Abstract
Rosiglitazone, a synthetic peroxisome proliferator-activated receptor-γ (PPARγ) agonist, prevents cell death after cerebral ischemia in animal models, but the underlying mechanism has not been clarified. In this study, we examined how rosiglitazone protects neurons against ischemia. Mice treated with rosiglitazone were subjected to 60 minutes of focal ischemia followed by reperfusion. Rosiglitazone reduced infarct volume after ischemia and reperfusion. We show that this neuroprotective effect was reversed with a PPARγ antagonist. Western blot analysis showed a significant increase in expression of phosphorylated stress-activated protein kinases (c-Jun N-terminal kinase (JNK) and p38) in ischemic brain tissue. Rosiglitazone blocked this increase. Furthermore, we observed that rosiglitazone increased expression of the dual-specificity phosphatase 8 (DUSP8) protein and messenger RNA in ischemic brain tissue. Dual-specificity phosphatase 8 is a mitogen-activated protein kinase phosphatase that can dephosphorylate JNK and p38. Another key finding of the present study was that knockdown of DUSP8 in primary cultured cortical neurons that were subjected to oxygen–glucose deprivation diminished rosiglitazone's effect on downregulation of JNK phosphorylation. Thus, rosiglitazone's neuroprotective effect after ischemia is mediated by blocking JNK phosphorylation induced by ischemia via DUSP8 upregulation.
Keywords: cerebral ischemia, c-Jun N-terminal kinase, dual-specificity phosphatase 8, p38 mitogen-activated protein kinase, rosiglitazone
Introduction
The peroxisome proliferator-activated receptor-γ (PPARγ) is a member of the PPAR family, which belongs to the nuclear receptor superfamily. Peroxisome proliferator-activated receptor-γ has a significant role in glucose and lipid homeostasis. Two synthetic PPARγ ligands, rosiglitazone and pioglitazone, which are thiazolidinediones (TZDs), are approved by the Food and Drug Administration as treatments for type 2 diabetes. Recent studies have shown that both rosiglitazone and pioglitazone are neuroprotective in animal models of acute ischemic injury;1, 2, 3 however, the mechanisms underlying these effects at the cellular level are not fully understood.
Cerebral ischemia induces neuronal damage whereby various mechanisms of cell death and survival are evoked. Several studies have shown that inhibition of the cell death mechanism or activation of the cell survival mechanism can be protective after stroke. Among these mechanisms, stress-activated protein kinases (SAPKs), including c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), are activated by cerebral ischemia.4 Inhibition of either activated JNK or p38 MAPK, by drugs or by gene therapy, can protect against cell death after ischemia.5, 6, 7
Stress-activated protein kinase signaling pathways contain various checkpoints for regulation. Many studies have demonstrated that phosphorylation of upstream kinases is significant in regulating the signaling cascade. Dephosphorylation of MAPKs also has a key role in determining the magnitude and duration of kinase activation. Mitogen-activated protein kinase phosphatases, also known as dual-specificity phosphatases (DUSPs), can inactivate MAPKs via dephosphorylation.8 Dual-specificity phosphatase 8 is abundantly expressed in the brain, heart, and lungs.9 DUSP8 is reported to act specifically via JNK and p38 MAPK, unlike extracellular signal-regulated kinase.8
Recent reports show that TZDs modulate JNK expression and activity in cardiac ischemia reperfusion injury10 and in insulin-resistant brains, which results in reduction of tau phosphorylation.11 Hence, we examined JNK and DUSP8 as possible mechanisms for the neuroprotective effect observed after treatment with rosiglitazone after cerebral ischemia. In this study, we investigated whether JNK signaling pathways are modulated by rosiglitazone to inhibit cell death in mice after transient focal cerebral ischemia. We also investigated whether DUSP8 expression is involved in the neuroprotection mechanism conferred by rosiglitazone. We found that the neuroprotective effect of rosiglitazone is involved in the prevention of JNK signaling activation. Furthermore, we provide evidence that inhibition of JNK activation was due to DUSP8 activation induced by rosiglitazone.
Materials and methods
Animals
All animals were treated in accordance with Stanford University guidelines, and the animal protocols were approved by Stanford University's Administrative Panel on Laboratory Animal Care. Male C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) weighing 30 to 35 g were used in this study.
Middle Cerebral Artery Occlusion Mouse Model
The mice were subject to 60 minutes of transient focal cerebral ischemia as described.12 Rectal temperature was controlled with a homeothermic blanket and kept at 37°C. A coated 5-0 surgical monofilament nylon suture was introduced into the left internal carotid artery through the external carotid artery stump. After 60 minutes of occlusion, cerebral blood flow was resumed by the careful removal of the suture. Physiological parameters were monitored throughout the surgeries. Sham controls underwent the same procedure without insertion of the suture or occlusion of the vessels. In the sham-operated mice, the filament was not advanced to occlude the middle cerebral artery (MCA). Blood samples were collected before MCA occlusion and right after reperfusion for measurement of pH, pO2, pCO2, and blood glucose level.
Drug Treatment
The PPARγ ligand rosiglitazone and the PPARγ antagonist GW9662 (Cayman Chemical Company, Ann Arbor, MI, USA) were dissolved in dimethyl sulfoxide (DMSO). As a preliminary study to determine the optimal dose, either the vehicle (25% DMSO in physiological saline) or rosiglitazone (1, 3, and 6 mg/kg) was injected twice intraperitoneally 1 hour before and 1 hour after the induction of ischemia. Administration of 3 mg/kg of rosiglitazone significantly reduced infarct size, so this dose was chosen for subsequent studies. In the PPARγ inhibition study, 4 mg/kg of GW9662 were injected intraperitoneally 1.5 hours before the induction of ischemia.
Measurement of Infarct Volume
For the studies to determine the dose to use and to confirm the inhibitory effect of the PPARγ antagonist, infarct volume was calculated by 2,3,5-triphenyltetrazolium hydrochloride (TTC) staining. Brains were quickly removed 24 hours after ischemia, sectioned coronally at 1-mm intervals, and stained by immersion in 2% TTC for 20 minutes at 37°C.
In subsequent studies, the percentage of infarct area was measured by cresyl violet staining as described earlier.13 Fifty-micrometer cryosections were processed for staining with a solution of 0.1% cresyl violet for 45 minutes, then rinsed in water, and mounted. The area of the infarct was determined in each slice using the ImageJ 1.43 program (NIH, Bethesda, MD, USA). The infarct volume was calculated by summing infarction areas of all sections and multiplying by slice thickness.
Terminal Deoxynucleotidyl Transferase-Mediated Uridine 5′-Triphosphate-Biotin Nick End Labeling Staining
Twenty-micrometer cryosections were processed for terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) staining. DNA fragmentation was detected in situ by TUNEL staining using a fluorescent method (In Situ Cell Death Detection Kit TMR red; Roche Applied Sciences, Indianapolis, IN, USA) and the manufacturer's protocol. Red TUNEL-positive cells with apoptotic nuclear features were counted manually. The percentage of TUNEL-positive cells was calculated by dividing their number by the number of 4′,6 diamidino-2-phenylindole-positive cells.
Protein Extraction
After subjecting the animals to ischemia and 3, 24, and 48 hours of reperfusion, the brains were removed under anesthesia with isoflurane (n=4 each group). For whole cell lysate, the cortex and basal ganglia tissue was obtained from the MCA territory on the ischemic side, and homogenized and sonicated in ice-cold 1 × lysis buffer (Cell Signaling Technology, Beverly, MA, USA) with 1% protease inhibitor mixture (Sigma-Aldrich, St Louis, MO, USA). The homogenate was centrifuged at 900 g for 10 minutes at 4°C and the resulting supernatant was used for quantification. The nuclear fraction was prepared from the whole ipsilateral hemisphere of each mouse after 3 and 24 hours of reperfusion using the protocol supplied with the Nuclear Extraction Kit (Cayman Chemical Company).
Western Blotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on a NuPAGE gel (Invitrogen, Carlsbad, CA, USA) and then immunoblotted. Anti-spectrin (1:5,000, #MAB1622; Millipore, Billerica, MA, USA), anti-phospho-JNK (1:1,000, #4668; Cell Signaling Technology), anti-JNK (1:1,000, #9258; Cell Signaling Technology), anti-phospho-p38 (1:1,000, #4511; Cell Signaling Technology), anti-p38 (1:1,000, #9212; Cell Signaling Technology), anti-DUSP8 (1:2,000, #AP8451a; Abgent, San Diego, CA, USA), or anti-β-actin (1:100,000, #A5441; Sigma-Aldrich) primary antibodies were used for western blotting using whole cell lysate. Anti-phospho-c-Jun (S73) (1:2,000, #3270; Cell Signaling Technology) or anti-histone H1 (1:500, #ab11079; Abcam, Cambridge, MA, USA) primary antibodies were used for western blotting using the nuclear fraction. After incubation with an appropriate horseradish peroxidase-conjugated secondary antibody, the bound antibodies were detected by a chemiluminescence system (ECL Plus, Roche Applied Sciences). Images were scanned and the results were quantified using Multi-Analyst software (Bio-Rad Laboratories, Hercules, CA, USA).
JNK Activity Assay
A JNK activity assay was performed following the procedures described by the manufacturer (Cell Signaling Technology). Whole cell lysate protein was obtained from the whole ipsilateral hemisphere of each mouse injected with DMSO, rosiglitazone, or GW9662/rosiglitazone after 3 hours of reperfusion (n=4 each group). The level of c-Jun phosphorylated by JNK was determined using an anti-phospho-c-Jun (Ser63) antibody. The intensity of the phosphorylated c-Jun band represented the relative JNK activity precipitated by c-Jun fusion protein beads.
DUSP8 Activity Assay
DUSP8 activity assay was performed using a phosphatase activity assay kit from AnaSpec (Fremont, CA, USA). A DUSP8 antibody was used to immunoprecipitate ischemic and control samples. Fifty microliters of p-nitrophenyl phosphate, a colorimetric substrate for measuring phosphatase activity, was added to 50 μL of the ischemic and control samples. Upon dephosphorylation, p-nitrophenyl phosphate turns yellow and was detected at 405 nm absorbance.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction Analysis of DUSP8
Total RNA was isolated from the ipsilateral hemisphere after 3 hours of reperfusion according to the manufacturer's protocol (RNeasy Mini kit; Qiagen, Valencia, CA, USA). One microgram of isolated total RNA was reverse transcribed using SuperScript VILO cDNA Synthesis Kit (Invitrogen). Polymerase chain reaction (PCR) analysis was performed with a Stratagene Mx3000p QPCR System (Stratagene, La Jolla, CA, USA) using RT2 qPCR Primer Assays (SABiosciences, Frederick, MD, USA) according to the manufacturers' specifications. Primers used for the real-time qPCR Primer Assay were mouse DUSP8 (#PPM28642A), 18SrRNA (#PPM57735E), and SYBR Green qPCR Master Mixes (all from SABiosciences). Duplicate reactions were performed for each template. Polymerase chain reaction cycling conditions were as follows: initial denaturation at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, and 1 minute annealing at 60°C. Data analysis was performed using M × 3000 software (Stratagene).
Double Immunofluorescent Staining and Immunohistochemical Detection of DUSP8
To evaluate DUSP8 expression and detect a change in expression with rosiglitazone treatment, we performed double immunohistochemical staining. Anti-DUSP8 (1:50, #AP8451a; Abgent) primary antibody was applied to 20-μm cryosections. For immunohistochemical detection of DUSP8, the incubated sections were processed for avidin–biotin immunohistochemical staining using a Vector ABC kit (Vector Laboratories, Burlingame, CA, USA) and visualized with diaminobenzidine with the nuclei counterstained with hematoxylin. For double immunofluorescent staining, the incubated sections were processed by Alexa Fluor 546-conjugated goat anti-rabbit antibody (1:200, #A21207; Invitrogen). The sections were then incubated with a mouse anti-NeuN (neuronal marker) Alexa Fluor 488-conjugated monoclonal antibody (1:200, #MAB377X; Chemicon International, Temecula, CA, USA) for double staining and then covered with mounting medium with 4′,6 diamidino-2-phenylindole.
Primary Cortical Neuron Culture
The protocol we followed was adapted from a procedure described previously.12 Cortical neurons were prepared from brains of 16-day-old C57BL/6 mouse embryos, plated on poly-𝒟-lysine-coated dishes and cultured in minimum essential medium (Invitrogen) containing glucose, 5% horse serum, glutamine (2 mmol/L), penicillin (50 U/ml), and streptomycin (50 g/mL). The next day, the medium was changed to Neurobasal medium (Invitrogen) containing B-27. Neurons were cultured at 37°C in a humidified 5% CO2 atmosphere and used after 7 days in vitro.
Small Interfering RNA Transfection
To knockdown DUSP8, we used small interfering RNA (siRNA) probes targeted to mouse DUSP8 and nontargeting siRNA as a control (Qiagen). The target sequences for the mouse-specific DUSP8 siRNA mixture were as follows: 5′-CGGAATAAGCTATGTCCTCAA-3′ (SI04920125), 5′-AGCGTGGAGGTCATCGAAGTA-3′ (SI04920132), 5′-CAGGCCAGGTATAAATATATA-3′ (SI02668176), and 5′-AAGGTGATGCACGCAAAGAAA-3′ (SI02734137). The nontargeting siRNA (SI03650318) control was used in all siRNA transfection experiments. Primary cortical neurons were transfected with HiPerFect Transfection Reagent (Qiagen) according to the manufacturer's instructions. Primary cortical neurons grown on 24-well plates (1 × 105 cells/well) or 6-mm dishes (1 × 106 cells/plate) previously coated with poly-𝒟-lysine were treated with 10 nmol/L siRNA per well, and after 24 hours of incubation, they were analyzed for various experiments.
Oxygen–Glucose Deprivation and Reoxygenation
The primary neuronal cultures were subjected to oxygen–glucose deprivation (OGD) by replacing the medium with buffered salt solution without glucose and were placed in a gas-tight humidified anoxic chamber at 37°C for 4 hours. Under these conditions, PO2 in the chamber was zero after 10 minutes and PO2 in the medium was 15 to 20 mm Hg. After OGD, the cell medium was changed to a neuronal medium with glucose and reoxygenated for 3 to 24 hours.
Lactate Dehydrogenase Assay
Cell viability after 24 hours of reoxygenation was quantified by standard measurement of lactate dehydrogenase (LDH) release with the use of a LDH kit (BioVision Research, Mountain View, CA, USA). The percentage death (% of LDH release) was calculated as 100 × (control−treated)/control.
Cell Death Detection
Cell death after 24 hours of reoxygenation was measured by a quantitative sandwich enzyme immunoassay using mouse monoclonal antibodies directed against DNA and histones according to the manufacturer's instructions (cell death detection ELISA PLUS kit; Roche Applied Sciences). This assay is used to quantify DNA fragmentation of mono- and oligonucleosomes in the cytoplasm, which indicates apoptotic cell death.
Statistical Analysis
The data are expressed as mean±s.d. Statistical analysis was performed using SPSS 12.0.1 software (SPSS, Chicago, IL, USA). Comparisons among multiple groups were performed using one-way analysis of variance followed by post hoc Tukey's test. Comparisons between two groups were achieved using Student's t-test. Significance was accepted with P<0.05.
Results
Rosiglitazone Protects Brain Against Ischemia Reperfusion Injury in a Mouse MCA Occlusion Model
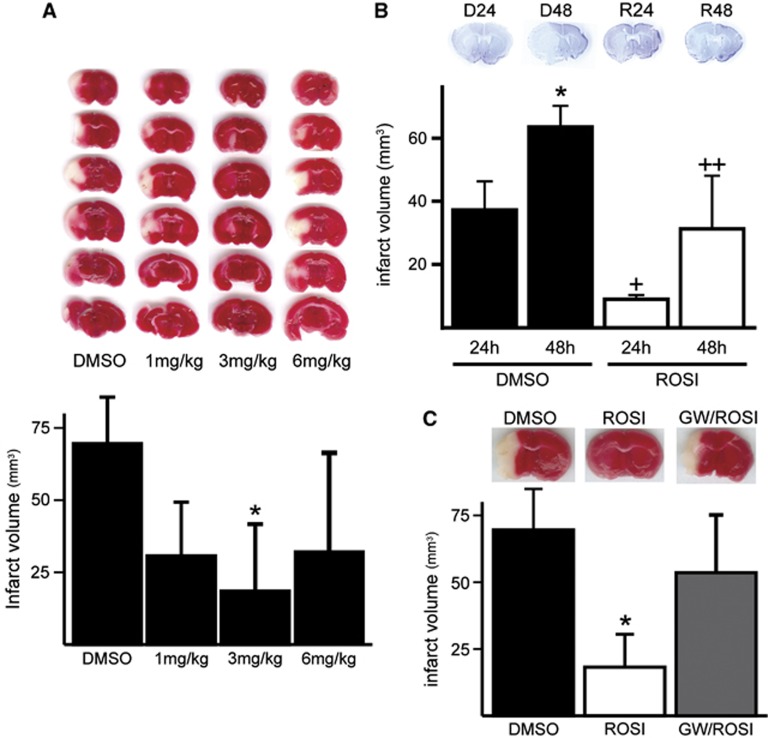
To evaluate the in vivo neuroprotective effect of rosiglitazone, it was injected twice intraperitoneally 1 hour before and 1 hour after ischemia, and the infarct volume of the ipsilateral brain was measured 24 hours later with TTC staining. There was no significant difference in physiological variables, including blood glucose level, between the vehicle control and the rosiglitazone-treated groups (data not shown). 2,3,5-Triphenyltetrazolium hydrochloride staining showed that a maximal reduction occurred at 3 mg/kg, whereas at a higher concentration (6 mg/kg), rosiglitazone's protective effect was reduced. Infarct volumes were 69.5±7.5, 30.6±8.4, 18.4±10.4, and 32.1±16.3 mm3, for the DMSO group and in the 1, 3, and 6 mg/kg groups, respectively (n=5 for each group, Figure 1A). We used 3 mg/kg of rosiglitazone in the remaining studies. Administration of rosiglitazone significantly reduced infarct volume after 24 and 48 hours of reperfusion. Infarct volumes were 39.6±5.3 and 67.3±2.7 for 24 and 48 hours of reperfusion in the controls, and were reduced to 10.4±0.6% and 37.3±8.5% for 24 and 48 hours of reperfusion with treatments of 3 mg/kg rosiglitazone, respectively. Thus, the infarct volumes were reduced by 73.8% and 44.6% after 24 and 48 hours of reperfusion, respectively (n=4 each group, P<0.05, Figure 1B). The protective effect of rosiglitazone was blocked by administration of GW9662, a PPARγ antagonist. Infarct volumes were 64.6±13.9, 17.1±5.8, and 50.2±10.1 mm3 for control, rosiglitazone, and GW9662/rosiglitazone treatment groups, respectively (n=4 each group, P<0.05, Figure 1C).
Figure 1.
Rosiglitazone treatment decreased infarct volume after middle cerebral artery occlusion and reperfusion. (A) 2,3,5-Triphenyltetrazolium hydrochloride staining of brain sections after 24 hours of reperfusion in mice with rosiglitazone treatment. Infarct volume was significantly reduced after two injections of 3 mg/kg rosiglitazone (n=5 per group, *P<0.05 versus dimethyl sulfoxide (DMSO) treatment group). (B) Cresyl violet staining of brain sections after 24 and 48 hours of reperfusion with DMSO (D24 and D48) or rosiglitazone (R24 and R48) treatment. Infarct volume was significantly reduced with rosiglitazone treatment by 75.8% and 50.8% after 24 and 48 hours of reperfusion, respectively (n=4 each group, *P<0.05, +P<0.05 versus 24 hours of reperfusion in DMSO treatment group, ++P<0.05 versus 48 hours of reperfusion in DMSO treatment group). The neuroprotective effect of rosiglitazone was reversed by administration of the peroxisome proliferator-activated receptor-γ antagonist GW9662. (C) 2,3,5-Triphenyltetrazolium hydrochloride staining of brain sections after 24 hours of reperfusion in mice with DMSO, rosiglitazone, and GW9662/rosiglitazone treatment. Infarct volume was significantly reduced with rosiglitazone treatment and this neuroprotective effect was reversed by administration of GW9662 (n=4 per group, *P<0.05 versus DMSO treatment group). GW, GW9662; ROSI, rosiglitazone.
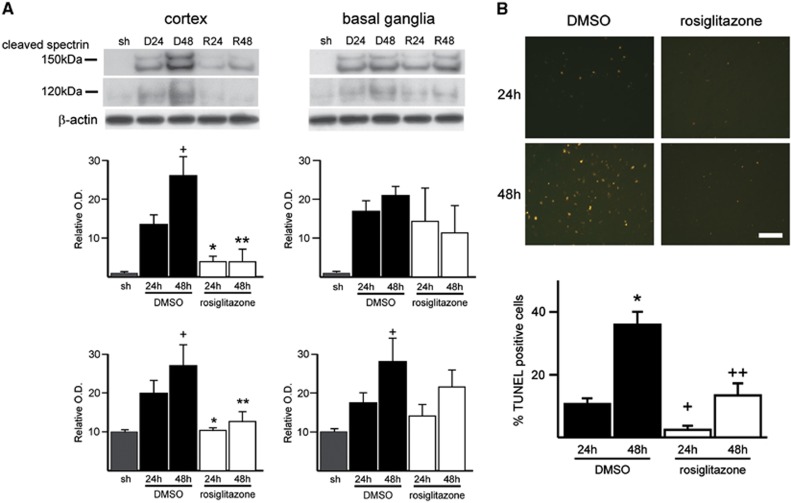
We examined cleaved spectrin, an apoptotic marker, after MCA occlusion and reperfusion. The spectrin antibody is known to recognize both caspase—(150 and 120 kDa) and calpain—(150 kDa) cleaved products and is used to assess brain injury after MCA occlusion.14 After 24 and 48 hours of reperfusion, cleaved spectrin products (both 150 and 120 kDa) were observed to be significantly higher in the cortex of control mice than in the cortex of the rosiglitazone-treated mice. There was no significant difference between the two groups in the basal ganglia tissue (Figure 2A). To detect DNA fragmentation in situ, we performed TUNEL staining in brain cryosections. Rosiglitazone reduced ischemia-induced DNA damage after 24 and 48 hours of reperfusion. In contrast to the control group, in which TUNEL-positive cells were densely distributed in the ischemic cortex, the rosiglitazone-treated group exhibited a smaller amount of TUNEL-positive cells after both 24 and 48 hours of reperfusion (Figure 2B).
Figure 2.
Rosiglitazone treatment attenuated cleaved spectrin immunoreactivity in the cortex tissue of ischemic mouse brains, but not in the basal ganglia tissue. (A) Western blot of spectrin and densitometric analysis of the calpain-mediated 150-kDa fragment and the caspase-mediated 120-kDa fragment bands. Expression of cleaved spectrin was significantly increased after 24 and 48 hours of reperfusion with dimethyl sulfoxide (DMSO) treatment (D24 and D48, respectively). With rosiglitazone treatment (R24 and R48, respectively), the increased expression of cleaved spectrin following middle cerebral artery occlusion was significantly attenuated in the cortex tissue, but not in the basal ganglia tissue (n=4 each group, +P<0.05, *P<0.05 versus D24 group, **P<0.05 versus D48 group). (B) Rosiglitazone treatment also resulted in a reduction in transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL)-positive cells in the ischemic cortex. Photomicrographs show TUNEL-positive cells in the ischemic cortex of the DMSO- or rosiglitazone-treated mice after 24 and 48 hours of reperfusion. Transferase-mediated uridine 5′-triphosphate-biotin nick end labeling-positive cells were observed in the entire ischemic cortex in the DMSO treatment group, but were fewer in the rosiglitazone treatment group; quantitative analysis of TUNEL positivity confirmed this (n=4 each group, +P<0.05, *P<0.05 versus D24 group, ++P<0.05 versus D48 group). Bar: 100 μm. O.D., optical density; sh, sham.
Western Blot Analysis Identifies Rosiglitazone-Inhibited SAPK Phosphorylation in Ischemic Mouse Brains
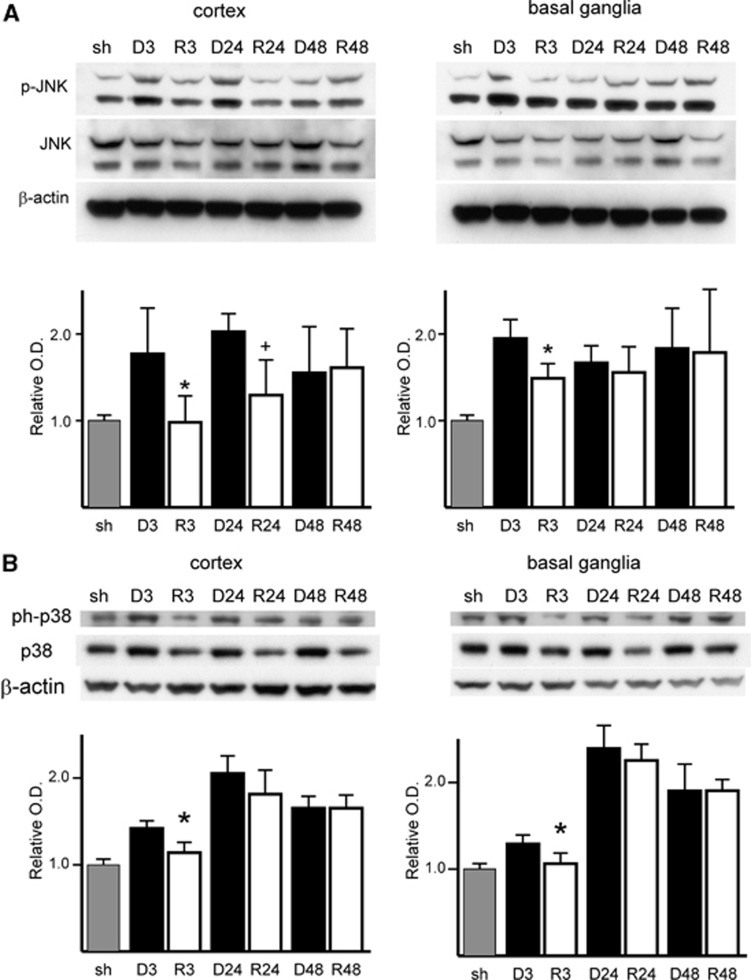
To identify whether SAPK signaling is involved in the neuroprotective effect of rosiglitazone, we analyzed ischemic brain tissue by western blot using whole cell lysate. Rosiglitazone treatment blocked the increase in phosphorylation of JNK and p38 at early reperfusion time points. In the rosiglitazone treatment group, JNK phosphorylation decreased significantly after 3 and 24 hours of reperfusion in cortex tissue (P=0.043 for the 3-hour reperfusion DMSO group (D3) versus the rosiglitazone-treated group (R3) and P=0.028 for the 24-hour reperfusion DMSO group (D24) versus the rosiglitazone-treated group (R24)) and after 3 hours of reperfusion in basal ganglia tissue (P=0.018 for D3 versus R3). There was no significant difference after 48 hours of reperfusion in cortex tissue or after 24 and 48 hours of reperfusion in basal ganglia tissue (Figure 3A). The level of phosphorylated p38 MAPK decreased after 3 hours of reperfusion in cortex tissue (P=0.014 for D3 versus R3) and after 3 hours of reperfusion in basal ganglia tissue (P=0.032 for D3 versus R3). There was no significant difference at other time points in the cortex or basal ganglia (Figure 3B).
Figure 3.
Rosiglitazone treatment attenuated increased phosphorylation of c-Jun N-terminal kinase (JNK) (p-JNK) and p38 mitogen-activated protein kinase (MAPK) induced by middle cerebral artery occlusion at early reperfusion time points. (A) Western blot and densitometric analysis of p-JNK, JNK, and β-actin expression. Rosiglitazone restored JNK phosphorylation after 3 and 24 hours of reperfusion in the cortex tissue and after 3 hours of reperfusion in the basal ganglia tissue. There was no significant difference after 48 hours of reperfusion in either the cortex or basal ganglia tissue and after 24 hours of reperfusion in the basal ganglia tissue (n=4 each group, *P<0.05 versus 3 hours of reperfusion with dimethyl sulfoxide (DMSO) treatment, +P<0.05 versus 24 hours of reperfusion with DMSO treatment). (B) Western blot of phospho-p38 MAPK (ph-p38), p38 MAPK, and β-actin, and densitometric analysis of band expression. Rosiglitazone restored p38 MAPK phosphorylation after 3 hours of reperfusion in both the cortex and basal ganglia tissue (n=4 each group, *P<0.05 versus 3 hours of reperfusion with DMSO treatment). O.D., optical density; sh, sham.
Rosiglitazone Inhibits Upregulation of JNK Activity Induced by Ischemia and Reperfusion
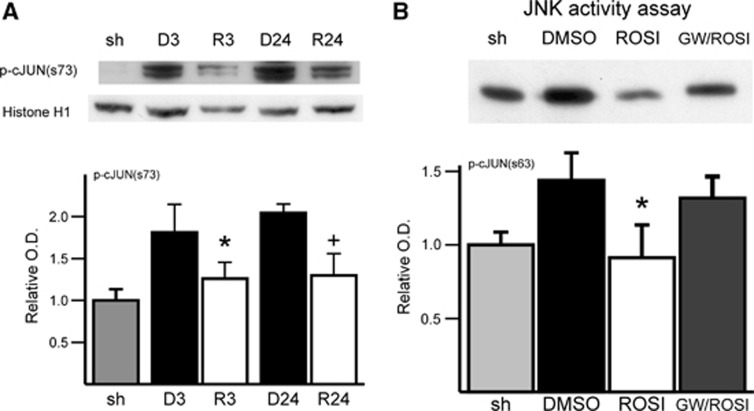
To confirm the effect of rosiglitazone on the JNK pathway, we examined JNK activity in two different ways. First, western blot analysis using the nuclear fraction showed that phosphorylation of c-Jun, a substrate of JNK, was significantly reduced by rosiglitazone after 3 and 24 hours of reperfusion (n=4 each group, Figure 4A). Analysis of JNK activity by kinase assay indicated that rosiglitazone suppressed JNK activity evoked by ischemia and reperfusion. Rosiglitazone's effect was reversed by the PPARγ antagonist, GW9662 (n=4 each group, Figure 4B). Western analysis showed a decrease in phosphorylated c-Jun in rosiglitazone-treated ischemic tissue, which was reversed by GW9662.
Figure 4.
Rosiglitazone treatment attenuated increased phosphorylation of c-Jun (p-cJun) in the nuclear fraction after middle cerebral artery (MCA) occlusion after 3 and 24 hours of reperfusion. (A) Western blot and densitometric analysis of p-cJun (s73) and histone H1 expression (n=4 each group, *P<0.05 versus 3 hours of reperfusion with dimethyl sulfoxide (DMSO) treatment, +P<0.05 versus 24 hours of reperfusion with DMSO treatment). (B) Representative blot of c-Jun N-terminal kinase (JNK) activity assay where p-cJun by JNK was measured. The p-cJun band represents relative JNK activity precipitated by cJun fusion proteins. c-Jun N-terminal kinase activity induced by MCA occlusion and 3 hours of reperfusion was ameliorated with rosiglitazone treatment. The effect of rosiglitazone was reversed by GW9662 treatment (n=4 each group, *P<0.05 versus DMSO treatment group). GW, GW9662; O.D., optical density; ROSI, rosiglitazone; sh, sham.
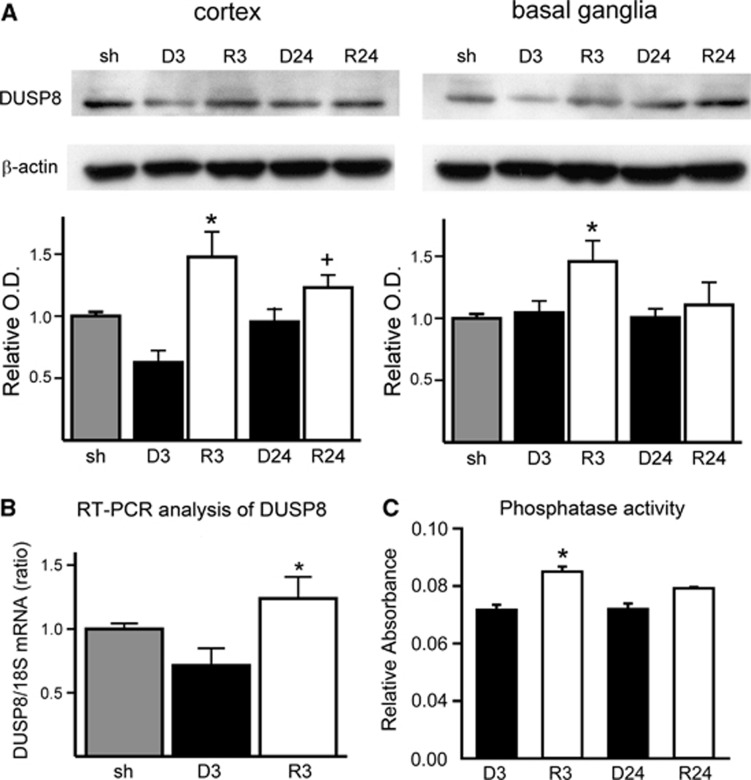
Rosiglitazone Increases Expression of DUSP8 in the Ischemic Mouse Cortex at both the Protein and Messenger RNA Levels
As JNK is dephosphorylated by DUSP8,15 we further examined the level of DUSP8 expression by western blot analysis using whole cell lysate. The levels after 3 and 24 hours of reperfusion in cortex tissue (P<0.01 for D3 versus R3 and P<0.01 for D24 versus R24) and after 3 hours of reperfusion in basal ganglia tissue (P=0.012 for D3 versus R3) were elevated by rosiglitazone treatment (n=4 each group, Figure 5A). To confirm whether this elevation of DUSP8 occurred at the transcriptional level, we analyzed ischemic brain tissue by reverse transcriptase-PCR. The level of DUSP8 messenger RNA (mRNA) was increased with rosiglitazone treatment (n=4 each group, Figure 5B). A DUSP8 activity assay was also performed to show that increased activity correlated with increased DUSP8 expression. Phosphatase activity was determined in ischemic brains treated with rosiglitazone (R3 and R24) and DMSO (D3 and D24) after 3 and 24 hours of reperfusion (Figure 5C). The rosiglitazone-treated samples after 3 hours of reperfusion showed a significant increase in phosphatase activity compared with non-treated ischemic brains (n=4 each group, Figure 5C).
Figure 5.
Rosiglitazone treatment increased dual-specificity phosphatase 8 (DUSP8) expression at both protein and messenger RNA (mRNA) levels. (A) Western blot and densitometric analysis of DUSP8 and β-actin expression (n=4 each group, *P<0.05 versus 3 hours of reperfusion with dimethyl sulfoxide (DMSO) group, +P<0.05 versus 24 hours of reperfusion with DMSO group). Dual-specificity phosphatase 8 was significantly upregulated in the cortex after 3 and 24 hours. (B) Comparison of the effect of rosiglitazone on DUSP8 mRNA expression in mouse brains after 3 hours of reperfusion with DMSO and rosiglitazone treatments (n=4 each group, *P<0.05 versus DMSO treatment group). (C) Phosphatase activity assay of ischemic brains treated with rosiglitazone (R3 and R24) and DMSO (D3 and D24) after 3 and 24 hours of reperfusion. The rosiglitazone-treated samples after 3 hours of reperfusion showed a significant increase in phosphatase activity compared with non-treated ischemic brains (n=4 each group, *P<0.05 versus DMSO-treated group). O.D., optical density; sh, sham; 18S, control mRNA.
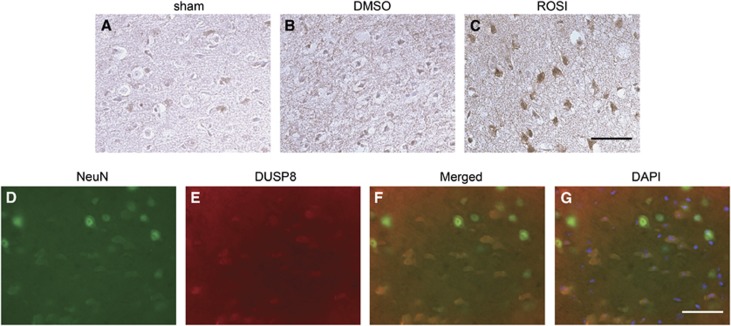
DUSP8 Expresses in Neurons and Increases Immunoreactivity with Rosiglitazone Treatment
To determine the expression of DUSP8, we performed immunohistochemistry on sham controls and DMSO-treated and rosiglitazone-treated mice. Dual-specificity phosphatase 8 was constitutively expressed in the normal mouse brain (Figure 6A). After 24 hours of reperfusion, DUSP8 expression was slightly reduced in the ischemic cortex of the DMSO-treated mice (Figure 6B), but was significantly increased in the cortex of rosiglitazone-treated ischemic mice (Figure 6C). Double immunofluorescence for DUSP8 and NeuN demonstrated that DUSP8-positive cells were expressed mainly in neurons, but not in astrocytes in the cerebral cortex (Figure 6D–G).
Figure 6.
Dual-specificity phosphatase 8 (DUSP8) expression was increased in rosiglitazone-treated animals after middle cerebral artery (MCA) occlusion and colocalization to neurons. (A–C) Immunohistochemistry for DUSP8 in the cortex of the MCA territory after 24 hours of reperfusion. With rosiglitazone treatment, DUSP8 expression was intensely increased compared with the same region of the mouse brains with dimethyl sulfoxide (DMSO) treatment. (D–G) Representative photomicrographs show fluorescent double staining of DUSP8 (red) and NeuN (green), in mouse brain cryosections. Nuclei were counterstained with 4′,6 diamidino-2-phenylindole (DAPI) (blue) (G). Dual-specificity phosphatase 8-positive cells were observed in the cortex (D). Merged images in F and G show that DUSP8-positive cells were colocalized in neurons. Bars: 100 μm. ROSI, rosiglitazone.
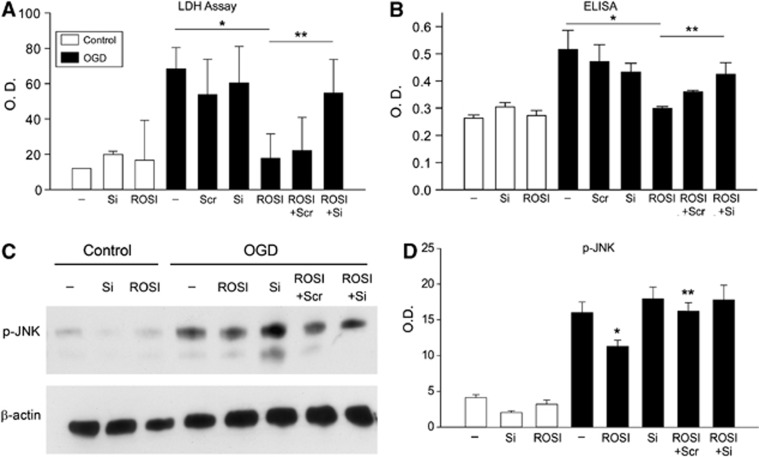
Rosiglitazone Prevents Neuronal Cell Death via DUSP8
To confirm whether inhibition of phosphorylated JNK by rosiglitazone is mediated via DUSP8 upregulation, we transfected the cells with DUSP8 siRNA or control scrambled siRNA, and subjected them to OGD with or without rosiglitazone. We subjected primary cultured neurons to 4 hours of OGD followed by reoxygenation for 3 to 24 hours. Cell viability was analyzed by LDH assay and in situ cell death assay (Roche Applied Sciences). About 20% of cells were viable after 24 hours of reoxygenation (Figure 7A and 7B). Pretreatment of the cells with rosiglitazone (100 μmol/L) significantly increased their viability at 24 hours of reoxygenation. The protective effect of rosiglitazone was abolished by knockdown of DUSP8 (Figure 7A and 7B), while knockdown of DUSP8 without rosiglitazone treatment per se did not alter cell viability. Western blot and densitometric analysis showed that knockdown of DUSP8 with siRNA abolished the effect of rosiglitazone on phosphorylation of JNK (Figure 7C and 7D), demonstrating that rosiglitazone acted via DUSP8 to inhibit JNK phosphorylation.
Figure 7.
Cell death assays demonstrating dual-specificity phosphatase 8 (DUSP8)'s role in inhibiting c-Jun N-terminal kinase (JNK) phosphorylation (p-JNK) after rosiglitazone treatment. (A) Lactate dehydrogenase (LDH) cell death assay of neurons subjected to 3 hours of oxygen–glucose deprivation (OGD) and 24 hours of reoxygenation demonstrated that rosiglitazone acted via DUSP8 in blocking phosphorylation of JNK. (B) Cell death enzyme-linked immunosorbent assay (ELISA) confirmed this (n=6). *P<0.05, **P<0.05. Western analysis (C) and densitometric analysis (D) showed that with knockdown of DUSP8, the ability of rosiglitazone to phosphorylate JNK was reduced. O.D., optical density; ROSI, rosiglitazone; Scr, scrambled siRNA; Si, small interfering RNA (siRNA).
Discussion
This study demonstrates the neuroprotective effect of rosiglitazone in a mouse model of cerebral ischemia and reperfusion. Rosiglitazone reduced infarct volume after ischemia and reperfusion, independent of any alterations in cerebral blood flow and other physiological parameters. The study also shows that this protective effect was reversed by the PPARγ antagonist GW2992. This was confirmed in primary cortical neuronal cultures. The present results provide evidence that rosiglitazone blocks phosphorylation of JNK induced by ischemia.
Recent studies have shown that both rosiglitazone and pioglitazone are neuroprotective in animal models of acute focal ischemia.3 Studies have also shown that neuroprotection by TZDs following ischemia may involve: (a) a reduction in the inflammatory response, such as by upregulation of nuclear factor-κB, intercellular adhesion molecule-1, or cyclooxygenase;16, 17, 18, 19 (b) activation of an anti-inflammatory mediator, such as synthesis of lipoxin A4 by 5-lipoxygenase expression;20 (c) activation of superoxide dismutase;21, 22 or (d) prevention of harmful neuronal responses to ischemic insult, such as enhanced 14-3-3ɛ expression.23 There is significant evidence from these studies that PPARγ activation is involved in neuroprotection. Although the anti-inflammatory action of PPARγ is known to have a major role in neuroprotection, other beneficial effects of PPARγ and its agonists, such as reducing oxidative stress and preventing apoptosis after ischemia, have been reported.24, 25 Our study demonstrates a mechanism by which rosiglitazone can block phosphorylation of JNK and hence reduce cell death. In our present study, we examined the SAPK signaling pathway underlying the effect of rosiglitazone and activation of PPARγ.
Our results showing decreased cleaved spectrin and TUNEL staining in rosiglitazone-treated mice compared with untreated mice subjected to ischemia suggest that prevention of apoptosis is one of the key mechanisms that contributes to the protective effect of TZDs after ischemic brain injury. This confirms a report by Lin et al26 showing that rosiglitazone treatment reduced apoptotic cell death after focal ischemia by reduction of caspase-3, which was prevented by GW9662. One of the mechanisms involved in this anti-apoptotic effect is the phosphorylation of the kinases JNK and p38 that is induced by ischemia. In the present study, we demonstrate the decrease in JNK phosphorylation by rosiglitazone. This reduction was significant in the ischemic cortex at early reperfusion periods. Furthermore, the enzymatic activity of JNK was reduced in mouse brain tissue treated with rosiglitazone. This was further confirmed by examination of phosphorylation levels of c-Jun, one of the JNK substrates. The kinase assay analysis also demonstrated that the decrease in JNK phosphorylation by rosiglitazone was reversed by treatment with the PPARγ antagonist. Our data are in agreement with a previous study that showed that rosiglitazone prevented phosphorylation of p38 MAPK induced by global ischemia.24 The relationship between high-reactive oxygen species and activation of downstream signaling cascades has been clearly shown. Collino et al24 have also suggested the ability of rosiglitazone to reduce oxidative stress via its action on signaling pathways. Activation of either JNK or p38 MAPK is well documented in cerebral ischemia models.4 Reports have shown several agents that prevent the activation of JNK5, 7, 27 or p38 MAPK6, 28 and that improve the outcome of ischemic brain injury by reducing apoptosis signaling.
The MAPK cascades contain various checkpoints for regulatory factors. Protein kinases have been a major focus of recent molecular targets, and MAPKs have been widely demonstrated to have pivotal roles in mitogenic signal transduction, survival, stress response, and programed cell death. Studies have led to recognizing the importance of protein tyrosine phosphatases in controlling the phosphorylation state of the protein kinases.29, 30, 31 Dual-specificity phosphatases are protein tyrosine phosphatases that inactivate or modulate MAPKs via dephosphorylation of both tyrosine and serine–threonine residues. Dual-specificity phosphatase 8, also referred to as hVH5, is highly expressed with a wide distribution in the central and peripheral nervous system.9 Dual-specificity phosphatase 8 is reported to be specific for JNK and p38 MAPK, but not for extracellular signal-regulated kinase.8 Hence, we hypothesized that the blocking effect of rosiglitazone on JNK activation was modulated by DUSP8 upregulation. It has recently been reported that MAPK phosphatase-1 (a DUSP family member) is upregulated in the rat after MCA occlusion and that conditional overexpression of MAPK phosphatase-1 suppressed phosphorylation of JNK as well as cell death in neuroblastoma cells subjected to hypoxia reoxygenation.32 Our results corroborate these findings of an association between DUSP8 and JNK.
In this study, we demonstrate that DUSP8 expression increased at 3 hours of reperfusion and continued to be upregulated after 24 hours of reperfusion in the ischemic cortex tissue after rosiglitazone treatment. It was upregulated at 3 hours of reperfusion in the basal ganglia, but was not sustained. Dual-specificity phosphatase 8 expression was also upregulated by rosiglitazone treatment at the mRNA level. Rosiglitazone increased expression of DUSP8 (a phosphatase) mRNA in ischemic brain tissue. Dual-specificity phosphatase 8 activity was also increased in rosiglitazone-treated animals subjected to ischemia. Furthermore, in primary cortical neurons, using siRNA to knockdown DUSP8, we confirmed that the rosiglitazone effect dephosphorylated activated JNK and was correlated with upregulation of DUSP8. Knockdown of DUSP8 using siRNA in primary neuronal cultures resulted in the reduction of the protective effect of rosiglitazone. In the neuronal studies, reduced cell viability after OGD was overcome by rosiglitazone treatment and this protective effect was abolished in DUSP8 knockdown neurons. We also demonstrated that phosphorylation of JNK induced by OGD was blocked by rosiglitazone and this effect was reversed by knockdown of DUSP8. These results show that the neuroprotective effect of rosiglitazone involved in prevention of JNK pathway activation is via upregulation and activation of DUSP8. Thus, we have elucidated that the protective effect of rosiglitazone against ischemic brain injury is likely owing to the prevention of JNK phosphorylation through activation of DUSP8. Moreover, we have elucidated downstream signaling mechanisms that contribute to the protective effects of rosiglitazone against ischemic brain injury, which are likely owing to the prevention of JNK phosphorylation through activation of DUSP8.
In summary, we demonstrate a new mechanism of protection against neuronal cell death after ischemia. Rosiglitazone mediates a decrease in the phosphorylation of JNK induced by ischemia, which is via DUSP8 upregulation. Although rosiglitazone has been used for type 2 diabetes, it is a promising therapeutic molecule for stroke. Our results offer a mechanistic insight into the possible role of rosiglitazone as a new therapeutic approach for ischemic stroke.
The authors declare no conflict of interest.
Footnotes
This work was supported by grants PO1 NS014543, RO1 NS025372, and RO1 NS038653 from the National Institutes of Health, and by the James R. Doty Endowment. The authors thank Liza Reola and Bernard Calagui for technical assistance.
References
- Ji S, Kronenberg G, Balkaya M, Färber K, Gertz K, Kettenmann H, et al. Acute neuroprotection by pioglitazone after mild brain ischemia without effect on long-term outcome. Exp Neurol. 2009;216:321–328. doi: 10.1016/j.expneurol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A. Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. J Cereb Blood Flow Metab. 2009;29:1683–1694. doi: 10.1038/jcbfm.2009.87. [DOI] [PubMed] [Google Scholar]
- White AT, Murphy AN. Administration of thiazolidinediones for neuroprotection in ischemic stroke: a pre-clinical systematic review. J Neurochem. 2010;115:845–853. doi: 10.1111/j.1471-4159.2010.06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PGH, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Piao CS, Kim J-B, Han P-L, Lee J-K. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J Neurosci Res. 2003;73:537–544. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin X-M, Sun F, et al. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Martell KJ, Seasholtz AF, Kwak SP, Clemens KK, Dixon JE. hVH-5: a protein tyrosine phosphatase abundant in brain that inactivates mitogen-activated protein kinase. J Neurochem. 1995;65:1823–1833. doi: 10.1046/j.1471-4159.1995.65041823.x. [DOI] [PubMed] [Google Scholar]
- Khandoudi N, Delerive P, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A. Rosiglitazone, a peroxisome proliferator–activated receptor-γ, inhibits the Jun NH2-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51:1507–1514. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- Yoon S-Y, Park J-S, Choi J-E, Choi J-M, Lee W-J, Kim S-W, et al. Rosiglitazone reduces tau phosphorylation via JNK inhibition in the hippocampus of rats with type 2 diabetes and tau transfected SH-SY5Y cells. Neurobiol Dis. 2010;40:449–455. doi: 10.1016/j.nbd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Kim GS, Chan PH. The PIDDosome mediates delayed death of hippocampal CA1 neurons after transient global cerebral ischemia in rats. Proc Natl Acad Sci USA. 2008;105:16368–16373. doi: 10.1073/pnas.0806222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia–reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-γ ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cárdenas A, Boscá L, Castillo J, Dávalos A, et al. Rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2 cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J Cereb Blood Flow Metab. 2006;26:218–229. doi: 10.1038/sj.jcbfm.9600182. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernández-López D, Pradillo JM, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARγ-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, et al. A peroxisome proliferator-activated receptor-γ agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- Schock SC, Xu J, Duquette PM, Qin Z, Lewandowski AJ, Rai PS, et al. Rescue of neurons from ischemic injury by peroxisome proliferator-activated receptor-γ requires a novel essential cofactor LM04. J Neurosci. 2008;28:12433–12444. doi: 10.1523/JNEUROSCI.2897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-S, Cheung W-M, Tsai Y-S, Chen Y-T, Fong W-H, Tsai H-D, et al. Ligand-activated peroxisome proliferator–activated receptor-γ protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3ɛ upregulation. Circulation. 2009;119:1124–1134. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Gallicchio M, Rosa AC, Dianzani C, et al. Modulation of the oxidative stress and inflammatory response by PPAR-γ agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol. 2006;530:70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- Vemuganti R. Therapeutic potential of PPARγ activation in stroke. PPAR Res. 2008;2008:461981. doi: 10.1155/2008/461981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-N, Cheung W-M, Wu J-S, Chen J-J, Lin H, Chen J-J, et al. 15d-Prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nito C, Kamada H, Endo H, Niizuma K, Myer DJ, Chan PH. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood–brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28:1686–1696. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu B-E, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Pulido R, Hooft van Huijsduijnen R. Protein tyrosine phosphatases: dual-specificity phosphatases in health and disease. FEBS J. 2008;275:848–866. doi: 10.1111/j.1742-4658.2008.06250.x. [DOI] [PubMed] [Google Scholar]
- Koga S, Kojima S, Kishimoto T, Kuwabara S, Yamaguchi A. Over-expression of map kinase phosphatase-1 (MKP-1) suppresses neuronal death through regulating JNK signaling in hypoxia/re-oxygenation. Brain Res. 2012;1436:137–146. doi: 10.1016/j.brainres.2011.12.004. [DOI] [PubMed] [Google Scholar]