Abstract
Ischemic stroke is a devastating condition lacking effective therapies. A promising approach to attenuate ischemic injury is mild hypothermia. Recent studies show that adenosine nucleotides can induce hypothermia in mice. The purpose of the present study was to test the hypothesis that adenosine 5′-triphosphate (ATP) induces mild hypothermia in rats and reduces ischemic brain injury. We found that intraperitoneal injections of ATP decreased core body temperature in a dose-dependent manner; the dose appropriate for mild hypothermia was 2 g/kg. When ATP-induced hypothermia was applied to stroke induced by middle cerebral artery occlusion, however, a neuroprotective effect was not observed. Instead, the infarct volume grew even larger in ATP-treated rats. This was accompanied by an increased rate of seizure events, hemorrhagic transformation, and higher mortality. Continuous monitoring of physiologic parameters revealed that ATP reduced heartbeat rate and blood pressure. ATP also increased blood glucose, accompanied by severe acidosis and hypocalcemia. Western blotting showed that ATP decreased levels of both phospho-Akt and total-Akt in the cortex. Our results reveal that, despite inducing hypothermia, ATP is not appropriate for protecting the brain against stroke. Instead, we show for the first time that ATP treatment is associated with exaggerated ischemic outcomes and dangerous systemic side effects.
Keywords: acidosis, ATP, brain ischemia, hyperglycemia, hypothermia
Introduction
Ischemic stroke is a common neurologic disorder with long-term medical, social, and economic burdens, partially because of lack of effective treatment.1 Mild hypothermia, or a core body temperature (CBT) between 32°C and 24°C, has shown remarkable neuroprotective effects against a number of ischemic models across several animal species.2, 3, 4 Mild hypothermia also yielded encouraging results in human patients with cardiac arrest and resuscitation.5, 6 Although optimal cooling methods still need to be improved, the application of mild hypothermia to stroke patients has also been proposed.4, 7 Traditional methods such as immersion in ice-cold water or alcohol spray have been used in patients under coma or general anesthesia, but they are not applicable to conscious patients. Therefore, less invasive approaches or, preferably, pharmacological inductions of hypothermia are being sought.4, 7
A recent study reports that AMP has a role in thermoregulation.8 Constant darkness, a component of hibernation, increases AMP levels in blood and is associated with specific gene expression signatures.8, 9 Furthermore, injection of exogenous AMP to mice and rats induces hypothermia and a hibernation-like condition.8, 10, 11 However, AMP-induced mild hypothermia in rats failed to protect the brain from stroke because of multiple side effects, including hypotension and hyperglycemia.10
Interestingly, adenosine 5′-triphosphate (ATP) has also been reported to induce hypothermia in mice.11 Considering that ATP is the primary source of cellular energy, it could serve as a potential therapy against stroke by providing cellular fuel and decreasing body temperature simultaneously. To test this novel hypothesis, we assessed whether ATP can induce mild hypothermia in rats and protect the brain from ischemia injury.
Materials and methods
Monitoring of Physiologic Parameters
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. The manuscript is written in accordance with the ARRIVE (Animal Research: Reporting in vivo experiments). Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 280 to 310 g were housed at room temperature (22°C to 24°C) under a 12:12 hour light-dark cycle, with free access to food and water. The rats were randomly assigned to vehicle and ATP groups through the use of a lottery-drawing box. All outcome assessments, including the measurement of temperature, evaluation of ischemic infarction and neurologic performance, monitoring of physiologic parameters, and gel analysis, were performed by investigators who were blinded to the group assignment.
Rectal temperature was measured every 30 minutes with an Omega 450 AET Thermocouple Thermometer (OMEGA Engineering, Inc., Stamford, CT, USA) while the rats were awake unless indicated otherwise.10 Brain temperature was monitored in a separate cohort using an Omega HH-25TC Thermometer (OMEGA Engineering, Inc.), as previously described.10 In brief, rats were anesthetized with isoflurane. After a midline incision was made over the scalp, a burr hole was drilled at the following coordinates: AP +0.5 mm and lateral 3.0 mm from the Bregma. A 29-Ga thermocouple was then inserted via the burr hole into the caudate-putamen and fixed in place with dental cement.
Blood pressure and heartbeat rates were continuously monitored in select rats via left femoral arterial catheterization, using a Power Lab system (AD Instruments, Colorado Springs, CO, USA). At indicated time points, arterial blood was drawn from the femoral catheterization and analyzed for blood gas and electrolytes using an iSTAT Portable Clinical Analyzer (Abbott Point-of-Care, East Windsor, NJ, USA) combined with CG8+ cartridges.10
Preparation and Administration of Adenosine 5′-Triphosphate Solution
Adenosine 5′-triphosphate powder was purchased from Sigma (St. Louis, MO, USA) and dissolved in sterile distilled water. For hypothermic studies, the freshly prepared ATP solution was injected intraperitoneally at doses of 0.5, 1, 1.5, or 2 g/kg body weight. In ATP-treated ischemia groups, 2 g/kg of ATP was intraperitoneally injected 60 minutes after the onset of cerebral ischemia (see below). In selected rats, 2 g/kg ATP was intravenously injected via the left femoral vein. Animals were randomly assigned to ATP or vehicle groups. Control animals were injected with equal volumes of sterile saline through the same route of administration.
Rat Model of Transient Focal Cerebral Ischemia
Middle cerebral artery occlusion (MCAO) was used to induce transient focal cerebral ischemia in rats as described previously.10 In brief, rats were anesthetized with isoflurane in a mixture of 30% O2:70% N2O via a facemask at 5% for induction and 1.5% to 2.0% for maintenance of anesthesia. Under a surgical microscope, the left external, internal, and common carotid arteries were exposed through a midline neck incision. After coagulating and cutting of the branches of the external carotid arteries, a 3-0 monofilament nylon suture with a blunted tip was inserted in the lumen of the external carotid artery and advanced to the origin of the middle cerebral artery via the internal carotid artery. The duration of ischemia was 2 hours and was followed by reperfusion. As mentioned above, ATP was injected 60 minutes after the onset of ischemia. The success of MCAO was confirmed by the measurement of regional cortical cerebral blood blow (rCBF), examination of neurologic dysfunction and the formation of infarct (see below). Neurologic dysfunction was assessed in all rats once they fully recovered from anesthesia, while the measurements of rCBF and infarct were performed in randomly selected rats as described below. Rat would be excluded from the study if their rCBF failed to reduce to <30% of baseline or showed no neurologic deficits after anesthesia recovery.
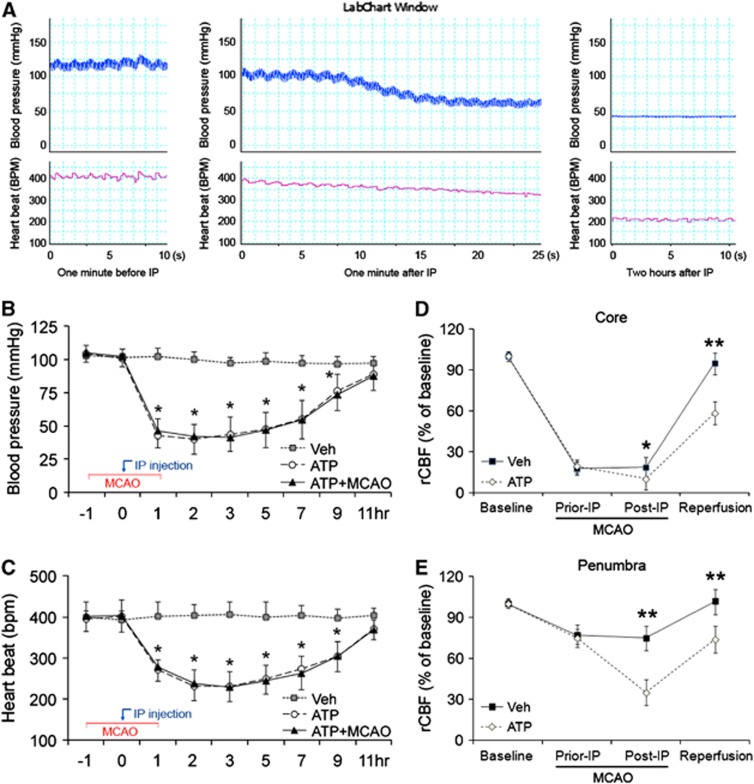
To measure CBF changes after MCAO and ATP injection, rCBF in the ischemic core (bregma 1.0 mm, lateral 3.0 mm) and penumbra (bregma −3.0 mm, lateral 4.0 mm) was measured using laser-Doppler flowmetry in randomly selected rats. Four sequential measurements were performed on each rat at: (1) 5 minutes before MCAO as baseline, (2) 30 minutes after the onset of MCAO (30 minutes before intraperitoneal injection), (3) 90 minutes after the onset of MCAO (30 minutes after intraperitoneal injection), and (4) 15 minutes after the onset of reperfusion.
Evaluation of Ischemic Outcomes
Ischemic outcomes were assessed by neurobehavioral tests, infarct volumes, as well as the rates of hemorrhagic transformation (HT), seizure, and death. Rats were randomly assigned to vehicle- and ATP-treated groups, with 7 to 10 animals per group. Neurologic dysfunction was evaluated at 24 hours after ischemia and scored on a 0 to 4 scale. Briefly, 0: no deficit; 1: forelimb flexion deficit on contralateral side; 2: decreased resistance to lateral push and torso turning to the ipsilateral side when held by tail; 3: very significant circling to affected side and reduced capability to bear weight on the affected side; 4: rarely moves spontaneously and prefer to stay in rest. To quantify infarct volumes, rats were killed at 24 hours after MCAO, and the brains were removed and sliced into 2 mm sections. The sections were immediately incubated with 2% 2, 3, 5-triphenyltetrazolium chloride (TTC). Infarct area in each section was measured using NIH Image J (NIH, Bethesda, MD, USA) and calculated using the following equation: healthy area of contralateral hemisphere−healthy area of ipsilateral hemisphere; the infarct area on every section was then used to calculate the infarct volume by multiplying the thickness of the sections. Hemorrhagic transformation was also evaluated on TTC-stained brain sections, which was determined based on the following simplified criteria: (1) multiple dark hemorrhagic spots (at least two) in a brain and (2) the hemorrhagic spots were located within the middle cerebral artery territory.
Fluoro-Jade B (FJB; Millipore, Billerica, MA, USA) staining was also used to detect early neuronal degeneration after ischemia.12, 13 Briefly, brains were harvested 4 hours after MCAO in rats. Paraffin sections were cut and immersed in 100% alcohol for 3 minutes and 70% alcohol for 1 minute and then washed with distilled water. After incubation in 0.06% potassium permanganate for 15 minutes and distilled water washes, sections were stained with 0.001% FJB in 0.09% acetic acid for 20 minutes. Stained sections were observed under a fluorescence microscope (Nikon TE2000 fluorescent microscope; Nikon Instruments, Melville, NY, USA), and positive cells were counted under × 20 magnification.
Primary Cortical Neuronal Cultures, Adenosine 5′-Triphosphate Treatment, and Oxygen-Glucose Deprivation
Primary cortical neurons were cultured from E18 embryos of Sprague-Dawley rats as described previously.14 In brief, cortical tissues were dissociated, suspended in Neurobasal medium supplemented with B27, and then plated in 24-well dishes at 1.6 × 105 cells/cm2. Experiments were performed on day in vitro (DIV) 12 at which time point cultures consisted of >97% neurons. Oxygen-glucose deprivation (OGD) was used as an in vitro model of ischemia to assess the effects of ATP on neuronal death. Briefly, culture medium was replaced with medium lacking glucose. Plates were placed in an airtight chamber (Billups-Rothenberg, Del Mar, CA, USA) and flushed with 100% argon gas for 3 minutes; neurons were then incubated at 37°C for 60 minutes. Control cultures were incubated at 37°C in humidified 95% air and 5% CO2 for equivalent periods. Adenosine 5′-triphosphate was then added to the medium after OGD to a final concentration of 4 mmol/L.
Neuronal viability was assessed with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, MAP2 and Hoechst 33342 staining. In brief, neurons were treated with 0.5 mg/mL MTT for 1 hour at 37°C. Then, 100 μL dimethyl sulfoxide was added. Absorbance of the reaction was measured at 570 nm over a period of 5 minutes. Data were presented as percentages of the value of control cells. Nuclear staining with Hoechst 33258 was used to confirm the results of the MTT assays. Neurons were fixed with 4% paraformaldehyde for 10 minutes, washed six times with phosphate-buffered saline, stained with Hoechst for 15 minutes, and washed three more times in phosphate-buffered saline. Cells with normal and with condensed fragmented nuclei were counted and considered as viable and nonviable, respectively. The MAP2 staining was applied to display the structure of the cytoplasm. Neurons were fixed and incubated with MAP2 antibody (Santa Cruz, Santa Cruz, CA, USA, 1:500) for 2 hours. Then, cells were incubated with Cy3-labeled secondary antibodies (Jackson Laboratory, Bar Harbor, ME, USA, 1:300) for 1 hour after rinsing with phosphate-buffered saline. All images were captured with a Nikon TE2000 fluorescent microscope (Nikon Instruments).
Western Blot Analysis
Western blots were performed using standard methods described previously.10, 14 Rats were killed at 1, 4, and 8 hours after MCAO, or 8 hours after sham operation (n=3 to 4 per experimental condition). In ATP-treated groups, 2 g/kg of ATP was intraperitoneally injected 30 minutes after the onset of MCAO. Cortical tissues from ischemic regions were collected, snap-frozen on dry ice and stored at −80°C until use. After thawing, brain tissues were homogenized in lysis buffer (Cell Signaling Technologies, Danvers, MA, USA) containing 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The suspension was then sonicated and centrifuged. Total protein extracts were separated using 4% to 15% gradient Ready Gels (Bio-Rad, Hercules, CA, USA) and subjected to western blot analysis. Blots were probed with antibodies (Cell Signaling Technologies) recognizing total Akt (t-Akt) and phosphorylated Akt (p-Akt) at Ser473 and total mammalian target of rapamycin (t-mTor) and phosphorylated mTor (p-mTor) at Ser2448. Optical density of bands was measured with the assistance of NIH image J (NIH, Bethesda, MD, USA). The optical density of each band was first expressed as a fraction of the corresponding β-actin band, and then compared with the optical density of sham-operated animals on the same gel.
Statistical Analysis
Data on temperature, neuronal death counts, MTT assay, heartbeat, blood pressure, blood glucose, and electrolytes were analyzed with ANOVA followed by post hoc Scheffe's tests and are presented as mean±s.d. Data on infarct volume were analyzed with the t-test and are presented as mean±s.d. Data on animal death rate, seizure rate, and HT rate were analyzed with the χ2 test and are presented as ratios. Data on optical density of western blots bands were analyzed with ANOVA followed by post hoc Scheffe's tests and are presented as mean±s.e.m. The significance level of all tests (α) was 0.05 and the power of the tests was 0.8 (1−β).
Results
Adenosine 5′-Triphosphate Induces Hypothermia in Rats in a Dose-Dependent Manner
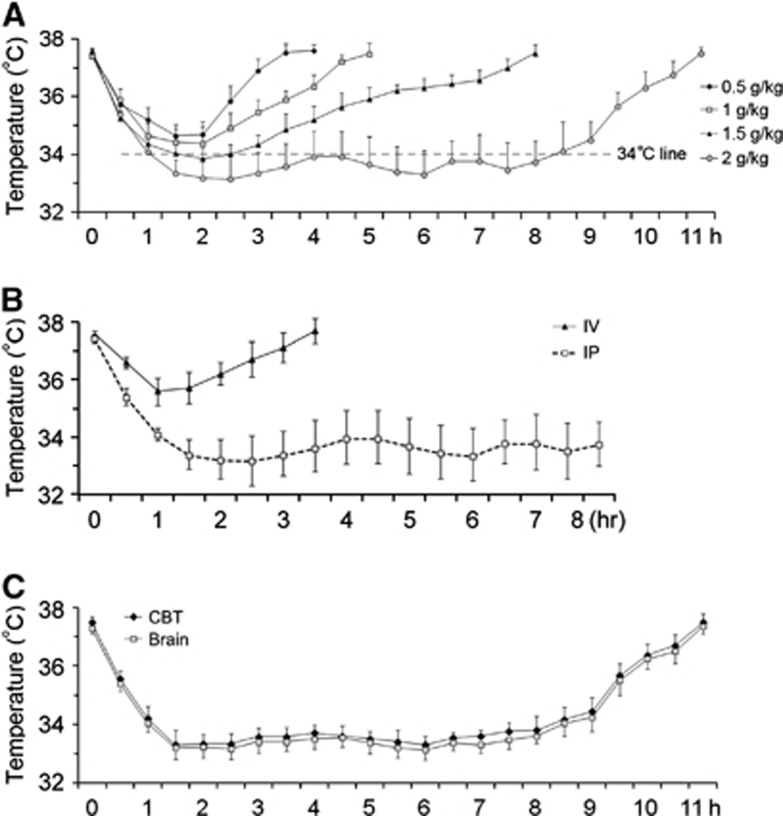
To detect if ATP induces hypothermia in rats, we dissolved ATP in sterile water, intraperitoneally injected the solution, and then measured rectal temperature. As shown in Figure 1A, ATP decreased rat CBT at a dose range of 0.5 to 2 g/kg, supporting previous reports that ATP decreases body temperature in mice.11 However, the temperature did not decrease <34°C when 0.5 or 1 g/kg ATP was injected. When 1.5 g/kg ATP was injected, the temperature was slightly <34°C but this only lasted 1 hour. Therefore, 0.5 to 1.5 g/kg ATP failed to reduce CBT enough to reach the mild hypothermia range. When ATP was injected at 2 g/kg, it did induce mild hypothermia in rats (Figure 1A), which started 1 hour after the injection and lasted ∼7 hours, with a trough of 33.2°C. We therefore chose 2 g/kg of ATP for subsequent experiments. During hypothermia, all rats were awake and ambulatory without any shivering.
Figure 1.
Intraperitoneal injection of adenosine 5′-triphosphate (ATP) induces mild hypothermia in rats. ATP was dissolved in sterile distilled water and intraperitoneally injected at indicated doses. Core body temperature (CBT) was measured every 30 minutes via the rectum. (A) ATP induced mild hypothermia in a dose-dependent manner. (B) Intravenous injection of ATP failed to induce hypothermia. (C) Brain temperature was parallel to CBT. n=4, CBT and brain temperature were presented as mean±s.d.
To ascertain that ATP administration decreases brain temperature in a similar temporal profile as CBT, we measured brain temperature and CBT simultaneously in a separate cohort of rats. As shown in Figure 1C, brain temperature was 0.1°C to 0.2°C lower than CBT before ATP injection. As CBT decreased after ATP injection, brain temperature decreased concurrently during the same time course, indicating that intraperitoneal ATP administration induced hypothermia in brain and CBT at similar profiles.
To determine if intravenous injection, a common route in clinical practice, is also effective in inducing mild hypothermia, we injected ATP at 2 g/kg via the left femoral vein and monitored body temperature changes. However, as shown in Figure 1B, intravenous injection was less effective than intraperitoneal injection in inducing hypothermia.
Adenosine 5′-Triphosphate Exacerbates Ischemic Brain Injury after Transient Focal Ischemia in Rats
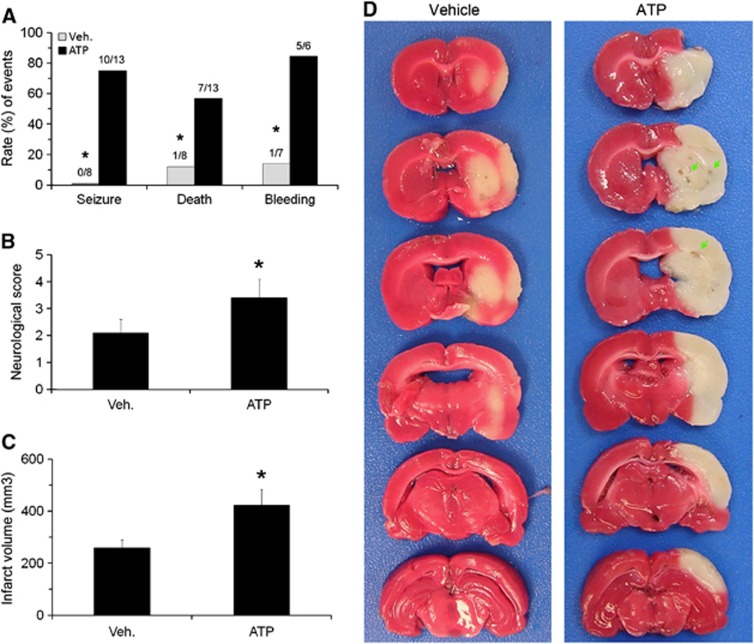
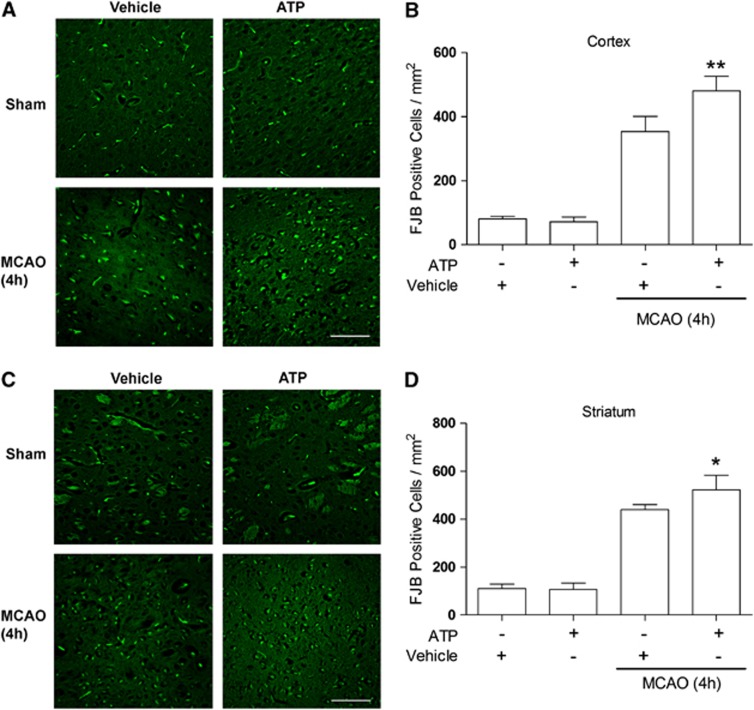
We then detected the effects of ATP-induced hypothermia on ischemic outcomes. Transient focal ischemia was induced by 2 hours MCAO and ATP (2 g/kg) was intraperitoneally injected at 60 minutes after the onset of ischemia. This was performed so that the mild hypothermia could be achieved at the onset of reperfusion and lasts for 7 hours. Contrary to our expectations, we noticed increased rates of seizure (0/8 in vehicle group and 10/13 in ATP group, P<0.01 versus vehicle group) and death (1/8 in vehicle group and 7/13 in ATP group, P<0.01 versus vehicle group) in ATP-treated rats (Figure 2A). In the survivors, neurologic function was worse than the vehicle group (P<0.01) (Figure 2B). The TTC staining showed that infarct volumes in the ATP-treated group were significantly larger than the vehicle group (P<0.01) (Figures 2C and 2D). This was accompanied by an increased rate of HT (1/7 in vehicle group and to 5/6 in ATP group, P<0.01 versus vehicle group) (Figures 1A and 1D). To assess whether ATP also accelerates neuronal death early after ischemia, we performed FJB staining on brain sections collected 4 hours after MCAO. In this histologic assay, degenerating neurons express green fluorescence. As shown in Figure 3, fluorescent neurons were largely absent in the sham-operated group and in the ATP only group. The background green fluorescence observed in these groups is from artifactual staining of blood vessels and myelinated fiber bundles of the internal capsule in the striatum. After ischemia, cortical and striatal neurons started dying, as indicated by condensed, triangular FJB-positive neuronal profiles. The numbers of dying neurons were higher in the ATP-treated group (P<0.05 versus vehicle group), indicating that ATP treatment exacerbated early neuronal death after brain ischemia. Collectively, our results show that ATP treatment worsened ischemic outcome.
Figure 2.
Adverse effect of systemic adenosine 5′-triphosphate (ATP) treatment on ischemic outcomes in rats. Vehicle or ATP solution was intraperitoneally injected 60 minutes after onset of 2 hours middle cerebral artery occlusion (MCAO). (A) ATP increased the rates of seizure, death, and hemorrhagic transformation (HT) in rats after MCAO. *P<0.01 versus vehicle group. ATP also worsened neurologic dysfunction (B) and increased infarct volume (C) after focal ischemia. n=7 in vehicle group and 6 in ATP group, data are presented as mean±s.d., *P<0.01 versus vehicle group. (D) Representative pictures of 2,3,5-triphenyltetrazolium chloride (TTC) staining of rat brains after focal ischemia, showing increased infarct volume and bleeding in ATP-treated rats.
Figure 3.
Accelerated neuronal death in adenosine 5′-triphosphate (ATP)-treated group. Fluoro-Jade B (FJB) staining of cortical (A) and striatal (C) sections 4 hours after middle cerebral artery occlusion (MCAO). (A) Representative photomicrographs and (B) quantitative analysis of FJB-positive neurons in ispilateral cortex, indicating increased cortical neuronal degeneration in ATP-treated group. Note the nonspecific, staining of rope-like blood vessels and myelinated fiber bundles, in addition to the specific staining of neuron-shaped cells with dendritic processes. Scale bar=100 μmol/L, n=3, data are presented as mean±s.d., **P<0.01 versus vehicle group. (C) Representative photomicrograph and (D) quantitative analysis of FJB-positive neurons in ipsilateral striatum, indicating increased striatal neuronal degeneration in ATP-treated group. Scale bar=100 μmol/L, n=3, data are presented as mean±s.d., *P<0.05 versus vehicle group.
Direct Effect of Adenosine 5′-Triphosphate on Neuronal Death In Vitro
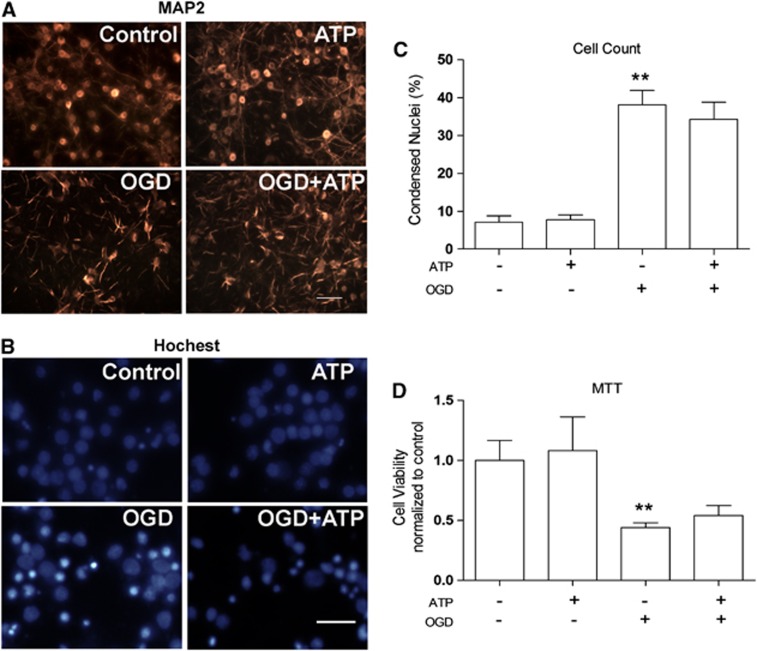
There are at least two possibilities for the adverse effects of ATP on stroke: the first is that ATP directly kills neurons or increases neuronal death after ischemic injury. The second is that ATP causes a systemic disorder that indirectly exaggerates ischemic brain injury. To investigate if ATP directly kills neurons, we treated rat primary cortical neurons with ATP at a final concentration of 4 mmol/L, a comparable concentration to in vivo studies. As shown in Figure 4, ATP treatment alone for 24 hours did not cause neuronal death, as indicated by morphologic tests and the MTT assay (P>0.05 versus OGD group). We also assessed the effects of ATP on cell death induced by OGD, an in vitro ischemic model. One hour OGD killed 38.15% of neurons, indicated by condensed nuclei (Figures 4B and 4C). Adenosine 5′-triphosphate (4 mmol/L) treatment did not increase or decrease neuronal death induced by OGD (Figures 4A to 4D), suggesting that ATP itself did not augment neuronal death induced by OGD (P<0.05 versus OGD group). These results support the notion that ATP causes a systemic disorder that indirectly exacerbates ischemic neuronal injury.
Figure 4.
Effects of adenosine 5′-triphosphate (ATP) on cultured neuronal death after oxygen-glucose deprivation (OGD). Rat cortical neuronal cultures were challenged by 60 minutes OGD, followed by vehicle or ATP (4 mmol/L) treatments. Neuronal viability was assessed 24 hours later by MAP2 staining, Hoechst staining, and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. (A) Representative MAP2 immunocytochemistry. Scale bar=100 μm. (B) Representative Hoechst stain. Scale bar=50 μm. (C) Counts of dead neurons based on Hoechst staining. Data are presented as mean±s.e.m., **P<0.01 versus normal group. (D) 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, showing that ATP treatment did not exacerbate neuronal death induced by OGD. Data are presented as mean±s.e.m., **P<0.01 versus normal group.
Adenosine 5′-Triphosphate Causes Cardiosuppression and Cerebral Hypoperfusion
Deterioration in vital signs and blood parameters was previously noticed in animals treated with AMP, a metabolite of ATP.10, 11 To detect if ATP has similar effects, we monitored heartbeat rate and arterial blood pressure in rats treated with vehicle or ATP. Body temperature was maintained in a physiologic range in the vehicle group, but was reduced in the ATP-treated groups in accordance with the temperature curve of Figure 1A. In the vehicle group, both blood pressure and heartbeat also remained in the physiologic range (Figures 5A to 5C). In the ATP-treated groups, however, blood pressure started decreasing within a minute after the injection, and decreased abruptly to 55 mm Hg within 90 seconds after the injection (Figure 5A). Blood pressure remained low (40 to 50 mm Hg) (P<0.05 versus vehicle group) for the next 6 hours and then gradually increased to the normal range (Figure 5B). Although it did not shift as dramatically, heartbeat rate also sharply decreased to 200 to 250 beats per minute after ATP injection (P<0.05 versus vehicle group). The bradycardia remained for the next 6 hours and was followed by recovery (Figure 5C). Blood pressure and heartbeat changed similarly when MCAO was induced, indicating that ischemia did not affect the changes in blood pressure and heartbeat rate once ATP was injected (Figures 5B and 5C).
Figure 5.
Adenosine 5′-triphosphate (ATP) caused cardiosuppression in rats independent of middle cerebral artery occlusion (MCAO). (A) Representative recordings of blood pressure and heartbeat in rats before and after the injection of ATP, showing the rapid and long-lasting hypotension and bradycardia. (B) Arterial blood pressure changes after ATP injection with or without MCAO. n=4, data are presented as mean±s.d., *P<0.01 versus vehicle group and P<0.05 versus ATP group. (C) Heartbeat rate change after ATP injection with or without MCAO. n=4, data are presented as mean±s.d., *P<0.01 versus vehicle group and P<0.05 versus ATP group. (D) ATP reduced cerebral perfusion in ischemic core during and after ischemia, where regional cerebral blood flow (rCBF) was measured by laser Doppler flowmetry. n=4, data are presented as mean±s.d., *P<0.05 and **P<0.01 versus vehicle group. (E) ATP reduced cerebral perfusion in penumbra. n=4, data are presented as mean±s.d., **P<0.01 versus vehicle group.
Hypotension may exaggerate cerebral ischemia via further decreasing cerebral blood flow. To detect if this was the case in rats after ATP injection, we measured rCBF in the ischemic core and penumbra after MCAO. As shown in Figure 5D, rCBF in the ischemic core of both groups decreased to around 20% of the baseline after MCAO. Injection of ATP further decreased rCBF in the ischemic core (P<0.05 versus vehicle group) during ischemia and reduced rCBF even when the reperfusion (P<0.01 versus vehicle group) was established. Worse yet, rCBF in the penumbra also decreased to dangerous levels during ischemia as well as early reperfusion (Figure 5E). Collectively, these findings indicate that ATP treatment causes a fast-onset, long-lasting and severe cardiosuppression, leading to cerebral hypoperfusion in both ischemic core (P<0.01 versus vehicle group) and penumbra (P<0.01 versus vehicle group).
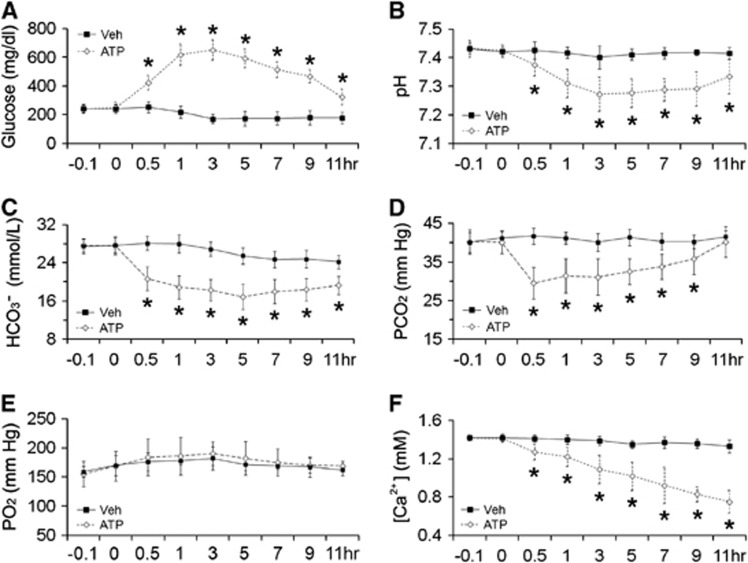
Adenosine 5′-Triphosphate Causes Severe Hyperglycemia and Acidosis in Rats
To investigate if ATP causes a disturbance in blood chemicals, we collected arterial blood samples via a femoral cannulation. As shown in Figure 6A, blood glucose concentration doubled shortly (30 minutes) after ATP injection, peaked at 3 hours and then subsided gradually (P<0.05 versus vehicle group). With a similar temporal profile, blood pH decreased after ATP injection, with a trough at 3 hours (Figure 6B) (P<0.05 versus vehicle group). This acidosis was accompanied by decreased levels of bicarbonate and PCO2 (Figures 6C and 6D) while PO2 remained steady (Figure 6E), indicating severe metabolic acidosis. To detect if ATP induced hypocalcemia, a risk factor for seizures,10, 15 we measured free ionized calcium in blood. As shown in Figure 6F, ATP caused a gradual but severe hypocalcemia (P<0.05 versus vehicle group). This hypocalcemia may contribute to the increased seizure rate in ATP-treated rats after MCAO.
Figure 6.
Effects of adenosine 5′-triphosphate (ATP) on blood parameters in rats. Arterial blood samples were collected from femoral cannulation in rats treated with vehicle or ATP and analyzed with a portable iSTAT Analyzer. (A) ATP induced hyperglycemia. (B) ATP caused acidosis with decreased bicarbonate (C) and PCO2 (D) levels while PO2 (E) remained unchanged. (F) ATP caused hypocalcemia. n=3 to 6, data are presented as mean±s.d., *P<0.05 versus vehicle group.
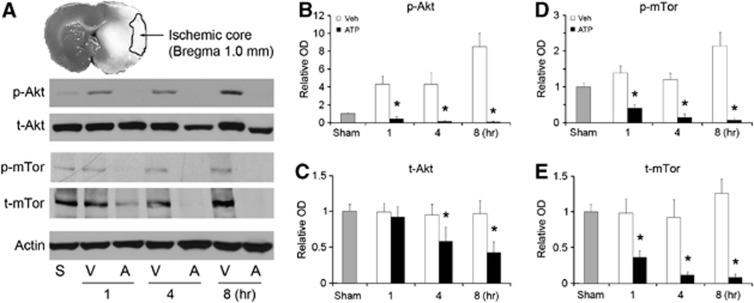
Adenosine 5′-Triphosphate Treatment Decreases the Levels of Phosphorylated Akt and Total Akt in Rat Brain after Middle Cerebral Artery Occlusion
Hypothermia may protect against stroke by increasing the phosphorylation state of Akt.16 We investigated Akt changes in the brain after MCAO and ATP treatment by standard western blotting on cortical lysates. As shown in Figure 7A, p-Akt levels were increased in the cortex of vehicle-treated rats 1 to 8 hours after MCAO while t-Akt remained unchanged (Figures 7A to 7C). These data support previous reports on compensatory changes in Akt after stroke.17, 18 In the ATP-treated group, however, p-Akt levels were significantly decreased, beginning as early as 1 hour after MCAO (Figures 7A and 7B). More surprisingly, t-Akt was also decreased in ATP group (Figures 7A and 7C). To confirm these findings, we also detected the phosphorylation state of mTor, a downstream substrate of Akt. In support of the Akt data, p-mTor was increased after MCAO in vehicle group, but decreased in the ATP group (Figures 7A, 7D and ) (P<0.01 versus vehicle group). These results reveal that ATP treatment decreases both p-Akt and t-Akt after ischemia.
Figure 7.
Adenosine 5′-triphosphate (ATP) treatment profoundly decreases levels of both phosphorylated Akt (p-Akt) and total Akt (t-Akt) in rat brains. Cortical tissues were collected from rats after middle cerebral artery occlusion (MCAO) at indicated times and subjected to protein extraction and western blot analysis. (A) Representative western blots, showing decreased levels of Akt and mammalian target of rapamycin (mTor) in ATP-treated groups. S, sham-operated; V, vehicle-treated, and A, ATP-treated. Semiquantitative levels of (B) p-Akt, (C) t-Akt, (D) phosphorylated mTor (p-mTor), and (E) t-mTor showing decreased Akt and mTor in the cortex. n=3, data are presented as mean±s.e.m., *P<0.01 versus sham and vehicle groups.
Discussion
Mild hypothermia has been considered as an effective and powerful neuroprotective strategy in the management of ischemic brain injury.2, 4 In the current study, we found that single intraperitoneally injection of ATP reproducibly induced mild hypothermia in rats for up to 7 hours. However, surprisingly ATP-induced hypothermia was associated with larger infarct volume and augmented neurologic deficits after MCAO. These findings suggest that the putative neuroprotection of ATP-induced hypothermia was counteracted by the severe side effects, such as cardiovascular depression and hyperglycemia, which might have partly mediated the hypothermia-inducing effect by ATP but essentially worsened the outcome.
Purinergic compounds (adenosine, AMP, ADP, and ATP) all have the capacity to lower body temperature in mice and rats.8, 10, 11 However, the exact mechanisms involved in the hypothermic response are still unclear. Mammalian thermoregulatory circuits are composed of three basic components: (1) sensory afferents: these neurons deliver the information on environmental temperatures from thermoreceptors to the brain; (2) integration hub: the hypothalamic preoptic area functions as the thermoregulatory center; (3) motor efferents: these neurons adjust body temperature by modulating brown adipose thermogenesis, cardiovascular activity, and muscle shivering.19 A previous report shows that disrupting the preoptic area with monosodium glutamate does not block the hypothermic effect of AMP, indicating that purinergic compounds induce hypothermia independent of the thermoregulatory center in the preoptic area.11 Theoretically, it therefore remains possible that motor efferents or effectors are responsible for the hypothermia induced by purinergic compounds. When used exogenously, these compounds may activate purinergic receptors, which could contribute to their hypothermic effects. Currently, four adenosine receptors (A1, A2a, A2b, A3) and five ATP receptor (P2X, P2Y, P2U, P2T, P2Z) have been identified. It was reported that pretreatment of mice with aminophylline, an adenosine receptor antagonist, attenuated the hypothermic effects of adenosine and AMP11 and hypoxia,20 suggesting important roles for adenosine receptors. However, the role of other purinergic in hypothermia is not clear.
Cardiovascular depression has been associated with AMP-induced mild hypothermia in rats.10 The current study also showed a relationship between ATP-induced hypothermia and decreased heartbeat rate and blood pressure. These effects lasted several hours after intraperitoneal injection, in accordance with the kinetic profile of hypothermia. Thus, we speculate that the hypothermia caused by purinergic compounds is probably dependent on the lower heart output and vasodilation. Although adenosine may be a mediator, it is unclear how purinergic compounds cause deep cardiovascular depression. Adenosine stimulates adenosine receptors in the heart and blood vessels,11, 21 leading to cardiovascular depression and AMP, ADP, and ATP can all be degraded to adenosine. Additionally, ATP can suppress the conduction of the atrioventricular node22 while luminal perfusion of ATP and ADP can dilate the middle cerebral artery in rats.23 Thus, it also seems that ATP can directly cause cardiovascular depression. It is also possible that ATP acts as a neurotransmitter that inhibits cardiovascular activity by affecting cardiovascular nuclei in the central nervous system.24
Hypothermia can also be the result of hypometabolism of glucose. The AMP-induced hypothermia was associated with hypometabolism in mouse liver, one of the major organs for thermogenesis.25 However, the liver may not be the only organ affected, because hyperglycemia was commonly noticed under hypothermic conditions in the laboratory10, 26 and in clinical studies.27, 28 The cause of the hyperglycemia is not fully understood, although insulin deficiency may be a culprit. Hypothermia increases insulin resistance,29 decreases insulin levels in blood,10 and decreases insulin receptor numbers in liver, fat, and muscle.29 Insulin deficiency limits glucose entry into cells, leading to hypometabolism. Because AMP can decrease serum levels of insulin,30 ATP, an AMP precursor, may have a similar effect. Adding to this trauma, cardiosuppression causes systemic hypoperfusion, thereby reducing glucose delivery and further exacerbating hypometabolism.
It remains unclear why ATP is less effective in inducing hypothermia when it is intravenously injected. As shown in Figure 1B, the hypothermic effect of 2 g/kg intravenous ATP is only similar to 0.5 g/kg of intraperitoneal injection. One possible reason may be that ATP is rapidly degraded when it is infused directly into blood, because both blood and blood vessels show high levels of enzymes that metabolize purinergic chemicals. For instance, triphosphate diphosphohydrolase, which converts ATP to ADP or ADP to AMP, is rich in endothelia.31 Ecto-nucleotide pyrophosphatase/phosphodiesterase, which converts ATP to AMP, is rich in platelets.32 Purine nucleoside phosphorylase, which degrades inosine, a breakdown product of adenosine, is rich in neutrophils.33 These enzymes quickly degrade the ATP that enters the blood, decreasing its working concentration. In contrast, ATP infused intraperitoneally may also act directly on abdominal organs such as the liver to induce hypothermia through unknown mechanisms before it is finally degraded in blood.
ATP can be a double-edge sword to neurons. On one hand, neurons need ATP as an energy source. On the other hand, ATP can also kill neurons under certain conditions when the concentration exceeds a critical threshold. Intracellular ATP concentrations are in the millimolar (mmol/L) range under physiologic conditions, whereas extracellular ATP is quite low, ranging from nanomolar (nmol/L) to micromolar (μmol/L) concentrations. Extracellular ATP has an important role in cell-to-cell signaling by acting on ionotropic P2X and G protein-coupled P2Y receptors in the central nervous system. However, if extracellular ATP concentrations are too high, ATP becomes toxic. An example of such a condition is brain ischemia where injured and dying cells release ATP into the extracellular space through their damaged cell membranes. Focal ischemia in rats increases striatal ATP levels to 50 nmol/L.34 At this low concentration, however, ATP may not directly kill neurons. The ATP alone evokes neurotoxicity at 100 μmol/L in cerebellar granular cells, 500 μmol/L in striatum neurons, 2 mmol/L in hippocampus cultures, and 45 mmol/L in retina.35, 36 In this study, we used primary cortical neurons and did not observe neurotoxicity at 4 mmol/L ATP alone or when ATP was added to the media during OGD. These data suggest that the detrimental effects of ATP on stroke in rats may not result from a direct action of ATP on neurons, but a systemic or peripheral action. Intraperitoneally injected ATP might reach peripheral organs more effectively than the neurons in the ischemic core because blood flow to the ischemic core is so severely reduced. Furthermore, if ATP is rapidly degraded in the blood, it may not have a chance to get to the brain in sufficient quantities to directly kill neurons.
The exact mechanisms for the adverse effects of ATP observed in the present study are not fully understood. However, ATP induces cardiosuppression and hypoperfusion, which further decreases cerebral blood flow and may thereby directly increase the infarct size. The ATP induces severe hyperglycemia, a notoriously adverse factor for stroke that is also associated with HT and metabolic acidosis. The ATP also increases seizure incidence, probably because of hypocalcemia. It has been long held that hyperphosphatemia causes hypocalcemia.37 Therefore, ATP infusion may lead to profound hypocalcemia by raising blood phosphorus. These adverse factors are similar to what we observed in the AMP study, where they have been extensively discussed.10 However, a recent study in rats reported that hypothermia induced by AMP benefits myocardial infarction models.38 In heart ischemia, the bradycardia and hypothermia induced by AMP can be beneficial, as they reduce oxygen consumption and metabolic stress. However, it is the case for brain ischemia, as the bradycardia and hypotension induced by ATP or AMP cannot decrease the metabolic needs of brain; instead cardiosuppression decreases the nutrition or oxygen supply to the injured brain tissue. Although hypothermia is thought to be protective against brain injury, it probably cannot counteract the severe cardiovascular depression caused by ATP or AMP. This is a possible reason contributing to the discrepancy brain and heart.
Finally, another negative effect of ATP on brain ischemia is that ATP decreases Akt levels. Akt is a master kinase promoting neuronal survival and hypothermia might protect through activation of Akt.16 Both ATP and adenosine can increase Akt phosphorylation in neurons and astrocytes.39, 40 In the current study, however, ATP treatment decreased both p-Akt levels and t-Akt levels in ischemic cortex. We propose that this is the result of early and accelerated infarction of brain tissues, leading to degradation of total Akt protein.
In summary, intraperitoneal injection of ATP induces hypothermia in rats in a dose-dependent manner. When this type of hypothermia is applied to brain ischemia, no neuroprotection is achieved. Instead, the adverse consequences of the ischemic event are greatly magnified. The results of this study suggest that the precise means of inducing hypothermia and systemic side effects determine whether the treatment will actually protect the brain. Thus, the present study is the first demonstration that ATP-induced hypothermia is associated with a striking exacerbation of ischemic outcome and its behavioral manifestations. Worse yet, ATP-induced hypothermia is linked to enhanced mortality. This unexpected finding cautions that pharmacological CBT regulators need to be carefully evaluated for adverse systemic reactions and indirect effects on the brain before they can be effectively exploited in the clinic.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH grants (NS36736, NS43802 and NS45048 to JC), the VA Merit Review Grant (to JC) and the American Heart Association (10SDG2560122 to FZ).
References
- Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care. 2010;16:525–533. [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. The Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31:2041–2051. doi: 10.1097/01.CCM.0000069731.18472.61. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- White RD, Goodman BW, Arendt CJ. Neurologically intact survival following prolonged cardiac arrest monitored with continuous capnography and subsequent treatment with therapeutic hypothermia. Mayo Clin Proc. 2011;86:1124–1125. doi: 10.4065/mcp.2011.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oommen SS, Menon V. Hypothermia after cardiac arrest: Beneficial, but slow to be adopted. Cleve Clin J Med. 2011;78:441–448. doi: 10.3949/ccjm.78a.10157. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Petersson J, Norrving B, Hacke W, Dirnagl U, Wagner M, et al. Hypothermia for stroke: call to action 2010. Int J Stroke 2010. 5:489–492. doi: 10.1111/j.1747-4949.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- Lee CC. Is human hibernation possible. Annu Rev Med. 2008;59:177–186. doi: 10.1146/annurev.med.59.061506.110403. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Luo Y, Ji X, Nemoto EM, Chen J. When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29:1022–1034. doi: 10.1038/jcbfm.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol. 2007;293:R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ, Fluoro-Jade B. a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Belluzzo M, Monti F, Pizzolato G. A case of hypocalcemia-related epilepsia partialis continua. Seizure. 2011;20:720–722. doi: 10.1016/j.seizure.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguls B, Justicia C, Pallas M, Planas AM. Focal cerebral ischemia causes two temporal waves of Akt activation. Neuroreport. 2001;12:3381–3384. doi: 10.1097/00001756-200110290-00046. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Warita H, Sasaki C, Ri Zhang W, Sakai K, Shiro Y, et al. Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci Lett. 1999;274:45–48. doi: 10.1016/s0304-3940(99)00676-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Barros RC, Branco LG, Carnio EC. Respiratory and body temperature modulation by adenosine A1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir Physiol Neurobiol. 2006;153:115–125. doi: 10.1016/j.resp.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Stange K, Lagerkranser M, Rudehill A, Sollevi A. Effects of adenosine-induced hypotension on cerebral blood flow and metabolism in the pig. Acta Anaesthesiol Scand. 1989;33:199–203. doi: 10.1111/j.1399-6576.1989.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Pelleg A, Kutalek SP, Flammang D, Benditt D. ATPace: injectable adenosine 5′-triphosphate: Diagnostic and therapeutic indications. Purinergic Signal. 2012;8 (Suppl 1:57–60. doi: 10.1007/s11302-011-9268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Johnson TD, Childres WF, Bryan RM. Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. Am J Physiol. 1997;273 (3 Part 2:H1472–H1477. doi: 10.1152/ajpheart.1997.273.3.H1472. [DOI] [PubMed] [Google Scholar]
- Passamani LM, Pedrosa DF, Mauad H, Schenberg LC, Paton JF, Sampaio KN. Involvement of the purinergic system in central cardiovascular modulation at the level of the nucleus ambiguus of anaesthetized rats. Exp Physiol. 2011;96:262–274. doi: 10.1113/expphysiol.2010.054882. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Miki T, Van Oort-Jansen A, Matsumoto T, Loose DS, Lee CC. Hepatic gene expression profiling of 5′-AMP-induced hypometabolism in mice. Physiol Genomics. 2011;43:325–345. doi: 10.1152/physiolgenomics.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N. Management of pitfalls for the successful clinical use of hypothermia treatment. J Neurotrauma. 2009;26:445–453. doi: 10.1089/neu.2008.0648. [DOI] [PubMed] [Google Scholar]
- Cueni-Villoz N, Devigili A, Delodder F, Cianferoni S, Feihl F, Rossetti AO, et al. Increased blood glucose variability during therapeutic hypothermia and outcome after cardiac arrest. Crit Care Med. 2011;39:2225–2231. doi: 10.1097/CCM.0b013e31822572c9. [DOI] [PubMed] [Google Scholar]
- Melhuish T. Linking hypothermia and hyperglycemia. Nurs Manage. 2009;40:42–45. doi: 10.1097/01.NUMA.0000365472.26379.be. [DOI] [PubMed] [Google Scholar]
- Torlinska T, Perz M, Madry E, Hryniewiecki T, Nowak KW, Mackowiak P. Effect of hypothermia on insulin-receptor interaction in different rat tissues. Physiol Res. 2002;51:261–266. [PubMed] [Google Scholar]
- Cai Y, Wang Q, Ling Z, Pipeleers D, McDermott P, Pende M, et al. Akt activation protects pancreatic beta cells from AMPK-mediated death through stimulation of mTOR. Biochem Pharmacol. 2008;75:1981–1993. doi: 10.1016/j.bcp.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and Characterization of CD39/Vascular ATP Diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Furstenau CR, Trentin Dda S, Barreto-Chaves ML, Sarkis JJ. Ecto-nucleotide pyrophosphatase/phosphodiesterase as part of a multiple system for nucleotide hydrolysis by platelets from rats: kinetic characterization and biochemical properties. Platelets. 2006;17:84–91. doi: 10.1080/09537100500246641. [DOI] [PubMed] [Google Scholar]
- Moriwaki Y, Yamamoto T, Higashino K. Enzymes involved in purine metabolism—a review of histochemical localization and functional implications. Histol Histopathol. 1999;14:1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]
- Melani A, Corti F, Stephan H, Müller CE, Donati C, Bruni P, et al. Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp Neurol. 2012;233:193–204. doi: 10.1016/j.expneurol.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Amadio S, D'Ambrosi N, Cavaliere F, Murra B, Sancesario G, Bernardi G, et al. P2 receptor modulation and cytotoxic function in cultured CNS neurons. Neuropharmacology. 2002;42:489–501. doi: 10.1016/s0028-3908(01)00197-6. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Fletcher E. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J Comp Neurol. 2009;513:430–440. doi: 10.1002/cne.21964. [DOI] [PubMed] [Google Scholar]
- Burke MD. Calcium and phosphorus studies: interpretation of results and strategies for further testing. Postgrad Med. 1980;68:69–72. doi: 10.1080/00325481.1980.11715488. [DOI] [PubMed] [Google Scholar]
- Tao Z, Zhao Z, Lee CC. 5′-Adenosine monophosphate induced hypothermia reduces early stage myocardial ischemia/reperfusion injury in a mouse model. Am J Transl Res. 2011;3:351–361. [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006;26:3798–3804. doi: 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, et al. P2 × 7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol. 2004;141:1106–1117. doi: 10.1038/sj.bjp.0705685. [DOI] [PMC free article] [PubMed] [Google Scholar]