Abstract
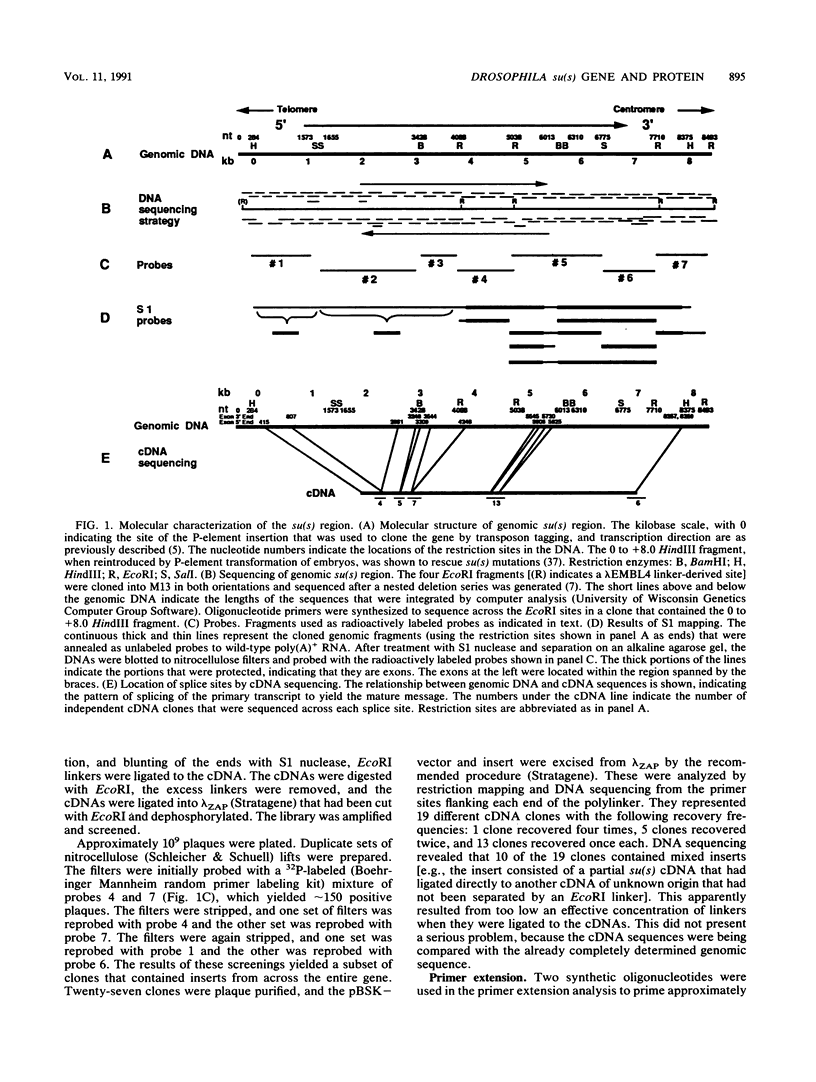
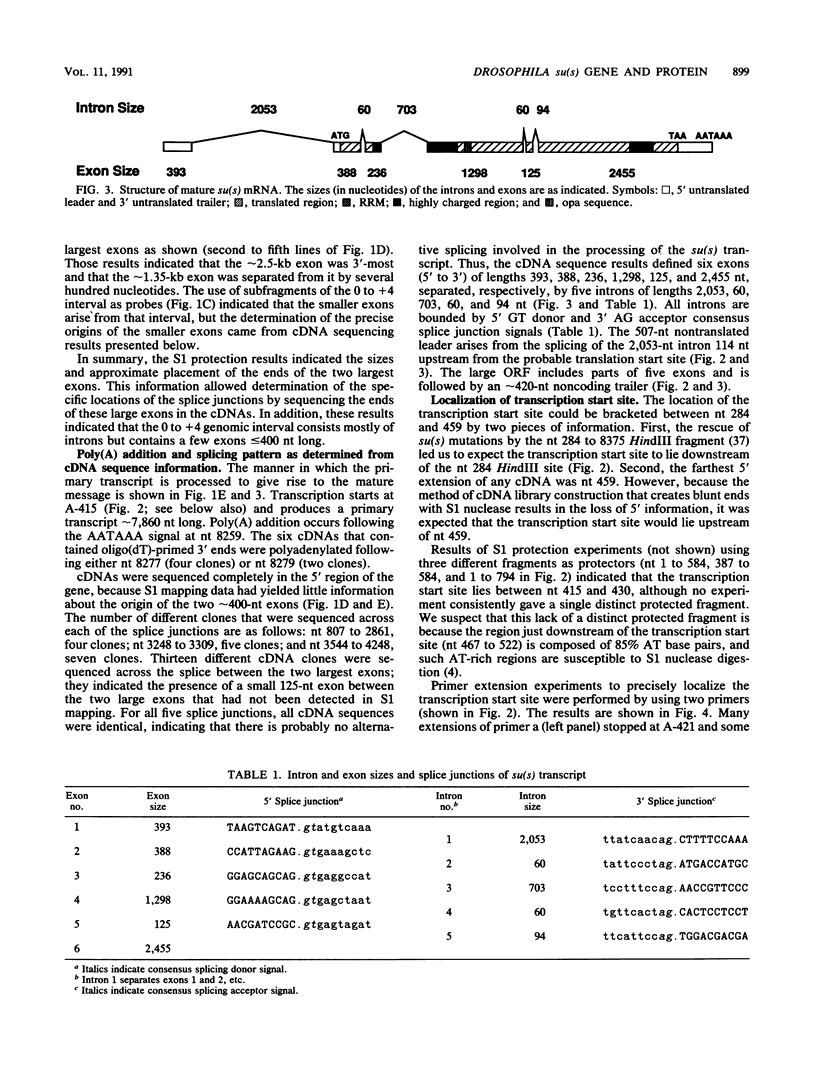
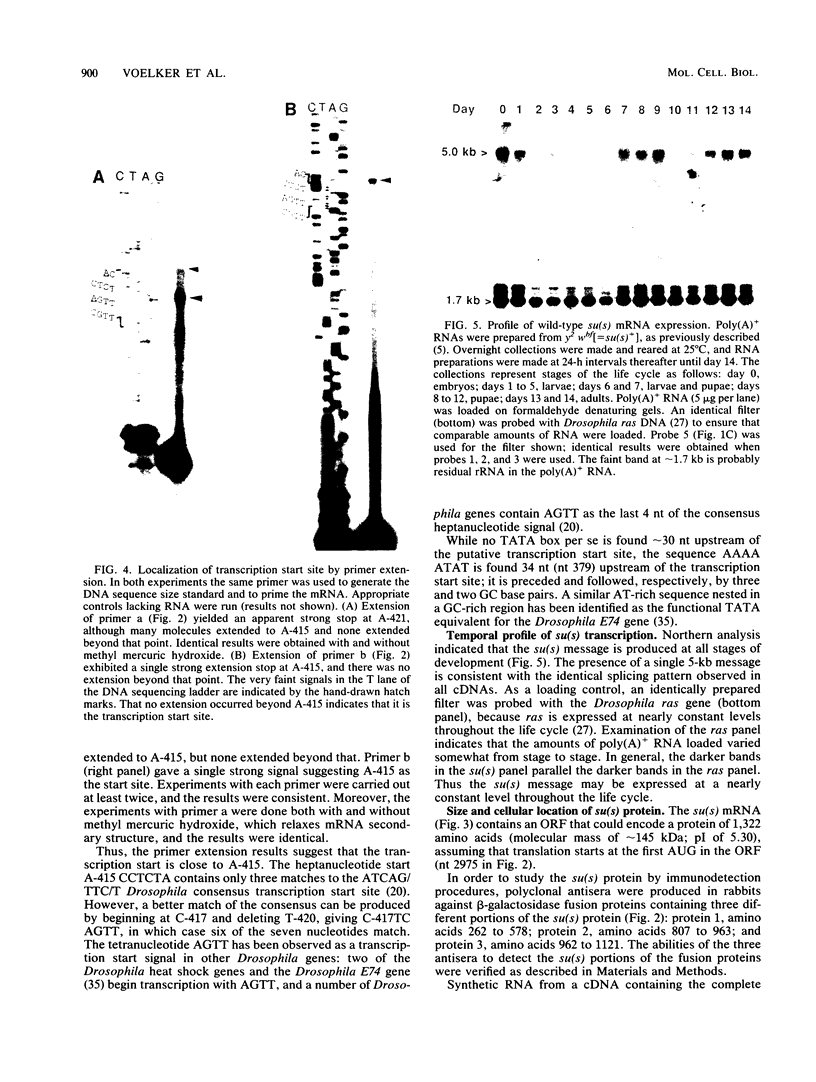
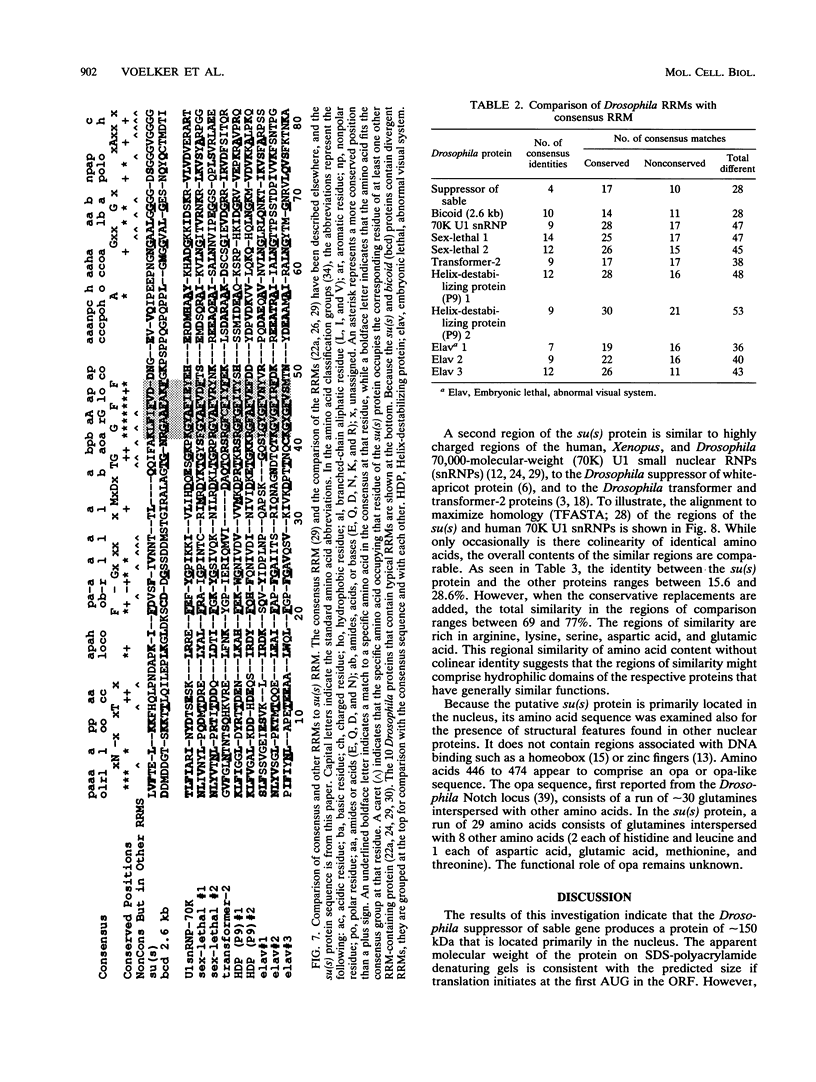
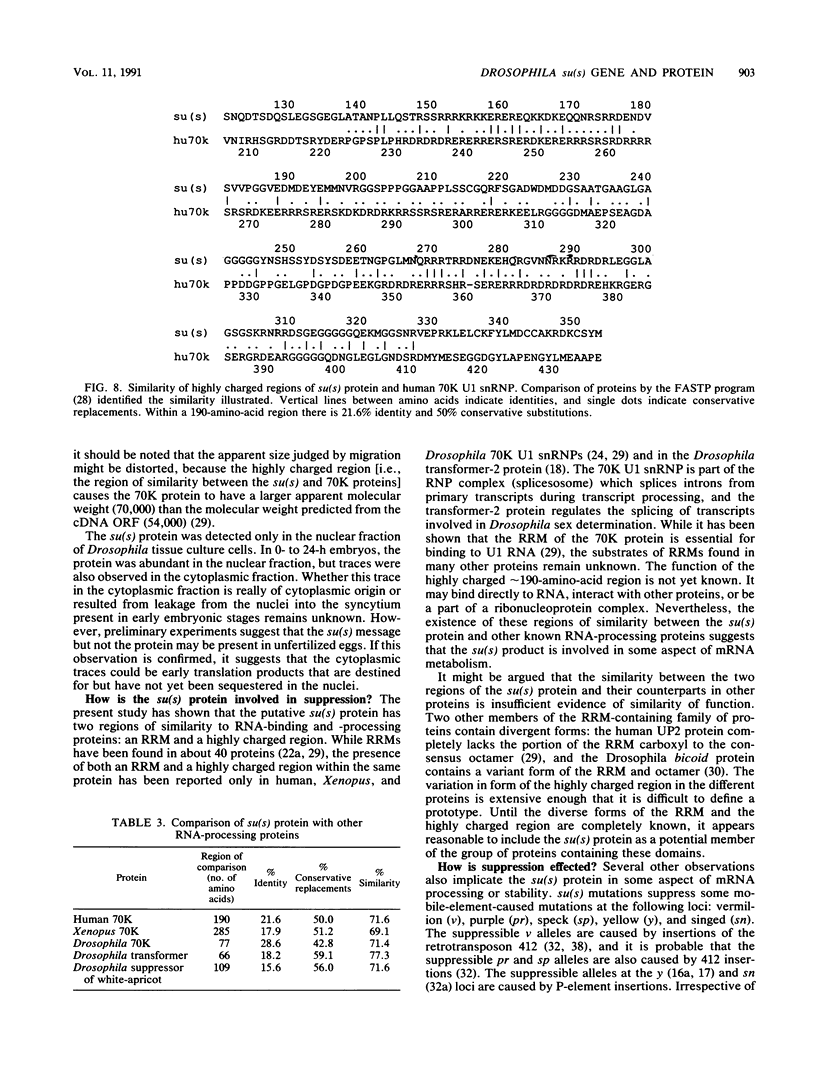
The nucleotide sequence of the Drosophila melanogaster suppressor of sable [su(s)] gene has been determined. Comparison of genomic and cDNA sequences indicates that an approximately 7,860-nucleotide primary transcript is processed into an approximately 5-kb message, expressed during all stages of the life cycle, that contains an open reading frame capable of encoding a 1,322-amino-acid protein of approximately 150 kDa. The putative protein contains an RNA recognition motif-like region and a highly charged arginine-, lysine-, serine-, aspartic or glutamic acid-rich region that is similar to a region contained in several RNA-processing proteins. In vitro translation of in vitro-transcribed RNA from a complete cDNA yields a product whose size agrees with the size predicted by the open reading frame. Antisera against su(s) fusion proteins recognize the in vitro-translated protein and detect a protein of identical size in the nuclear fractions from tissue culture cells and embryos. The protein is also present in smaller amounts in cytoplasmic fractions of embryos. That the su(s) protein has regions similar in structure to RNA-processing protein is consistent with its known role in affecting the transcript levels of those alleles that it suppresses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Chang D. Y., Wisely B., Huang S. M., Voelker R. A. Molecular cloning of suppressor of sable, a Drosophila melanogaster transposon-mediated suppressor. Mol Cell Biol. 1986 May;6(5):1520–1528. doi: 10.1128/mcb.6.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. B., Zachar Z., Bingham P. M. Developmental expression of a regulatory gene is programmed at the level of splicing. EMBO J. 1987 Dec 20;6(13):4095–4104. doi: 10.1002/j.1460-2075.1987.tb02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Swanson M. S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci. 1988 Mar;13(3):86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Etzerodt M., Vignali R., Ciliberto G., Scherly D., Mattaj I. W., Philipson L. Structure and expression of a Xenopus gene encoding an snRNP protein (U1 70K). EMBO J. 1988 Dec 20;7(13):4311–4321. doi: 10.1002/j.1460-2075.1988.tb03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fridell R. A., Pret A. M., Searles L. L. A retrotransposon 412 insertion within an exon of the Drosophila melanogaster vermilion gene is spliced from the precursor RNA. Genes Dev. 1990 Apr;4(4):559–566. doi: 10.1101/gad.4.4.559. [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147–173. doi: 10.1146/annurev.ge.20.120186.001051. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. Rapid purification of a cloned gene product by genetic fusion and site-specific proteolysis. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4692–4696. doi: 10.1073/pnas.81.15.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Richardson K. L., Corces V. G., Green M. M. Genetic instability in Drosophila melanogaster: P-element mutagenesis by gene conversion. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6455–6459. doi: 10.1073/pnas.85.17.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goralski T. J., Edström J. E., Baker B. S. The sex determination locus transformer-2 of Drosophila encodes a polypeptide with similarity to RNA binding proteins. Cell. 1989 Mar 24;56(6):1011–1018. doi: 10.1016/0092-8674(89)90634-x. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Stepień P. P., Tso J. Y., Brousseau R., Narang S., Thomas D. Y., Wu R. Synthesis of human insulin gene. VIII. Construction of expression vectors for fused proinsulin production in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):251–254. doi: 10.1016/0378-1119(84)90186-0. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Yim J. J., Grell E. H., Wobbe C. R. Mechanism of suppression in Drosophila: evidence for a macromolecule produced by the su(s)+ locus that inhibits sepiapterin synthase. Cell. 1982 Oct;30(3):817–823. doi: 10.1016/0092-8674(82)90286-0. [DOI] [PubMed] [Google Scholar]
- Mahaffey J. W., Kaufman T. C. Distribution of the Sex combs reduced gene products in Drosophila melanogaster. Genetics. 1987 Sep;117(1):51–60. doi: 10.1093/genetics/117.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancebo R., Lo P. C., Mount S. M. Structure and expression of the Drosophila melanogaster gene for the U1 small nuclear ribonucleoprotein particle 70K protein. Mol Cell Biol. 1990 Jun;10(6):2492–2502. doi: 10.1128/mcb.10.6.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. M., Stone K. L., Cobianchi F., Wilson S. H., Williams K. R. Phenylalanines that are conserved among several RNA-binding proteins form part of a nucleic acid-binding pocket in the A1 heterogeneous nuclear ribonucleoprotein. J Biol Chem. 1988 Mar 5;263(7):3307–3313. [PubMed] [Google Scholar]
- Mozer B., Marlor R., Parkhurst S., Corces V. Characterization and developmental expression of a Drosophila ras oncogene. Mol Cell Biol. 1985 Apr;5(4):885–889. doi: 10.1128/mcb.5.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. An RNA recognition motif in the bicoid protein. Cell. 1989 Jul 28;58(2):231–232. doi: 10.1016/0092-8674(89)90834-9. [DOI] [PubMed] [Google Scholar]
- Searles L. L., Voelker R. A. Molecular characterization of the Drosophila vermilion locus and its suppressible alleles. Proc Natl Acad Sci U S A. 1986 Jan;83(2):404–408. doi: 10.1073/pnas.83.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D. Interacting gene systems: I. The regulation of tryptophan pyrrolase by the vermilion-suppressor of vermilion system in Drosophila. Genetics. 1969 Jul;62(3):781–795. [PMC free article] [PubMed] [Google Scholar]
- Thummel C. S. The Drosophila E74 promoter contains essential sequences downstream from the start site of transcription. Genes Dev. 1989 Jun;3(6):782–792. doi: 10.1101/gad.3.6.782. [DOI] [PubMed] [Google Scholar]
- Voelker R. A., Graves J., Gibson W., Eisenberg M. Mobile element insertions causing mutations in the Drosophila suppressor of sable locus occur in DNase I hypersensitive subregions of 5'-transcribed nontranslated sequences. Genetics. 1990 Dec;126(4):1071–1082. doi: 10.1093/genetics/126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., Huang S. M., Wisely G. B., Sterling J. F., Bainbridge S. P., Hiraizumi K. Molecular and genetic organization of the suppressor of sable and minute (1) 1B region in Drosophila melanogaster. Genetics. 1989 Jul;122(3):625–642. doi: 10.1093/genetics/122.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]