smooth muscle thin filaments by double gold immunolabeling. Am J Physiol Cell Physiol. 1991;260:C1332–C1340. doi: 10.1152/ajpcell.1991.260.6.C1332. [DOI] [PubMed] [Google Scholar]
- Chen X, Pavlish K, Benoit JN. Myosin phosphorylation triggers actin polymerization in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2008;295:H2172–H2177. doi: 10.1152/ajpheart.91437.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gunst SJ. Dynamic association between α-actinin and β-integrin regulates contraction of canine tracheal smooth muscle. J Physiol. 2006;572:659–676. doi: 10.1113/jphysiol.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöblom B, Salmazo A, Djinović-Carugo K. α-Actinin structure and regulation. Cell Mol Life Sci. 2008;65:2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Siegman MJ. Chemical energy usage and myosin light chain phosphorylation in mammalian smooth muscle. Fed Proc. 1983;42:57–61. [PubMed] [Google Scholar]
- Hellstrand P.Energetics of smooth muscle contractionIn: Bárány M (ed.). Biochemistry of Smooth Muscle Contraction Academic Press: San Diego, CA; 1996379–392. [Google Scholar]
- Hai CM, Murphy RA. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol Cell Physiol. 1988;254:C99–C106. doi: 10.1152/ajpcell.1988.254.1.C99. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Homma K, White HD, Yanagida T, Ikebe M. Smooth muscle myosin phosphorylated at single head shows sustained mechanical activity. J Biol Chem. 2008;283:15611–15618. doi: 10.1074/jbc.M710597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Perkins WJ, Lorenz RR, Prakash YS, Sieck GC, Warner DO. F-actin stabilization increases tension cost during contraction of permeabilized airway smooth muscle in dogs. J Physiol. 1999;519:527–538. doi: 10.1111/j.1469-7793.1999.0527m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo Saez A, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol. 2004;286:C433–C447. doi: 10.1152/ajpcell.00030.2003. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wu Y, Wu C, Gunst SJ. Integrin-linked kinase (ILK) regulates N-WASp-mediated actin polymerization and tension development in tracheal smooth muscle. J Biol Chem. 2007;282:34568–34580. doi: 10.1074/jbc.M704966200. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, McDonald PC, Walsh MP, Dedhar S. Integrin-linked kinase: not so ‘pseudo' after all. Oncogene. 2011;30:4375–4385. doi: 10.1038/onc.2011.177. [DOI] [PubMed] [Google Scholar]
- Kovalevich J, Tracy B, Langford D. PINCH: More than just an adaptor protein in cellular response. J Cell Physiol. 2011;226:940–947. doi: 10.1002/jcp.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda JL, Wu C. The parvins. Cell Mol Life Sci. 2006;63:25–35. doi: 10.1007/s00018-005-5355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Tang DD, Gunst SJ. Roles of focal adhesion kinase and paxillin in the mechanosensitive regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:1452–1459. doi: 10.1152/jappl.2001.91.3.1452. [DOI] [PubMed] [Google Scholar]
- Tang DD, Wu M-F, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542:510–513. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a ‘molecular switch' for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD, Tan J. Downregulation of profilin with antisense oligodeoxynucleotides inhibits force development during stimulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H1528–H1536. doi: 10.1152/ajpheart.00188.2003. [DOI] [PubMed] [Google Scholar]
- Anfinogenova Y, Wang R, Li Q, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res. 2007;101:420–428. doi: 10.1161/CIRCRESAHA.107.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res. 2005;97:829–836. doi: 10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]
- Li X, Earp HS. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- Keogh RJ, Houliston RA, Wheeler-Jones CP. Human endothelial Pyk2 is expressed in 2 isoforms and associates with paxillin and p130Cas. Biochem Biophys Res Commun. 2002;290:1470–1477. doi: 10.1006/bbrc.2002.6350. [DOI] [PubMed] [Google Scholar]
- Gutsche-Perelroizen I, Lepault J, Ott A, Carlier MF. Filament assembly from profilin-actin. J Biol Chem. 1999;274:6234–6243. doi: 10.1074/jbc.274.10.6234. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Ducruix A, Pantaloni D. Signalling to actin: the Cdc42-N-WASP-Arp2/3 connection. Chem Biol. 1999;6:R235–R240. doi: 10.1016/s1074-5521(99)80107-0. [DOI] [PubMed] [Google Scholar]
- Ridley A, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Zeidan A, Nordström I, Albinsson S, Malmqvist U, Swärd K, Hellstrand P. Stretch-induced contractile differentiation of vascular smooth muscle: sensitivity to actin polymerization inhibitors. Am J Physiol Cell Physiol. 2003;284:C1387–C1396. doi: 10.1152/ajpcell.00508.2002. [DOI] [PubMed] [Google Scholar]
- Dubroca C, Loyer X, Retailleau K, Loirand G, Pacaud P, Feron O, et al. RhoA activation and interaction with caveolin-1 are critical for pressure-induced myogenic tone in rat mesenteric resistance arteries. Cardiovasc Res. 2007;73:190–197. doi: 10.1016/j.cardiores.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Numaguchi K, Eguchi S, Yamakawa T, Motley ED, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem. 2008;283:36522–36531. doi: 10.1074/jbc.M805294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem. 1995;270:17582–17587. doi: 10.1074/jbc.270.29.17582. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Iida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr Biol. 2002;12:1704–1710. doi: 10.1016/s0960-9822(02)01184-3. [DOI] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Gohla A, Birkenfield J, Bokoch GM. Chronophin a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by slingshot via calcineurin. J Biol Chem. 2005;280:12683–12689. doi: 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, et al. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24:473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. Actin dynamics: tropomyosin provides stability. Curr Biol. 2002;12:R523–R525. doi: 10.1016/s0960-9822(02)01028-x. [DOI] [PubMed] [Google Scholar]
- DesMarais V, Ichetovkin I, Condeelis J, Hitchcock-DeGregori SE. Spatial regulation of actin dynamics: a tropomyosin-free, actin-rich compartment at the leading edge. J Cell Sci. 2002;115:4649–4660. doi: 10.1242/jcs.00147. [DOI] [PubMed] [Google Scholar]
- Albinsson S, Nordström I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem. 2004;279:34849–34855. doi: 10.1074/jbc.M403370200. [DOI] [PubMed] [Google Scholar]
- Dai YP, Bongalon S, Mutafova-Yambolieva V, Yamboliev IA. Distinct effects of contraction agonists on the phosphorylation state of cofilin in pulmonary artery smooth muscle. Adv Pharmacol Sci. 2008;2008:362741. doi: 10.1155/2008/362741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer WT, Gunst SJ. Focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolishes its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Gaestel M, Schröder W, Benndorf R, Lippmann C, Buchner K, Hucho F, et al. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J Biol Chem. 1991;266:14721–14724. [PubMed] [Google Scholar]
- Maizels ET, Peters CA, Kline M, Cutler RE, Shanmugam M, Hunzicker-Dunn M. Heat-shock protein-25/27 phosphorylation by the δ isoform of protein kinase C. Biochem J. 1998;332:703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol Lung Cell Mol Physiol. 1997;273:L930–L940. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- Ibitayo AI, Sladick J, Tuteja S, Louis-Jacques O, Yamada H, Groblewski G, et al. HSP27 in signal transduction and association with contractile proteins in smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 1999;277:G445–G454. doi: 10.1152/ajpgi.1999.277.2.G445. [DOI] [PubMed] [Google Scholar]
- Komalavilas P, Penn RB, Flynn CR, Thresher J, Lopes LB, Furnish EJ, et al. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L69–L78. doi: 10.1152/ajplung.00235.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Vemuri B, Gunst SJ. Phosphorylation of vasodilator-stimulated phosphoprotein (VASP) regulates contractility of airway smooth muscle tissues by regulating actin dynamics. Am J Respir Crit Care Med. 2010;181:A5310. [Google Scholar]
- Walders-Harbeck B, Khaitlina SY, Hinssen H, Jockusch BM, Illenberger S. The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett. 2002;529:275–280. doi: 10.1016/s0014-5793(02)03356-2. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Jouvenal K, Tripier D, Walter U. Identification, purification and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein) Proc Natl Acad Sci USA. 1995;92:7956–7960. doi: 10.1073/pnas.92.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M, Rüdiger M, Jockusch BM, Walter U. VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. FEBS Lett. 1996;399:103–107. doi: 10.1016/s0014-5793(96)01295-1. [DOI] [PubMed] [Google Scholar]
- Lambrechts A, Verschelde JL, Jonckheere V, Goethals M, Vandekerckhove J, Ampe C. The mammalian profilin isoforms display complementary affinities for PIP2 and proline-rich sequences. EMBO J. 1997;16:484–494. doi: 10.1093/emboj/16.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, et al. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, et al. Differential VASP phosphorylation controls remodelling of the actin cytoskeleton. J Cell Sci. 2009;122:3954–3965. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck B, Hüttelmaier S, Schlüter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J Biol Chem. 2000;275:30817–30825. doi: 10.1074/jbc.M005066200. [DOI] [PubMed] [Google Scholar]
- Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, et al. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron. 2000;26:143–154. doi: 10.1016/s0896-6273(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Larsen B, Skriver EB, Herning M, Enevoldsen E, Lassen NA. Focal cerebral hyperemia in acute stroke. Incidence, pathophysiology and clinical significance. Stroke. 1981;12:598–607. doi: 10.1161/01.str.12.5.598. [DOI] [PubMed] [Google Scholar]
- Smeda JS. Stroke development in stroke-prone spontaneously hypertensive rats alters the ability of cerebrovascular muscle to utilize internal Ca2+ to elicit constriction. Stroke. 2003;34:1491–1496. doi: 10.1161/01.STR.0000073797.91891.9E. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- Sonoyama K, Greenstein A, Price A, Khavandi K, Heagerty T. Vascular remodeling: implications for small artery function and target organ damage. Ther Adv Cardiovasc Dis. 2007;1:129–137. doi: 10.1177/1753944707086358. [DOI] [PubMed] [Google Scholar]
- Khavandi K, Greenstein AS, Sonoyama K, Withers S, Price A, Malik RA, et al. Myogenic tone and small artery remodeling: insight into diabetic nephropathy. Nephrol Dial Transplant. 2009;24:361–369. doi: 10.1093/ndt/gfn583. [DOI] [PubMed] [Google Scholar]
- Heagerty AM, Heerkens EH, Izzard AS. Small artery structure and function in hypertension. J Cell Mol Med. 2010;14:1037–1043. doi: 10.1111/j.1582-4934.2010.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C, Carlier M-F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]