Abstract
Mitochondria support the energy-intensive functions of brain endothelium but also produce damaging-free radicals that lead to disease. Previously, we found that estrogen treatment protects cerebrovascular mitochondria, increasing capacity for ATP production while decreasing reactive oxygen species (ROS). To determine whether these effects occur specifically in endothelium in vivo and also explore underlying transcriptional mechanisms, we studied freshly isolated brain endothelial preparations from intact and ovariectomized female mice. This preparation reflects physiologic influences of circulating hormones, hemodynamic forces, and cell–cell interactions of the neurovascular unit. Loss of ovarian hormones affected endothelial expression of the key mitochondrial regulator family, peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1), but in a unique way. Ovariectomy increased endothelial PGC-1α mRNA but decreased PGC-1β mRNA. The change in PGC-1β correlated with decreased mRNA for crucial downstream mitochondrial regulators, nuclear respiratory factor 1 and mitochondrial transcription factor A, as well as for ATP synthase and ROS protection enzymes, glutamate-cysteine ligase and manganese superoxide dismutase. Ovariectomy also decreased mitochondrial biogenesis (mitochondrial/nuclear DNA ratio). These results indicate ovarian hormones normally act through a distinctive regulatory pathway involving PGC-1β to support cerebral endothelial mitochondrial content and guide mitochondrial function to favor ATP coupling and ROS protection.
Keywords: brain endothelial cells, mitochondrial biogenesis, ovariectomy, PGC-1β, ROS
Introduction
Endothelial cells in the cerebral vasculature are distinguished by unique properties, especially pertaining to maintenance of the blood–brain barrier and active transport of a number of substances in and out of the brain.1 Given the high demand for ATP, cerebral endothelial cells exhibit a greater mitochondrial content than endothelial cells elsewhere in the body.2 Mitochondria are now understood to have central roles in a wide variety of crucial physiologic and pathophysiologic processes.3 They are the sites of numerous biosynthetic processes, regulate apoptosis, and generate a large portion of cellular ATP by oxidative phosphorylation (OXPHOS). Their central role in cellular metabolism also means that they produce a substantial portion of cellular reactive oxygen species (ROS). Accumulated mitochondrial damage by ROS can hamper cellular metabolism, induce cellular apoptosis and, eventually, cause tissue and organism pathology. The accumulation of ROS damage in endothelial cells is considered as an important pathologic mechanism for progression of atherosclerosis and incidence of stroke.4, 5 The potential consequences of mitochondrial ROS require careful regulation of mitochondrial function to meet cellular needs.
Regulation of OXPHOS capacity and creation of new mitochondria (mitochondrial biogenesis) require the coordinated synthesis of nuclear- and mitochondrial-encoded genes.6 A family of coactivators, so-called ‘master regulators' of mitochondrial function, has been described that includes peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β). These coactivators initiate expression of transcription factors, such as nuclear respiratory factor 1 (NRF1), that selectively target mitochondrial-related genes.7 Nuclear respiratory factor 1 appears to be a member of the final common pathway for initiating mitochondrial biogenesis by activating transcription of nuclear genes encoding mitochondrial proteins, while also increasing the expression of mitochondrial transcription factor A (TFAM).8 The TFAM translocates to the mitochondria and initiates transcription and replication of mitochondrial DNA.9
Most studies of the regulation of mitochondrial biogenesis have focused on peripheral oxidative tissues such as liver, brown fat, and skeletal muscle.7 Much less is known regarding mitochondrial regulation and biogenesis in vascular tissue in general and brain endothelium, in particular. Previously, we have shown that estrogen has protective effects on mitochondria in cerebral blood vessels and cultured endothelial cells,10, 11, 12 likely contributing to protective effects of estrogen in vascular disease and stroke.13 In cerebral blood vessels, estrogen treatment elevates the levels of proteins involved in OXPHOS and suppresses mitochondrial ROS, thus protecting mitochondria from oxidative stress.12 However, it is not known whether these effects occur under physiologic hormone exposure and specifically in brain endothelial cells and, if so, what underlying transcriptional mechanisms may be involved.
The preparations used to date for studying mitochondrial regulation in cerebral vascular tissue, i.e., isolated cerebral blood vessels and cultured cells, are not ideal for studying transcriptional regulation of mitochondria under physiologic conditions. Studies of cerebral blood vessels are confounded by the multiple cell types present, and, in culture, brain endothelial cells do not fully retain all of the unique properties induced by the brain environment.14 To study mRNA changes in cerebral endothelium, we have adapted and validated a method of obtaining an enriched preparation of freshly isolated brain endothelial cells.14 These cells were isolated from either intact or ovariectomized (OVX) female mice to determine whether endogenous ovarian hormones have an effect on mitochondrial biogenesis in brain endothelium. To accomplish this goal, we measured mRNA and protein for key transcription factors important in regulation of mitochondrial biogenesis and also determined the mitochondrial/nuclear DNA ratio, an indicator of mitochondrial content. Additionally, we evaluated the expression of specific proteins regulated during the process of mitochondrial biogenesis. It is well known that loss of ovarian hormones because of menopause or surgery increases risk for endothelial inflammation, atherosclerosis progression, cardiovascular disease, and stroke.15, 16 Therefore, we hypothesized that ovariectomy, with its associated loss of ovarian hormones, would cause a decrease in endothelial mitochondrial biogenesis and alter mitochondrial function.
Materials and methods
Animals
All animal procedures were conducted in an AAALAC-accredited facility in accordance with protocols approved by the UC Irvine Institutional Animal Care and Use Committee. Female C57BL/6 mice were obtained from Charles River, either ovariectomized by the vendor or left intact. Animals were fed ad libitum and kept under diurnal lighting conditions (12 hours light/12 hours dark). Animals were 3 to 4 months old at the time of euthanasia, and OVX mice were 2 to 3 weeks after surgery. Animals were anesthetized using isoflurane, decapitated, and then the brains were removed for endothelial cell isolation. To confirm loss of ovarian hormones after ovariectomy, the uteri were dissected out, dried, and weighed. Uteri from OVX animals were significantly smaller than those of intact animals (0.11±0.009 versus 0.71±0.06 mg/g body weight, P<0.001).
Reagents
Unless otherwise noted, chemicals were purchased from Sigma (St Louis, MO, USA). BSA (Fraction V, cold alcohol precipitated), Halt protease inhibitor cocktail, and methanol were purchased from Fisher Scientific (Pittsburgh, PA, USA). M-450 epoxide-coated Dynabeads, Trizol, 4% to 12% Bis-Tris NuPAGE Gels, MES running buffer, LDS sample loading buffer, sample reducing agent and nuclease-free distilled water were purchased from Invitrogen (Carlsbad, CA, USA). Collagenase/dispase was from Roche (Indianapolis, IN, USA), and PECAM1/CD31 antibody was purchased from Abcam (Cambridge, MA, USA). Western antibodies were Complex 5 (ATP synthase), Mitosciences/Abcam (Eugene, OR, USA), Vinculin, Abcam (Cambridge, MA, USA), GCLm, gift from Dr Ulrike Luderer, University of California, Irvine (described in Thompson et al17), PGC-1β and TFAM, Aviva Systems Biology (San Diego, CA, USA). Nuclease-free glycogen was purchased from Ambion (Life Technologies, Carlsbad, CA, USA), Maxima First Strand cDNA Synthesis and Maxima SYBRgreen/ROX qPCR kits were from Fermentas (Pittsburgh, PA, USA)/Fisher Scientific. Immobilon-FL PVDF membranes were purchased from Millipore (Cambridge, MA, USA).
Endothelial Isolation
For each cerebral endothelial preparation, the forebrains of four mice were pooled and gently homogenized in 10 mL physiologic buffer solution (PBS1; mmol/L): 138 NaCl, 4 KCl, 10 HEPES, 2.2 CaCl2, pH 7.4) using a loose fitting Dounce homogenizer to preserve blood vessel integrity.
Crude brain preparation
Samples (100 μL) of the initial brain homogenate were centrifuged at 4,000 × g for 5 minutes and resuspended in 1 mL Trizol for later analysis.
Crude vessel preparation
The initial brain homogenate was centrifuged at 800 × g for 5 minutes at 4°C; the supernatant was then discarded, and the pellet used for vessel isolation. The pellet was resuspended in 10 mL 15% dextran (mol wt range: 25,000 to 35,000) in PBS1 and centrifuged at 20,000 × g for 10 minutes at 4°C. The dextran, along with the tissue layer floating on top, was removed while the pellet, containing blood vessels, was resuspended in 10 mL PBS1. This crude vessel preparation was then washed three times by resuspension in PBS1 and centrifugation at 600 × g for 10 minutes at 4°C. The final pellet was resuspended in 1 mL PBS1. A sample (100 μL) of the crude vessel fraction was centrifuged at 4,000 × g for 5 minutes, then resuspended and homogenized in Trizol for later analysis.
Endothelial-enriched preparation
The crude vessel preparation was subjected to digestion in 3 mL 2% collagenase/dispase (w/v) in PBS1 for 30 minutes at 37°C. In all, 10 mL of cold Wash Buffer (PBS1 with 0.1% BSA and 2 mmol/L EDTA, pH 7.2) was then added to suppress collagenase/dispase activity. Digested vessels were filtered sequentially through 70 μm (BD Falcon, Cell Strainer, BD Biosciences, San Jose, CA, USA) and 20 μm filters (Partec, Celltrics, Swedesboro, NJ, USA) on ice to obtain a cell suspension. This cell suspension was centrifuged at 600 × g for 10 minutes at 4°C, the supernatant removed, and the pellet resuspended in 10 mL Wash Buffer. This step was repeated two times. After the final centrifugation, the pellet was resuspended in 1 mL of Wash Buffer containing magnetic 2 × 107 Dynabeads (M-450 epoxide) coated with mouse anti-CD31/PECAM1 antibody. Beads had been prepared by incubating 2 × 107 beads in 50 μL incubation buffer (100 mmol/L Na2PO4 pH 7.4 containing 0.1% BSA), with 10 μg antibody at 4°C overnight according to manufacturer's specifications. The endothelial preparation and antibody-coated Dynabeads were mixed by gentle inversion at 4°C for 20 minutes, and then this mixture was placed in a magnet to separate out the beads. Beads were then washed with 1 mL Wash Buffer and reexposed to the magnet; this step was repeated three times. After the final wash, the beads with antibody-attached cells were resuspended in either 1 mL Trizol (for RNA measurement) or 300 μl Puregene Cell Lysis Solution (for DNA measurement), incubated for 10 minutes and placed into the magnet to remove the Dynabeads. For protein determinations, Dynabeads with attached cells from two preparations (four brains each) were combined and sonicated in 40 μL of protein lysis buffer (50 mmol/L β-glycerophosphate, 100 μmol/L Na3VO4, 2 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L dithiothreitol, 0.5% Triton X-100, 0.5% SDS, and 1 × Halt Protease Inhibitors). After 15 minutes incubation on ice, the Dynabeads were removed by magnet. The cell lysate was spun at 12,000 × g for 5 minutes to remove any insoluble matter; supernatants were stored at −20°C until analyzed by western blot.
Quantitative Real-time PCR
RNA was isolated using Trizol according to manufacturer's protocol with nuclease-free glycogen as a coprecipitant. Reverse transcription was performed using a Maxima First Strand cDNA Synthesis Kit, including random hexamer and oligodT primers. DNA was isolated using a Qiagen (Valencia, CA, USA) Puregene kit according to manufacturer's specifications with nuclease-free glycogen as a coprecipitant and RNAse A added to remove any contaminating RNA.
Primers were generated using the NCBI primer creation tool and were synthesized by Integrated DNA Technology (San Diego, CA, USA), primer sequences are supplied in Supplementary data. Primer efficiency was established using template dilutions over 5 log units. All primers used in experiments had efficiencies between 1.96 and 2.03, and primers for cDNA failed to amplify genomic DNA samples or non Reverse Transcribed controls. Appropriate dilutions were made of cDNA and genomic DNA to ensure even loading across samples. Samples were run using Maxima SYBRgreen/ROX kit in an ABI Prism 7000 (Invitrogen-Life Sciences, Carlsbad, CA, USA). Relative mRNA levels were compared using the delta delta Ct method18 with TATA binding protein as the reference gene. Each tissue preparation was assayed in triplicate and the results averaged to obtain the value for that preparation. Mitochondrial/Nuclear DNA ratios were established by establishing relative abundance of the mitochondrial DNA marker ND1 and the nuclear DNA marker 18S rRNA, using the delta delta Ct method.18
Western Blot Analysis
The protein concentration of endothelial cell lysates was assessed using the Bradford assay (protein was diluted to keep detergent concentration <0.1%). In all, 20 μg of protein in NuPage LDS Sample Buffer was loaded into 4% to 12% Bis-Tris NuPage gels, and these were run at 120 V for 60 minutes. Proteins were transferred using Transfer Buffer (25 mmol/L Tris base, 200 mmol/L glycine, 20% v/v methanol) onto Immobilon-FL PVDF membrane at 50 V for 90 minutes. Membranes were blocked with 5% skim milk in PBS for 1 hour. Primary antibodies were diluted with 5% milk in PBS with 0.1% Tween-20 (PBST). Blots were incubated in primary antibody overnight at 4°C with shaking. Blots were washed with PBST three times for 5 minutes and then incubated with fluorescent-labeled secondary antibodies diluted 1:10,000 in 5% milk in PBST for 1 hour at room temperature with shaking. Blots were washed again three times with PBST for 10 minutes and twice with PBS for 5 minutes. Blots were imaged using a LI-COR Biosciences Odyssey Infrared Fluorescent scanner (Lincoln, NE, USA) and quantified using vinculin as a loading control.
Statistical Analysis
Data are presented as mean±s.e. N indicates the number of tissue preparations (each obtained by pooling tissue from 4 to 8 animals) that were assayed. Statistical differences were assessed using Student's unpaired t-test with significance at P<0.05.
Results
Endothelial Cell Enrichment
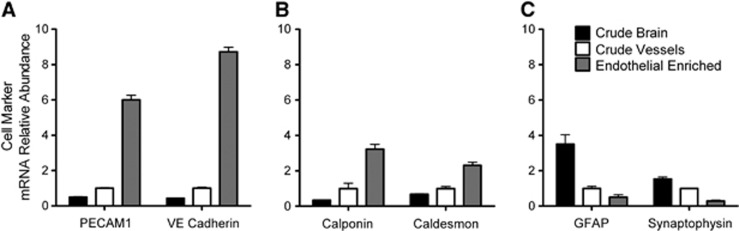
We first validated whether our isolation method produced a consistent endothelial cell enrichment. As shown in Figure 1, mRNA levels of endothelial cell markers (PECAM1 and VE cadherin) were compared with markers for vascular smooth muscle/pericytes (calponin and caldesmon) and brain cells (GFAP for glial cells and synaptophysin for neurons). To establish increasing endothelial cell content during the isolation process, we show data from three phases of the isolation as defined in Materials and Methods: crude brain, crude vessels, and endothelial-enriched preparations. The relative abundance of mRNA for endothelial markers was clearly increased by the isolation process. Endothelial fractions consistently showed levels of PECAM1 and VE cadherin that were 6- and 9-fold higher, respectively, than what was found in the crude vessel preparations and 12- and 20-fold higher, respectively, than the crude brain preparations (Figure 1A). In contrast, mRNA levels of markers for glia and neurons markedly declined during endothelial cell enrichment (Figure 1C). Markers for vascular smooth muscle/pericytes also increased from the crude brain to crude vessels to the endothelial-enriched preparations (Figure 1B). The mRNA levels of calponin and caldesmon in the endothelial-enriched preparation showed a more modest change as compared with endothelial markers, i.e., 2- and 3-fold increase, respectively, over crude vessels. Together, the data indicate that our protocol preferentially enriches endothelial cells. While the continued presence of nonendothelial cell markers indicates that the enzyme digest of the blood vessels was not complete, there was a tradeoff between the completeness of the digest and RNA integrity. Samples that underwent 45 minutes of enzyme digest at 37°C showed tailing of the rRNA bands (visualized by ethidium bromide staining in nondenaturing agarose gel electrophoresis). In contrast, samples digested for only 30 minutes did not, indicating little or no RNA degradation with the 30-minute digest. All endothelial preparations were obtained using the latter conditions.
Figure 1.
Effect of purification on cell marker mRNA levels plotted as relative abundance normalized to the crude vessel preparation. Crude brain, crude vessel, and endothelial-enriched preparations are compared. The mRNA levels of (A) endothelial, (B) vascular smooth muscle, and (C) glial and neuronal cell markers are shown. TATA binding protein (TBP) was used as an internal control. Values are means±s.e., N=4 to 6.
Enrichment of Endothelial Nitric Oxide Synthase mRNA and Effect of Ovariectomy
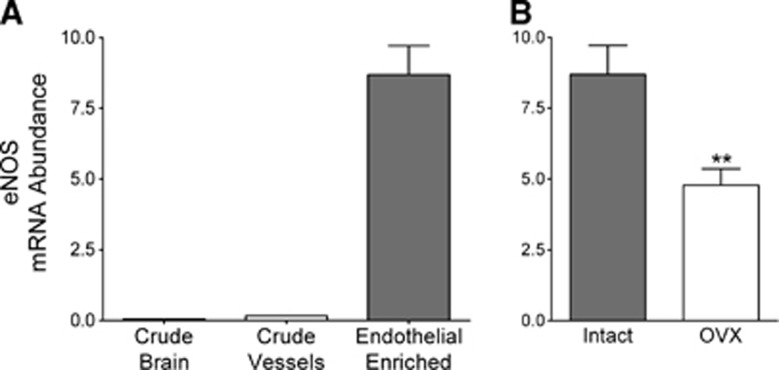
Endothelial nitric oxide synthase (eNOS) in the brain is present only in endothelial cells,19 and we have previously shown that gonadal steroids, specifically estrogen, increase eNOS mRNA and protein in cerebral blood vessels.20, 21 To further establish the appropriateness of our endothelial cell preparation, eNOS mRNA levels were measured in samples taken throughout the isolation process. As shown in Figure 2A, eNOS mRNA measurements confirm significant and consistent enrichment in our endothelial cell preparations.
Figure 2.
Effects of (A) endothelial enrichment and (B) ovariectomy on endothelial nitric oxide synthase (eNOS) mRNA levels. Levels of eNOS mRNA in (A) different purification preparations and (B) endothelial-enriched preparations from intact and ovariectomized (OVX) animals are shown. mRNA abundance is expressed as a ratio to the internal control gene, TATA binding protein (TBP). Values are means±s.e., N=7 to 9. **P<0.01 compared with intact.
We then investigated whether endogenous ovarian hormones would alter levels of eNOS mRNA by comparing endothelial cell preparations from intact and OVX mice. The significantly lower level of eNOS mRNA in the cerebral endothelial preparations from OVX compared with intact female mice (Figure 2B) further supports the efficacy of the endothelial cell isolation procedure in that the endothelial preparation accurately reflects hormonal changes occurring in the animal.
Estrogen Receptors α and β
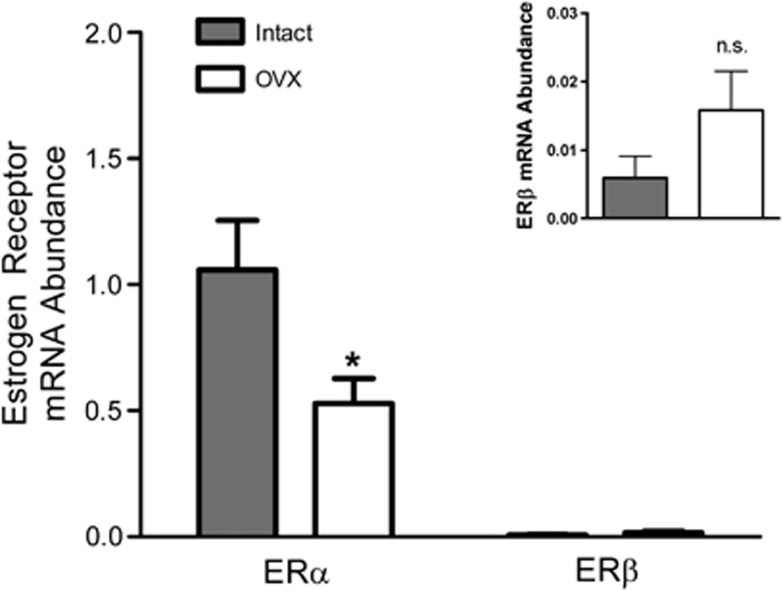
The brain endothelial preparations also expressed mRNA for both estrogen receptor subtypes, ERα and ERβ (Figure 3). Interestingly, mRNA levels for ERα were much greater than those for ERβ. We previously showed that estrogen increases levels of ERα in cerebral blood vessels,22 so we hypothesized that there would be a difference between intact and OVX animals. The mRNA levels of ERα in the endothelial-enriched preparation decreased significantly with ovariectomy (53%). There was no significant effect on ERβ (Figure 3, insert).
Figure 3.
Effects of ovariectomy on estrogen receptor (ER) mRNA levels. The mRNA levels of ERα and ERβ in endothelial-enriched preparations from intact and ovariectomized (OVX) animals are shown. The mRNA abundance is expressed as a ratio to internal control gene, TATA binding protein (TBP). N=6 to 8. *P<0.05 compared with intact. Inset magnifies ERβ mRNA abundance data.
Mitochondria-Regulating Transcription Factors: mRNA Levels
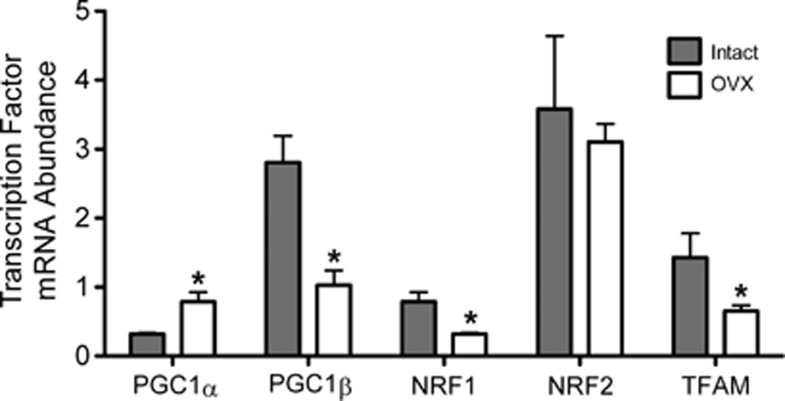
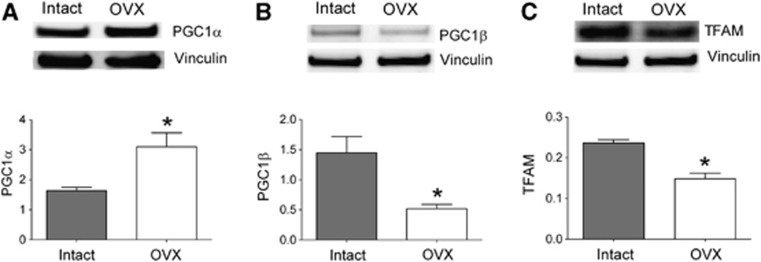
Our laboratory has previously shown that long-term estrogen treatment increases protein levels of the mitochondrial-regulating transcription factor, NRF1, in cerebral blood vessels of OVX animals.12 Estrogen also increases mitochondrial OXPHOS protein levels and activities in isolated cerebral blood vessel preparations.12 Using the enriched endothelial preparation, we mapped a transcription pathway beginning with the mitochondrial-regulating transcriptional coactivators, PGC-1α and PGC-1β, leading to NRF1 and then to TFAM. The mRNA levels of these factors were compared between intact and OVX animals to assess the effects of endogenous hormones. Surprisingly, we found significant, but opposite, effects of ovariectomy on the two PGC-1 transcripts. As shown in Figure 4, PGC-1α levels were higher by 148% while PGC-1β levels were lower by 63% in preparations from OVX animals compared with intact. Each member of the PGC-1 family has been shown to be individually capable of increasing NRF1 and TFAM, as well as promoting mitochondrial biogenesis, in many cell types and tissues.9, 23 However, the overall outcome on the mitochondrial-regulating transcription factor cascade when PGC-1α and PGC-1β change in opposite directions has, until now, been unknown. Interestingly, we found that both NRF1 and TFAM mRNAs decreased with ovariectomy; effects that correlated with the decrease in PGC-1β. Levels of NRF2 mRNA were unaffected by ovariectomy.
Figure 4.
Effects of ovariectomy on mRNA levels of mitochondrial-regulating transcription factors. Transcription factor mRNA levels in endothelial-enriched preparations from intact and ovariectomized (OVX) animals are shown. The mRNA abundance is expressed as a ratio to the internal control gene, TATA binding protein (TBP). Values are means±s.e., N=7 to 9. *P<0.05 compared with intact. PGC1, peroxisome proliferator-activated receptor γ coactivator 1; NRF1, nuclear respiratory factor 1; TFAM, mitochondrial transcription factor A.
Mitochondria-Regulating Transcription Factors: Protein Levels
To confirm that ovariectomy also changed protein levels of transcription factors responsible for initiating and guiding mitochondrial biogenesis, we analyzed key proteins in this pathway by western blot. Protein levels of PGC-1α, PGC-1β, and TFAM were measured in endothelial cell preparations isolated from intact and OVX animals. As shown in Figure 5, the levels of PGC-1α increased by 189%, which parallels the increase in PGC-1α mRNA observed in preparations from OVX animals. In contrast, ovariectomy decreased protein levels of both PGC-1β and TFAM, by 64% and 37%, respectively. The protein analysis confirms that the PGC-1 isoforms are affected differently by ovariectomy as seen with mRNA changes. The protein data provide further confirmation that the loss of ovarian hormones affects the primary transcriptional pathway governing mitochondrial biogenesis in cerebral endothelial cells. The TFAM is often used as a protein marker for changes in mitochondrial abundance.24
Figure 5.
Effects of ovariectomy on protein levels of mitochondrial-regulating transcription factors. Representative western blots and quantifications are shown for (A) PGC1α (90 kDa), (B) PGC1β (112 kDa), and (C) TFAM (29 kDa). Protein quantification for each band of interest has been corrected for gel loading by normalizing to vinculin (130 kDa) measured within the same lane. Values are means±s.e., N=2 to 5, *P<0.05 compared with intact. PGC1, peroxisome proliferator-activated receptor γ coactivator 1; TFAM, mitochondrial transcription factor A.
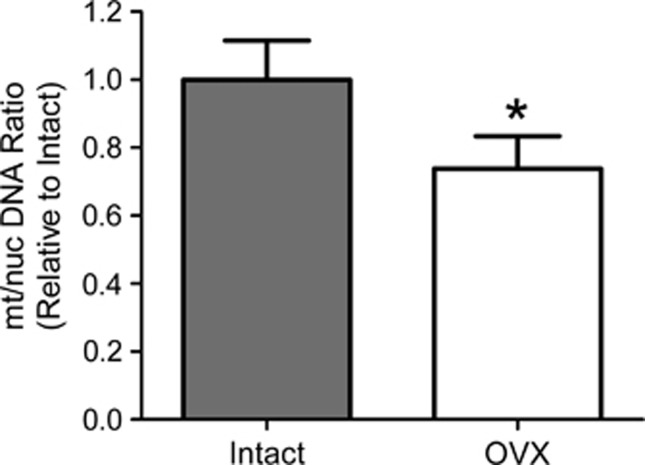
Mitochondrial/Nuclear DNA Ratios
Mitochondria vary greatly in size and shape, constantly combining and separating, being destroyed and created, to ensure that the cell efficiently meets its energy needs without producing excess ROS. The variability in size, shape, and exact composition makes counting mitochondria difficult and often misleading. At present, one of the most reliable methods for determining changes in mitochondrial content is measurement of the ratio between mitochondrial and nuclear DNA.24 To assess this, representative markers from the two genomes were determined in qPCR experiments, 18S rRNA for nuclear DNA and ND1 for mitochondrial DNA. Then, the ratio of mitochondrial genomes per nuclear genome was calculated. As shown in Figure 6, using this approach we found that mitochondrial DNA content was significantly lower (29%) in endothelial-enriched preparations from OVX animals compared with intact.
Figure 6.
Effects of ovariectomy on mitochondrial/nuclear DNA ratios. Ratios of the mitochondrial genome marker (gene ND1) to nuclear genome marker (gene 18S Ribosomal RNA) were calculated and then normalized to intact. Values are mean±s.e., N=4 to 7. *P<0.05 compared with intact. OVX, ovariectomized.
ATP Synthase
Although PGC-1β has not been as well studied as PGC-1α, it was shown in cultured myotubes that increasing expression of PGC-1β, but not PGC-1α, elevated levels of both ATP synthase subunits (Complex 5) and antioxidant enzymes.25 This expression profile would increase mitochondrial efficiency while suppressing mitochondrial ROS. Because of the showed increases in ATP synthase associated with increased PGC-1β, we measured mRNA and protein levels of ATP synthase subunit 1α. Endothelial levels of mRNA were decreased 23% after ovariectomy (Figure 7A). As shown in Figure 7B, protein levels of ATP synthase subunit 1α were also decreased (39%) in preparations from OVX animals when compared with intact. These data confirm that the loss of ovarian hormones results in a lower level of OXPHOS subunits and provide further evidence for a decrease in mitochondrial biogenesis, as ATP synthase subunit levels are used as protein markers for changes in mitochondrial abundance.24
Figure 7.
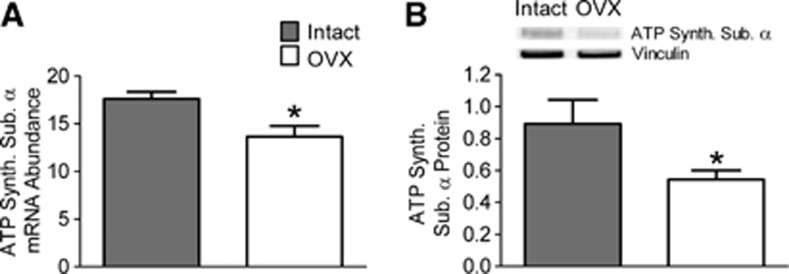
Effects of ovariectomy on (A) mRNA and (B) protein levels of ATP synthase (ATP synthase subunit α, Complex 5). Values from endothelial preparations from intact and ovariectomized (OVX) animals are compared. The mRNA abundance is expressed as a ratio to the internal control gene, TATA binding protein (TBP). Protein quantification for ATP synthase, subunit α (55 kDa) has been corrected for gel loading by normalizing to vinculin. Values are means±s.e., N=3 to 4. *P<0.05 compared with intact.
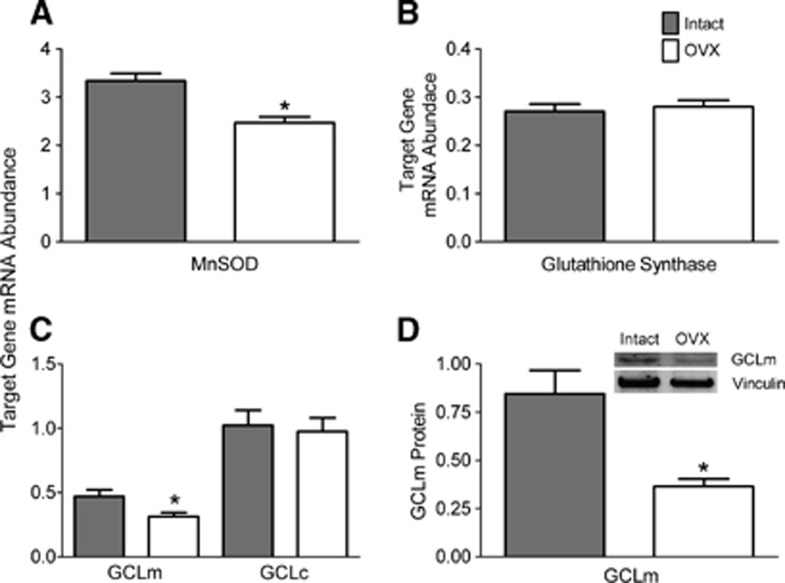
Antioxidant Enzymes
Management of ROS is crucial for cell survival. This process involves many different constituents, but the two most critical for detoxifying ROS in the mitochondria are manganese superoxide dismutase (MnSOD) and the glutathione system.26, 27 Therefore, we assessed levels of mRNA transcripts relevant to these two components to determine the effect of ovariectomy.
The MnSOD is located exclusively in the mitochondria and converts superoxide produced from electron transport chain activity and other sources to less reactive hydrogen peroxide that is then further detoxified.27 As shown in Figure 8A, ovariectomy significantly decreased levels of endothelial MnSOD mRNA by 26%.
Figure 8.
Effects of ovariectomy on reactive oxygen species (ROS) detoxification enzymes. Levels of mRNA are shown for (A) manganese superoxide dismutase (MnSOD), (B) glutathione synthase, and (C) glutamate-cysteine ligase modulatory (GCLm) and catalytic (GCLc) subunits. Protein levels of GCLm (31 kDa) are shown in (D). Values from endothelial preparations from intact and ovariectomized (OVX) animals are compared. The mRNA abundance is expressed as a ratio to the internal control gene, TATA binding protein (TBP). Protein quantifications are corrected for loading using vinculin. Values are means±s.e., N=4 to 5. *P<0.05 compared with intact.
Glutathione, one of the most critical cellular antioxidants, is synthesized in two steps.28 The first of these, glutamate-cysteine ligase (GCL), is the rate-limiting step. GCL is a two-part enzyme, consisting of an essential catalytic subunit and a modulatory subunit that increases activity of the catalytic subunit. The second phase in glutathione synthesis is the enzyme glutathione synthase, which creates the final tripeptide, glutathione. Therefore, we measured the effect of ovariectomy on transcript levels of these three components of the glutathione synthesis pathway. As shown in Figures 8B and 8C, the mRNA abundances of glutathione synthase and the GCL catalytic domain were unaffected by ovariectomy. However, mRNA levels of the GCL modulatory subunit were significantly lower (33%) in endothelial preparations from OVX animals (Figure 8C). Moreover, protein levels of the GCL modulatory subunit also were decreased 57% after ovariectomy (Figure 8D).
Discussion
Our study shows that removal of ovarian hormones affects transcription factors that regulate mitochondrial function in brain endothelium in vivo. A major strength is the use of endothelial preparations isolated from intact and OVX female mice to discern the effects of ovarian hormones under physiologic conditions. The two major findings of this study are (1) loss of ovarian hormones results in a reduction in mitochondrial biogenesis in cerebral endothelial cells and (2) this occurs because of decreases in a unique transcriptional pathway that also induces antioxidant enzymes that suppress mitochondrial ROS. Surprisingly, ovariectomy increased PGC-1α mRNA and protein levels but decreased PGC-1β mRNA and protein. Opposite changes in members of the PGC-1 master mitochondrial regulator family comprise a novel and intriguing finding. It appears PGC-1β is the primary mediator of mitochondrial changes related to ovarian hormones in cerebral endothelium. Ovariectomy decreased the entire transcriptional pathway beginning with PGC-1β, then NRF1 and TFAM, with a final result of decreased capacity for mitochondrial ATP synthesis and ROS inactivation. The results of ovariectomy suggest that ovarian hormones normally support mitochondrial function in cerebral endothelial cells via a unique effect on PGC-1β. Our current findings reinforce the hypothesis that ovarian hormones protect against a variety of age-related diseases by increasing mitochondrial efficiency while decreasing mitochondrial oxidative stress.12
To study the hormonal influence of the ovaries on cerebral endothelial cells in vivo, we adapted a protocol for freshly isolating an enriched endothelial preparation from mouse brains. The importance of examining endothelial cells directly from the animal, without growing them in culture, is highlighted by studies that indicate brain endothelial cells change rapidly when they are removed from the neurovascular unit.14 We show that our isolated preparations consistently exhibit elevated levels of endothelial cell markers eNOS, PECAM1, and VE-cadherin, consistent with a high enrichment of endothelial cells. However, it should be noted that we cannot rule out a small contribution from nonendothelial cells, in particular smooth muscle cells and pericytes. We also wanted to verify that the isolation procedure was sufficiently reproducible to show differences between the OVX and intact animal groups. As expected, brain endothelial preparations from OVX animals had significantly lower levels of mRNA for eNOS and ERα, two proteins known to be regulated by estrogen in endothelium and cerebral vessels.13, 21, 22, 29, 30 We also validated that there was no effect of ovariectomy on ERβ. However, it was surprising to find relatively low levels of ERβ mRNA compared with ERα mRNA in freshly isolated brain endothelial cells. This comparison has not been done before, and it differs from our finding in cultured brain endothelial cells that ERβ mRNA levels are higher and more similar to those of ERα (Kemper MF, unpublished observations).
Mitochondrial biogenesis is a tightly regulated and orchestrated event, and coordinated synthesis of nuclear and mitochondrial encoded proteins is essential to maintain proper mitochondrial function.3 The creation of new mitochondria requires synthesis of new membranes and mitochondrial targeted proteins as well as replication of mitochondrial DNA. Quantification of any of these processes can be used to quantify mitochondrial biogenesis. However, the abundance of mitochondrial DNA, as determined by the ratio between mitochondrial and nuclear DNA, is one of the more trusted measures, as it is largely independent of mitochondrial size.24 We show that the loss of ovarian hormones by ovariectomy causes a decrease in the mitochondrial/nuclear DNA ratio, indicating a lower level of mitochondrial DNA per cell and thus less mitochondria. An effect on mitochondrial biogenesis is further confirmed by our finding of decreases in protein levels of TFAM, a key mitochondrial targeted transcription factor, and ATP synthase subunit 1α. These markers are both well-established alternative methods for determining mitochondrial abundance.24 Together, these data suggest that ovarian hormones normally act to maintain mitochondrial abundance in brain endothelium.
The transcriptional machinery that guides the creation of new mitochondria has been extensively studied. While many different cellular inputs and signals can initiate biogenesis, the transcription factors that are directly responsible for regulating electron transport proteins and mitochondrial DNA transcription and replication form a relatively small group. It is well established that activation of PGC-1α or PGC-1β leads to increases in NRF1,23 which in turn initiates the transcription of a wide variety of nuclear-encoded, mitochondrial targeted proteins, such as cytochrome c, electron transport chain complex subunits, and antioxidant proteins.31, 32 Additionally, NRF1 stimulates transcription of TFAM that then translocates to the mitochondria and initiates transcription and replication of the mitochondrial genome.8, 9, 33 We show that this pathway in brain endothelial cells, starting with PGC-1β, followed by NRF1 and TFAM, is suppressed after the loss of ovarian hormones.
The increase in levels of PGC-1α in OVX animals, however, was an unexpected result considering the decreases observed in mitochondrial biogenesis. This PGC-1 isoform is well known to activate mitochondrial transcription factors and biogenesis in other tissues.23 The consequences of differential regulation of the PGC-1 isoforms are relatively unknown. What could be the benefit for the endothelial cell of increasing PGC-1β and decreasing PGC-1α? Mitochondrial biogenesis in vascular endothelium is not well studied. However, just as cellular metabolism is not the same across different cell types, mitochondrial function within various cells is not identical.32, 34 Each cell type must tailor its mitochondrial function to best meet particular metabolic needs. One of the most obvious mitochondrial characteristics that vary between cell types is the coupling of electron flow to ATP synthesis. Certain cells in the body, such as brown adipose tissue and skeletal muscle, have less coupled electron transport.35 This functions as an adaptation to allow heat generation on demand and also to protect the cells against large changes in energy demand that can cause large increases in ROS, potentially leading to mitochondrial and cellular damage.36 Cells such as the cerebral endothelia we examined would be expected to have a relatively large but constant energy demand because of active transport across the blood–brain barrier and other functions. Thus, a more coupled metabolism would be advantageous to minimize waste and nutrient consumption.
The two different PGC-1 isoforms, PGC-1α and PGC-1β, may guide the mitochondrial biogenesis process to create either coupled or uncoupled mitochondria. One particularly enlightening study by St-Pierre et al.25 showed in cultured myotubes that increasing levels of either isoform with viral vectors stimulated mitochondrial biogenesis via the transcriptional pathway described above, but the metabolic characteristics of the mitochondria created were strikingly different depending on the particular isoform present. While either coactivator could increase total cellular oxygen consumption, PGC-1α alone increased the portion of total oxygen consumption not coupled to ATP production, thus indicating divergent roles for PGC-1α and β in producing uncoupled or coupled mitochondria. If similar mechanisms operate in brain endothelial cells, then the dominant role of PGC-1β in intact females would favor more coupled mitochondria.
However, as mentioned above, highly coupled electron transport can be problematic because of increases in ROS production when the electrochemical gradient becomes too large without an outlet through uncoupling proteins.36 The potential for ROS damage with highly coupled electron transport can be offset by increases in antioxidant mechanisms, such as through MnSOD activity or glutathione synthesis. Interestingly, myotube overexpression of PGC-1β, as compared with PGC-1α, preferentially induced genes involved in these two ROS detoxifying pathways.25 Similarly in the brain endothelial preparations, we found that ovariectomy decreased levels of MnSOD and the modulatory subunit for GCL in parallel with the decrease in PGC-1β. The data suggest that ovarian hormones may normally suppress mitochondrial ROS in brain endothelium by maintaining PGC-1β expression and induction of antioxidant-related proteins.
In comparison with OVX animals, the mitochondrial measures in brain endothelial preparations from intact animals correlate well with what we found previously in cerebral blood vessels from estrogen-treated OVX rodents.12 Long-term estrogen exposure increased protein levels of NRF1, mitochondrial complex subunits, and MnSOD in cerebral vessels.12 We also have shown that estrogen decreases mitochondrial ROS in cultured cerebral endothelial cells by acting through ERα.11 Estrogen also increases levels of NRF1, TFAM, and mitochondrial DNA in MCF7 cultured breast cancer cells, but the role of the PGC-1 isoforms in this process is unknown.37 Macari et al.38 showed that estrogen decreases PGC-1α in the uterus, but effects on PGC-1β or its downstream targets remain unexplored. While our current data are consistent with effects of estrogen acting on ER expressed in brain endothelium, it is possible that removal of progesterone contributes to the effects of ovariectomy. A study of whole brain mitochondria found that both estrogen and progesterone can increase mitochondrial DNA content, decrease electron leak, and increase levels of complex 5 subunit α.39 Further investigation into ovarian hormone protection of endothelial mitochondrial function is needed to determine which ovarian hormone(s) is responsible and which receptors are involved.
The current study highlights the involvement of ovarian hormones in creating a protective state for mitochondria in brain endothelial cells. Together, the data support the concept that in intact females, ovarian hormones normally act through PGC-1β to support both metabolic capacity (mitochondrial biogenesis) and efficiency (improved ROS management and ATP coupling). These actions would ultimately decrease accumulation of mitochondrial ROS damage and dysfunction over the long term, likely contributing to endothelial protection in the female brain.
Acknowledgments
The authors acknowledge the support of the National Heart Lung and Blood Institute grant RO1HL-50775 and the technical assistance of Mengying Guo, Hakop Aladzhadzhyan, Dr Gregory Parks and Dr Zhiwei Wang.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Edvinsson L, Krause DN.Cerebral blood flow and metabolism2nd edn.Lippincott Williams & Willkins: Philadelphia, PA; 2002 [Google Scholar]
- Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep. 2011;13:200–207. doi: 10.1007/s11906-011-0195-x. [DOI] [PubMed] [Google Scholar]
- Yu E, Mercer J, Bennett M. Mitochondria in vascular disease. Cardiovasc Res. 2012;95:173–182. doi: 10.1093/cvr/cvs111. [DOI] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional Paradigms in Mamalian Mitochondrial Biogenesis and Function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Guo J, Krause DN, Horne J, Weiss JH, Li X, Duckles SP. Estrogen-receptor-mediated protection of cerebral endothelial cell viability and mitochondrial function after ischemic insult in vitro. J Cereb Blood Flow Metab. 2010;30:545–554. doi: 10.1038/jcbfm.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, et al. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharmacol Exp Ther. 2008;325:782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN, Stirone C, Procaccio V. Estrogen and mitochondria: a new paradigm for vascular protection. Mol Interv. 2006;6:26–35. doi: 10.1124/mi.6.1.6. [DOI] [PubMed] [Google Scholar]
- Lyck R, Ruderisch N, Moll AG, Steiner O, Cohen CD, Engelhardt B, et al. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J Cereb Blood Flow Metab. 2009;29:1491–1502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Surgical menopause and cardiovascular risks. Menopause. 2007;14 (3 Pt 2:562–566. doi: 10.1097/gme.0b013e318038d333. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD. Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19:272–277. doi: 10.1097/gme.0b013e31822a9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, White CC, Krejsa CM, Diaz D, Woods JS, Eaton DL, et al. Induction of glutamate-cysteine ligase (gamma-glutamylcysteine synthetase) in the brains of adult female mice subchronically exposed to methylmercury. Toxicol Lett. 1999;110:1–9. doi: 10.1016/s0378-4274(99)00133-2. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, et al. Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci. 1999;19:RC30. doi: 10.1523/JNEUROSCI.19-19-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol. 2005;67:105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- Stirone C, Chu Y, Sunday L, Duckles SP, Krause DN. 17 Beta-estradiol increases endothelial nitric oxide synthase mRNA copy number in cerebral blood vessels: quantification by real-time polymerase chain reaction. Eur J Pharmacol. 2003;478:35–38. doi: 10.1016/j.ejphar.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:E184–E192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros DM. Assessing mitochondria biogenesis. Methods. 2008;46:288–294. doi: 10.1016/j.ymeth.2008.09.026. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, et al. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Davidge ST. High glucose-induced oxidative stress alters estrogen effects on ERalpha and ERbeta in human endothelial cells: reversal by AMPK activator. J Steroid Biochem Mol Biol. 2009;117:99–106. doi: 10.1016/j.jsbmb.2009.07.007. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33:1685–1691. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Scarpulla RC. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter. Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J Biol Chem. 1989;264:14361–14368. [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann NY Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Mitochondrial metabolic function assessed in vivo and in vitro. Curr Opin Clin Nutr Metab Care. 2010;13:511–517. doi: 10.1097/MCO.0b013e32833cc93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari C, Teyssier C, Tribollet V, Mouzat K, Forcet C, Horard B, et al. Estrogens repress PGC1-alpha expression in the uterus. Mol Cell Endocrinol. 2010;330:33–40. doi: 10.1016/j.mce.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149:3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.