Abstract
A combination of student interest, a grant for computers, and a suggestion from a colleague led me to convert the printed lab manual for my neurophysiology course into an online version. The web-based version incorporates videos of procedures, color photos, diagrams, links to articles, and other supplementary materials. This paper discusses designing for the web, retaining compatibility with print, layering information, making videos in digital format, and sharing self-published resources. The labs themselves (appendix) are traditional “wet” experiments with crayfish that have been used in many neurobiology courses.
Keywords: web, online, video, QuickTime, crayfish
In the spring semester of 2003, I transformed an often-revised series of illustrated, printed lab handouts for my neurophysiology course into a web-based online lab manual. The online version provides videos of procedures, color diagrams, large photographs, and links to supplementary layers of information that go far beyond what was feasible in a printed manual. Each lab module is self-contained, and although the modules are copyrighted, they are intended for use without restriction by colleagues at other colleges. This paper describes the project to create those modules. I hope it will encourage others to take similar steps in their own courses, and I hope also that sharing these self-published materials will contribute to an increasing trend of sharing online resources within the neuroscience teaching community. Each lab module is described briefly in the appendix, and the modules are available to view and download at my course’s web site: http://www.science.smith.edu/departments/NeuroSci/courses/bio330/labs.html
HOW THIS PROJECT BEGAN
Three events led to the creation of an online lab manual. The first was a student’s question several years ago as she struggled to follow written directions for a dissection: “Couldn’t we have this as a video?” Her question led to a grant from our college committee on educational technology to buy a digital video camera and two Macintosh computers for editing and display. Within a few years, we had produced QuickTime videos for each lab experiment showing the procedure and sample results. Students no longer asked, “Is this the nerve cord?”
The second event was a grant to the Smith College Neuroscience Program from the AV Davis Foundation. The grant provided the neurophysiology course with iMac computers for each of the six student set-ups. Students used the iMacs to view the QuickTime videos, starting and stopping them at their own pace as they reached different phases of the experiment. The videos seemed an easy and natural adjunct to the printed handouts.
The third event was a comment by a colleague who taught the neurophysiology course when I was on leave. “Your lab manual is too wordy,” she reported. “Students don’t want to read so much text.” When I re-examined the lab handouts, I saw she was right. In spite of my polishing and repolishing the handouts over the years, they needed to be tightened up.
My first plan was a simple rewrite that would also incorporate references to the videos. Then I realized that I could instead create web-based documents that linked directly to the videos and also provided still frames as color illustrations of the procedures. Adding other color and grayscale images about the experiments and links to supplementary information was an obvious next step. The page design would have to be compatible with black-and-white printing (it seemed premature to discard printed handouts entirely, an issue discussed in detail below), but the printed version and especially the web version could be much richer than the previous handouts.
SPECIAL FEATURES OF AN ONLINE MANUAL
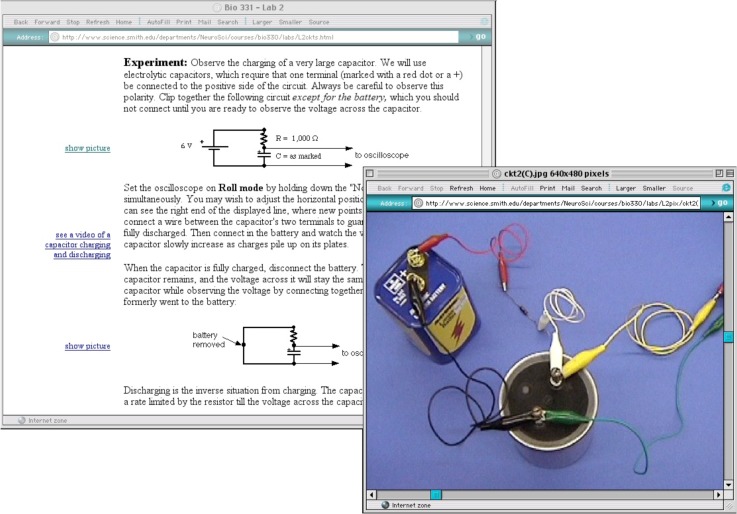
An online manual provides features that could not exist in a printed manual. Videos of procedures are an obvious example, but another is the presentation of multiple layers of information. The top-level description of a procedure or concept can link to more detailed descriptions and images. For example, Lab 2 develops ideas about resistance and capacitance that are relevant to electrical recordings from neurons. Students make measurements from simple circuits that they clip together. The circuits are drawn with the usual electrical symbols, but many students have difficulty transforming the diagrams into circuits with real components. In the online manual, the circuit diagrams link to large color images of the actual components (Fig 1). Students may choose to look at some or all of these supplementary images, depending on their needs. In other labs, the illustrations of equipment and dissections appear initially as small thumbnail images, in color, which for some students will be enough. If they need to see more detail, each image is a link to an enlarged version of itself. Thus, students get to choose whether to go to a deeper layer or not.
Figure 1.
An example of circuit diagrams from Lab 2 with an overlapping second window showing how to arrange real components for the top circuit. See the Supplementary Materials (www.funjournal.org/materials2.asp) for links to Lab 2 and other labs.
In presenting information in layers, it is important to be aware of the “optional reserve reading rule,” which states that no student ever looks at a course’s reserve reading unless it is required for a test or paper. Similarly, one cannot expect that most students will follow links to buried information. An image that is essential (for example, the neural circuitry of the swimmeret system in Lab 8) needs to be presented the first time in a full-sized version. Other images are sufficiently readable as thumbnails (for example, cross-sections of axons in a nerve, also in Lab 8), and the link to a full-sized version serves students who are intrigued to look more closely. Similarly, appendices can be left as links if they are either items that few students will care about (but are easy to provide -- for example, the specifications of the preamplifiers or the recipes for saline solutions, both of which were in the old printed manual), or if the appendix is essential and related to several different experiments (for example, the method for capturing screen shots and pasting them into AppleWorks documents). Students will always go to essential appendices when they need them.
Since links to deeper layers take students away from the page they are on, it is important to keep the students from getting lost or wandering away. This requires consistent design conventions that conform to current expectations for using the web. When our lab computers start up, they launch a browser that automatically displays the table of contents for the labs as its home page. Students click the link to that week’s experiment, which takes them to instructions that generally stay on the screen for the rest of the afternoon. Links within the instructions launch second windows: small windows for playing the videos, large ones for displaying full-sized images, appendices, and other ancillary information. The only exceptions are two Supplements connected with the lab projects at the end of the semester. The Supplements are extensive and need to launch second windows themselves. To avoid confusion, they are formatted as top-layer pages, like the lab exercises. The Supplement that gives abstracts of research articles for the lab project links to online papers that open in a second window. The supplement on crayfish neuroanatomy launches enlargements and videos in second windows. By linking down to other layers, the supplements behave like the weekly lab instructions that they displace in the main window. (The supplements do provide links back to the labs that call them.) Generally, there are never more than two windows open at the same time.
THE PROBLEM OF SIMULTANEOUS PRINT AND SCREEN VERSIONS
While a web page invites presentation of information in layers, there are two relevant corollaries of the “reserve reading rule” that constrain the design of an online manual. These are that (1) students are unlikely to read a lengthy description if it is only on the web, and more generally, (2) no one likes reading lengthy text from a screen. Thus, it seemed essential to distribute a printed handout for each lab, which students could take home to prepare for lab. Even though most students have computers in their rooms to access the campus network, I was not confident that they would read the background information essential to understanding a lab’s conceptual basis if it was available only online. On the other hand, I did not want them reading the background material for the first time in lab. This meant that the design of the web pages had to be compatible with printing the pages to serve as a lab handout. Of course, the printed version would lack color, and the material that was found only through links to other levels would not appear. This meant that certain information had to remain at the top level, some figures had to appear at full size, and links to material the students might like to know existed had to announce themselves explicitly (“click images for enlarged views”). Figure 2 shows an excerpt of introductory material from the lab on the crayfish electroretinogram. Three small figures appear, each of which is readable on its own in a printed version of the page, but each of which links to expanded figures in the online version.
Figure 2.
An example of background material in web-page format to show the level of information that will appear in the printed lab handout. The small images in the left margin link to larger versions of themselves, which open in a second window that is available only online. The pair of traces showing an intracellular recording and the ERG link to a large figure with many pairs of similar traces for different stimulus intensities, from which this pair is an excerpt. From Lab 6: Electroretinogram of the crayfish eye.
The downside of having a format that works on both a screen and a page is that the two media are quite different. A few tricks can make the design work better in both media. For example, colored backgrounds under parts of the on-screen text tie together sections that scroll out of sight, something that is not necessary in a printed version. The backgrounds can be suppressed when the web pages are printed as handouts. But as “Corollary 2” (above) indicates, lots of text is not good web design, which favors brevity, headlines, and color. Yet brief text (even with pictures) usually does not convey enough information. The tension between optimal formats for the web and for print is a continuing problem that is not easily solved.
One solution would be to have two versions, one for the web, one to print. I rejected that path in favor of a single version that I printed in its entirety, largely because of the difficulties of maintaining two parallel versions of material that is constantly being revised. However, I did discuss the issue with my class partway through the semester. The class in general liked the online materials and videos (in a survey at the end of the semester, every student rated them 6 or 7 on a scale where 7 was highest). In our discussion, two of the students who were taking a microbiology course advocated a simple printed checklist of the steps to be done in lab. It was an interesting idea, with observation of the results days later Electrophysiology, on the other hand, requires quick decisions based on the preparation’s current state. It is not usually feasible to collect data and then figure out later what it means. Consequently, I want my students to know as much as possible about the experiment before they set foot in lab. At present, this seems to require extensive introductory text and figures on paper, even if it does not look web-like on the screen, but this may soon change.
Many technologists believe we will soon have thin digital tablets that are as comfortable to read as paper and as versatile as screens. We will then routinely read text with video embedded in it, and screen and print formats will merge. Furthermore, today’s students are increasingly comfortable reading information on conventional computer screens without first printing it. Studying background material online may become routine for them. (Chunking the text into web-sized portions with little quizzes embedded at various checkpoints could further encourage their online reading.) I don’t think we have reached either stage yet, however, and for now my online manual is a hybrid that tries to bridge both web and print media.
TECHNICAL DETAILS
For those who wish to make their own videos or create online materials, some technical details will be helpful. The miniDV digital video format has made editing video considerably easier than it once was. I used a Sony miniDV camera on a modestly priced tripod to make the videos, and iMovie 2 software on a G3 Macintosh to edit them. Other miniDV cameras and Windows-based software would offer similar capabilities.
In selecting a miniDV camera, there were several important criteria. The camera had to accept an external microphone, so I could narrate as I filmed, using an inexpensive headset microphone for consistent sound; additional voice-over narration was added during computer editing using the same microphone and a Griffin Technology adapter for amplification. The camera needed an A/V connector for audio and video input/output, for exchanging signals with VHS VCRs, and potentially for digital recording of video from other sources such as microscope cameras. Finally, the camera had to be able to fill the frame with a very small object, while still having as large a working distance as possible between the object and the lens. T his last feature requires visiting a video store and trying out cameras; it is not in a camera’s list of specifications. A long working distance was necessary because I planned to photograph close-ups of dissections without a microscope. Reasonably priced miniDV cameras do not have removable lenses, so I could not fit the camera to a microscope adapter. Instead, I made a holder for the camera that fit on a microscope stand, and I dissected preparations while looking at the camera’s screen. This was definitely awkward. A better approach, if one has access to a microscope with a video camera, is to convert the microscope camera’s analog video signal to a digital signal. An inexpensive way of doing this is to use the miniDV camera’s A/V connector to record analog video on miniDV tape.
Choosing good software is much easier. Video clips need to be trimmed and resequenced; audio may have to be extracted, silenced, or supplemented with voice-over narration. Adding titles and transitions (dissolves and fades) will make the video look more professional. Apple’s iMovie does all of these things easily, and I would recommend it over more complex software like Final Cut Pro. However, Final Cut Pro and its sibling Final Cut Express do offer additional features and potentially better results. Video-editing applications at various levels of complexity are also available on the Windows platform. The platforms used for video editing and for web viewing by students need not be the same, as long as a cross-platform format like QuickTime is chosen.
The next step, converting the edited digital videos into QuickTime movies for uploading to a server, requires harder choices. iMovie offers various preselected QuickTime formats, but I used the “Expert” option to select a frame size of 320×240, Sorenson-3 compression, 15-fps frame-rate, and key frames every 150 frames. The sound track is 16-bit mono, 11.025 kHz, and Qualcomm PureVoice compression. These are not frugal settings, and the resulting files are huge (from 4 to 70 MB). A smaller frame size and lower frame rate would decrease the file size, but at the cost of lower visual quality. The videos are compiled as progressive downloads, which means they start to play before the entire file has been downloaded. In spite of the huge file sizes, videos play almost immediately within our campus network.
The other component of this project, authoring HTML pages for the web, will already be familiar to many readers of this journal. My first web-authoring application was Claris HomePage (now discontinued), which I still use because of its simplicity in laying out pages, and the ease with which HTML code can be inserted for special tasks (like embedding video). DreamWeaver is a current HTML-authoring application for designing web pages that would be a good choice on both Mac and Windows platforms. Images were edited with an excellent shareware program, GraphicConverter 4; Photoshop is the standard commercial alternative. The web pages were designed for display on a 1024×768 screen in Internet Explorer, which is a denser screen than many users choose. One final design point I followed was “keep it simple.” It is wise to avoid any format that requires a plug-in, with the necessary exception of QuickTime for videos. Readers interested in thoughtful advice about designing academic web sites will find Lynch and Horton’s (2001) book useful.
Since not every campus offers fast networks and a computer for each lab group, it is important to note that not every step in a project like this has to happen at once. Our original plan was to make digital videos and play them “on demand” on a single fast computer at the front bench. This would still be a good first step. When a grant provided computers for each workgroup, we moved the “digital VCR” capability to the student benches, allowing each group to start and stop the videos as they needed. This meant loading the software and videos on the hard drive of each lab computer, which was a bit of a chore, but this also had an upside: the files could be even larger than our current web-based ones, allowing full-screen video. Moving everything to the web provided the benefit of easier maintenance of materials that were subject to constant revision, since the files existed in only one location, on the web server. It also meant that the students could consult the same version in their rooms as they did in lab. However, if a fast campus network is not available, it would also be possible to copy the entire web site to CD-ROMs to be distributed to the class. The CD-ROM would not require special software, since the HTML text, images, and videos would be displayed just like online materials by a standard web browser. The downside, of course, would be duplicating many CD-ROMs and not being able to make revisions once the materials had been handed out.
SHARING SELF-PUBLISHED RESOURCES
One goal of this paper is to let colleagues know that these materials exist, and that they are welcome to use them. The appendix describes each lab briefly. The online text and videos are copyrighted so that I can retain control, but they are available to any non-profit educational institution for internal use without fee or explicit permission. I do ask that colleagues who use the materials credit the original source, even if they modify them, and I enjoy hearing from anyone who uses a module or video in their own course.
Online listings have started to appear for materials developed by faculty members with the intention of sharing them with others, a trend that should make such materials more widely available. Two examples of biology sites that list neuroscience materials are Merlot (www.merlot.org) and BiosciEdNet (www.biosciednet.org; see supplementary materials at www.funjournal.org/materials2.asp for these and other links). This journal provides listings and reviews of materials that are hosted elsewhere (such as Betty Zimmerberg’s animations of synaptic transmission; see Ramirez, 2003) and it also hosts materials on its own site (e.g., Paul et al., 2002). As web listings of teaching materials proliferate, it will be increasingly important for the lists to be peer-reviewed, annotated, and organized for browsing.
Another goal of this paper is to encourage others to move their own materials online by describing a completed project. Especially for laboratory directions, self-publishing is an extremely viable alternative to commercial publishing. Commercial lab manuals for advanced courses have never been very successful; teachers want to mix and match lab exercises to suit their own courses, which means published lab manuals either have to be encyclopedic (and thus expensive) or designed as separate offprints (difficult for a publisher to manage). Even then, the instructions can never be exactly suited to the equipment at a particular institution. This problem is exacerbated for videos of procedures, because visuals are very “literal” by nature, and an oscilloscope or an amplifier that looks different from a student’s own equipment may be bewildering. With the increased ease of video editing, more instructors will be making their own lab videos. Putting course materials online and sharing them freely with other neuroscientists is also increasing. With luck, we will soon have a large community of teacher-scholars who provide open access to their neuroscience courseware.
APPENDIX. ORIGIN AND CONTENT OF LABS
Smith College’s neurophysiology course is an upper-level undergraduate course for students who have taken an intermediate course in animal physiology or cell biology. The syllabus follows a conventional sequence from membrane potentials through synapses to motor control and visual processing (the full syllabus is available at www.science.smith.edu/departments/NeuroSci/courses/bio330/syllabus.html). The required laboratory course is coupled fairly tightly to the main course’s syllabus. For the first seven weeks, the labs consist of prescribed electrophysiological experiments. The last five weeks are devoted to a lab project that students help design themselves. All experiments involve invertebrate preparations (crayfish and earthworms), a tradition which I learned about from the late John Welsh at Harvard University (Welsh et al., 1968), and which was expanded through experiments on crayfish developed by Donald Kennedy at Stanford University in the 1960s. In first devising my own versions of these experiments, I was also aware of a neurobiology course using crayfish that Alan Gelperin taught at Princeton University. The lab on resting potentials (Lab 3) was adapted from a version published by Atwood and Parnas (1968) at the University of Toronto, and the experiment on the electroretinogram was inspired by a demonstration at the Grass Instruments’ booth at a Neuroscience Annual Meeting. Finally, although it did not influence my own versions, the recent “Crawdad” CD-ROM from Cornell University (Wyttenbach et al, 1999; see review by Parfitt, 2002) has given the entire neuroscience community access to a full lab manual and videos for similar experiments. These are robust preparations that many faculty members have adopted because they work well in the hands of undergraduates.
Week 1: Using the Oscilloscope
The first week’s lab is a short one. Students meet the oscilloscope that they will use in all future labs, and are led through it one control at a time. The video is a condensed version of the same grand tour. It serves for review rather than for preparation, since some students do this lab on the same day that classes begin.
Week 2: Circuits and Amplifiers
Although many students have had physics, they often lack intuitive understanding of resistors and capacitors. This lab attempts to redress that problem, emphasizing topics important to neurophysiology.
Week 3: Effect of potassium concentration on the resting potential
Our first “wet” experiment uses microelectrodes to record from crayfish muscle cells. The topic and technique closely complement the accompanying lecture concepts. Although students do mash a few electrodes at first, the cells are so large that every group develops an adequate technique and acquires plausible data. One accompanying video shows the preparation; another shows how microelectrodes are made, something that would not have been included in a print-only manual.
Week 4: Action potentials in earthworm giant axons
Using extracellular techniques (simple hook electrodes), students record huge single spikes and observe threshold, measure conduction velocity, and examine stimulus parameters. Conceptually, this is a far better preparation than old standbys like the frog sciatic nerve, where the compound action potential gets bigger as the stimulus voltage is raised, and seems anything but all-or-none. The earthworm video is one of our nicest in showing the dissection and the experiment.
Week 5: Computer simulations of action potentials and synaptic potentials
Instructions for conducting this lab are online, but the actual simulation software is not (we install it on each lab computer). The software was distributed in Mac and Windows versions to anyone adopting the “shorter” Kandel textbook (Kandel et al, 1995), a textbook we used for several years. In my view, the software is very well designed for our level of students, but in using it they need to be guided to explore interesting questions.
Week 6: Electroretinogram of the crayfish eye
Originally, I conceived of this lab as an example of a graded receptor potential, which of course it still is. But it now comes at a time in the course when we are learning about metabotropic synapses, and I have rewritten the introduction to emphasize the role of second-messengers in visual transduction. The delay between the brief strobe flash and the prolonged depolarization serves to illustrate vividly the time-course of activating a second messenger system. The experiment also produces quantitative data on the relation between light intensity and the magnitude of the visual response. The video shows the preparation and some examples of responses.
Week 7: Motor units in the crayfish nerve cord
This lab has always been a favorite, as one by one groups succeed in hearing spontaneous spikes recorded from motoneurons in the abdominal nerve cord. I view the lab more as experiential than focused on data collection, although it does teach students a technique they can use in their lab projects. The video shows the experiment and its results, and there is also an extensive supplementary page on anatomy that incorporates magnificent videos from Brian Mulloney’s group in which serial sections glide through a crayfish ganglion in each of the three planes (Mulloney et al., 2003).
Weeks 8–12: Laboratory Projects
The semester’s last five weeks are devoted to a project where the class uses the same basic preparation, but each group designs its own variations on the experiment. In recent years, we have investigated the pharmacology of the crayfish swimmeret system, for which we rely heavily on papers published by Brian Mulloney and his colleagues at the University of California at Davis (see Lab 8 on the web site for extensive references and abstracts). In the first week (Lab 8), students come to lab with a paper they have written based on several assigned readings. We discuss the system and try to make sense of the sites where various transmitters may act. For the next three labs, students set up, carry out, and refine their experiments. In the final lab, each group presents the data they have collected in a poster session. A video on the preparation helps students do their first experiment, but by the end of the projects they have become quite expert on their own.
REFERENCES
- Atwood H, Parnas I. Recording from the crayfish abdominal extensor muscle preparation with microelectrodes. In: Kerkut GA, editor. Experiments in physiology and biochemistry. Vol. 1. New York: Academic; 1968. Chapter 16. [Google Scholar]
- Kandel E, Schwartz J, Jessell T. Essentials of neural science and behavior. Norwalk CT: Appleton and Lange; 1995. [Google Scholar]
- Lynch PJ, Horton S. Web style guide. 2nd ed. New Haven, CT: Yale University Press; 2001. www.webstyleguide.com. [Google Scholar]
- Mulloney B, Naranzogt T, Hall WM. Architectonics of crayfish ganglia. Microsc Res Tech. 2003;60:253–265. doi: 10.1002/jemt.10265. [DOI] [PubMed] [Google Scholar]
- Parfitt K. Designing an effective, affordable laboratory course in neurophyiology. J Undergraduate Neurosci Ed. 2002;1:R5–R6. [Google Scholar]
- Paul CA, Goergen EM, Beltz BS. Exploring neurogenesis in crustaceans. J Undergraduate Neurosci Ed. 2002;1:A18–A22. [PMC free article] [PubMed] [Google Scholar]
- Ramirez JJ. Synaptic transmission: a four step process by Betty Zimmerberg. J Undergraduate Neurosci Ed. 2003;1:R9. [PMC free article] [PubMed] [Google Scholar]
- Welsh JH, Smith RI, Kammer AE. Laboratory exercises in invertebrate physiology. 3rd ed. Minneapolis: Burgess; 1968. [Google Scholar]
- Wyttenbach R, Johnson BR, Hoy RR. Crawdad: a CD-ROM lab manual for neurophysiology. Sunderland, MA: Sinauer; 1999. [Google Scholar]