Abstract
Second-generation radioligands for translocator protein (TSPO), an inflammation marker, are confounded by the codominant rs6971 polymorphism that affects binding affinity. The resulting three groups are homozygous for high-affinity state (HH), homozygous for low-affinity state (LL), or heterozygous (HL). We tested if in vitro binding to leukocytes distinguished TSPO genotypes and if genotype could affect clinical studies using the TSPO radioligand [11C]PBR28. In vitro binding to leukocytes and [11C]PBR28 brain imaging were performed in 27 human subjects with known TSPO genotype. Specific [3H]PBR28 binding was measured in prefrontal cortex of 45 schizophrenia patients and 47 controls. Leukocyte binding to PBR28 predicted genotype in all subjects. Brain uptake was ∼40% higher in HH than HL subjects. Specific [3H]PBR28 binding in LL controls was negligible, while HH controls had ∼80% higher binding than HL controls. After excluding LL subjects, specific binding was 16% greater in schizophrenia patients than controls. This difference was insignificant by itself (P=0.085), but was significant after correcting for TSPO genotype (P=0.011). Our results show that TSPO genotype influences PBR28 binding in vitro and in vivo. Correcting for this genotype increased statistical power in our postmortem study and is recommended for in vivo positron emission tomography studies.
Keywords: neuroinflammation, PBR28, schizophrenia, translocator protein
Introduction
Translocator protein 18 kDa (TSPO) is a proposed biomarker for neuroinflammation. Positron emission tomography (PET) can measure the density of this biomarker using radioligands that bind to TSPO.1 The prototypical radioligand [11C](R)-PK 11195 has been used to detect increased TSPO density in diverse disorders characterized by neuroinflammation, including Alzheimer's disease, stroke, multiple sclerosis, rheumatoid arthritis, and frontotemporal dementia.2, 3, 4, 5, 6 However, [11C](R)-PK 11195 has several disadvantages, including low specific-to-nonspecific binding.7 These limitations prompted the development of radioligands with greater specific signal, including [11C]PBR28, [18F]PBR06, [11C]DAA1106, [11C]DPA713, [18F]PBR111, [18F]FEPPA, and [11C]AC-5216.8, 9, 10, 11, 12, 13, 14
Unfortunately, quantification of binding for all tested second-generation TSPO radioligands is confounded by the expression of two different forms of TSPO, coded by the rs6971 single-nucleotide polymorphism (SNP).15 This SNP in exon 4 of the TSPO gene causes a nonconservative alanine-to-threonine substitution in position 147. Among persons of European ancestry, the predominant form (alanine) has a prevalence of ∼70% and high-affinity binding; the polymorphism (threonine) has a prevalence of ∼30% and low-affinity binding. The prevalence of three resulting combinations is: 49% for homozygous high-affinity (HH); 9% for homozygous low-affinity (LL); and 42% for heterozygotes (HL) (http://hapmap.ncbi.nlm.nih.gov/cgi-perl/snp_details_phase3?name=rs6971&source=hapmap28_B36&tmpl=snp_details_phase3).
The existence of these two forms of TSPO was first suggested by PET scans using [11C]PBR28, which has about 50-fold differential affinity in HH compared with LL subjects.7, 16 Furthermore, we now know that all second-generation TSPO radioligands have differential affinity, ranging from fourfold to 50-fold.17 A recent study by Mizrahi et al18 using [18F]FEPPA was the first to demonstrate the association of TSPO genotype and variability in PET imaging. To decrease variability in our PET studies, we routinely exclude LL subjects (about 9% of the population) based on low-affinity binding to peripheral leukocytes. Nevertheless, our PET subjects are a mixture of HH and HL genotypes, which adds variability to measurements. Furthermore, PET studies using other second-generation radioligands include all three genotypes (HH, HL, and LL).
The differential affinity of TSPO confounds the clinical application of TSPO radioligands because it adds variance to the measurements. However, the degree to which TSPO genotype influences in vivo binding measured by PET is unknown. This study sought to assess the utility of in vitro receptor binding to distinguish these three groups (HH, LL, and HL) and to extrapolate its impact on the sensitivity of future clinical PET studies. More specifically, we sought to determine: (1) if TSPO genotype correlates with in vitro PBR28 binding using peripheral leukocytes and in vivo using brain PET imaging; (2) if differential affinity exists for PBR28 both in controls and in disease states (in this case, schizophrenia); and (3) if correcting for genotype improves the sensitivity of PBR28 to detect group differences in TSPO density measured in postmortem brain from schizophrenia subjects and healthy controls. Finally, we estimated the nondisplaceable uptake of [11C]PBR28 to further characterize the potential clinical utility of this radioligand. Since HH and HL subjects are expected to have different contributions of specific binding, we used the measured total brain binding with PET (in vivo study) and the specific binding in postmortem brain tissue (in vitro study) to estimate nondisplaceable uptake. Because LL subjects are excluded from our PET scans, we used only HH and HL subjects for leukocyte binding and PET imaging studies in live subjects. For the postmortem study conducted to assess between-group differences, all three genotypes were included.
Materials and methods
This study was approved by the NIMH Combined Neurosciences Institutional Review Board and all volunteers gave their informed consent before inclusion in the study.
In Vitro Study
In Vitro Leukocyte Assay
Leukocytes (white blood cells) were collected from 32 healthy volunteers (27 were used in the in vivo study) and 9 patients with Alzheimer's disease. Leukocytes were isolated and homogenized as previously described.7
To determine whether subjects were high- or mixed-affinity binders, heterologous receptor binding assays were performed using the racemic radioligand [3H]PK 11195 (3.2 GBq/μmol; Perkin-Elmer Life and Analytical Sciences, Boston, MA, USA), with cold PBR28 as the displacer. In brief, 100 μL [3H]PK 11195 (∼0.9 nmol/L) was added to each assay tube, followed by 100 μL of 12 cold PBR28 concentrations (0.01 to 3,000 nmol/L), 50 mmol/L HEPES (pH=7.4) buffer (to determine total binding), or 10 μmol/L PBR28 (to determine nonspecific binding). In all, 100 μL leukocyte homogenate suspension (20 μg/mL protein) was added to each tube and incubated for 30 minutes in a shaking water bath at 23°C. Samples were filtered with a Brandel cell harvester (Gaithersburg, MD, USA) through Whatman GF/A filter paper (pretreated with 0.5% polyethyleneimine), followed by three washes of 1 mL ice-cold 50 mmol/L HEPES buffer (pH=7.4; 4°C). Radioactivity was measured with liquid scintillation counting for five minutes using 4 mL of Ultima-Gold (Perkin-Elmer, Chicago, IL, USA).
Data for heterologous binding assays were analyzed using nonlinear regression curve-fitting software provided by GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). A mean KD of 4.7 nmol/L for [3H]11195 was used as the dissociation constant to calculate Ki.7 One- and two-site fitting were compared to determine which equation fits best. Two-site fitting was the preferred model when the P value for null hypothesis (the one-site fitting) was <0.05. The HH were identified as subjects whose equation fit best to one-site fitting with a Ki <1 nmol/L. HL were identified as subjects whose data best fit the two-site equation.
Genetic Analysis
Genomic DNA from 49 subjects was included to genotype the SNP, rs6971, within the TSPO gene on chromosome 22q13.2. This technique integrates a polymerase chain reaction (PCR)-based assay with laser scanning technology to excite fluorescent dyes present in the specially designed TaqMan probes (Applied Biosystems,Carlsbad, CA, USA). One probe perfectly matched to the common (G) allele labeled with VIC, and the second probe matched to the variant (A) allele labeled with 6-carboxyfluorescein (FAM). Genomic DNA was extracted from blood using the AutoGen automated systems and AutoGenFlex STAR Whole Blood Extraction kit, catalog # AGKT-WB-640 (Holliston, MA, USA). In all, 6 μL of human genomic DNA, at 2.5 ng/μL, from each subject, was applied to 48 wells of a 96-well PCR plate and allowed to dry at 50°C for ∼3 hours. A working master mix was prepared that contained: 0.25 μL of TaqMan genotyping probe mix (40 × ), 2.25 μL of TaqMan Genotyping Master Mix (Applied Biosystems), and 2.5 μL of water. Ultimately, a total of 5 μL of this mix was added to the dry DNA samples along with 10 μL of mineral oil to avoid evaporation during PCR cycling. The context sequence [VIC/FAM] was as follows:
CCCCTACCTGGCCTGGCTGGCCTTC[A/G]CGACCACACTCAACTACTGCGTATG
Among 11 control samples genotyped, 4 of 4 A/A, 4 of 4 G/A, and 3 of 3 G/G samples produced the expected genotypes.
Postmortem Brain Samples
Postmortem samples of dorsolateral prefrontal cortex (DLPFC) tissue from 45 patients with schizophrenia and 47 healthy controls were collected as previously described.19 Informed consent was obtained from family members according to established guidelines. Medical, psychiatric, and substance use history, smoking status, and demographic information were collected by telephone interview with next-of-kin within 1 week of donation (Supplementary Table 1).
For individuals with schizophrenia, each case was reviewed by two board-certified psychiatrists to establish DSM-IV Axis I lifetime psychiatric diagnoses, using psychiatric record reviews and/or family informant interviews.19 Normal controls had no history of significant psychological problems or care, psychiatric admissions, lifetime history of substance abuse or dependence, or acute substance intoxication. Toxicology testing was conducted on every case to screen for ethanol and illicit drugs. For individuals with schizophrenia, additional testing was performed by National Medical Services (Willow Grove, PN, USA) to assess antipsychotic medication use at the time of death. Whenever possible, use of antipsychotic medications was culled from available medical records and converted to CPZ (chlorpromazine) equivalent (CPZE) doses in milligrams.
Gray matter tissue from the crown of the middle frontal gyrus was obtained from the coronal slab midway between the frontal pole and the most anterior extent of the genu of the corpus callosum. Dorsolateral prefrontal cortex corresponding to Brodmann's areas 9 and 46 was dissected on dry ice using a hand-held dental drill and immediately stored at −80°C.
All brain samples had been genotyped for multiple loci, including the rs6971 SNP, using Human 1 M duo v3 chip via standard procedures (Illumina, San Diego, CA, USA).
In Vitro [3H]PBR28 Receptor Binding Assay
Tissue was homogenized in buffer (20 mmol/L HEPES, 5 mmol/L MgCl2, 1 mmol/L EDTA, pH 7.4) with a Teflon pestle using a Glas-Col Homogenizing System and centrifuged at 25,000 g for 25 minutes at 4°C. The pellet was resuspended, aliquotted, and stored at −80°C. Protein concentration was determined using the Micro BCA Protein Assay Kit (Thermo-Scientific, Rockford, IL, USA).
To determine the specific binding of PBR28, a two-point binding assay was performed in triplicate. In all, 100 μL [3H]PBR28 (specific activity 2.3 GBq/μmol; Amersham, UK) was added to each assay tube, followed by 100 μL of buffer (to determine total binding), or 10 μmol/L cold PBR28 (to determine nonspecific binding). In all, 100 μL DLPFC homogenate suspension (∼20 μg/mL) was added last to initiate the incubation. Specific binding was calculated by subtracting nonspecific binding from total binding.
Positron Emission Tomography Imaging with [11C]PBR28
Twenty-seven healthy volunteers (45.7±17.9 years) underwent [11C]PBR28 PET imaging. Supplementary Table 2 shows demographic information for each subject.
[11C]PBR28 was synthesized as described in Investigational New Drug Application #76 441, a copy of which is available at: http://pdsp.med.unc.edu/snidd/. At the time of injection, [11C]PBR28 had high radiochemical purity (>99%) and high-specific activity (122±67 GBq/μmol). The injected dose of [11C]PBR28 was 658±62 MBq.
The procedures for scan acquisition and image analysis are similar to those previously described for [11C]PBR28;16 these are described in detail in the Supplementary Material. Total distribution volume (VT, mL/cm3) was calculated for each brain region. We followed proposed consensus nomenclature for reversibly binding radioligands,20 where VT is the sum of both specific and nondisplaceable uptake, which equals the ratio of radioligand concentration in the brain to that in arterial plasma at equilibrium and is proportional to receptor density.
Estimation of Nondisplaceable Uptake of [11C]PBR28
We used the postmortem results to estimate the relative expression of H and L forms in HL subjects, and then used total uptake (VT) measured in HH and HL subjects from the PET data to estimate specific (VS) and nondisplaceable uptake (VND) using the following equations.
Where x was determined by dividing the specific binding of [3H]PBR28 in postmortem tissue in HL subjects by that in HH subjects. By combining equations (1 and 2), and assuming VND is uniform across subjects:
Using the measured values of VT from the PET data of HH and HL subjects, we then solved for VND to estimate the nonspecific uptake of [11C]PBR28.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 17.0. Differences in specific binding of [3H]PBR28 (in vitro study) and distribution volume (VT) of [11C]PBR28 (in vivo study) were compared between HH and HL groups by independent sample t-tests, and among all three genotypes by one-way analysis of variance (ANOVA). Differences in the specific binding of [3H]PBR28 between schizophrenia patients and controls was compared using factorial ANOVA with genotype as a fixed factor. Associations between [3H]PBR28 binding and demographic variables were evaluated using Pearson correlation analysis. Observed P values from t-tests were corrected for multiple comparisons using the false discovery rate,21 with P value threshold for significance adjusted to=(n/7) × 0.05, where n is the rank of the observed P value for each of the seven brain regions tested. Effects of demographic variables were evaluated with factorial ANOVA.
Data are given as mean±s.d.
Results
In Vitro Leukocyte Assay Results Predicted Translocator Protein Genotype
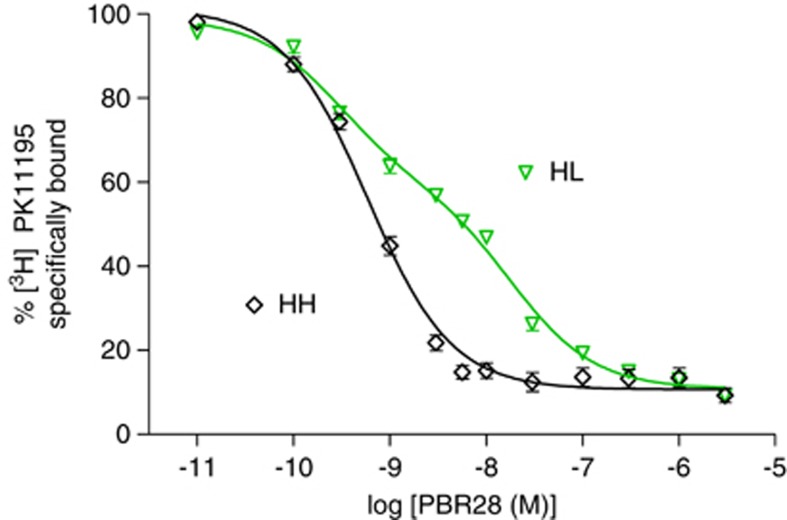
To determine if the HH and HL genotypes of TSPO correlated with in vitro PBR28 binding, leukocytes were collected from 32 healthy volunteers and 9 patients with Alzheimer's disease. In 19 of these subjects, the displacement curve best fit a one-site model, suggesting that these subjects express TSPO with high affinity for PBR28 (HH; Figure 1). The average Ki for these subjects was 0.52±0.21 nmol/L. For the remaining 22 subjects, the displacement curve best fit a two-site model, suggesting that these subjects expressed two variants of TSPO (HL), one with high affinity for PBR28 (Ki of 0.22±0.13 nmol/L) and one with low affinity for PBR28 (Ki of 24.0±17.5 nmol/L). All subjects were genotyped for the Ala147Thr polymorphism, and one- and two-site fits correctly identified the genotype in all subjects. That is, all 22 subjects with a two-site fit of binding to leukocytes were heterozygous, and all of the 19 HH subjects with a one-site fit were homozygous, for the G-allele.
Figure 1.
Representative displacement by PBR28 of [3H]PK 11195 binding to leukocyte membranes. PBR28 showed a one-site displacement in high-affinity state (HH) subjects (n=17) but a two-site displacement in heterozygous (HL) subjects (n=17). One- and two-site fitting was determined by GraphPad Prism 5.0 and was performed without knowledge of genotype. Symbols represent mean±s.d., which was sometimes smaller than the symbol itself.
The Ala147Thr Polymorphism Reduced [11C]PBR28 Binding In Vivo
To determine if TSPO genotype correlated with in vivo PBR28 binding, 27 healthy volunteers from the in vitro study (9 HH and 18 HL) also underwent PET imaging with [11C]PBR28. The HH subjects had ∼40% greater [11C]PBR28 binding than HL subjects in all regions measured (P<0.02; Table 1). The greater brain uptake in HH versus HL subjects was not due to differential peripheral factors of metabolism or plasma protein binding of the radioligand. Brain uptake was measured as VT, which corrected for differences in metabolism by normalizing brain uptake to exposure from only the parent radioligand, separated from radiometabolites. Even though the modeling corrects for differences in the arterial input function, the time-activity curves for parent radioligand were very similar for both HH and HL groups (Supplementary Figure 1A). In contrast, the brain time-activity curves were different, with HH subjects showing slower washout than HL subjects (Supplementary Figure 1B). In addition, the plasma-free fraction (fP) of [11C]PBR28 was not statistically different between the HH and HL groups (4.6% versus 3.8%, P=0.19, overall mean=4.1±1.4%).
Table 1. Total [11C]PBR28 binding (V T, mL/cm3).
| Region | HH (n=9) | HL (n=18) | P value | Adjusted P value threshold (FDR corrected) |
|---|---|---|---|---|
| Medial temporal cortex | 4.6 (1.5) | 3.0 (0.8) | 0.001 | 0.007 |
| Cingulate cortex | 4.5 (0.6) | 3.0 (0.8) | 0.003 | 0.014 |
| Temporal cortex | 4.6 (1.5) | 3.2 (0.9) | 0.007 | 0.021 |
| Whole brain | 4.5 (1.4) | 3.2 (0.9) | 0.008 | 0.029 |
| Frontal cortex | 4.5 (1.4) | 3.3 (0.9) | 0.008 | 0.036 |
| Parietal cortex | 4.4 (1.3) | 3.3 (0.9) | 0.014 | 0.043 |
| Occipital cortex | 4.5 (1.4) | 3.3 (0.9) | 0.018 | 0.050 |
HH, high-affinity binder; HL, mixed-affinity binder; FDR, false discovery rate.
Data are given as mean with s.d. below in parenthesis.
Translocator Protein Showed Differential Affinity in Brain Tissue from Both Control and Schizophrenia Subjects
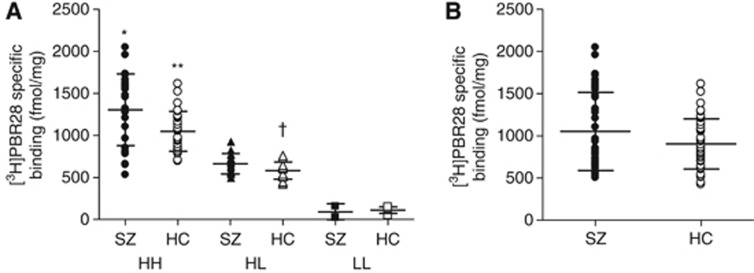
To simulate the effect of genotype on a potential clinical PET study of TSPO, we measured [3H]PBR28 binding in postmortem samples of DLPFC from 45 patients with schizophrenia and 47 control subjects, all with known TSPO genotype. Among the controls, specific binding was higher in HH subjects (1,047±237 fmol/mg) than HL (582±101 fmol/mg) and LL subjects (111±38 fmol/mg) (P<0.001; Figure 2A). Although LL subjects had completely separate specific binding values, HH and HL subjects had overlapping values, showing that binding was affected by factors other than genotype. Specific binding in HL controls was, on average, 56% of that in HH controls. Individuals with schizophrenia had a similar distribution of binding among the three genotypes (Figure 2A). Specific binding was greater in HH patients (1,303±426 fmol/mg) than in HL (664±121 fmol/mg) and LL patients (91±95 fmol/mg, P<0.001). No interaction was noted between specific [3H]PBR28 binding and age at death, gender, race, height, weight, or body mass index.
Figure 2.
Specific [3H]PBR28 binding in dorsolateral prefrontal cortex (DLPFC) from schizophrenia patients (SZ) and healthy controls (HC) with (A) and without (B) stratification based on genotype. (A) Using a one-way analysis of variance (ANOVA), specific binding in the high-affinity state (HH) group was statistically significant as follows: *P<0.001 versus heterozygous (HL) and low-affinity state (LL) SZ, and P=0.007 versus HH HC. **P<0.001 versus HL and LL HC, †P<0.001 versus LL HC. (B) The distribution of specific binding for HH and HL subjects shown in this panel would mimic positron emission tomography (PET) imaging results in living patients, after our typical exclusion of LL subjects. One-way ANOVA resulted in nonsignificant differences between HC and SZ groups (P=0.085). When genotype was added to the statistical model as a fixed factor; however, SZ patients had significantly greater specific binding than HC subjects (P=0.011).
Notably, HH schizophrenia patients had greater specific [3H]PBR28 binding than HH controls (Figure 2A; P=0.007); however, HL schizophrenia patients did not have significantly higher binding than HL controls (P=0.060), suggesting a blunted association between schizophrenia and TSPO density in the HL subjects. Moreover, when HH and HL subjects were grouped together, brain tissue from individuals with schizophrenia showed 16% greater specific binding of [3H]PBR28 than brain tissue from healthy controls (Figure 2B). By itself, this difference was initially not statistically significant (P=0.085). However, when binding affinity status was included as a covariate in the statistical model, this difference reached significance (P=0.011).
Schizophrenia patients were older at age of death (55.3±14.0 versus 41.6±13.7 years), which could explain the difference in [3H]PBR28 binding between patient and controls. A significant correlation was noted in HH schizophrenia patients between [3H]PBR28 binding and age of death (r=0.44, P=0.023); however, no correlation was seen in HH controls or in HL subjects of either group. In addition, a stronger correlation was seen between [3H]PBR28 binding and duration of illness among HH patients (r=0.54, P=0.006), suggesting that age at death is a confounding factor related to the length of the course of schizophrenia.
Because the difference in [3H]PBR28 binding could also be explained by premortem antipsychotic medication use in schizophrenia patients, we investigated the possible association between [3H]PBR28 binding and antipsychotic use. We found that specific binding was the same in schizophrenia patients taking antipsychotics at the time of death (1,028±445 fmol/mg, n=34) as those not taking antipsychotics (1,136±545 fmol/mg, n=9), even after correcting for TSPO genotype (P=0.687). Specific [3H]PBR28 binding did not correlate with median, lifetime, or last CPZE dose in HH or HL patients (P>0.21). When the statistical model included TSPO genotype and antipsychotic medication use as covariates, schizophrenia patients still had significantly greater specific [3H]PBR28 binding (P=0.015).
Schizophrenia patients had lower measures of brain pH (6.2±0.2 versus 6.5±0.3), shorter brain storage time before analysis (8.1±2.8 versus 10.1±3.2 years), and greater prevalence of premortem nicotine use (79.1% versus 31.0%) than controls (Supplementary Table 1); however, neither brain pH nor brain storage time significantly correlated with specific [3H]PBR28 binding in either controls or schizophrenia patients (P>0.1). In addition, nicotine users did not have different specific binding than nonusers (P=0.312, LL subjects excluded).
Estimation of Nondisplaceable Uptake of [11C]PBR28
We used the postmortem results to estimate the relative expression of H and L forms in HL subjects, and then used total uptake (VT) in HH and HL subjects to estimate specific (VS) and nondisplaceable uptake (VND).
We assumed that binding to the L form is negligible (2%) compared with that to the H form and that VND is uniform across all subjects. The postmortem study showed that specific binding in HL subjects was 56% of that in HH subjects (Figure 2).
VT for whole brain in HH subjects, as measured by PET, was 1.4 times that in HL subjects (Table 1).
The solution of all four equations predicts that VND=1.6 mL/cm3.
Discussion
The results of this study demonstrate that both in vitro and in vivo binding of PBR28 correlated with the Ala147Thr polymorphism in the TSPO gene. In vitro displacement assays with peripheral leukocytes accurately predicted TSPO genotype in all subjects. Thus, it appears that either leukocyte binding or genotyping can be used to accurately separate TSPO genotypes. In vivo, brain uptake of [11C]PBR28 was on average 40% higher in HH than in HL subjects, but with significant overlap. In addition, TSPO binding in postmortem brain from individuals with schizophrenia was 16% higher than in control brain, an effect that was statistically significant only after correcting for TSPO genotype. Our results strongly suggest that clinical studies with [11C]PBR28 will have increased statistical power and require smaller sample sizes if they incorporate the genotype of the subjects. Thus, we recommend measuring the Ala147Thr polymorphism in all future studies using [11C]PBR28, as well as in other second-generation TSPO radioligands.
The results of this study are particularly important because they demonstrate that the Ala147Thr polymorphism is associated with reduced binding affinity to PBR28 in vitro as well as total radioligand binding in vivo. This indicates that PET data from clinical studies using [11C]PBR28 are influenced by the presence or absence of this TSPO polymorphism, underscoring the importance of determining genotype in studies using this radioligand. Based on our results, displacement binding assay results are equivalent to those from genotype analysis using PCR, allowing flexibility for investigators interested in using [11C]PBR28.
It is important to note that although we have treated this Ala147Thr polymorphism as a nuisance variable that adds noise to PET imaging, it might also have clinical significance. Translocator protein in the outer mitochondrial membrane transports cholesterol to CYP11A1 on the inner mitochondrial membrane to synthesize pregnenolone, a precursor for steroids. Thus, increased expression of TSPO in macrophages and activated microglia appear critical for the increased production of steroids by these inflammatory cells. We are aware of only one prior study on the effect of this Ala147Thr polymorphism on steroid production, in which Costa et al22 found that Thr147 homozygous or heterozygous volunteers had less pregnenolone in peripheral lymphocytes than Ala147 homozygous volunteers. Therefore, it is possible that HL and LL individuals have a reduced ability to respond to host cell injury via TSPO-mediated pathways.
Translocator Protein Binding in Schizophrenia
The finding that schizophrenic brain has greater specific binding of [3H]PBR28 than control brain was not a working hypothesis of this study; however, this result is consistent with the hypothesis that inflammatory processes are associated with schizophrenia. These results are particularly important given conflicting results seen in prior studies on schizophrenia. While some studies reported no significant differences in microglial cell density or TSPO binding sites between patients with schizophrenia and controls,23, 24, 25 others found elevated microglial density in DLPFC, temporal gyrus, temporal cortex, and frontal cortex of schizophrenia patients.26, 27, 28 Still others reported decreased microglical activation or TSPO specific binding in schizophrenia patients compared with controls.29, 30 A study using [11C](R)-PK 11195 found a 16% increase in total gray matter binding in schizophrenia patients;31 however, all 10 patients included were taking antipsychotic medication and the effect of antipsychotics on microglial activation is unknown. In our study, specific [3H]PBR28 binding was not greater in schizophrenic DLPFC taking antipsychotics at time of death than in those not taking antipsychotics, when differences in TSPO genotype were taken into account. Moreover, specific binding was greater for schizophrenia patients than controls even after accounting for antipsychotic use in the statistical model. While we cannot conclude definitively that antipsychotic use does not influence TSPO density, the results from our study show no evidence of such an association.
Using Genotype with Other Translocator Protein Radioligands
While this study was specific to PBR28, all other second-generation TSPO radioligands examined to date have differential affinity for the target protein.17 Thus, the Ala147Thr polymorphism may confound results using any of these second-generation TSPO radioligands by increasing variance or by the differential inclusion of the three genotypes in experimental and control groups. In fact, a recent report demonstrated that TSPO genotype was associated with differences in brain uptake of [18F]FEPPA in human subjects.18 Therefore, we recommend genotyping subjects before prospective clinical studies using any second-generation TSPO radioligands. Furthermore, the original and prototypical ligand [11C](R)-PK 11195 may also have differential affinity, with conflicting results reported using in vitro32 and in vivo7 methods. Thus, additional in vitro and in vivo studies are necessary to determine the utility of genotype in studies using [11C](R)-PK 11195.
One of the most important attributes of a PET radioligand is its ratio of specific to nondisplaceable brain uptake, the latter of which is the sum of nonspecific binding and free radioligand in tissue water. For targets like TSPO, which are widely distributed in brain, nondisplaceable uptake of the cognate radioligand can only be measured after pharmacological blockade by nonradioactive ligand. Such blockade is often impossible to perform in humans because the drug is not available or because of pharmacological effects. Our postmortem and in vivo results provide a relatively unique opportunity to estimate nondisplaceable uptake without pharmacological blockade. We used the postmortem results to estimate the relative expression of H and L forms in HL subjects, and then used total uptake (VT) in HH and HL subjects to estimate specific (VS) and nondisplaceable uptake (VND).
Our estimation of VND yielded two interesting results. First, the postmortem study showed that specific binding in HL subjects was 56% (and not 50%) of that in HH subjects (Figure 2). These results suggest that 56% of TSPO in heterozygotes is in the H form, not the 50% that would be expected if HL subjects were to express the H and L versions of the TSPO gene in equal amounts. Such a disparity could occur by differential expression of the two genes or differential turnover of two TSPO proteins. Second, our calculations predict that VND of [11C]PBR28 is 1.6 mL/cm3. Thus, the ratio of specific to nondisplaceable uptake of [11C]PBR28 in HH subjects is 1.8/1 (=(4.5–1.6)/1.6). This estimated value of VND and VND/fP in human subjects (1.6 and 39.0 mL/cm3, respectively) is very similar to that measured in monkey brain (1.6 and 28.6 mL/cm3, respectively) after pharmacological blockade.33 Although specific binding often differs between species, nonspecific binding (after correcting for plasma protein binding) is often thought to be similar across species, because of the similar chemical composition of brain tissue.
These calculations show that in vitro binding and in vivo brain uptake in a situation of altered receptor binding (i.e., affected by genotype) can be used to calculate nondisplaceable uptake in human brain. Similar measurements could be made for other TSPO radioligands to compare their relative ratios of specific to nondisplaceable uptake. This method is preferred over simply using VT of LL subjects as an estimation of VND. Prior studies using [11C]PBR28 have shown that kinetic modeling using either the one- and two-tissue compartment models provides poor fitting with measured brain PET data in LL subjects.16 Therefore, VT of LL subjects cannot be accurately calculated using kinetic modeling and cannot be used to estimate VND of [11C]PBR28. Our method of calculating VND is admittedly speculative and requires the assumption that (1) VND is uniform among subjects, (2) the ratio of specific binding between HH and HL subjects is the same in vitro and in vivo, and (3) specific binding of PBR28 to the L version of TSPO is negligible. However, these are reasonable assumptions given (1) the similar chemical composition of brain tissue among human subjects, (2) TSPO density is not expected to change disproportionately between HH and HL subjects postmortem, and (3) results from our postmortem study show dramatically lower specific binding in LL subjects than HH subjects.
In conclusion, the Ala147Thr TSPO polymorphism was associated with reduced in vivo binding with [11C]PBR28. The leukocyte binding assay accurately predicted the Ala147Thr polymorphism and may be used as an alternative to genotyping. Controlling for this polymorphism will not only provide more accurate quantitation of TSPO density but also increase statistical power and reduce the necessary sample size of clinical studies using [11C]PBR28. Patients with schizophrenia had greater TSPO binding in DLPFC. Whether this truly reflects a neuroinflammatory process in schizophrenia is unknown, but could be explored with TSPO PET imaging in live subjects after correcting for the Ala147Thr polymorphism.
Acknowledgments
This study represents the work of the Foundation for the National Institutes of Health Biomarkers Consortium Project ‘Measuring neuroinflammation in Alzheimer's disease and mild cognitive impairment with [11C]PBR28 PET.' This project was submitted to the Biomarkers Consortium Neuroscience Steering Committee for execution and was managed by a Biomarkers Consortium Project Team that includes members from academia, government, and the pharmaceutical industry. We thank the Project Team for their contributions: Edilio Borroni (Roche), Linda Brady (NIMH), Thomas Finn (FDA), Richard Hargreaves (Merck), Robert Innis (NIMH), Walter Koroshetz (NINDS), William Kreisl (NIMH), Timothy McCarthy (Pfizer), P David Mozley (Merck), Susanne Ostrowitzki (Roche), Victor Pike (NIMH), Eugenni Rabiner (GSK), Mark Shearman (EMD Serono), Judith Siuciak (FNIH), Cyrille Sur (Merck), Johannes Tauscher (Lilly). We thank Yi Zhang, PhD, for assistance in the production of radioligands, David Luckenbaugh for assistance with statistical analysis, Iolene Henter for assistance in editing the manuscript, and Maria D Ferraris-Araneta, C-RNP, Barbara Scepura, C-RNP, Gerald Hodges, RN, and the NIH PET Department for assistance in successfully completing the PET studies.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This project was funded by the Intramural Research Program of the National Institute of Mental Health-National Institutes of Health (IRP-NIMH-NIH), and as a public–private partnership supported by the NIMH and the Foundation for the NIH Biomarkers Consortium (www.biomarkersconsortium.org). This work was supported by EMD Serono, Glaxo Smith Kline, Lilly, Merck, Pfizer, Inc., and Roche. Additional support was provided by the American Academy of Neurology Foundation (to WCK).
Supplementary Material
References
- Scarf AM, Kassiou M. The translocator protein. J Nucl Med. 2011;52:677–680. doi: 10.2967/jnumed.110.086629. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;24:591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, et al. Microglia, amyloid, and cognition in Alzheimer's disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10:257–264. doi: 10.1046/j.1468-1331.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- Kropholler MA, Boellaard R, Elzinga EH, van der Laken CJ, Maruyama K, Kloet RW, et al. Quantification of (R)-[11C]PK11195 binding in rheumatoid arthritis. Eur J Nucl Med Mol Imaging. 2009;36:624–631. doi: 10.1007/s00259-008-0987-7. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Rossor M, Sampson EL, Mackinnon T, Banati RB. In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol. 2004;56:894–897. doi: 10.1002/ana.20332. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, et al. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage. 2010;49:2924–2932. doi: 10.1016/j.neuroimage.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y, Zoghbi SS, Simeon FG, Taku A, Pike VW, Innis RB, et al. Quantification of translocator protein (18 kDa) in the human brain with PET and a novel radioligand, 18F-PBR06. J Nucl Med. 2009;50:1047–1053. doi: 10.2967/jnumed.108.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, et al. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Zhang MR, Okauchi T, Yasuno F, Ikoma Y, et al. Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: an imaging tool for glial cells in the brain. Synapse. 2004;52:283–291. doi: 10.1002/syn.20027. [DOI] [PubMed] [Google Scholar]
- Doorduin J, Klein HC, Dierckx RA, James M, Kassiou M, de Vries EF. [11C]-DPA-713 and [18F]-DPA-714 as new PET tracers for TSPO: a comparison with [11C]-(R)-PK11195 in a rat model of herpes encephalitis. Mol Imaging Biol. 2009;11:386–398. doi: 10.1007/s11307-009-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MR, Kumata K, Maeda J, Yanamoto K, Hatori A, Okada M, et al. 11C-AC-5216: a novel PET ligand for peripheral benzodiazepine receptors in the primate brain. J Nucl Med. 2007;48:1853–1861. doi: 10.2967/jnumed.107.043505. [DOI] [PubMed] [Google Scholar]
- Van Camp N, Boisgard R, Kuhnast B, Theze B, Viel T, Gregoire MC, et al. In vivo imaging of neuroinflammation: a comparative study between [18F]PBR111, [11C]CLINME and [ 11C]PK11195 in an acute rodent model. Eur J Nucl Med Mol Imaging. 2010;37:962–972. doi: 10.1007/s00259-009-1353-0. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, Houle S, et al. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol. 2008;35:305–314. doi: 10.1016/j.nucmedbio.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I, et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [18F]-FEPPA. J Cereb Blood Flow Metab. 2012;32:968–972. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, et al. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, et al. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009;150:5438–5445. doi: 10.1210/en.2009-0752. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG, et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- Ritsner M, Modai I, Gibel A, Leschiner S, Silver H, Tsinovoy G, et al. Decreased platelet peripheral-type benzodiazepine receptors in persistently violent schizophrenia patients. J Psychiatr Res. 2003;37:549–556. doi: 10.1016/s0022-3956(03)00055-4. [DOI] [PubMed] [Google Scholar]
- Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res. 2000;93:103–110. doi: 10.1016/s0165-1781(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E, et al. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol. 2005;43:81–89. [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett. 1999;271:126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Kosno-Kruszewska E, Lechowicz W, Pasennik E, Schmidt-Sidor B, et al. Degeneration of microglial cells in frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol. 2004;42:157–165. [PubMed] [Google Scholar]
- Kurumaji A, Wakai T, Toru M. Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm. 1997;104:1361–1370. doi: 10.1007/BF01294737. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30:1608–1618. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi M, Briard E, Zoghbi SS, Gourley JP, Hong J, Musachio JL, et al. Kinetic evaluation in nonhuman primates of two new PET ligands for peripheral benzodiazepine receptors in brain. Synapse. 2007;61:595–605. doi: 10.1002/syn.20394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.