Abstract
Rationale
Matrix metalloproteinase (MMP)-28 regulates the inflammatory and extracellular matrix (ECM) responses in cardiac aging, but the roles of MMP-28 after myocardial infarction (MI) have not been explored.
Objective
To determine the impact of MMP-28 deletion on post-MI remodeling of the left ventricle (LV)
Methods and Results
Adult C57BL/6J wild type (WT, n=76) and MMP null (MMP-28−/−, n=86) mice of both sexes were subjected to permanent coronary artery ligation to create MI. MMP-28 expression decreased post-MI, and its cell source shifted from myocytes to macrophages. MMP-28 deletion increased day 7 mortality as a result of increased cardiac rupture post-MI. MMP-28−/− mice exhibited larger LV volumes, worse LV dysfunction, a worse LV remodeling index, and increased lung edema. Plasma MMP-9 levels were unchanged in the MMP-28−/− mice but increased in WT mice at day 7 post-MI. The mRNA levels of inflammatory and ECM proteins were attenuated in the infarct regions of MMP-28−/− mice, indicating reduced inflammatory and ECM responses. M2 macrophage activation was impaired when MMP-28 was absent. MMP-28 deletion also led to decreased collagen deposition and fewer myofibroblasts. Collagen cross-linking was impaired, due to decreased expression and activation of lysyl oxidase in the infarcts of MMP-28−/− mice. The LV tensile strength at day 3 post-MI, however, was similar between the two genotypes
Conclusions
MMP-28 deletion aggravated MI induced LV dysfunction and rupture, due to defective inflammatory response and scar formation by suppressing M2 macrophage activation.
Keywords: Myocardial infarction, MMP-28, fibroblast, macrophage phenotype, inflammation
INTRODUCTION
Following myocardial infarction (MI), the left ventricle (LV) undergoes molecular, cellular, and extracellular matrix changes that dramatically alter the size, shape, and function at the organ level.1 Macrophages phagocytose necrotic myocytes, apoptotic neutrophils, and extracellular matrix (ECM) debris, as well as produce cytokines and growth factors that stimulate the activation of fibroblasts.2, 3 Activated fibroblasts (myofibroblasts) promote abundant ECM synthesis and scar formation in the infarct area.4 Matrix metalloproteinases (MMPs) are activated to degrade ECM components to facilitate the remodeling process. The balance of ECM turnover is critical for stable scar repair. Excessive ECM deposition increases wall stiffness and diastolic dysfunction, while insufficient ECM accumulation and impaired collagen cross-linking contribute to cardiac rupture post-MI.5, 6 The inflammatory component regulates ECM synthesis, by promoting fibroblast proliferation and secretion, and ECM degradation, by increasing MMP activity. Therefore, understanding the coordination between inflammation and ECM turnover may represent a promising strategy for preventing adverse LV remodeling.
Multiple MMPs mediate post-MI LV remodeling and repair in part by regulating ECM turnover and inflammation.7 MMP-2, -7, and -9 null mice showed improved survival and attenuated LV remodeling post-MI, albeit by different mechanisms.8–10 MMP-28, also known as epilysin, was first cloned in 2001.11, 12 Intracellularly, the furin-like proprotein convertase activates the 58 kDa pro-form of MMP-28 to its 48 kDa active form, which is then secreted into the extracellular matrix environment.13 In vitro, MMP-28 has been shown to proteolytically process casein, Nogo-A (a myelin component), and neural cell adhesion molecule-1.12, 14 MMP-28 is highly expressed in many normal adult tissues and organs, including the heart, suggesting roles in normal tissue homeostasis. MMP-28 expression is upregulated in cancers, multiple sclerosis, and cardiac aging.15, 16 MMP-28 promotes transforming growth factor (TGF)-β triggered epithelial to mesenchymal transition in lung carcinoma cells.17 MMP-28 deletion also increases macrophage infiltration to the lung during pneumonia and facilitates airway epithelial cell apoptosis induced by influenza challenge, suggesting protective roles following injury.18, 19 Our previous study showed that MMP-28 deletion augmented the inflammatory and ECM responses to cardiac aging.15 However, the functional significance of MMP-28 post-MI remains to be elucidated.
Accordingly, the objective of this study was to determine the impact of MMP-28 deletion on MI induced LV remodeling and dysfunction. We hypothesized that MMP-28 deletion would exacerbate post-MI LV remodeling and dysfunction by modulating inflammatory and ECM responses. We used the permanent occlusion model in this study to induce a robust remodeling response, in order to dissect the potential molecular mechanisms in a model that has a large effect size.20 Of note, approximately 20% of MI patients are actually not reperfused, translating to about 250,000 patients each year in the US that have permanent occlusion MIs.21
METHODS
Detailed descriptions of the materials and methods are available in the Supplemental Material. The experimental design is given in Online Table I. A brief description of the methods used is given below.
Mice
All animal procedures were performed following the “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. Adult C57BL/6J wild type (WT, n=76) and MMP-28−/−(n=86) mice of both sexes were used in this study.
MI surgery
MI was induced by permanent occlusion of the left coronary artery, as previously described.22, 23
Echocardiography
LV function was evaluated by using the Vevo 770 system (VisualSonics) as previously described.15 The LV remodeling index was calculated as the ratio of end diastolic volume to LV mass.23
Survival analysis and autopsy
The mice were checked daily for survival. Cardiac rupture was confirmed by autopsy.
Tissue harvest and infarct area evaluation
The heart was collected as described previously.15 The LV infarct region (LVI) was separated from the remote region (LVC). Infarct area was calculated as the percentage of infarct area to total LV area.23 The edema index was calculated as the ratio of lung weight to tibia length.
Immunoblotting
Immunoblotting was performed as described previously.15 ImageJ was used to measure densitometry, and the total protein was used as the internal loading control for each lane.
Immunofluorescence
Immunofluorescence was performed as described previously.24 Co-localization was performed using either an antibody for MMP-28 and phalloidin or antibodies for MMP-28 and Mac-3 to determine the cellular localization of MMP-28. The α-smooth muscle actin (SMA) antibody was used to visualize myofibroblasts.
Plasma proteomic profiling
Plasma proteomic profiling of 58 analytes were measured as described previously.15
Real time RT2-PCR
Real Time RT2-PCR gene array for Inflammatory Cytokines and Receptors (Qiagen, PAMM-011A) and for Extracellular Matrix and Adhesion Molecules (Qiagen, PAMM-013A) were performed to quantify gene expression levels.15 Gene levels were normalized to the reference gene hprt1, and the data are reported as 2−ΔCt values × 100±SEM.
Immunohistochemistry
As described previously, macrophage staining was performed by using a specific antibody against Mac-3, and the percentage of positively stained area to total area was quantified.15
LV macrophage phenotype determination
RNA was extracted from infarct LVs of WT and MMP-28−/− mice. M1 and M2 markers were evaluated by quantitative real time-PCR (qRT-PCR).
Peritoneal macrophage isolation and stimulation
Peritoneal macrophages were isolated from WT and MMP-28−/− mice and stimulated with interferon (IFN)-γ+lipopolysaccharide (LPS) or interleukin (IL)-4.25 MMP-28, M1 and M2 markers were measured by qRT-PCR.
Cardiac fibroblast isolation and stimulation
Cardiac fibroblasts from WT and MMP-28−/− mice were isolated and stimulated with TGF-β1 (10 ng/mL) for 24 h. The levels of α-SMA, fibronectin1, and connective tissue growth factor (CTGF) were measured by qRT-PCR.
Collagen cross-linking
Hydroxylysyl pyridinoline and lysyl pyridinoline in day 7 infarct regions of WT and MMP-28−/− mice were measured.
Statistical analyses
Data are presented as mean±SEM. Two group comparisons were analyzed by unpaired t-test. Multiple group comparisons were performed using the one-way ANOVA, followed by the Student Newman-Keuls post-test (when the Bartlett’s variation test passed), or using the nonparametric Kruskal-Wallis test followed by Dunn post-test (when the Bartlett’s variation test did not pass). The survival rate was analyzed by Kaplan-Meier survival analysis and compared by the log-rank test. A value of p<0.05 was considered statistically significant.
RESULTS
MMP-28 cell source post-MI shifted from the myocyte to the macrophage
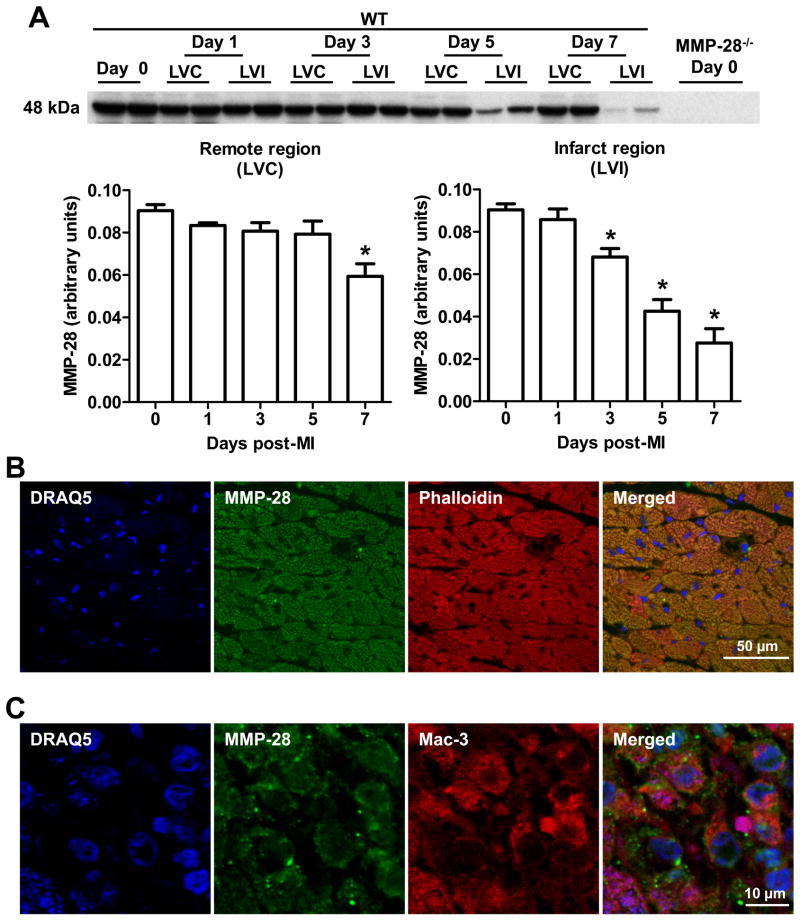
Previous studies report that MMP-28 is expressed in adult mouse hearts, and we confirmed high expression pre-MI (Figure 1A).15, 16 Post-MI, total MMP-28 levels were significantly decreased in both remote and infarct regions. In the remote region, the decrease was not seen until the day 7 post-MI time point; however, in the infarct region, MMP-28 expression was reduced at days 3, 5, and 7 post-MI. The decrease in MMP-28 was consistent with the pattern of cardiomyocyte loss.
Figure 1. Expression patterns and cellular source of MMP-28 at baseline and post-MI.
A, MMP-28 expression decreased in both remote and infarct regions post-MI. MMP-28−/− day 0 hearts were used as negative controls. n=4–6/group. *p<0.05 vs. day 0. B, MMP-28 co-localized with phalloidin, a marker of myocytes, at baseline (day 0). C, MMP-28 from macrophages increased in the infarct region post-MI. MMP-28 co-localized with Mac-3, a marker of macrophages, at day 5 post-MI. Representative images from n=3 stained LVs for B and C. Blue=DRAQ5, Green=MMP-28, Red=phalloidin or Mac-3.
We investigated the cellular source of MMP-28 by dual immunofluorescence staining. At day 0 pre-MI, MMP-28 co-localized with the myocyte marker phalloidin (Figure 1B), indicating that cardiomyocytes are a major source of MMP-28 under the normal setting. In agreement with a previous study showing that macrophages can secrete MMP-28, MMP-28 co-localized with Mac-3 (Figure 1C), a marker of macrophages, following MI.18 These findings indicate that myocyte-derived MMP-28 decreases, whereas macrophage-derived MMP-28 increases post-MI.
MMP-28 deletion decreased survival and increased cardiac rupture rates
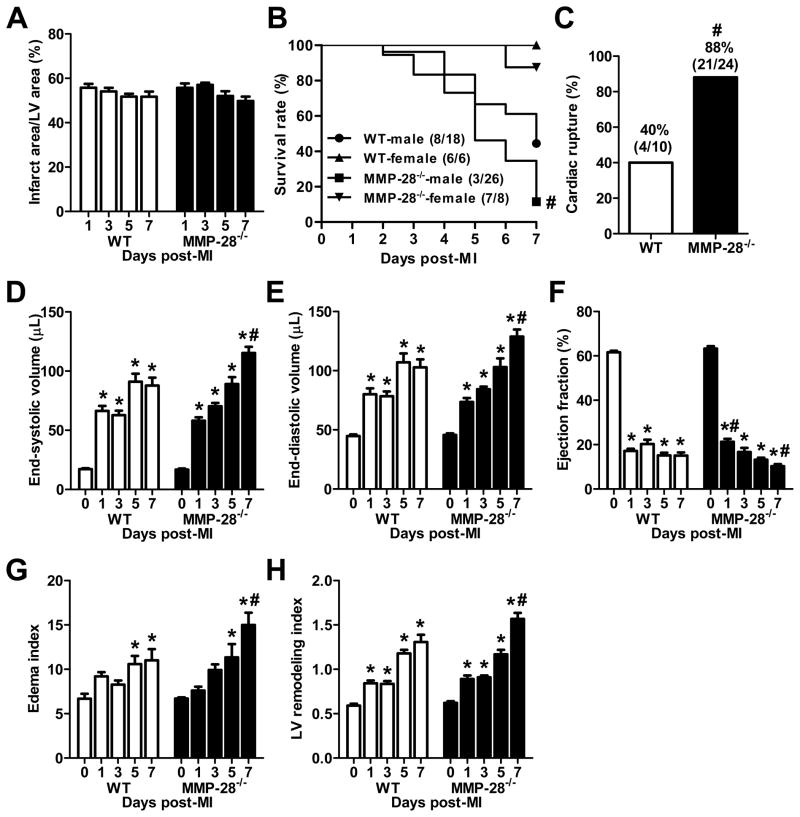
Infarct areas were similar between WT and MMP-28−/− mice for all time points examined (Figure 2A), demonstrating that MMP-28 deficiency had no effect on the acute ischemia response. We attempted to perform a 28 day post-MI survival analysis, but all male MMP-28−/− mice with MI died before day 28. For this reason, we performed a 7 day post-MI survival rate analysis. As shown in Figure 2B, 8 out of 18 male WT mice (44%) survived to 7 day post-MI. In contrast, 3 out of 26 male MMP-28−/− mice (12%) survived to 7 day after MI (p<0.05). Female WT and MMP-28−/− mice showed similar survival rates. At autopsy, we found that 40% of the non-surviving WT mice (4/10) died of cardiac rupture, while 88% of the non-surviving MMP-28−/− mice (21/24) died of cardiac rupture (p<0.05, Figure 2C). We did not observe any difference in rupture time kinetics between the two genotypes. Cardiac rupture primarily occurred between 3–7 days post-MI, consistent with previous reports.26, 27
Figure 2. MMP-28 deletion decreased survival, increased cardiac rupture, and exacerbated LV dysfunction post-MI.
A, Infarct areas were similar between WT and MMP-28−/− mice. n=8–14/group. B, MMP-28 deletion reduced 7 day post-MI survival. n=6–26/group. C, Cardiac rupture post-MI was increased in MMP-28−/− mice. n= 10–24/group. D and E, LV end systolic and diastolic volumes at day 7 post-MI were higher in MMP-28−/−, compared with WT. F, Ejection fraction in MMP-28−/− mice was attenuated at day 1, but exacerbated at day 7 post-MI, compared with WT. G, The ratio of lung weight to tibia length (edema index) at day 7 post-MI was higher in MMP-28−/− than in WT. H, MMP-28 deletion showed higher EDV/ LV mass (LV remodeling index) at day 7 post-MI. n=8–14/group for D through H. *p<0.05 vs. day 0 and #p<0.05 vs. WT.
MMP-28 deletion deteriorated post-MI LV function
As expected, the LV end systolic and diastolic volumes increased in WT mice post-MI (Figure 2D and E). Interestingly, the decrease in ejection fraction at day 1 post-MI was attenuated in the absence of MMP-28, compared to WT (Figure 2F, p<0.05), indicating that cardiac function was actually better at day 1 when MMP-28 was absent. By day 7, the end systolic and diastolic volumes in MMP-28−/− mice were 31% and 25% higher respectively, compared to WT (both p<0.05). The increases in volumes translated to a reduced ejection fraction with MMP-28 deletion, compared to day 7 post-MI WT (p<0.05). These data suggest that MMP-28 deletion improves the early cardiac functional response while exacerbating cardiac dysfunction at later times, suggesting a complex interplay of MMP-28 functions in the post-MI setting.
Post-MI, pump dysfunction leads to increased LV end diastolic pressure and subsequent pulmonary edema. In Figure 2G, the ratio of lung weight to tibia length (edema index) markedly increased at days 5 and 7 post-MI in both WT and MMP-28−/− mice, compared to day 0 controls (all p<0.05). The edema index was 22% higher in MMP-28−/− mice, compared to WT at day 7 post-MI (p<0.05), indicating worse lung edema.
The ratio of end diastolic volume to LV mass represents the LV remodeling index, which increases during cardiac remodeling and repair.23 Our data demonstrated that the LV remodeling index progressively increased from day 0 to day 7 post-MI in WT mice (Figure 2H, all p<0.05 vs. day 0). MMP-28−/− mice showed similar increases in LV remodeling index at days 1, 3 and 5 post-MI, but displayed a 20% higher remodeling index at day 7 post-MI than WT mice (Figure 2H, p<0.05), indicating that adverse LV remodeling was accentuated.
MMP-28 deletion did not influence TNF-α level in plasma and LV at day 1
TNF-α is involved in the pathogenesis and progression of MI, ischemia/reperfusion, and heart failure.28–30 Plasma TNF-α levels increased at day 1 in WT and MMP-28−/− mice (both p<0.05 vs. day 0), and returned towards baseline by day 7 post-MI (Online Figure IA). In the LV, TNF-α mRNA content was not significantly upregulated in either group, compared to respective day 0 levels (Online Figure IB).
Post-MI plasma proteomic profiling
To determine the impact of MMP-28 deletion on plasma biomarkers, we conducted plasma proteomic profiling of 58 analytes (Table 1 and Online Table II). The 13 analytes shown in Table 1 were significantly upregulated at day 7 post-MI in WT mice, as expected for an MI response. In the WT group, basic fibroblast growth factor was the only factor decreased at day 7 post-MI (p<0.05 vs. day 0). In the MMP-28−/− mice, 10 analytes were statistically increased at day 7 after MI (all p<0.05), all of which overlapped with analytes increased in WT. These factors, therefore, are MI but not MMP-28 dependent. The three factors that did not increase in MMP-28−/− mice were Factor VII, fibrinogen, and MMP-9, suggesting these 3 may be downstream signaling effectors of MMP-28. Basic fibroblast growth factor was downregulated in MMP-28−/− mice similar to WT, and macrophage-derived chemokine was also decreased, compared to day 0 controls (both p<0.05).
Table 1.
Plasma proteomic profiling reveals MI and MMP-28 effects.
| WT | MMP-28−/− | |||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | |
| Apolipoprotein A-I (μg/mL)a | 28±1 | 155±14* | 25±1 | 158±10* |
| CD40 (pg/mL)a,b | 26±1 | 38±2* | 31±2# | 41±1* |
| Endothelin-1 (pg/mL)c | 40±5 | 30±3 | 42±2 | 51±4# |
| Factor VII (ng/mL)a | 33±5 | 60±5* | 38±4 | 65±5 |
| Fibrinogen (mg/mL)a | 87±7 | 146±19* | 87±10 | 126±9 |
| Fibroblast growth factor basic (ng/mL)a | 115±14 | 35±4* | 130±10 | 40±2* |
| Interleukin-18 (ng/mL)a | 7.3±0.3 | 33.3±1.0* | 7.7±0.4 | 34.6±2.3* |
| Macrophage colony-stimulating factor-1 (ng/mL)b | 5.5±0.3 | 4.8±0.2 | 4.6±0.2# | 4.8±0.2 |
| Macrophage-derived chemokine (ng/mL)a | 2.0±0.2 | 1.8±0.2 | 2.4±0.1 | 1.9±0.1* |
| Macrophage inflammatory protein-1α (ng/mL)a | 3.6±0.4 | 7.4±0.4* | 4.0±0.4 | 8.0±0.5* |
| Macrophage inflammatory protein-1β (pg/mL)c | 92±11 | 71±6 | 109±14 | 143±14# |
| Macrophage inflammatory protein-3β (ng/mL)a | 1.5±0.1 | 3.4±0.2* | 1.9±0.1 | 3.8±0.2* |
| Matrix metalloproteinase-9 (ng/mL)c | 81±6 | 118±10* | 68±7 | 85±5# |
| Myeloperoxidase (ng/mL)c | 84±8 | 126±10* | 109±12 | 171±15*# |
| Thrombopoietin (ng/mL)c | 19±3 | 51±5* | 24±2 | 67±8*# |
| Tissue factor (ng/mL)a | 6.7±0.7 | 13.3±1.1* | 8.2±0.8 | 12.6±0.8* |
| Tissue inhibitor of metalloproteinases-1 (ng/mL)a | 1.7±0.2 | 3.2±0.6* | 1.3±0.2 | 2.7±0.3* |
| Von Willebrand factor (ng/mL)a | 248±26 | 429±63* | 300±29 | 459±46* |
The data are reported as mean±SEM. Of a total of 58 analytes measured, the 18 listed here were significantly different among the groups. In the absence of MI, MMP-28−/− mice showed higher CD40 but lower macrophage colony stimulating factor-1 levels, compared to WT mice (both p<0.05). n=8–12/group.
p<0.05 vs. respective day 0 and
p<0.05 vs. WT.
Effects that are MI-dependent;
effects that are MMP-28-dependent; and
effects that are MI+MMP-28-dependent.
Interestingly, at day 7 post-MI, 4 analytes in the MMP-28−/− mice were higher than WT MI counterparts (endothelin-1, macrophage inflammatory protein-1β, myeloperoxidase, and thrombopoietin, all p<0.05).
Reduced inflammatory factors in the MMP-28−/− Mice
The inflammatory response regulates post-MI LV remodeling. For this reason, we explored the effect of MMP-28 deletion on inflammation by examining gene levels of 84 inflammatory cytokines and receptors (Table 2 and Online Table III). At day 7 post-MI, 36 genes were significantly upregulated and 7 genes were downregulated in the infarct region of WT day 7 MI mice, compared to day 0 (all p<0.05); whereas 25 genes in the infarct region of MMP-28−/− mice were statistically increased by MI, and 9 decreased, compared to day 0 (all p<0.05). These changes were consistent with a robust inflammatory response in the infarct region. In the remote area of day 7 post-MI WT mice, the expression of 10 genes were increased and 5 decreased, compared with day 0 (all p<0.05). Only 3 genes in the remote region of MMP-28−/− mice were upregulated, and 11 downregulated compared with day 0 (all p<0.05).
Table 2. MMP-28 deletion decreased the post-MI inflammatory (Inf) and ECM responses.
Only genes affected by both MI and MMP-28 deletion are displayed here.
| WT | MMP-28−/− | |||||
|---|---|---|---|---|---|---|
| Inf | Day 0 | Day 7 | Day 0 | Day 7 | ||
| LV | LVC | LVI | LV | LVC | LVI | |
| Ccl2 | 4.6±0.5 | 10.2±0.9 | 17.3±3.5*^ | 6.3±1.2 | 9.2±1.3 | 10.6±1.9# |
| Ccl6 | 24±2 | 51±14 | 94±34* | 14±2 | 19±4 | 35±11# |
| Ccl9 | 2.1±0.2 | 8.0±1.1* | 18.1±3.2^* | 1.7±0.1 | 3.4±0.2# | 9.8±1.7*^# |
| Ccl17 | 0.16±0.04 | 1.49±0.23 | 4.11±0.68*^ | 0.24±0.06 | 0.52±0.06 | 2.46±0.68*^# |
| Ccr2 | 1.8±0.4 | 5.5±1.3* | 15.7±0.7*^ | 1.6±0.3 | 4.5±1.0* | 7.7±0.7*^# |
| Ccr3 | 3.5±0.6 | 11.7±1.9* | 33.5±4.4*^ | 2.1±0.4 | 8.0±0.9 | 19.8±2.3*^# |
| Ccr5 | 2.5±0.3 | 10.7±1.5 | 33.9±5.2*^ | 1.6±0.3 | 7.8±0.8 | 23.3±2.6*^# |
| Il1b | 0.81±0.07 | 1.57±0.33 | 2.94±0.28*^ | 0.98±0.11 | 0.73±0.12 | 1.49±0.20# |
| Il2rg | 7.4±0.6 | 10.7±2.0 | 15.7±2.4*^ | 7.8±0.7 | 7.6±0.5 | 10.5±1.7# |
| Il10ra | 1.24±0.19 | 3.83±0.48 | 12.70±1.83*^ | 0.95±0.13 | 3.00±0.47 | 9.43±1.07*^# |
| Il18 | 0.54±0.02 | 1.88±0.40* | 5.75±0.47*^ | 0.56±0.07 | 1.16±0.10 | 3.97±0.51*^# |
| Itgb2 | 4.1±0.4 | 16.6±3.2 | 58.9±3.6*^ | 4.2±0.6 | 10.3±1.7 | 43.9±8.8*^# |
| Mif | 177±12 | 239±15* | 196±12^ | 179±9 | 155±12# | 135±15# |
| Pf4 | 18±1 | 26±7 | 76±18*^ | 14±1 | 13±1 | 34±8# |
| Tgfb1 | 19±2 | 43±3* | 70±6*^ | 18±1 | 30±2*# | 56±4*^# |
| Tnfrsf1a | 13±1 | 25±2* | 33±3*^ | 16±1 | 15±1# | 20±3# |
| ECM | ||||||
| Cdh3 | 0.03±0.01 | 0.78±0.20 | 3.51±0.59*^ | 0.04±0.01 | 0.31±0.05 | 2.05±0.31*^# |
| Col1a1 | 31±3 | 891±156 | 3066±483*^ | 27±3 | 636±105 | 2340±284*^# |
| Col3a1 | 195±21 | 3125±501* | 7240±874*^ | 192±21 | 2236±399 | 4516±1090*^# |
| Col5a1 | 16±1 | 156±21* | 350±44*^ | 17±2 | 109±11 | 244±48*^# |
| Ctgf | 119±12 | 1241±168* | 2050±246*^ | 141±22 | 667±90# | 1344±236*^# |
| Ctnna1 | 296±13 | 270±24 | 174±6*^ | 309±14 | 214±16*# | 154±16*^ |
| Ecm1 | 24±2 | 96±15* | 210±13*^ | 30±2 | 74±11 | 160±28*^# |
| Emilin1 | 9.4±0.5 | 59.5±7.2* | 119.0±14.5*^ | 10.4±1.0 | 43.3±4.3* | 91.3±11.1*^# |
| Fn1 | 13±1 | 360±75 | 1225±233*^ | 13±1 | 245±41 | 870±163*^# |
| Itga4 | 0.45±0.05 | 0.94±0.16 | 2.58±0.28*^ | 0.39±0.08 | 0.86±0.16 | 1.87±0.40*^# |
| Itga5 | 10±1 | 45±6* | 48±5* | 11±2 | 30±1*# | 33±3*# |
| Itgal | 1.10±0.11 | 1.25±0.22 | 2.29±0.16*^ | 1.46±0.29 | 0.79±0.13 | 1.72±0.15^# |
| Itgb1 | 223±16 | 381±17* | 502±31*^ | 231±10 | 311±12*# | 387±23*^# |
| Itgb2 | 5.1±0.8 | 18.2±3.5 | 64.3±6.0*^ | 4.3±0.6 | 11.7±2.1 | 47.6±8.8*^# |
| Mmp2 | 34±3 | 99±10 | 176±30*^ | 32±4 | 66±9 | 91±25# |
| Mmp9 | 0.46±0.03 | 0.26±0.11 | 2.24±0.70*^ | 0.64±0.27 | 0.10±0.03 | 1.15±0.35# |
| Postn | 10±1 | 495±65* | 857±138*^ | 8±1 | 363±59*# | 522±115*# |
| Selp | 0.58±0.12 | 2.73±0.56* | 4.43±0.42*^ | 0.83±0.07 | 1.72±0.15# | 3.19±0.43*^# |
| Tgfbi | 38±3 | 96±21 | 301±27*^ | 35±3 | 57±8 | 172±34*^# |
| Thbs2 | 17±1 | 89±17 | 331±57*^ | 20±2 | 60±10 | 238±51*^# |
| Tnc | 0.4±0.1 | 40.0±6.4* | 66.9±6.5*^ | 1.0±0.2 | 24.0±2.7*# | 45.4±9.5*^# |
The data are reported as 2−ΔCt values × 100±SEM. Of the 168 genes evaluated, 37 Inf genes and 44 ECM genes were MI-dependent; and 3 Inf genes were MMP-28 dependent (shown in Online Tables III and IV).
LVC=remote region, LVI=infarct region. n=6/group.
p<0.05 vs. respective day 0,
p<0.05 vs. respective LVC, and
p<0.05 vs. WT.
When compared with WT day 7 MI, 16 genes in the infarct region and 4 genes in the remote region were lower in the MMP-28−/− mice (all p<0.05), indicating an overall decreased inflammatory response in the absence of MMP-28.
M2 macrophage polarization was impaired by MMP-28 deletion
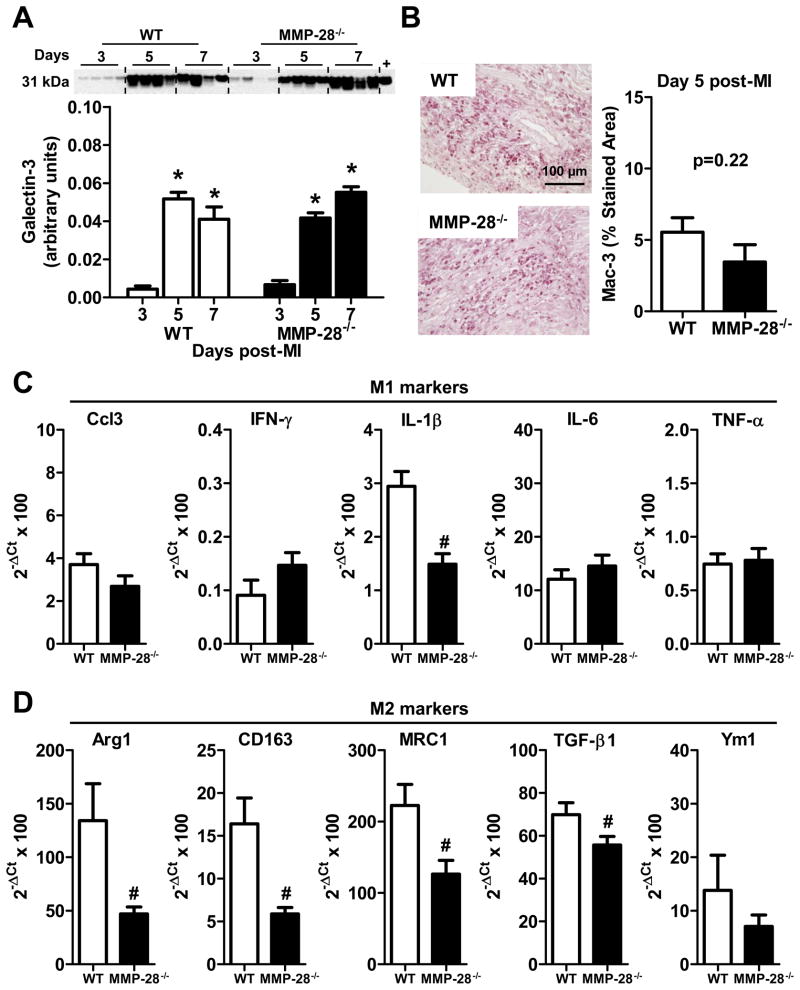
Macrophages are a major source of inflammatory factors post-MI. Therefore, we explored the impact of MMP-28 deletion on macrophage infiltration and phenotype. The expression of galectin-3, a macrophage marker, was induced in WT infarct LV at day 3 and peaked at day 5 post-MI (Figure 3A, p<0.05 vs. day 3), consistent with our previously published time course.7 MMP-28−/− mice showed similar increases in macrophage content in the post-MI infarct region, compared to WT counterparts at the same time points (Figure 3A). Immunohistochemistry staining for Mac-3 also demonstrated similar macrophage infiltration rates at day 5 post-MI in WT and MMP-28−/− mice (Figure 3B). Macrophage infiltration, therefore, was not altered by MMP-28 deletion. Pro-inflammatory M1 macrophages promote ECM degradation, while anti-inflammatory M2 macrophages facilitate the ECM reconstruction and reparative response. Our qRT-PCR data showed that at day 7 post-MI, IL-1β was the only M1 marker reduced with MMP-28 deletion (Figure 3C). Of the M2 markers measured, all except Ym1 were significantly reduced in the absence of MMP-28 (Figure 3D). These results suggest that M2 macrophage activation was compromised in the MMP-28−/− infarct.
Figure 3. M2 macrophage activation was impaired with MMP-28 deletion.
A, Galectin-3 expression was similarly induced in WT and MMP-28−/− mice post-MI. Isolated peritoneal macrophages were used as positive controls. n=4/group. B, Macrophage numbers infiltrated into infarct area at day 5 post-MI showed no difference between WT and MMP-28−/− mice. n=5–7/group. C, MMP-28 deletion did not affect M1 macrophage markers (except IL-1β) in day 7 infarct. D, MMP-28 deletion attenuated M2 macrophage markers in day 7 infarct. n=6/group for C and D. *p<0.05 vs. day 3 and #p<0.05 vs. WT.
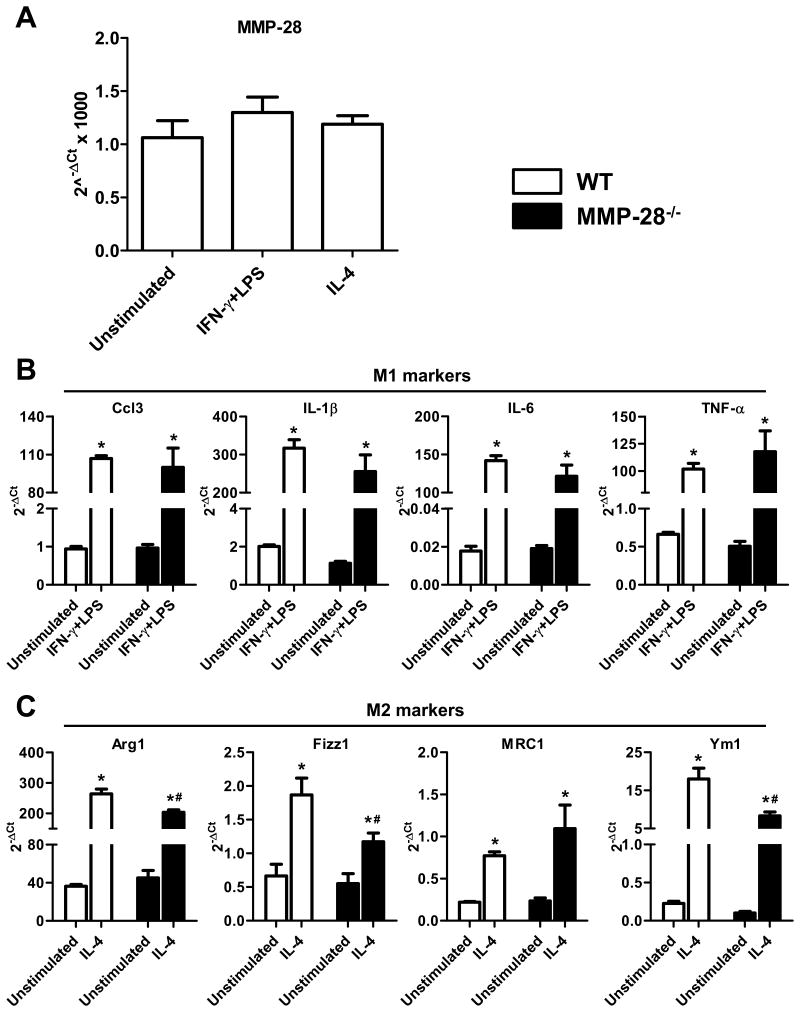
In order to further investigate the impact of MMP-28 deletion on macrophage phenotype, peritoneal macrophages isolated from WT and MMP-28−/− mice were stimulated with IFN-γ+LPS or IL-4. MMP-28 expression in macrophages was not altered after stimulation with IFN-γ+LPS or IL-4 (Figure 4A), suggesting that distinct macrophage phenotypes have no difference in MMP-28 expression. All M1 markers measured here showed similar upregulation in both WT and MMP-28−/− macrophages with IFN-γ+LPS stimulation, compared to unstimulated macrophages (Figure 4B, all p<0.05). This indicates that the in vivo environment is not completely recapitulated by IFN-γ+LPS stimulation. In agreement with the in vivo data, MMP-28−/− macrophages showed reduced response to IL-4 stimulation. Compared with WT, MMP-28−/− macrophages stimulated with IL-4 showed reduced expression of M2 markers (Figure 4C, all p<0.05), signifying impaired M2 macrophage polarization.
Figure 4. MMP-28 deletion attenuated macrophages polarization towards M2 subtype.
Peritoneal macrophages were stimulated with IFN-γ+LPS or IL-4. A, MMP-28 expression was similar among different macrophage subtypes. B, MMP-28 deletion did not affect macrophage activation towards M1 subtype. C, MMP-28 deletion attenuated macrophage polarization towards M2 phenotype. n=4/group for A through C. *p<0.05 vs. unstimulated and #p<0.05 vs. WT.
Reduced ECM transcription in the MMP-28−/− mice
To explore mechanisms of increased rupture in the MMP-28−/− mice, we performed gene array analysis for 84 ECM and adhesion molecules at days 0 and 7 post-MI (Table 2 and Online Table IV). In the infarct area of WT, 51 genes increased and 8 genes decreased, compared to day 0 (all p<0.05). In contrast, in the infarct region of MMP-28−/− mice, 45 genes showed increase and 9 genes decrease, compared with MMP-28−/− day 0 (all p<0.05). In the non-infarct remote region of WT mice, 27 genes were upregulated and 2 genes downregulated, compared with WT day 0 (all p<0.05). In the remote area, MMP-28−/− mice displayed upregulation in 15 genes and downregulation in 4 genes after MI (all p<0.05).
In line with the increased rupture rates in the MMP-28−/− mice, 20 ECM genes in the infarct region and 7 ECM genes in the remote region were decreased compared with the day 7 WT MIs. The genes decreased in the infarct region of the MMP-28−/− mice included Col1a1, Col3a1, Col5a1, MMP-2, and MMP-9, indicating defective ECM transcription in the absence of MMP-28. Decreased ECM transcription, especially for collagen I and III genes, may contribute to the increased rupture rates observed in the MMP-28−/− mice.6
Impaired collagen deposition and cross-linking in the MMP-28−/− mice
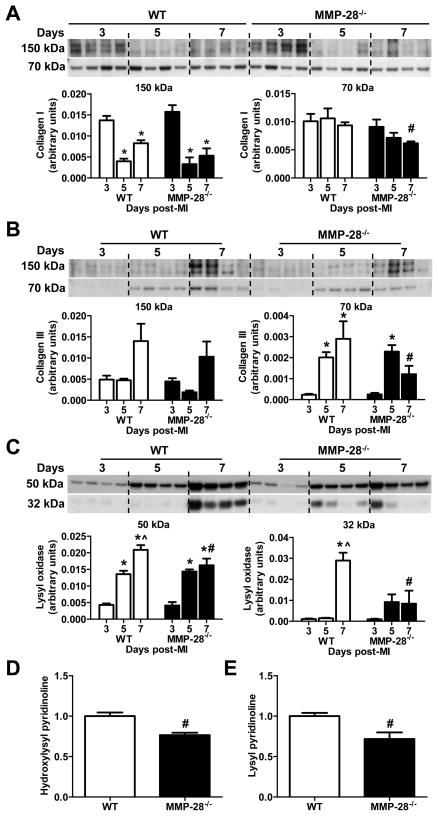
Post-MI, collagen I and III are upregulated and constitute major components of the reparative infarct scar. Insufficient collagen accumulation, excessive collagen degradation, or disorganized collagen cross-linking each could contribute to infarct-related cardiac rupture.31, 32 Compared to elevated collagen I levels in WT MI, collagen I expression in MMP-28−/− mice was 34% lower at day 7 post-MI (Figure 5A, p<0.05). In contrast to WT, the collagen III content at day 7 post-MI was 26% lower in MMP-28−/− mice (Figure 5B, p<0.05).
Figure 5. Collagen deposition, lysyl oxidase content, and collagen cross-linking were reduced with MMP-28 deletion.
A and B, MMP-28 deletion led to reduced collagen I and collagen III accumulation at day 7 post-MI. C, Both precursor and active forms of lysyl oxidase at day 7 post-MI were suppressed with MMP-28 deletion. n=4/group for A through C. Dand E, Hydroxylysyl pyridinoline and lysyl pyridinoline in day 7 infarct were reduced with MMP-28 deletion. n=3/group. *p<0.05 vs. day 3, ^p<0.05 vs. day 5, and #p<0.05 vs. WT day 7.
Lysyl oxidase is a copper-dependent extracellular enzyme that mediates cross-linking in collagen and elastin.33, 34 Our immunoblotting data revealed that in the WT, both the precursor (50 kDa) and active (32 kDa) forms were gradually upregulated from days 3 to 7 post-MI (Figure 5C, both p<0.05). However, in the MMP-28−/− mice, both forms of lysyl oxidase were partially inhibited by MMP-28 deletion (Figure 5C, both p<0.05). At day 7 post-MI, there was notable inhibition of the active form. Lysyl oxidase catalyzes the telopeptidyl hydroxylysyl residues to form hydroxylysyl pyridinoline and lysyl pyridinoline, which are directly involved in the collagen cross-linking.35 The day 7 infarct regions in MMP-28−/− mice had reduced hydroxylysyl pyridinoline and lysyl pyridinoline, compared to WT (Figure 5D and E, both p<0.05). MMP-28 deletion, therefore, suppressed collagen synthesis and cross-linking, which could explain the increase in cardiac rupture in the absence of MMP-28.
Myofibroblast numbers were lower in the MMP-28−/− mice
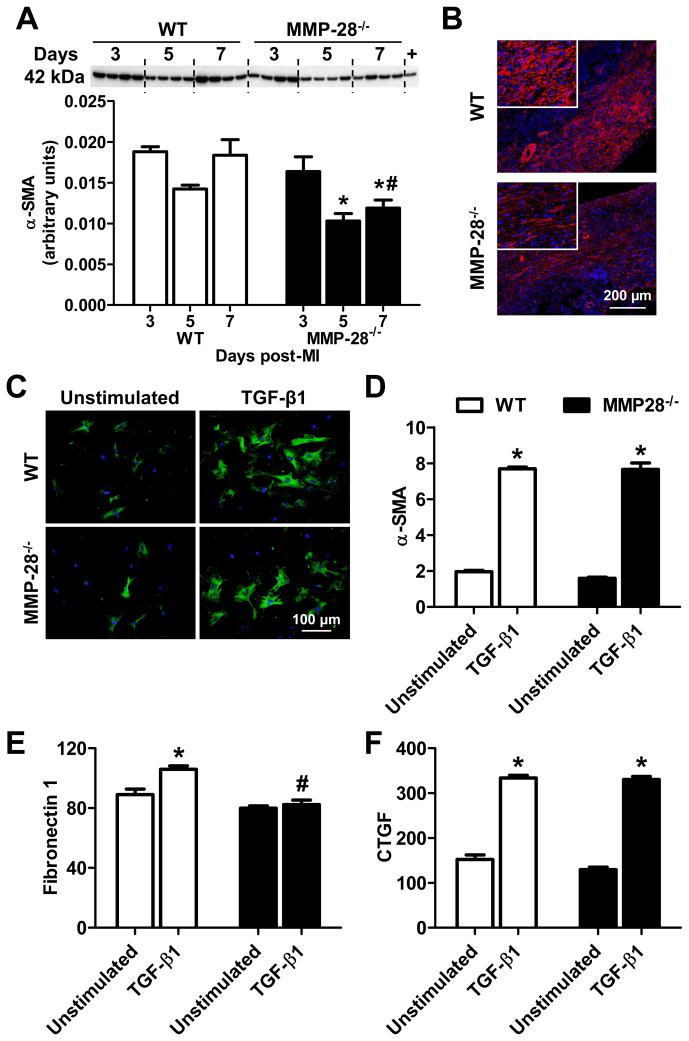
Following MI, fibroblasts transdifferentiate into myofibroblasts that express α-SMA. Further, myofibroblasts are a primary source of ECM in the infarcted myocardium. To explore whether decreased ECM synthesis in MMP-28−/− mice was the result of reduced myofibroblast numbers, we measured myofibroblast content in the infarct hearts. As displayed in Figure 6A, α-SMA was highly expressed in the infarct regions of both WT and MMP-28−/− mice. At days 3 and 5 post-MI, there were no significant differences between the two genotypes. However, α-SMA levels at day 7 were markedly lower in MMP-28−/− mice than WT (Figure 6A, p<0.05). Immunofluorescence staining confirmed reduced α-SMA positive myofibroblast numbers in the non-vessel interstitium of the infarct region in MMP-28−/− mice at day 7 post-MI (Figure 6B).
Figure 6. MMP-28 deletion reduced myofibroblast numbers at day 7 post-MI and affected myofibroblast function.
A, α-SMA expression at day 7 post-MI was reduced in MMP-28−/− mice, compared to WT. Isolated cardiac fibroblasts were used as positive controls. n=4/group. B, Immunofluorescence staining showed that MMP-28−/− mice had fewer myofibroblast numbers in the non-vessel interstitial area at day 7 after MI. Blue=DAPI, red=α-SMA. Representative images from n=3 stained LVs. C and D, TGF-β1 induced similar upregulation in α-SMA in both WT and MMP-28−/−cardiac fibroblasts. E, Fibronectin 1 was lower in MMP-28−/− fibroblasts stimulated with TGF-β1, compared to WT. F, TGF-β1 induced similar upregulation in CTGF in both genotypes. n=4/group for C through F. *p<0.05 vs. unstimulated and #p<0.05 vs. WT.
MMP-28 deletion affected myofibroblast phenotype
In order to explore if MMP-28 mediates TGF-β1-induced fibroblast transdifferentiation and secretion, we isolated cardiac fibroblasts from both WT and MMP-28−/− hearts, and stimulated the cells with TGF-β1. As shown in Figure 6C and D, α-SMA protein and mRNA expression were upregulated in the WT and MMP-28−/− cells after stimulation with TGF-β1 (both p<0.05). This result indicates that fibroblast to myofibroblast transdifferentiation was not influenced by the deletion of MMP-28. Both fibronectin 1 and CTGF mRNA were significantly induced with TGF-β1 stimulation in WT cells (Figure 6E and F, both p<0.05). Interestingly, the upregulation of fibronectin 1, but not CTGF, was blunted in the MMP-28−/−fibroblasts (p<0.05), suggesting the myofibroblast phenotype was altered by MMP-28 deletion.
MMP-28 deletion did not affect LV tensile strength at day 3 post-MI
Mechanical tensile strength of the infarct scar was determined in mice at day 3 post-MI, the time when ruptures are first seen and infarct tissue shows significant impairment in tensile strength.26 Stretch ratios at rupture, expressed as the ratio of the circumferential length when the infarct tissue first breaks to the initial length, were calculated. There were no significant differences in the stretch ratios (Online Figure IIA), suggesting that the scar stiffness at day 3 post-MI was similar between the two genotypes. Ultimate tensile strength multiplied by the wall thickness provided a good indicator for rupture vulnerability. Compared to WT counterparts, MMP-28−/− mice showed similar tensile strengths at day 3 post-MI (Online Figure IIB). These results indicate that MMP-28 effects on rupture manifest after day 3 post-MI.
Ruptured hearts showed Worse LV dilatation than surviving hearts
By LaPlace’s law, higher LV volumes should correspond to increased LV wall stress. To explore the impact of MMP-28 deletion on LV wall stress, we measured day 3 post-MI LV volumes for all mice used for the day 7 survival study. The mice were divided into 2 groups: those that would survive 7 days and those that ruptured at days 4–7 post-MI. In both WT and MMP-28−/− genotypes, the ruptured hearts showed higher end-diastolic volumes at day 3 post-MI, compared with mice that survived 7 days (Online Figure IIC, both p<0.05). This indicates that there was higher LV wall stress in the hearts that would go on to rupture. Of note, the end-diastolic volumes of the ruptured hearts were not different between WT and MMP-28−/− mice, indicating that the increased LV wall stress was not sufficient to explain the increased rupture rates in MMP-28−/− mice. These results indicate that infarct mechanics at day 3 post-MI do not explain or predict rupture at later time points.
MMP-28 deletion did not affect regulatory T cell content or PTEN expression
To investigate the impact of MMP-28 deletion on regulatory T (Treg) cells, we measured the mRNA expression of Foxp3 (a marker of Treg cells) in the day 7 infarct regions. Foxp3 expression showed no difference between WT and MMP-28−/− mice (Online Figure IIIA). Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is upregulated in infarct hearts and exacerbates post-MI LV remodeling by inhibition of Akt activity and IL-10 production.36 However, PTEN expression did not show any differences (Online Figure IIIB).
DISCUSSION
This study evaluated the impact of MMP-28 deletion on LV remodeling and dysfunction induced by MI, focusing on inflammatory and ECM reactions. The major findings were as follows: (1) Post-MI, the MMP-28 cell source switched from myocytes to macrophages; (2) MMP-28 deletion led to adverse LV remodeling and dysfunction, accompanied by increased mortality and rupture rates; (3) Inflammatory factors in the infarct hearts and M2 macrophage polarization were reduced with MMP-28 deletion; (4) ECM transcription, collagen deposition and cross-linking, and myofibroblast numbers were all reduced in MMP-28−/− mice; and (5) mechanical strength at day 3 post-MI was not different between the two genotypes. Taken together, these findings demonstrate that MMP-28 deficiency aggravates post-MI remodeling and dysfunction by inhibiting M2 macrophage activation, ECM synthesis, and collagen cross-linking. Importantly, this study challenges our preconceived idea that all MMPs play detrimental roles in the MI setting and should be inhibited. In the setting of MI, MMP-28 deletion had detrimental effects.
Consistent with past reports, we showed that MMP-28 is expressed by cardiomyocytes under normal conditions, suggesting a role in tissue homeostasis and turnover.15, 16 Following MI, myocytes in the infarct area undergo a dramatic loss; but MMP-28 was still robustly secreted, indicating inflammatory leukocytes could be the source. Our confocal staining confirmed that macrophages infiltrated into the infarct area served as a rich supply of MMP-28. Using a mouse model of pathogen induced pneumonia, Manicone and colleagues found that MMP-28 was expressed by bone marrow-derived macrophages, but not by alveolar macrophages.18 While macrophages were the predominant source of MMP-28 in the post-MI setting, we could not exclude the possibility that other cells, such as cardiac fibroblasts, endothelial cells and other inflammatory cells (e.g., T and B lymphocytes) could express MMP-28 in the infarcted LV. Nonetheless, this is the first report to document MMP-28 in macrophages in the post-MI LV.
Post-MI, the inflammatory response initially serves to remove unwanted tissue debris, supply growth factors, and promote neovascularization and granulation tissue formation.37 An increasing body of evidence has indicated that accentuation, prolongation, and expansion of the inflammatory reaction aggravates adverse remodeling and dysfunction after MI.2, 32, 38, 39 However, insufficient or impaired inflammation also contributes to worsened LV remodeling. The Matsui laboratory reported that syndecan-4 deficiency amplified post-MI LV dysfunction and increased cardiac rupture, which was strongly related to defective granulation tissue formation, as evidenced by reduced numbers of leukocytes, myofibroblasts, and collagen deposition in the absence of syndecan-4.5 Enhanced post-MI cardiac rupture could be attributed to mechanisms that impair and disorganize granulation tissue formation during the reparative phase of MI.
Likewise, a study by Nahrendorf and colleagues showed that the impaired inflammatory response promoted an increase in cardiac rupture and compromised wound healing in factor XIII deficient mice.40 Our array data revealed that MMP-28−/− mice had lower levels of inflammatory associated factors, which, at least in part, explains the increased rupture and worse dysfunction with MMP-28 deletion. In contrast, we previously showed that MMP-28 deletion augmented the inflammatory response to accelerate cardiac aging.15 There are two possible explanations for the differences seen between these two models (aging and MI). First, MMP-28 from different cell sources could exert distinct roles. In the aging setting, both myocytes and macrophages contribute MMP-28, but the myocyte contribution is much greater than that of the macrophage. In the post-MI setting, MMP-28 in the infarct area is mainly secreted by macrophages. Because of these differences, MMP-28 could have net roles on inflammation that are opposite. Second, depending on the different kinds of injury or stress, MMP-28 may exert differential effects on the inflammatory response. Collectively, the proper degree of inflammatory response and its timely resolution both favor stable wound repair and healing.41 In this study, only male, but not female, MMP-28−/− mice revealed higher rupture rates than WT counterparts. Estrogen supplementation has been shown to protect male mice from post-MI cardiac rupture by inhibiting MMP-9 activity and increasing Akt-Bcl-2 anti-apoptotic signaling.42 However, there has been no report that connects MMP-28 and estrogen to the inflammatory response.
Unexpectedly, MMP-28 deletion did not reduce macrophage numbers post-MI, indicating that the reduction in inflammatory factor levels was due to a differential macrophage polarization pattern rather than differences in cell infiltration. Troidl and colleagues demonstrated that macrophages are differentially activated during different phases of LV remodeling after MI.43 During the acute inflammatory phase, classical M1 macrophages predominate, while macrophages shift into an alternative anti-inflammatory M2 subtype during the scar formation and reparative period. In this study, we found that MMP-28 deletion impaired alternative M2 macrophage activation at day 7 post-MI, which could explain the increased cardiac rupture and worse remodeling observed in the MMP-28−/− mice. Similarly, in vitro experiment confirmed that MMP-28 deletion inhibited the macrophage polarization towards M2 subtype.
Because of the limited regenerative capacity of the mammalian myocardium, a collagen-based scar is required to replace the substantial loss of cardiomyocytes, both to fill the space and maintain normal wall stress.44 Thus, a balanced ECM turnover is of great importance to form a high-quality scar and to prevent progressive LV remodeling.45 In this study, we found that MMP-28 deletion led to reduced mRNA transcription of ECM genes at day 7 post-MI, including Col1a1, Col3a1, and Col5a1. Our immunoblotting data confirmed the decreased protein deposition of collagen I and III in the MMP-28−/−mice. These data indicate defective composition of the reparative scar in the absence of MMP-28. Interestingly, the absence of collagen VI attenuates LV remodeling and dysfunction post-MI,46 suggesting different functions of individual collagen subtypes. In our study, we did not find any differences in collagen VI between WT and MMP-28−/− mice.
Impaired TGF-β1/Smad signaling leads to inhibited collagen synthesis and facilitates cardiac rupture post-MI.31, 47 Our array data showed decreased TGF-β1 transcription in MMP-28−/− mice, supporting the elevated rupture when MMP-28 is absent. Increased MMP-9 levels post-MI could contribute to cardiac rupture by degrading ECM components.8, 10, 48, 49 Interestingly, MMP-28−/− mice showed lower levels of MMP-9 in the plasma and infarct regions, which suggests that increased cardiac rupture in MMP-28−/−mice after MI was not MMP-9 dependent. Following MI, cardiac fibroblasts differentiate into myofibroblasts, a central source of ECM. Myofibroblasts were involved in LV remodeling and repair by regulating ECM turnover.50 Further, we demonstrated that the reduced levels of ECM in MMP-28−/− mice were due to reduced numbers of myofibroblasts. In vitro experiments showed that MMP-28deletion impaired fibronectin 1 expression in fibroblasts after TGF-β1 stimulation, indicating an altered myofibroblast phenotype in the absence of MMP-28.
Not only scar quantity, but also quality, is crucial to prevent post-MI cardiac rupture. Collagen is a relatively stiff material with high tensile strength, and small alterations in its concentration, relative proportion of different types of collagen, the diameter of collagen fibers, or their spatial alignment and cross-linking are shown to have an important effect on cardiac mechanical characteristics.51, 52 Lysyl oxidase mediated cross-linking of collagen I and III fibers results in the deposition of insoluble collagen fibers and increased tensile strength to resist wall stress.33 We found that MMP-28 deletion led to reduced expression and activation of lysyl oxidase, as well as impairment in collagen cross-linking at day 7. However, mechanical testing did not show significant differences in tensile strength of day 3 infarct hearts between WT and MMP-28−/− mice, indicating that the role of crosslinking is a late event in remodeling.
Post-MI, Treg cells infiltrate into the infarct region.53 Treg cells attenuate LV remodeling and dysfunction by suppressing leukocyte infiltration, pro-inflammatory factor secretion, apoptosis, and MMP-2 activity, as well as upregulating IL-10 expression.53 Inhibition of PTEN has been shown to exert beneficial roles in multiple cardiac diseases, including MI, ischemia/reperfusion, and pressure overload models.36, 54, 55 MMP-28 deletion, however, did not affect Treg cell infiltration and PTEN expression, suggesting MMP-28 mediated LV remodeling may be Treg- and PTEN-independent.
Elevated cardiac TNF-α levels facilitate MI-induced LV dysfunction.28 In this study, TNF-α protein levels increased in the plasma, but levels were not affected by MMP-28 deletion. This indicates that differences in LV function between WT and MMP-28−/− mice were not likely mediated through TNF-α.
This study revealed a negative impact of MMP-28 deletion in the post-MI setting, indicating that MMP-28 inhibition strategies would likely be detrimental.15, 19 Therefore, MMP-28 treatment, rather than inhibition, may protect against MI induced LV adverse remodeling. Our data also highlight the concept that specific MMPs have much different functions post-MI, even when originating from the same cell source (e.g., macrophages).
In conclusion, MMP-28 deletion aggravated post-MI LV dysfunction and rupture by blunting the inflammatory and fibrotic responses that are necessary for high-quality scar formation and proper wound healing (Online Figure IV). Therefore, inhibiting MMP-28 and associated pathways in the post-MI setting would likely not provide a favorable approach to prevent abnormal cardiac remodeling and rupture post-MI.
Supplementary Material
Novelty and Significance.
What Is Known?
MMP-28 is constitutively expressed in the myocardium.
MMP-28 deletion augments the inflammatory and extracellular matrix responses to cardiac aging.
MMP-28 deletion enhances macrophage infiltration to the lung during pneumonia
What New Information Does This Article Contribute?
Post-myocardial infarction (MI), the MMP-28 cell source switches from myocytes to macrophages.
MMP-28 deletion contributes to adverse left ventricular remodeling and dysfunction, accompanied by increased mortality and rupture rates.
MMP-28 deletion inhibits M2 macrophage polarization.
MMP-28 mediates the inflammatory and extracellular matrix (ECM) responses in cardiac aging, but MMP-28 roles following MI were unknown. The present study determined the effect of MMP-28 deletion on post-MI remodeling. MMP-28 expression in the infarct region decreased post-MI due to the loss of myocytes, and its cell source shifted from myocytes to macrophages. MMP-28 null mice showed decreased survival due to increased rupture rates and worse cardiac function compared to wild type controls. MMP-28 deletion also resulted in a reduced inflammatory response as well as decreases in myofibroblast numbers, ECM deposition, and collagen cross-linking. The underlying mechanism involved impaired M2 macrophage activation in the absence of MMP-28. Our data highlight the concept that specific MMPs have much different functions post-MI, even when originating from the same cell source (e.g., macrophages). Therefore, inhibiting MMP-28 and associated pathways in the post-MI setting would likely not provide a beneficial approach to prevent adverse LV remodeling and rupture post-MI.
Acknowledgments
SOURCES OF FUNDING
We acknowledge the support from the National Institutes of Health, NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center; from NIH-National Center for Complementary and Alternative Medicine for 1K99AT006704-01 to GVH; from National Science Foundation 0649172, EB009496 and 1SC2 HL101430 to Y-FJ; from NIH R01HL095852 and NSF 0644646 to HCH; from NHLBI HL084385 and HHMI Physician-Scientist Early Career Award to AMM; and from NIH R01 HL075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran’s Administration (Merit) to MLL.
Non-standard Abbreviations
- α-SMA
α-smooth muscle actin
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- INF
inflammatory
- LV
left ventricle
- LVC
remote region
- LVI
infarct region
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
- TGF-β
transforming growth factor-β
- Treg cells
regulatory T cells
Footnotes
DISCLOSURES
None.
References
- 1.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Lindsey ML, Halade GV. Dha derivatives of fish oil as dietary supplements: A nutrition-based drug discovery approach for therapies to prevent metabolic cardiotoxicity. Expert Opin Drug Discov. 2012 doi: 10.1517/17460441.2012.694862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsui Y, Ikesue M, Danzaki K, Morimoto J, Sato M, Tanaka S, Kojima T, Tsutsui H, Uede T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ Res. 2011;108:1328–1339. doi: 10.1161/CIRCRESAHA.110.235689. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann U, Bonz A, Frantz S, Hu K, Waller C, Roemer K, Wolf J, Gattenlohner S, Bauersachs J, Ertl G. A collagen alpha2(i) mutation impairs healing after experimental myocardial infarction. Am J Pathol. 2012;180:113–122. doi: 10.1016/j.ajpath.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 8.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A. Targeted deletion of mmp-2 attenuates early lv rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1229–1235. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 9.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchenko GN, Strongin AY. Mmp-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265:87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 12.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (mmp-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276:10134–10144. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- 13.Illman SA, Keski-Oja J, Parks WC, Lohi J. The mouse matrix metalloproteinase, epilysin (mmp-28), is alternatively spliced and processed by a furin-like proprotein convertase. Biochem J. 2003;375:191–197. doi: 10.1042/BJ20030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner SR, Mescher AL, Neff AW, King MW, Chaturvedi S, Duffin KL, Harty MW, Smith RC. Neural mmp-28 expression precedes myelination during development and peripheral nerve repair. Dev Dyn. 2007;236:2852–2864. doi: 10.1002/dvdy.21301. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc Microanal. 2012;18:81–90. doi: 10.1017/S1431927611012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illman SA, Lohi J, Keski-Oja J. Epilysin (mmp-28)--structure, expression and potential functions. Exp Dermatol. 2008;17:897–907. doi: 10.1111/j.1600-0625.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 17.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (mmp-28) induces tgf-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci. 2006;119:3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 18.Manicone AM, Birkland TP, Lin M, Betsuyaku T, van Rooijen N, Lohi J, Keski-Oja J, Wang Y, Skerrett SJ, Parks WC. Epilysin (mmp-28) restrains early macrophage recruitment in pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manicone AM, Harju-Baker S, Johnston LK, Chen AJ, Parks WC. Epilysin (matrix metalloproteinase-28) contributes to airway epithelial cell survival. Respir Res. 2011;12:144. doi: 10.1186/1465-9921-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ. Animal models of heart failure: A scientific statement from the american heart association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 21.Gharacholou SM, Alexander KP, Chen AY, Wang TY, Melloni C, Gibler WB, Pollack CV, Jr, Ohman EM, Peterson ED, Roe MT. Implications and reasons for the lack of use of reperfusion therapy in patients with st-segment elevation myocardial infarction: Findings from the crusade initiative. Am Heart J. 2010;159:757–763. doi: 10.1016/j.ahj.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Salto-Tellez M, Yung Lim S, El-Oakley RM, Tang TP, ZAAL, Lim SK. Myocardial infarction in the c57bl/6j mouse: A quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–97. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 23.Zamilpa R, Kanakia R, Cigarroa Jt, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML. Cc chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300:H1418–1426. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Zhang X, Bao H, Mi S, Cai W, Yan H, Wang Q, Wang Z, Yan J, Fan G, Lindsey ML, Hu Z. Toll-like receptor (tlr) 2 and tlr4 differentially regulate doxorubicin induced cardiomyopathy in mice. PLoS One. 2012;7:e40763. doi: 10.1371/journal.pone.0040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit il-1beta release through pyrophosphates. Embo J. 2009;28:2114–2127. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: Time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65:469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Gao XM, Ming Z, Su Y, Fang L, Kiriazis H, Xu Q, Dart AM, Du XJ. Infarct size and post-infarct inflammation determine the risk of cardiac rupture in mice. Int J Cardiol. 2010;143:20–28. doi: 10.1016/j.ijcard.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Dorge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G. Coronary microembolization: The role of tnf-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 29.Thielmann M, Dorge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Buchert A, Kruger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: Signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002;90:807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 30.Kleinbongard P, Heusch G, Schulz R. Tnfalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of sparc results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsujita K, Kaikita K, Hayasaki T, Honda T, Kobayashi H, Sakashita N, Suzuki H, Kodama T, Ogawa H, Takeya M. Targeted deletion of class a macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation. 2007;115:1904–1911. doi: 10.1161/CIRCULATIONAHA.106.671198. [DOI] [PubMed] [Google Scholar]
- 33.Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: From basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010;299:H1–9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 34.Voloshenyuk TG, Hart AD, Khoutorova E, Gardner JD. Tnf-alpha increases cardiac fibroblast lysyl oxidase expression through tgf-beta and pi3kinase signaling pathways. Biochem Biophys Res Commun. 2011;413:370–375. doi: 10.1016/j.bbrc.2011.08.109. [DOI] [PubMed] [Google Scholar]
- 35.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 36.Parajuli N, Yuan Y, Zheng X, Bedja D, Cai ZP. Phosphatase pten is critically involved in post-myocardial infarction remodeling through the akt/interleukin-10 signaling pathway. Basic Res Cardiol. 2012;107:248. doi: 10.1007/s00395-012-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann DL. The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liehn EA, Postea O, Curaj A, Marx N. Repair after myocardial infarction, between fantasy and reality: The role of chemokines. J Am Coll Cardiol. 2011;58:2357–2362. doi: 10.1016/j.jacc.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of cd4+ t lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 40.Nahrendorf M, Hu K, Frantz S, Jaffer FA, Tung CH, Hiller KH, Voll S, Nordbeck P, Sosnovik D, Gattenlohner S, Novikov M, Dickneite G, Reed GL, Jakob P, Rosenzweig A, Bauer WR, Weissleder R, Ertl G. Factor xiii deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation. 2006;113:1196–1202. doi: 10.1161/CIRCULATIONAHA.105.602094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 42.Cao J, Zhu T, Lu L, Geng L, Wang L, Zhang Q, Yang K, Wang H, Shen W. Estrogen induces cardioprotection in male c57bl/6j mice after acute myocardial infarction via decreased activity of matrix metalloproteinase-9 and increased akt-bcl-2 anti-apoptotic signaling. Int J Mol Med. 2011;28:231–237. doi: 10.3892/ijmm.2011.681. [DOI] [PubMed] [Google Scholar]
- 43.Troidl C, Mollmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsasser A. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sane DC, Mozingo WS, Becker RC. Cardiac rupture after myocardial infarction: New insights from murine models. Cardiol Rev. 2009;17:293–299. doi: 10.1097/CRD.0b013e3181bf4ab4. [DOI] [PubMed] [Google Scholar]
- 45.Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci. 2010;87:391–400. doi: 10.1016/j.lfs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luther DJ, Thodeti CK, Shamhart PE, Adapala RK, Hodnichak C, Weihrauch D, Bonaldo P, Chilian WM, Meszaros JG. Absence of type vi collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res. 2012;110:851–856. doi: 10.1161/CIRCRESAHA.111.252734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Chilton RJ, Lindsey ML. Heart rate reduction: An old and novel candidate heart failure therapy. Hypertension. 2012;59:908–910. doi: 10.1161/HYPERTENSIONAHA.111.186494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 50.Bryant JE, Shamhart PE, Luther DJ, Olson ER, Koshy JC, Costic DJ, Mohile MV, Dockry M, Doane KJ, Meszaros JG. Cardiac myofibroblast differentiation is attenuated by alpha(3) integrin blockade: Potential role in post-mi remodeling. J Mol Cell Cardiol. 2009;46:186–192. doi: 10.1016/j.yjmcc.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Fomovsky GM, Rouillard AD, Holmes JW. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J Mol Cell Cardiol. 2012;52:1083–1090. doi: 10.1016/j.yjmcc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rouillard AD, Holmes JW. Mechanical regulation of fibroblast migration and collagen remodeling in healing myocardial infarcts. J Physiol. 2012 doi: 10.1113/jphysiol.2012.229484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X. Regulatory t cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 54.Keyes KT, Xu J, Long B, Zhang C, Hu Z, Ye Y. Pharmacological inhibition of pten limits myocardial infarct size and improves left ventricular function postinfarction. Am J Physiol Heart Circ Physiol. 2010;298:H1198–1208. doi: 10.1152/ajpheart.00915.2009. [DOI] [PubMed] [Google Scholar]
- 55.Oudit GY, Kassiri Z, Zhou J, Liu QC, Liu PP, Backx PH, Dawood F, Crackower MA, Scholey JW, Penninger JM. Loss of pten attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res. 2008;78:505–514. doi: 10.1093/cvr/cvn041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.