Abstract
The management of massively transfused trauma patients has improved with a better understanding of trauma-induced coagulopathy, the limitations of crystalloid infusion, and the implementation of massive transfusion protocols (MTPs), which encompass transfusion management and other patient care needs to mitigate the “lethal triad” of acidosis, hypothermia, and coagulopathy. MTPs are currently changing in the United States and worldwide because of recent data showing that earlier and more aggressive transfusion intervention and resuscitation with blood components that approximate whole blood significantly decrease mortality. In this context, MTPs are a key element of “damage control resuscitation,” which is defined as the systematic approach to major trauma that addresses the lethal triad mentioned above. MTPs using adequate volumes of plasma, and thus coagulation factors, improve patient outcome. The ideal amounts of plasma, platelet, cryoprecipitate and other coagulation factors given in MTPs in relationship to the red blood cell transfusion volume are not known precisely, but until prospective, randomized, clinical trials are performed and more clinical data are obtained, current data support a target ratio of plasma:red blood cell:platelet transfusions of 1:1:1. Future prospective clinical trials will allow continued improvement in MTPs and thus in the overall management of patients with trauma.
Approximately 5 million people worldwide die from injuries each year.1 In 2002, road traffic-related injuries, interpersonal violence, burns, and drowning were among the 15 leading causes of death for people between the age of 5 and 44 yr globally.2 In the United States, injury is the main cause of premature mortality, ahead of malignancy and heart disease, for those who die before the age of 65 yr.3 Indeed, the number of deaths from injury has not declined for more than a decade. Therefore, trauma is considered a significant public health concern, which continues despite efforts to affect its prevention.4 – 6 Thus, clinical and interventional efforts to reduce the high-mortality rate seen in massive trauma remain paramount.
Early trauma-related mortality is typically secondary to head injury (40%–50% of causes of death) or hemorrhage (20%–40%), which is worsened with attendant coagulopathy, whereas late mortality is usually secondary to multiorgan failure (MOF) (7%–9%). Increased risk of MOF has been associated with massive transfusion, which can itself contribute to coagulopathy via coagulation factor dilution.7 Coagulopathy is present in 65% of patients requiring massive transfusion because of hemorrhage secondary coagulation and platelet consumption as well as physical loss. Thus, hemorrhage may account for a third of the in-hospital deaths, particularly in the first 24 h after admission.7a In massive hemorrhage, mortality exceeds 50%.8 Coagulopathy after hemorrhage is thought to be a secondary event because of a triad of depletion and dilution of coagulation factors, acidosis, and hypothermia.9,10 Surgical control to arrest the cause of the hemorrhage is the main intervention, but even when surgery is performed aggressively, hemorrhage portends low survival.11 This review will address new information regarding trauma-induced coagulopathy and blood bank management of massively transfused trauma patients.
MASSIVE TRANSFUSION
Massive transfusion portends a high-mortality rate in patients with trauma. Those who received 10 or more red blood cell (RBC) units in their hospital stay (2.6% of all patients with trauma) had a mortality rate of 39% and patients who received 50 or more units of blood products in the first 24 h (0.6% of all patients with trauma) had a mortality rate of 57% in two separate retrospective studies.12,13 Massive transfusion is commonly defined as transfusion of 10 or more RBC products (which approximates the total blood volume of a recipient) within 24 h14; other definitions include 10 or more RBC products within the initial hospital stay or within 6 h, six or more RBC units within 12 h, or 50 or more blood products in the first 24 h.12,15,16 In pediatric patients, massive transfusion is defined as transfusion of one blood volume of RBC products in 24 h. The number of RBC products in one blood volume is approximately equal to one RBC product multiplied by the patient’s age in years; for example, a 4 yr old is massively transfused after four RBC products.17
TRAUMA-INDUCED COAGULOPATHY
Coagulopathy is recognized as a contributor to trauma-related mortality. It was traditionally thought to develop over time, usually hours, and thus considered a secondary entity.18,19 This secondary coagulopathy is a result of dilutional and consumptive loss of coagulation factors in addition to hypothermia and acidosis, termed “the blood vicious cycle” or “the triad of death.”19 Consequently, severely injured patients are treated with damage control measures that focus on preventing hypothermia and acidosis as well as controlling hemorrhage.5 Damage control surgery, which was introduced more than 20 yr ago, reduces mortality. However, in the past decade, trauma center mortality rates have not improved.5
Early trauma-induced coagulopathy (ETIC) is a new paradigm of trauma-induced coagulopathy as an early and primary event. Other terms which have been applied to the early trauma-associated coagulopathy are “acute traumatic coagulopathy” and more recently “acute coagulopathy of trauma-shock.”20 ETIC has yet to be supported by large prospectively designed studies, but data in three large retrospective trials support the concept of ETIC. These trials identified a prolonged prothrombin time (PT), which occurs early after trauma in up to 25% of patients, as a predictor of mortality. First, MacLeod et al.21 analyzed a trauma registry database and demonstrated that an abnormal PT independently predicted a 35% increase in the likelihood of mortality. Second, Brohi et al.22 retrospectively analyzed PT, partial thromboplastin time (PTT), and thrombin time, in conjunction with other clinical data and demonstrated that ETIC was associated with significantly higher mortality. Finally, a retrospective analysis of a German trauma database demonstrated that an increase in PT was predictive of increased mortality. The authors subsequently proposed a prognostic model based on age, Glascow Coma Scale (GCS), Injury Severity Score, base excess, and PT to more precisely predict mortality.23 Each of these studies attempted to control for factors known to increase mortality. MacLeod et al.21 showed the prolonged PT to be independent of the presence of head injury, time from injury or the presence of shock. Brohi et al.22 were able to control for the amount of fluid resuscitation in their helicopter-transported trauma cohort. The German study controlled for similar factors that were found in the study by Macleod et al.23 Therefore, trauma registry data has demonstrated that an increased PT predicts an increase in mortality and occurs early after trauma in a wider range of patients than was originally believed. However, additional testing to explore abnormal elements of coagulation cascade and fibrinolysis in patients with trauma (i.e., ETIC) in conjunction with complete prospective patient data have not been reported, nor has a unifying hypothesis for the mechanism of ETIC emerged.
There are multiple various risk factors, including age and GCS, that are associated with a poor outcome.24 A retrospective review performed in Germany found a worsening base deficit (BD) was correlated with a poorer outcome.25 The extent of anatomic injury, indicated by the Injury Severity Score, was developed to reflect outcome and control for heterogeneity of injury.26 The duration of hypotension in severely hemorrhaging patients, as well as the occurrence of episodes of hypotension, have shown to be associated with increased mortality in small cohorts of patients.27 Hypothermia, as was shown in a retrospective review by Jurkovich et al.,28 is correlated with mortality when the patient’s body temperature decreases below 34°C. A prospective randomized trial confirmed the relationship of a reduced period of hypothermia and improved survival. All these studies, including the three ETIC studies, share the same difficulty: the known risk factors are consistently analyzed in isolation. In the three ETIC articles, factors controlled for were predetermined by data availability and trauma registry collection, and therefore, there is no study that simultaneously controls for all of the pertinent factors within one cohort of patients.
Coagulation normally begins with an immediate vascular contraction and platelet plug formation followed by activation of thrombin and formation of a fibrin clot in conjunction with initial impairment of fibrinolysis.29 Subsequently, extra fibrin is removed by secondary fibrinolysis. At present, it appears that there are two possible theories regarding ETIC: 1) tissue factor generation leads to increase thrombin generation and ultimately a disseminated intravascular coagulopathy (DIC)-like pattern with altered fibrinolysis and 2) tissue hypoperfusion leads to activation of protein C and systemic anticoagulation.
DIC is a predictor of acute respiratory distress syndrome, MOF, and death in patients with trauma.30 The diagnosis of DIC is based on clinical symptoms (presence of bleeding and organ dysfunction) and laboratory results (elevated fibrinogen degradation products [FDP], low platelet count, low fibrinogen, and increased PT).31 The trigger for thrombin formation is tissue factor binding to factor VII resulting in activation of the coagulation cascade. Extensive activation of blood coagulation with impaired fibrinolysis results in the generation and deposition of fibrin, resulting in microvascular thrombi and subsequent development of multiple organ dysfunction syndrome.31 Gando et al.30,31 reported that trauma patients with DIC had lower platelet counts, PT, fibrinogen, antithrombin, and α 2 plasmin inhibitor levels and higher FDP and tissue factor levels than non-DIC patients with trauma during the first 4 days after trauma. In addition, they demonstrated that patients with DIC had higher levels of plasminogen activator inhibitor-1 (PAI-1) activity, which inhibits fibrinolysis, potentially resulting in microvascular thrombi, which correlated with increased MOF.30 Gando et al.31 suggested that DIC is associated with systemic inflammatory response syndrome and the subsequent development of MOF.
The second theory was proposed by Brohi et al.32 who, in a prospective study of 208 trauma patients, measured prothrombin fragment (coagulation activation), fibrinogen, soluble thrombomodulin (coagulation inhibition), protein C activity (coagulation inhibition), PAI-1 activity (inhibitor of fibrinolysis), and D-dimers (fibrinolysis) as well as PTT, PT, and BD (a measure of the degree of tissue hypoperfusion). The authors correlated these laboratory coagulation values to clinical factors and demonstrated prolongation of PT and PTT when both the BD and prothrombin fragment were high. In addition, they demonstrated that thrombomodulin increases and protein C decreases (theoretically because it is activated) in relation to the BD and mortality. They suggested that the cause of ETIC is systemic anticoagulation via inhibition of the coagulation cascade by activated protein C. In subsequent publications from the same patient cohort, the author determined that traumatic brain injury must be paired with hypoperfusion (defined as increased BD) to result in coagulopathy, and both complement and endothelial activation are correlated with hypoperfusion and coagulopathy.33–35 Brohi et al.20 concluded that the mechanism of acute trauma-induced coagulopathy is the activation of anticoagulant and fibrinolytic pathways through the thrombomodulin-protein C pathway.
CURRENT MANAGEMENT
Maintenance of adequate blood flow and arterial blood pressure by infusing a sufficient volume of crystalloid and/or colloid solution is vitally important for maximizing tissue perfusion and helping to ensure patient survival. RBC transfusion is critical to ensure adequate oxygen delivery. Circulation is dependent on cardiac output and both the RBC mass and hemoglobin. The ability of transfused RBCs to release oxygen optimally is dependent, at least in part, on the metabolic status of the patient and the length of storage of the RBC product.
Crystalloid Versus Colloid Replacement
Practice has changed to earlier use of colloids (especially plasma) and RBC transfusion while simultaneously decreasing the amount of crystalloids administered because of increasing evidence that large-volume crystalloid administration is associated with abdominal compartment syndrome as well as cardiac, pulmonary, gastrointestinal, coagulation, and other complications.36 In addition, the current goal of volume resuscitation is euvolemia, entailing moderate volume resuscitation with the possible use of vasopressor drugs to support hemodynamics and to avoid supranormal resuscitation.36
RBC Transfusion
When blood loss is excessive and there is not adequate time for pretransfusion testing, group O RBC and AB plasma products should be issued until the recipient’s blood type is known. Each institution should have well-developed policies for emergency release, issuance, and delivery of blood products and switching blood types (i.e., issuing D-positive RBC products to a D-negative individual, issuing antigen-positive or antigen-untested in a patient with the corresponding alloantibody, and issuing ABO incompatible plasma [i.e., an AB patient requiring large amounts of plasma]).37
Women of childbearing potential should receive D-negative RBCs, if their blood type is unknown or they are D-negative and an inventory of group O, D-negative products should be kept for these individuals. These products are important to prevent formation of alloanti-D, which can lead to future hemolytic disease of the fetus and newborn. When D-negative women of childbearing potential receive D-positive RBCs, the transfusion service should review with the trauma team therapies which may decrease the likelihood of anti-D formation, such as administering Rh immunoglobulin. Men and older women may receive D-positive RBCs if D-negative RBC products are not available or if the inventory is low and the arrival a woman of child bearing potential with severe trauma can be predicted by the acuity of the facility’s trauma and emergency services. A recent study demonstrated the anti-D formation rate for D-negative patients who received D-positive RBCs in urgent situations was 22%.38 Therefore, the anti-D formation rate in hospitalized acutely ill patients is significantly lower than the 80% anti-D formation rate in healthy volunteers.
A patient sample for blood typing and antibody screen should be obtained as soon as possible after patient arrival to facilitate receipt of type-specific products when available, thus preserving the often-limited group O RBC and AB plasma supply. Transfusion of type-specific products also avoids obfuscation of the patient’s true blood type because of the infusion of large amounts of group O RBCs and AB plasma. In addition, patients who receive large amounts of “out of group” components may be receiving ABO-incompatible plasma (such as a group A patient receiving group O RBCs or platelets), which may inhibit the transfusing of non-group O RBC products due to passively acquired anti-A and/or anti-B, or result in a positive direct antiglobulin test and/or hemolysis.
RBC Storage Lesion
The RBC storage lesion is a term that collectively refers to a number of biochemical and physical changes that occur in the RBCs themselves, as well as the resultant changes in the entire RBC product (RBCs and supernatant, which includes anticoagulant-preservative solution and plasma) during storage.39 The decreased 2,3-DPG results in a shift in the oxygen dissociation curve to the left, which leads to less oxygen release than normal RBCs at the same partial pressure oxygen. Therefore, stored RBCs may not deliver oxygen to the tissues as well as fresh RBCs. 2,3-DPG levels return to normal levels within 24 h after transfusion. In addition to biochemical changes, RBCs change from a deformable biconcave disk, to reversibly deformed echinocytes, to irreversibly deformed spherechinocytes with increased membrane stiffness. These morphological changes may also result in decrease oxygen transport because of the inability of the RBC to flow through the microcirculation and the increase in RBC and vascular endothelial interactions. Recent data suggest that stored RBCs have reduced nitric oxide (NO) bioavailability.40,41 Because NO plays a vital role in the vasodilation of blood vessels and, therefore, in oxygen delivery to tissues, in conjunction with loss of 2,3-DPG and changes in deformability, stored RBCs may not have optimal oxygen delivery.41,42
Primarily retrospective data have accumulated suggesting that RBC product transfusion itself may be associated with, or an independent predictor or risk factor for increased morbidity, including, particularly, acute respiratory distress syndrome and MOF, and mortality in the recipient.39 In addition, in retrospective studies the use of older RBC products (>14 days of storage) versus the use of fresh RBC products (<14 days of storage) is associated with increased morbidity and mortality.39,43 Therefore, the benefits and costs of RBC transfusion must be carefully weighed for each patient, and future prospective clinical trials need to compare fresh versus older RBC products, as well as the use of alternatives to RBC products (i.e., hemoglobin-based oxygen carriers [HBOCs]) or drugs to reduce hemorrhage and coagulopathy.44,45 Lastly, if available and appropriate, RBC salvage can be performed intra-operatively and postoperatively to decrease the amount of allogeneic stored RBC products transfused.46
MASSIVE TRANSFUSION PROTOCOL
A massive transfusion protocol (MTP) is necessary in treating the massively hemorrhaging patient undergoing massive transfusion to mitigate the lethal triad of acidosis, hypothermia, and coagulopathy; optimize the logistics of blood product delivery to the patient; effectively communicate between the patient care area and the transfusion service; prevent errors which occur in critical and fast-moving environments; and create standardization in patient care. Two goals of the MTP should be earlier and more aggressive transfusion intervention and resuscitation with blood components that approximate whole blood as a part of damage control resuscitation.47 There are multiple MTP models for blood product administration, which can be used singly or in combination, such as laboratory test result-based blood product administration (also known as the “component approach”), predetermined blood product administration, and real-time transfusion service physician involvement to oversee blood product administration. Each institution should create their MTP for their specific patient care needs with an understanding of available resources.
MTPs Define
1) Notification of the transfusion service and laboratory, 2) laboratory testing algorithms (e.g., PT, PTT, platelet count, fibrinogen, and hemoglobin), 3) blood product preparation (amount of plasma, RBCs, platelets, and cryoprecipitate to prepare and issue at set time intervals), and 4) other patient care needs (e.g., blood warmers). In addition, the creation or modification of MTPs should be part of a multidisciplinary quality improvement initiative to improve patient care.
Multidisciplinary Communication
The management of patients with acute blood loss requires concise and effective communication between the trauma team and the hospital transfusion service. The hospital transfusion service needs to be able to rapidly prepare blood products for issue, assess component inventory and participate in reconciling laboratory values.
Laboratory
MTPs require adequate laboratory support to evaluate the patient’s hemoglobin, platelet count, PT, PTT, fibrinogen level, ionized calcium, and pH to adequately address and correct these values. Some institutions use thrombeslastography, which provides a dynamic and global assessment of the coagulation process, including platelet function, coagulation cascade, and fibrinolysis, to guide transfusion management of these patients.48 Currently, sufficient data are lacking to support its routine use.
Quality Improvement
The creation of a MTP should be part of a quality improvement initiative to improve patient care. There should be well-defined quality initiatives that can be measured and tracked to determine if the MTP is obtaining the desired goals. Some examples for quality indicators include blood product turnaround time, wastage, and utilization, transfusion adverse events, and patient mortality and laboratory values.
MTP Models
Component Therapy-Based Approaches
In the recent past, resuscitation and transfusion protocols started with significant crystalloid or non-plasma (such as albumin) colloid infusion and many RBC products. This was followed by a component therapy-type approach using clinical findings and laboratory results to guide blood product choices, volumes, and timing. This approach requires that laboratory tests are both timely and reflective enough of the coagulation system to aid in guiding therapy. Conventional coagulation tests are likely not clinically relevant because they do not reflect the in vivo hemostatic capacity the results are delayed and therefore less relevant for the acute situation. An example of using component therapy is to base transfusion on hemoglobin <8 g/dL, PT >1.5 times normal, platelet count <50,000/μL, and fibrinogen <100 g/dL (Fig. 1). Using this type of approach, there typically is no set administration ratio of RBC products to plasma, platelets, and/or cryoprecipitate.49
Figure 1.
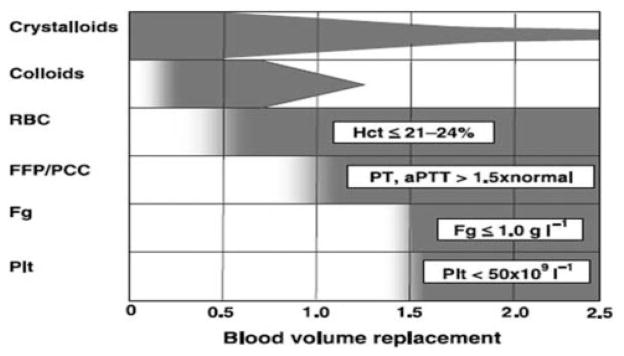
Laboratory-based blood product administration. Fluid and blood component treatment in major bleeding. Values of various parameters represent trigger points at which relevant blood components should be transfused. RBC = red blood cells; FFP = fresh frozen plasma; PCC = prothrombin complex concentrate; Fg = fibrinogen; Plt = platelets; Hct = hematocrit; PT = prothrombin time; aPTT = activated partial thromboplastin time. (Reprinted with permission from Spahn and Rossaint.29)
Predetermined Blood Product Administration
Recently, MTPs have shifted toward predetermined blood product administration in an effort to mitigate and treat coagulopathy by adequate coagulation factor administration early in the resuscitation, decreasing the amount of crystalloid administered and removing the delay in ordering, preparing, and subsequent administration of blood products.20 This is in contrast to the laboratory-based approach where there is a delay in the ordering and subsequent administration of platelet, plasma, and cryoprecipitate products because of delays due to laboratory turnaround time and product preparation time. Most MTPs using the predetermined approach have preset transfusion packages that are delivered consecutively until the patient either dies or their bleeding is under control (Fig. 2). In addition, predetermined blood product administration clearly defines the proportion of blood components to transfuse, decreasing the chances of less than optimal transfusion therapy (i.e., large volumes of RBC products with little or no transfusion of plasma or platelet products).48
Figure 2.
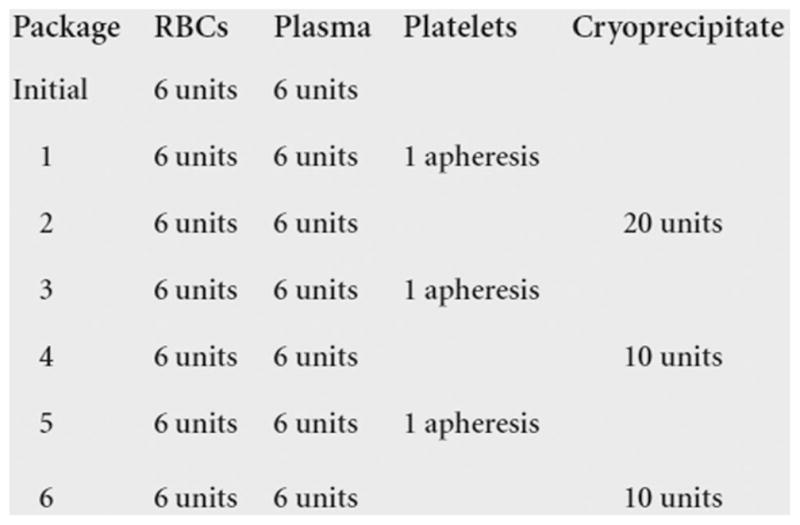
Predetermined blood product administration. The Grady Memorial Hospital/Emory University Massive Transfusion Protocol (modified from Dente et al.63).
Real-Time Transfusion Service Physician Involvement
In this approach, the transfusion service physician is notified when a patient has been massively transfused (or in some instances if a severely injured trauma patients has arrived). The transfusion service physician can then contact the trauma team to determine the status of the patient and suggest blood product administration. In addition, the transfusion service physician can take primary responsibility for monitoring the patient’s coagulation laboratory values.48 Lastly, the transfusion service physician can participate in inventory management to help ensure that adequate amounts of blood products are available and, if not, advise the trauma team as well as to the blood supplier.
Advantages of the Predetermined Ratio Approach
In 2005, a symposium of surgeons, anesthesiologists, hematologists, transfusion medicine specialists, epidemiologists, and others held at the United States Army Institute of Surgical Research resulted in general consensus to create guidelines for massive transfusion in the severely injured patient to transfuse RBC:plasma:platelets in a 1:1:1 ratio.50 This ratio corrects the coagulation factor loss resulting from early transfusion of crystalloids and RBC products.50 Therefore, to ensure a maximum plasma:RBC ratio of 1:2 is achieved in 98% of patients, the MTP should have a 1:1 goal.14 In addition, the use of MTP optimizes patient outcome and systemizes blood product administration.51
With this recommendation, MTPs were designed which attempted to “recapitulate” whole blood, i.e., to match the RBC, plasma, platelets, and cryoprecipitate ratio of whole blood, or “reconstitute” whole blood, which means to premix components that resemble whole blood into a single product or to use fresh whole blood. Based on the dramatic success of MTPs used in combat, recent studies show equivalent performance and patient benefit in the nonmilitary setting.14,52,53,63 Also, MTPs have demonstrated a decrease in overall blood use in some institutions.54,55
Early and Aggressive Blood Product Transfusion Therapy Improves Outcome
Mounting data demonstrates that the ratio of blood products transfused affects mortality both in military and civilian settings. First, early reports in the military setting showed significant reduction in mortality in 246 massively transfused (≥10 U of RBCs in 24 h) trauma patients (65% reduced to approximately 20%; P < 0.001; Fig. 3), with an optimal plasma to RBC product ratio of 1.4.52 Similar data of improved survival with plasma:RBC ratios approaching 1:1 in the civilian setting has also been reported.14,56 Indeed, in a study of 165 trauma patients pre- and post-implementation of a MTP, who received at least 10 U of RBCs within 24 h, at our level 1 civilian trauma center, one plasma product for two or fewer RBC products was associated with significantly improved survival versus one plasma product for two or more RBC products (30 day survival of 70% vs 47%; P = 0.010).53 One recent study used a level 1 civilian trauma center’s registry to determine the effect of the plasma: RBC ratio in 133 patients who received more than 10 U of RBCs in 6 h. The authors demonstrated a U-shaped association between plasma:RBC ratio and survival and concluded that the ideal ratio is likely between 1:2 and 1:3.15 Second, the ratio of platelet:RBC products transfused also appears to affect patient survival. In a retrospective study of 466 massively transfused (≥10 U of RBCs in 24 h) civilian patients, the group with a high plasma and platelet to RBC ratio (≥1 U of platelets and plasma to 2 U of RBCs) had the highest rate of 30 day survival (73%) compared with patients who received high plasma and low platelet (54%), low plasma and high platelet (67%), and low plasma and low platelet (<1 U of platelets and plasma to 2 U of RBCs; 43%) ratios (P < 0.001).14 Indeed, in the study from our institution, transfusion of one platelet-pheresis product (which is approximately equivalent to 6–7 whole blood derived units or 3.3–3.8 × 1011 platelets) for 20 or less RBC products was associated with significantly improved survival versus one platelet-pheresis product for 20 or more RBC products (30 day survival of 72% vs 13%; P = 0.0001) (unpublished data). Lastly, the fibrinogen:RBC ratio appears to influence patient survival. In a retrospective military study of 252 massively transfused patients (≥10 U of RBCs in 24 h), the amount of fibrinogen to RBC ratio transfused was calculated by adding the fibrinogen content of whole blood, platelet, plasma, RBC, and cryoprecipitate products in comparison to RBC products transfused. This study demonstrated the improved survival rates of a high (≥2 g fibrinogen/RBC U) versus low (<0.2 g fibrinogen/RBC U) fibrinogen:RBC ratio (survival 76% vs 48%; P < 0.001).57 Indeed, in the study from our institution, transfusion of one unit of cryoprecipitate for two or less RBC products was associated with significantly improved survival versus one unit of cryoprecipitate for two or more RBC products (30 day survival of 72% vs 35%; P = 0.003) (unpublished data). In conclusion, current data support the use of early and aggressive coagulation factor replacement through transfusion of plasma, platelet, and cryoprecipitate products. Although the optimal ratio is not precisely defined, these reports support an aggressive approach to transfusion.
Figure 3.
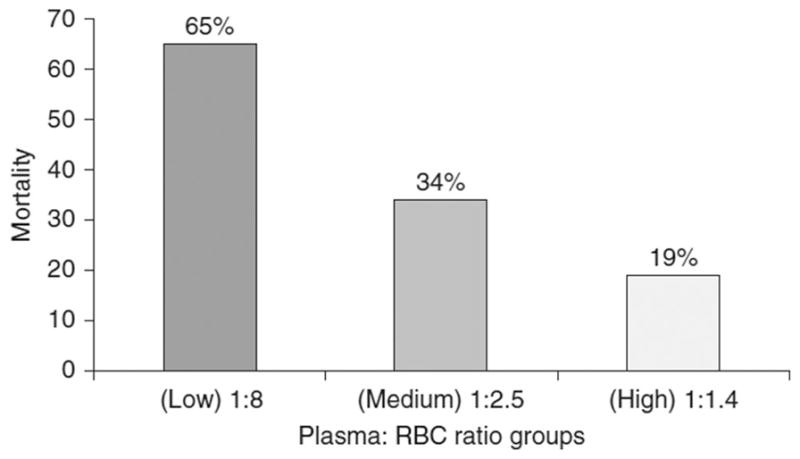
Ratio of blood products transfused affects mortality in patients receiving massive transfusions. Percentage mortality associated with low, medium, and high plasma to RBC ratios transfused at admission in a combat hospital. Ratios are median ratios per group and include units of fresh whole blood counted both as plasma and RBCs. (Reprinted with permission from Borgman et al.52)
All the studies cited above likely have survival bias in the data because those who survive longer are more likely to receive more coagulation factor therapies as opposed to patients who die early after admission and who may receive less coagulation factors because of delay in coagulation product transfusion.15 Randomized controlled trials are needed to determine whether 1:1:1 ratio of plasma:RBC:platelet is the optimal ratio in massively transfused severely injured patients with trauma.
Overall Blood Product Usage
In theory, if coagulopathy can be mitigated, then hemorrhage will cease earlier and overall blood product usage will be reduced. Data have demonstrated an improvement in coagulation factors with early and aggressive use of plasma.15 One institution correlated morbidity with intensive care unit (ICU) International Normalized Ratio (INR) using their trauma registry database and subsequently implemented early goal-directed therapy to achieve a plasma:RBC ratio of 1:1 to improve ICU admission INR and mortality.58 Recent data from this institution have demonstrated an improvement with the ICU admission INR (1.6 vs 1.48; P = 0.02) and survival (70% vs 85%; P = 0.02) pre versus post implementation of the 1:1 plasma:RBC ratio.59 Indeed, in the study from our institution, the implementation of a MTP with a 1:1 plasma:RBC ratio improved the ICU admission INR (pre-MTP 1.3 vs post-MTP 1.7; P = 0.04) and mortality (pre-MTP 50% vs post-MTP 38%; P = 0.14).63
The resultant decrease in blood product administration with mitigation of coagulopathy by increased coagulation factor therapy is less clear. Some civilian trauma centers have demonstrated a decrease in blood product administration with the implementation of a MTP; one center decreased the platelet use with their protocol although the RBC and plasma use remained similar and the center reported improved patient survival (pre-MTP 34% vs post-MTP 49%).55 Another center decreased RBC, plasma, and platelet use although significantly increasing recombinant factor VIIa use without improving survival (pre-MTP 50% vs post-MTP 48%).54 In contrast, the study from our institution demonstrated that RBC, platelet, and cryoprecipitate use pre- and post-MTP is similar, yet the plasma use has significantly increased.63 Therefore, it is not known whether implementation of a MTP results in decreased blood product use.
PEDIATRIC TRAUMA
The transfusion and coagulation management in children with traumatic injuries is largely unknown. The optimal MTP in children may differ from that for adults, as trauma-induced coagulopathy and MTPs are largely unstudied in the pediatric population. Two retrospective studies reviewed trauma-induced coagulopathy in pediatric patients. One study in patients with blunt trauma detected an elevated PT and/or PTT in 28% of patients and a markedly elevated PT and/or PTT in 6% of patients. Marked elevation correlated with GCS ≤13, low systolic blood pressure, open/multiple bony fractures, and major tissue wounds.60 The second study in patients with head injury correlated PT prolongation with increased risk of mortality.61 In addition, there may be unique transfusion issues in pediatric patients. For example, pediatric patients are more susceptible to hyperkalemia, which can be fatal, secondary to rapidly receiving large volumes of RBC products; the risk is increased when products are transfused through a central line and in patients with renal failure or low cardiac output.62 Pediatric-focused data on massive transfusion, including MTPs and transfusion ratios, and trauma induced coagulopathy, including ETIC, must be obtained through well-designed clinical studies to improve care in children with traumatic injuries.
FUTURE CONSIDERATIONS
Currently, the ideal transfusion ratios or models for transfusion and coagulation management of patients with trauma are unclear or controversial. What is apparent is that patients who die early after admission may receive fewer coagulation factors because of delay in coagulation product transfusion. It is unknown if early and aggressive coagulation factor replacement would improve survival in patients who die within hours of admission, as there may be a survival bias in the data wherein those who survive longer are more likely to receive more coagulation factor therapies.15 This question is best answered through a randomized, controlled clinical trial in which patients receive different, yet adequate, transfusion product ratios. Second, the pathogenic mechanism of ETIC and other trauma-related coagulopathies is not known. This information can only be gained through rigorous and prospective clinical trials to create a unifying hypothesis/mechanism for trauma-induced coagulopathy. Lastly, the effect of RBC storage time on patient outcome should be evaluated in a prospective, randomized, clinical trial. Subsequently, evidence-based therapies can emerge based on these clinical trials and their resulting outcomes.
SUMMARY
Trauma remains a leading cause of death worldwide and 30%– 40% of patients with trauma die secondary to hemorrhage. Trauma results in a primary and early coagulopathy which predicts for mortality, independent of head injury or injury severity. The pathophysiology of ETIC is currently unknown; it may result from hypoperfusion resulting in anticoagulation and hyperfibrinolysis or from tissue factor generation leading to thrombin generation and a DIC-like state. In addition, trauma-induced coagulopathy is secondary to hypothermia, acidosis, and coagulopathy resulting from massive transfusion with dilution of coagulation factors and consumption of coagulation factors. Therefore, to address the triad of death (i.e., acidosis, hypothermia, and coagulopathy), damage control resuscitation has been added to damage control surgery to stop the hemorrhage. Damage control resuscitation requires implementation of a MTP to ensure early and aggressive coagulation factor therapy as well as to limit crystalloid infusion, prevention of hypothermia and acidosis, and to permit moderate hypotension. MTPs require a multidisciplinary team to develop, implement, maintain, and continue to improve trauma patient care. The optimal amounts of plasma, platelet, cryoprecipitate, and other coagulation factors in relationship to the RBC transfusion volume are currently unknown, but current data support the use of plasma:RBC:platelet ratio of 1:1:1. Future prospective clinical trials will hopefully assist in continuing to improve the transfusion management of massively transfused patients with trauma.
References
- 1.Peden M, McGee K, Sharma G. The injury chart book: a graphical overview of the global burden of injuries. Geneva: World Health Organization; 2002. [Google Scholar]
- 2.Peden M, McGee K, Krug E. Injury: a leading cause of the global burden of disease, 2000. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.Office of Statistics and Programming, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS), National Vital Statistics System. Atlanta: Center for Disease Control & Prevention; 2005. [Google Scholar]
- 4.Shackford SR, Mackersie RC, Holbrook TL, Davis JW, Hollingsworth-Fridlund P, Hoyt DB, Wolf PL. The epidemiology of traumatic death. A population-based analysis. Arch Surg. 1993;128:571–5. doi: 10.1001/archsurg.1993.01420170107016. [DOI] [PubMed] [Google Scholar]
- 5.Reza A, Mercy JA, Krug E. Epidemiology of violent deaths in the world. Inj Prev. 2001;7:104–11. doi: 10.1136/ip.7.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demetriades D, Murray J, Sinz B, Myles D, Chan L, Sathyaragiswaran L, Noguchi T, Bongard FS, Cryer GH, Gaspard DJ. Epidemiology of major trauma and trauma deaths in Los Angeles County. J Am Coll Surg. 1998;187:373–83. doi: 10.1016/s1072-7515(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 7.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 7a.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–62. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Hewson JR, Neame PB, Kumar N, Ayrton A, Gregor P, Davis C, Shragge BW. Coagulopathy related to dilution and hypotension during massive transfusion. Crit Care Med. 1985;13:387–91. doi: 10.1097/00003246-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Faringer PD, Mullins RJ, Johnson RL, Trunkey DD. Blood component supplementation during massive transfusion of AS-1 red cells in trauma patients. J Trauma. 1993;34:481–7. doi: 10.1097/00005373-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara A, MacArthur JD, Wright HK, Modlin IM, McMillen MA. Hypothermia and acidosis worsen coagulopathy in the patient requiring massive transfusion. Am J Surg. 1990;160:515–18. doi: 10.1016/s0002-9610(05)81018-9. [DOI] [PubMed] [Google Scholar]
- 12.Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–13. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 13.Vaslef SN, Knudsen NW, Neligan PJ, Sebastian MW. Massive transfusion exceeding 50 units of blood products in trauma patients. J Trauma. 2002;53:291–6. doi: 10.1097/00005373-200208000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 15.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–71. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 16.Criddle LM, Eldredge DH, Walker J. Variables predicting trauma patient survival following massive transfusion. J Emerg Nurs. 2005;31:236–42. doi: 10.1016/j.jen.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Strauss RG, Hillyer CD, Luban NLC, editors. Handbook of pediatric transfusion medicine. San Diego: Academic Press; 2004. [Google Scholar]
- 18.McNamara JJ, Burran EL, Stremple JF, Molot MD. Coagulopathy after major combat injury: occurrence, management, and pathophysiology. Ann Surg. 1972;176:243–6. doi: 10.1097/00000658-197208000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddy VA, Morris JA, Cullinane DC. Hypothermia, coagulopathy, and acidosis. Surg Clin North Am. 2000;80:845–54. doi: 10.1016/s0039-6109(05)70099-2. [DOI] [PubMed] [Google Scholar]
- 20.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 21.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 22.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 23.Rixen D, Raum M, Bouillon B, Schlosser LE, Neugebauer E. Predicting the outcome in severe injuries: an analysis of 2069 patients from the trauma register of the German society of traumatology (DGU) Der Unfallchirurg. 2001;104:230–9. doi: 10.1007/s001130050719. [DOI] [PubMed] [Google Scholar]
- 24.Clark DE, Ryan LM. Concurrent prediction of hospital mortality and length of stay from risk factors on admission. Health Serv Res. 2002;37:631–45. doi: 10.1111/1475-6773.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rixen D, Raum M, Bouillon B, Lefering R, Neugebauer E. Base deficit development and its prognostic significance in post-trauma critical illness: an analysis by the trauma registry of the Deutsche Gesellschaft fur unfallchirurgie. Shock. 2001;15:83–9. doi: 10.1097/00024382-200115020-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kuhls DA, Malone DL, McCarter RJ, Napolitano LM. Predictors of mortality in adult trauma patients: the physiologic trauma score is equivalent to the trauma and injury severity score. J Am Coll Surg. 2002;194:695–704. doi: 10.1016/s1072-7515(02)01211-5. [DOI] [PubMed] [Google Scholar]
- 27.Zenati MS, Billiar TR, Townsend RN, Peitzman AB, Harbrecht BG. A brief episode of hypotension increases mortality in critically ill trauma patients. J Trauma. 2002;53:232–7. doi: 10.1097/00005373-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27:1019–24. [PubMed] [Google Scholar]
- 29.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–9. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 30.Gando S, Nanzaki S, Morimoto Y, Ishitani T, Kemmotsu O. Tissue factor pathway inhibitor response does not correlate with tissue factor-induced disseminated intravascular coagulation and multiple organ dysfunction syndrome in trauma patients. Crit Care Med. 2001;29:262–6. doi: 10.1097/00003246-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost. 2001;27:585–92. doi: 10.1055/s-2001-18864. [DOI] [PubMed] [Google Scholar]
- 32.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–18. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, Pittet J-F. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 34.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, Christiaans SC, Bir ND, Pittet JF. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–6. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 35.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet J-F. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma. 2007;63:1254–62. doi: 10.1097/TA.0b013e318156ee4c. [DOI] [PubMed] [Google Scholar]
- 36.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–21. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 37.AABB. Standards for blood banks and transfusion services. 25. Bethesda: AABB press; 2008. [Google Scholar]
- 38.Yazer MH, Triulzi DJ. Detection of anti-D in D− recipients transfused with D+ red blood cells. Transfusion. 2007;47:2197–201. doi: 10.1111/j.1537-2995.2007.01446.x. [DOI] [PubMed] [Google Scholar]
- 39.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 40.Winslow RM, Intaglietta M. Red cell age and loss of function: advance or SNO-job? Transfusion. 2008;48:411–14. doi: 10.1111/j.1537-2995.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 41.Bonaventura J. Clinical implications of the loss of vasoactive nitric oxide during red blood cell storage. Proc Natl Acad Sci USA. 2007;104:19165–6. doi: 10.1073/pnas.0708871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–47. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg JA, McGwin GJ, Marques MB, Cherry SAI, Reiff DA, Kerby JD, Rue LWI. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–8. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 44.Levy JH. Pharmacologic methods to reduce perioperative bleeding. Transfusion. 2008;48:31S–38S. doi: 10.1111/j.1537-2995.2007.01574.x. [DOI] [PubMed] [Google Scholar]
- 45.Estep T, Bucci E, Farmer M, Greenburg G, Harrington J, Kim HW, Klein H, Mitchell P, Nemo G, Olsen K, Palmer A, Valeri CR, Winslow R. Basic science focus on blood substitutes: a summary of the NHLBI division of blood diseases and resources working group workshop, March 1, 2006. Transfusion. 2008;48:776–82. doi: 10.1111/j.1537-2995.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 46.Jonathan HW. Indications and contraindications of cell salvage. Transfusion. 2004;44:40S–44S. doi: 10.1111/j.0041-1132.2004.04176.x. [DOI] [PubMed] [Google Scholar]
- 47.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 48.Johansson PI. The blood bank: from provider to partner in treatment of massively bleeding patients. Transfusion. 2007;47:176S–83S. doi: 10.1111/j.1537-2995.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 49.Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Neugebauer E, Spahn DR. Key issues in advanced bleeding care in trauma. Shock. 2006;26:322–31. doi: 10.1097/01.shk.0000225403.15722.e9. [DOI] [PubMed] [Google Scholar]
- 50.Holcomb JB, Hess JR. Early massive trauma transfusion: state of the art: editors’ introduction. J Trauma. 2006;60:S1–S2. [Google Scholar]
- 51.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–S6. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 52.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 53.Shaz BH, Young A, Harris R, Nicholas J, Hillyer CD, Dente C. Increased number of plasma products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2008:25a–26a. doi: 10.1111/j.1537-2995.2009.02414.x. (abstract) [DOI] [PubMed] [Google Scholar]
- 54.O’Keeffe T, Refaai M, Tchorz K, Forestner JE, Sarode R. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–91. doi: 10.1001/archsurg.143.7.686. [DOI] [PubMed] [Google Scholar]
- 55.Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr, St Jacques P, Young PP. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–83. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 56.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the trauma registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–19. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 57.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, Wolf SE, Wade CE, Holcomb JB. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–S86. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez EA, Jastrow K, Holcomb JB, Kao LS, Moore FA, Kozar RA. Early achievement of a 1:1 ratio of FFP:RBC reduces mortality in patients receiving massive transfusion. J Trauma. 2008;64:247. (abstract) [Google Scholar]
- 60.Holmes JF, Goodwin HC, Land CM, Kuppermann N. Coagulation testing in pediatric blunt trauma patients. Pediatr Emerg Care. 2001;17:324–8. doi: 10.1097/00006565-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Hymel KP, Abshire TC, Luckey DW, Jenny C. Coagulopathy in pediatric abusive head trauma. Pediatrics. 1997;99:371–5. doi: 10.1542/peds.99.3.371. [DOI] [PubMed] [Google Scholar]
- 62.Hume HA, Limoges P. Perioperative blood transfusion therapy in pediatric patients. Am J Ther. 2002;9:396–405. doi: 10.1097/00045391-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, Shah A, Vercruysse GA, Feliciano DV, Rozycki GS, Salomone JP, Ingram WL. Improvements in early mortality and coagulopathy are sustained better in blunt trauma patients after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. doi: 10.1097/TA.0b013e3181a59ad5. (in press) [DOI] [PubMed] [Google Scholar]


