Abstract
Purpose
The purpose of the study was to determine the sensitivity of the 18fluoro-dihydroxyphenylalanine positron emission tomography/computed tomography scan (18 F-PET/CT) in the diagnosis of focal congenital hyperinsulinism (HI).
Methods
A retrospective review of children with HI who underwent a preoperative 18 F-PET/CT scan was performed.
Results
Between 1/2008 and 2/2012 we performed 105 consecutive 18 F-PET/CT scans on infants with HI. Fifty-three patients had focal HI. Of those fifty-three patients, eight had a preoperative 18 F-PET/CT scan read as “diffuse disease”. The sensitivity of the study in the diagnosis of focal HI was 85%. The location of the eight missed focal lesions was: head (3), body (2), and tail (3). The 18 F-PET/ CT of the missed head lesions showed homogeneous tracer uptake (n =2) or heterogeneous uptake throughout the pancreas (n=1). The 18 F-PET/CT of the 2 missed body lesions and 1 missed tail lesion showed heterogeneous uptake throughout the pancreas. The 18 F-PET/CT of the other 2 missed tail lesions showed lesions adjacent to and obscured by the signal of the upper renal pole, identified retrospectively by closer observation. Fifty-two of the 105 patients had diffuse HI. Two of them had 18 F-PET/CT studies read as “focal disease”. Therefore, the specificity of the study was 96%. Of the forty-seven 18 F-PET/CT studies read as “focal disease”, forty-five had true focal HI. Therefore, the positive predictive value of the study in the diagnosis of focal HI was 96%.
Conclusion
The sensitivity and specificity of 18 F-PET/CT can be affected by certain anatomic features of the pancreas, by the location of the lesion, and by the reader’s experience.
Keywords: Congenital hyperinsulinism, Positron Emission Tomography, Pancreatectomy, Hypoglycemia
Radio-isotopic imaging modalities including positron emission tomography (PET) have been used to study pancreatic diseases since the early 1970’s [1]. The finding of the enzyme L-dihydroxyphenylalanine decarboxylase in cells of neuroendocrine origin such as the beta cells of the islets of Langerhans led to the development of radiotracers that were specific for endocrine tissues and therefore useful in the detection of pancreatic endocrine tumors [2,3]. It was hypothesized in 2005 that the radiotracer 18fluoro-L-dihydroxyphenylalanine (18 F-DOPA) would concentrate in the beta cells of the pancreas and could help distinguish between the focal and the diffuse forms of congenital hyperinsulinism (HI) [4]. The hypothesis was validated in several studies and the technique, known as 18fluoro-L-dihydroxyphenylalanine PET/computed tomography (18 F-PET/CT), was adopted in the few Hyperinsulinism Centers worldwide thereby replacing all previously used imaging modalities [5,6]. The purpose of this report is to present our most recent data on this subject and review the current literature.
1. Materials and methods
After obtaining Institutional Review Board approval, we performed a retrospective review of all patients with HI referred to the Congenital Hyperinsulinism Center at the Children’s Hospital of Philadelphia (CHOP) who underwent a preoperative 18 F-PET/CT scan. Between December 2004 and February 2012, we performed 207 18 F-PET/CT studies on patients with HI. During the first three years, the 18 F-PET/CT studies were done at the Hospital of the University of Pennsylvania adjacent to CHOP, using different protocols and more than one scanner. Some of these results have been previously reported [6,7]. The last 105 18 F-PET/CTs were consecutively performed at CHOP, on the same 16-slice Philips scanner, under the same acquisition protocol, and read by a single nuclear medicine physician, between January 2008 and February 2012. These 105 18 F-PET/ CTs are the object of this study.
The isotope 18 F-DOPA is administered in children at CHOP under a Food and Drug Administration-approved Investigational New Drug (IND) protocol and with the approval of the hospital’s Institutional Review Board. The isotope has a half-life of 110 min. It is manufactured on the day of the study at the Cyclotron Facility of the University of Pennsylvania, and used at a dose of 0.08–0.16 mCi/kg (2.96–5.92 MBq/kg) through a slow intravenous infusion within 1 to 3 h of its preparation. All glycemic medications are stopped prior to the study and a continuous intravenous glucose infusion maintains the plasma glucose concentration above 70 mg/dL (3.85 mmol/L). The 18 F-PET/CT is done under general anesthesia in a hybrid scanner that initially generates a non-contrast low-radiation CT scan of the abdomen, then captures the gamma-ray signal, and then generates a low-radiation contrast CT scan of the abdomen, all without changing the patient’s position. The study starts 10 min after the injection of the radiotracer. The nuclear signal is captured at 10-min intervals during the first 50 min post-injection because thereafter the radiotracer accumulates in the liver, gallbladder, biliary tree and duodenal lumen, potentially leading to false positive images of ectopic pancreatic activity. Focal lesions are seen as bright areas over a darker background. All studies were read by a single nuclear medicine physician who was blinded to genetic testing results.
2. Results
All 105 patients underwent pancreatectomy, and 53 patients had focal HI confirmed by pathologic analysis. All 53 focal lesions were completely removed by partial pancreatectomy with cure from HI. Of those 53 patients with focal HI, 8 had a preoperative 18 F-PET/CT scan read as “diffuse disease” so the sensitivity of the 18 F-PET/CT in the diagnosis of focal HI was 85%. The location of the 8 missed lesions was head (3), body (2) and tail (3) (Table 1). In two cases a mild increased uptake at the tail of the pancreas abutting the contour of the left kidney was not detected in the initial reading, but these areas were identified retrospectively on a more careful imaging analysis (Fig. 1). In two patients, the 18 F-PET/CT showed a faint homogeneous distribution of the tracer throughout the pancreas without any evidence of a focal lesion. One patient was found to have a focal lesion in the uncinate process (Fig. 2), and the other patient had a focal lesion in the pancreatic head. In the other 4 cases, the 18 F-PET/CT showed heterogeneous radiotracer activity throughout the pancreas without clear evidence of a focal lesion. For the 45 focal lesion patients with an identifiable lesion detected by 18 F-PET/CT scan, there was a 100% correlation between the location of focal lesion on PET scan and the actual location of the lesion at the time of surgery.
Table 1.
Cases of focal HI with a preoperative 18 F-PET/CT read as diffuse.
| Case | Location | 18 F-PET/CT description | Surgery |
|---|---|---|---|
| 1 | Head | Heterogeneous uptake throughout the pancreas | 2% pancreatectomy |
| 2 | Head | Faint homogeneous uptake throughout the pancreas | 10% pancreatectomy |
| 3 | Head | Faint homogeneous uptake throughout the pancreas | 70% pancreatectomy |
| 4 | Body | Heterogeneous uptake throughout the pancreas | 50% pancreatectomy |
| 5 | Body | Heterogeneous uptake throughout the pancreas | 2% pancreatectomy |
| 6 | Tail | Heterogeneous uptake throughout pancreas (mild increase at tail) a | 10% pancreatectomy |
| 7 | Tail | Heterogeneous uptake throughout pancreas (mild increase at tail) a | 2% pancreatectomy |
| 8 | Tail | Heterogeneous uptake throughout the pancreas | 2% pancreatectomy |
Cases with mild increased uptake at the tail of the pancreas overlapping with the signal of the upper renal pole, identified on retrospective readings.
Fig. 1.
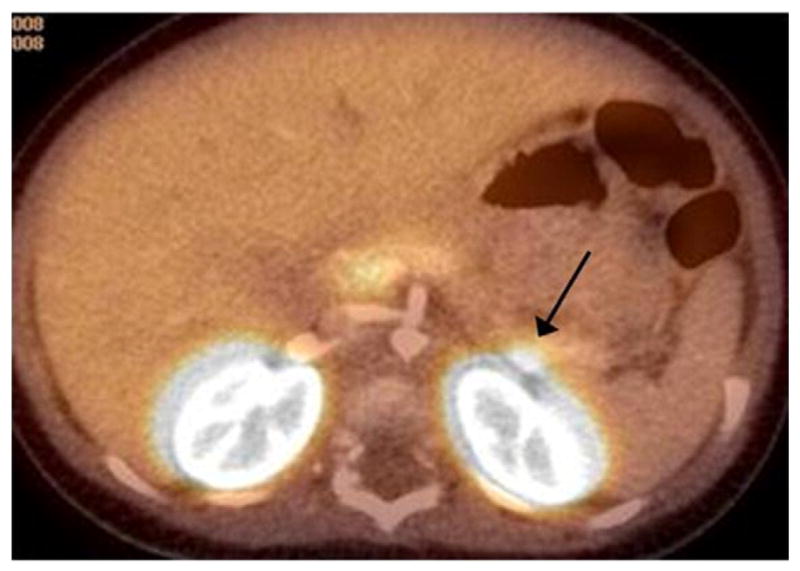
Focal lesion at the tail of the pancreas abutting the contour of the left kidney. It was not recognized as a discrete area of tracer uptake and the study was interpreted as diffuse HI.
Fig. 2.
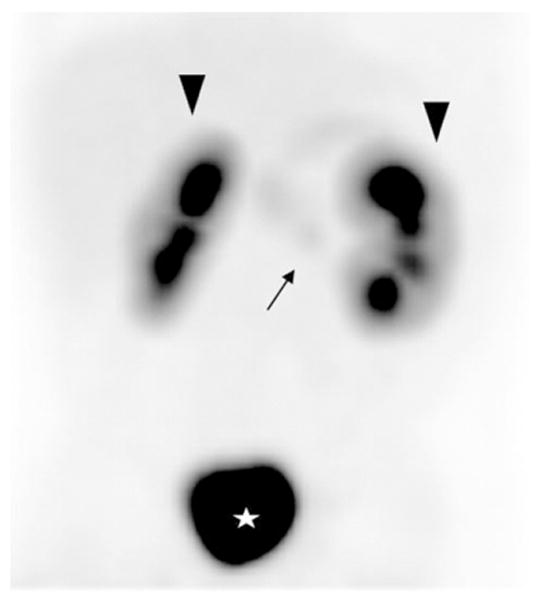
Diffuse homogeneous radiotracer distribution without evidence of a focal lesion. The uncinate process appears on the images with the same intensity as the rest of the pancreas. Patient had a focal lesion in the uncinate process (arrow) by pathology. Arrowheads: kidneys. Star: bladder.
Fifty two of the patients in our series had diffuse HI by pathologic analysis and 2 of them had 18 F-PET/CT scans read as “focal disease”, so the specificity of the 18 F-PET/CT in the diagnosis of focal HI was 96%. The two 18 F-PET/CT scans read as focal lesions in patients with diffuse disease showed a heterogeneous tracer uptake with a mildly brighter signal in the pancreatic head (one case) and body (one case). Since there were a total of 47 18 F-PET/CT scans read as “focal disease”, of which 45 were true focal lesions by pathologic analysis, the positive predictive value of the study was 96%.
3. Discussion
Positron emission tomography (PET) is a radio-isotopic imaging technique based on the detection of gamma rays generated by positron-emitting radionuclides. Neuroendocrine cells such as the beta cells of the islets of Langerhans have the ability to take up the catecholamine-precursor L-dihydroxyphenylalanine (L-DOPA) and convert it into dopamine by the enzyme L-DOPA decarboxylase. The rationale behind the use of PET techniques in the context of focal HI is that focal lesions contain a concentrated number of beta cells (secondary to adenomatous hyperplasia), so the area of the focal lesion generates a stronger gamma-ray signal compared to the rest of the pancreas, where the beta cells are not concentrated. Focal lesions are then seen as bright spots over a darker background, whereas cases of diffuse disease show homogeneous distribution of the tracer throughout the pancreas (Fig. 3). The radionuclide used in imaging of HI patients is 18-fluorine (18 F), which has a half-life of approximately 110 min.
Fig. 3.
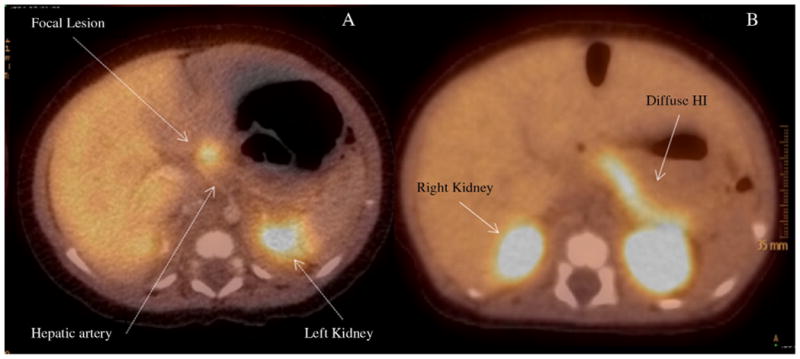
18 F-PET/CT scan of focal (A) and diffuse (B) HI.
The current management of patients with HI includes an 18 F-PET/CT when the disease does not respond to medical treatment and the genetic testing (if available) is consistent with focal disease or is inconclusive [8]. If patients who do not respond to medical treatment have diffuse disease by genetic testing, then imaging studies are not needed, and a near-total pancreatectomy is performed to help with patient management. Patients with focal disease, on the other hand, are treated by a complete resection of the focal lesion with cure of HI. The development of 18 F-PET/CT had a major impact in the preoperative evaluation of patients with HI given that before the 18 F-PET/CT era, patients had to undergo laborious invasive procedures to both distinguish between diffuse and focal HI and attempt to localize focal lesions. Those studies, namely “Arterial Stimulation with Venous Sampling” (ASVS) and “Transhepatic Portal Venous Sampling” (THPVS) required several hours under general anesthesia, multiple blood samples, a significant amount of radiation and had limited sensitivity and specificity for focal HI [9–11]. All conventional, non-invasive imaging studies like ultrasound, computed tomography, and magnetic resonance imaging have consistently failed to distinguish between focal and diffuse disease.
Despite its clear advantages, the sensitivity, specificity and positive predictive value of the 18 F-PET/CT study are not 100%. Most of the true focal lesions that were missed had an 18F-PET/CT study that showed either a homogeneous pattern without a discrete “hot spot” or a heterogeneous uptake pattern. The heterogeneous uptake pattern with irregular increased activity in the head, body and tail is very difficult to interpret and is likely related to the uneven volume of pancreatic tissue throughout the organ. This heterogeneous pattern can also be seen in diffuse HI (Fig. 4). The most commonly seen heterogeneous pattern is an increased uptake in the pancreatic head, which can be erroneously interpreted as a head focal lesion. We had 2 18 F-PET/CT with higher uptake in the head and body (one each) read as focal lesions that proved to be diffuse HI by pathologic analysis, which makes the false positive rate of the study (for focal HI) approximately 4%. A particular diagnostic challenge is presented by large focal lesions that occupy most of the pancreas and have the appearance of diffuse disease on the 18 F-PET/CT, [6,12]. The 18 F-PET/ CT has been helpful in the diagnosis and localization of rare cases of ectopic focal HI [13,14].
Fig. 4.
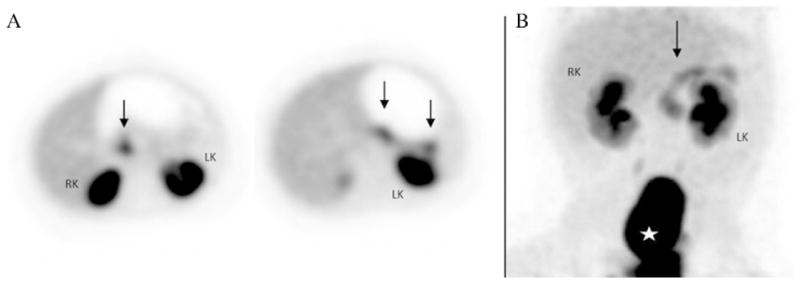
Heterogeneous radiotracer activity throughout the pancreas. (A) The head, body and tail showed increased patchy activity, without any area being particularly more intense than the remainder of the pancreas (black arrows). The patient had a focal lesion (by pathology) in the pancreatic body. (B) Similar heterogeneous pattern in a case of diffuse HI (black arrow). RK: right kidney. LK: left kidney. Star: bladder.
In cases with subtle differences in the signal intensity throughout the organ, a quantitative activity analysis using the ratio between the maximum intensity at the point of interest to the intensity at the background can be done in an attempt to improve diagnostic accuracy. The analysis is known as “standardized uptake value ratio” (SUVr). It has been suggested by some authors that an SUVr≥ 1.5 is diagnostic of focal disease, however cases of diffuse disease with a heterogeneous pattern can have SUVr of 1.4, which is too close to be considered definitive [5]. Other authors have suggested that the SUVr diagnostic of focal disease is 1.2, but that SUVr is lower than the SUVr of many cases of diffuse disease reported in the literature [15]. We did not measure SUVr in our patients. We believe that in the vast majority of cases careful visual analysis is adequate for interpretation and localization of a focal lesion, and in cases that are equivocal the areas of concern are communicated to the surgeon.
In most cases the focal lesions are resected with an area of normal tissue, so the size of the specimen does not correlate with the actual size of the lesion, and in some cases the lesion is present in more than one specimen. The specimens are usually sectioned by the pathologists in only one axis, so the actual tridimensional size of focal lesions is very difficult to determine except for rare pedunculated exophytic lesions. Because of these limitations, we do not know if there is a correlation between the size of the lesion and the intensity of the PET signal.
Several studies on the accuracy of 18 F-PET/CT for the diagnosis and localization of focal HI have been published with variable results (Table 2). The PET scan reader’s learning curve and intrinsic factors related to uneven pancreatic tissue distribution could explain, at least in our series, why the sensitivity is not 100%. We found no association between the actual location of the focal lesion and the likelihood of being missed by the 18 F-PET/CT. However, focal lesions in the pancreatic tail that overlap with the contour of the left kidney can be missed if the images are not carefully analyzed. In this series of 53 patients with focal HI there was a 100% accurate correlation between the location of focal lesions seen on 18 F-PET/CT scan and the actual location at the time of surgery. Finally, the eight 18 F-PET/CT studies that were read as diffuse HI did not lead us to perform unnecessary near-total pancreatectomies. Despite having no preoperative radiologic guidance, all focal lesions were identified by inspection, palpation and frozen section analysis prior to partial pancreatic resection. 18 F PET/CT is the most sensitive imaging study for the diagnosis and localization of focal lesions and is currently a crucial component of the preoperative management of patients with HI.
Table 2.
Studies on the accuracy of 18 F-PET/CT in the diagnosis and localization of focal HI published in the literature.
| Author | Year | Patients with focal HI by pathology | Focal lesions seen on 18 F-PET/CT | Sensitivity for the diagnosis of focal HI | Localization accuracy |
|---|---|---|---|---|---|
| Ribeiro [4] | 2005 | 5 | 5 | 100% | 100% |
| Otonkoski [5] | 2006 | 5 | 5 | 100% | 80% a |
| Hardy [6] | 2007 | 12 | 11 | 92% | 100% b |
| Ribeiro [16] | 2007 | 15 | 14 | 93% | 92% |
| Hardy [7] | 2007 | 24 | 18 | 75% | 100% |
| Barthlen [17] | 2008 | 9 | 9 | 100% | 90% |
| Capito [18] | 2009 | 16 | 16 | 100% | 81% |
| Masue [19] | 2011 | 9 | 3 | 33% | 100% |
| Zani [20] | 2011 | 14 | 14 | 100% | 64% |
| Our study | 2012 | 53 | 45 | 85% | 100% |
The neck of the pancreas is combined with the head of the pancreas for the study of localization accuracy.
One case reported as head lesion on 18 F-PET/CT and was a lesion in the uncinate process by pathology.
The case that showed diffuse uptake was a large focal lesion that occupied 80%–90% of the pancreas.
References
- 1.Kirchner PT, Ryan J, Zalutsky M, et al. Positron emission tomography for the evaluation of pancreatic disease. Semin Nucl Med. 1980;4:374–91. doi: 10.1016/s0001-2998(80)80039-0. [DOI] [PubMed] [Google Scholar]
- 2.Gazdar AF, Helman LJ, Israel MA, et al. Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res. 1988;48:4078–82. [PubMed] [Google Scholar]
- 3.Eriksson B, Bergström M, Sundin A, et al. The role of PET in localization of neuroendocrine and adrenocortical tumors. Ann N Y Acad Sci. 2002;970:159–69. doi: 10.1111/j.1749-6632.2002.tb04422.x. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro MJ, De Lonlay P, Delzescaux T, et al. Characterization of hyperinsulinism in infancy assessed with PET and 18F-fluoro-L-DOPA. J Nucl Med. 2005;46:560–6. [PubMed] [Google Scholar]
- 5.Otonkoski T, Näntö-Salonen K, Seppänen M, et al. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006;55:13–8. [PubMed] [Google Scholar]
- 6.Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. Diagnosis and localization of focal congenital hyperinsulinism by 18F-fluorodopa PET scan. J Pediatr. 2007;150:140–5. doi: 10.1016/j.jpeds.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. Accuracy of [18F]fluorodopa positron emission tomography for diagnosing and localizing focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2007;92:4706–11. doi: 10.1210/jc.2007-1637. [DOI] [PubMed] [Google Scholar]
- 8.Ismail D, Hussain K. Role of 18F-DOPA PET/CT imaging in congenital hyperinsulinism. Rev Endocr Metab Disord. 2010;11:165–9. doi: 10.1007/s11154-010-9145-1. [DOI] [PubMed] [Google Scholar]
- 9.Adzick NS, Thornton PS, Stanley CA, et al. A multidisciplinary approach to the focal form of congenital hyperinsulinism leads to successful treatment by partial pancreatectomy. J Pediatr Surg. 2004;39:270–5. doi: 10.1016/j.jpedsurg.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Stanley CA, Thornton PS, Ganguly A, et al. Preoperative evaluation of infants with focal or diffuse congenital hyperinsulinism by intravenous acute insulin response tests and selective pancreatic arterial calcium stimulation. J Clin Endocrinol Metab. 2004;89:288–96. doi: 10.1210/jc.2003-030965. [DOI] [PubMed] [Google Scholar]
- 11.deLonlay-Debeney P, Poggi-Travert F, Fournet JC, et al. Clinical features of 52 neonates with hyperinsulinism. N Engl J Med. 1999;340:1169–75. doi: 10.1056/NEJM199904153401505. [DOI] [PubMed] [Google Scholar]
- 12.Ismail D, Kapoor RR, Smith VV, et al. The heterogeneity of focal forms of congenital hyperinsulinism. J Clin Endocrinol Metab. 2012;97:E94–9. doi: 10.1210/jc.2011-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peranteau WH, Bathaii SM, Pawel B, et al. Multiple ectopic lesions of focal islet adenomatosis identified by positron emission tomography scan in an infant with congenital hyperinsulinism. J Pediatr Surg. 2007;42:188–92. doi: 10.1016/j.jpedsurg.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Hussain K, Seppänen M, Näntö-Salonen K, et al. The diagnosis of ectopic focal hyperinsulinism of infancy with [18F]-dopa positron emission tomography. J Clin Endocrinol Metab. 2006;91:2839–42. doi: 10.1210/jc.2006-0455. [DOI] [PubMed] [Google Scholar]
- 15.Arnoux JB, de Lonlay P, Ribeiro MJ, et al. Congenital hyperinsulinism. Early Hum Dev. 2010;86:287–94. doi: 10.1016/j.earlhumdev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro MJ, Boddaert N, Bellanné-Chantelot C, et al. The added value of [18F]fluoro-L-DOPA PET in the diagnosis of hyperinsulinism of infancy: a retrospective study involving 49 children. Eur J Nucl Med Mol Imaging. 2007;34:2120–8. doi: 10.1007/s00259-007-0498-y. [DOI] [PubMed] [Google Scholar]
- 17.Barthlen W, Blankenstein O, Mau H, et al. Evaluation of [18F]fluoro-L-DOPA positron emission tomography–computed tomography for surgery in focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2008;93:869–75. doi: 10.1210/jc.2007-2036. [DOI] [PubMed] [Google Scholar]
- 18.Capito C, Khen-Dunlop N, Ribeiro MJ, et al. Value of 18F-fluoro-L-dopa PET in the preoperative localization of focal lesions in congenital hyperinsulinism. Radiology. 2009;253:216–22. doi: 10.1148/radiol.2532081445. [DOI] [PubMed] [Google Scholar]
- 19.Masue M, Nishibori H, Fukuyama S, et al. Diagnostic accuracy of [18F]-fluoro-L-dihydroxyphenylalanine positron emission tomography scan for persistent congenital hyperinsulinism in Japan. Clin Endocrinol (Oxf) 2011;75:342–6. doi: 10.1111/j.1365-2265.2011.04072.x. [DOI] [PubMed] [Google Scholar]
- 20.Zani A, Nah SA, Ron O, et al. The predictive value of pre-operative fluorine-18-L-3,4-dihydroxyphenylalanine positron emission tomography–computed tomography scans in children with congenital hyperinsulinism of infancy. J Pediatr Surg. 2011;46:204–8. doi: 10.1016/j.jpedsurg.2010.09.093. [DOI] [PubMed] [Google Scholar]


