Summary
Stress-induced eating disorders cause significant health problems and are often comorbid with mood disorders. Emotional feeding, particularly in women, may be important for the development of obesity and failed attempts to lose weight. However, prospective studies assessing the effect of chronic psychosocial stress on appetite in different dietary environments in females are lacking. The present study tested the hypothesis that chronic psychosocial stress would increase consumption of high caloric diet and this emotional feeding would persist even when a healthier diet was available. Socially housed female rhesus monkeys were studied to address whether subordination increases caloric intake when a high fat and sugar diet (HFSD) was available concurrently with a low fat, high fiber diet (LCD). Cortisol responsivity and food intake were quantified during this choice phase and when only the LCD was available. The order of diet condition was counterbalanced to assess whether a history of HFSD would affect appetite. All females preferred the HFSD but subordinates consumed significantly more calories during the choice phase. The increased calorie intake was maintained in subordinate monkeys even after withdrawal of the HFSD. Subordinate females demonstrated reduced glucocorticoid negative feedback, with post dexamethasone serum cortisol levels significantly predicting intake of the HFSD but not the LCD during the choice condition. The cortisol response to an acute stressor significantly predicted subsequent intake of a HFSD in all females. Continual exposure to the psychosocial stress of subordination in female monkeys results in excess caloric intake of foods that mimic a western dietary environment. In addition, this social stressor interacts with a history of HFSD intake to promote increased feeding even in a healthy dietary environment.
Keywords: Psychosocial stress, Social subordination, Diet choice, Monkeys, Emotional feeding
1. Introduction
In 2009, nearly 73 million adults in the US were obese, representing 28% of the population and an increase of 7% over 2001 rates (CDC, 2010). Additionally, 34% of adults in the US are overweight (Flegal, 2005). Because the health (Hill, 2006) and economic burden (Withrow and Alter, 2010) imposed by obesity is enormous, effective programs to prevent or alleviate obesity are a high priority. While gene variants regulating satiety or metabolism are known to influence appetite and body weight control (Hinney et al., 2010), environmental triggers are likely key determinants of this phenotype (Font et al., 2010). Indeed, emotional feeding resulting from the chronic exposure to psychosocial stressors is a probable contributing factor for excess food intake (Bjorntorp, 2001; Dallman et al., 2005; Rosmond, 2004; Scott et al., 2008). Importantly, attempts to lose weight often fail (Kassirer and Angell, 1998), as eating behaviors become disinhibited and people overeat in response to emotional states (Hays and Roberts, 2008).
Emotional feeding is coincident with both periods of acute and chronic exposure to psychosocial stressors (Adam and Epel, 2007). Psychopathologies with etiologies related to chronic exposure to psychosocial stressors, such as depression and anxiety disorders, are highly comorbid with obesity (Simon and Arterburn, 2009; Werrij et al., 2006). It is uncertain which factors increase an individual's vulnerability to augment caloric intake under stressful circumstances. While intake of highly palatable foods is rewarding via activation of the dopaminergic reward system (Bassareo and Di Chiara, 1999; Blackburn et al., 1986; Johnson and Kenny, 2010; Small et al., 2003), it remains unclear how consumption of a calorically dense diet or one that is high fat and sugar diet (HFSD) alters physiological responses to both chronic and acute stressors.
There is a great deal of uncertainty surrounding the effects of HFSD consumption on the activity of the limbic–hypothalamic–pituitary–adrenal (LHPA) axis in the literature. This could be due to a number of factors including the specific animal model employed and whether a dietary choice was available as a part of the stress paradigm (Adam and Epel, 2007; Warne, 2009). Another important consideration for the study of stress-induced emotional feeding that is often neglected in animal models is gender. Indeed, emotional eating (Zellner et al., 2006, 2007) and an obese phenotype (Barry et al., 2008; Jones and Carney, 2006; Weissman and Olfson, 1995; Wurtman, 1993; Wurtman and Wurtman, 1995) occur significantly more often in women. The discrepancies in the current animal models available, and the alarming rate at which obesity epidemic is rising, necessitates that an appropriate animal model of stress-induced eating is developed so that the mechanisms responsible for stress-induced caloric intake can be elucidated.
Social subordination in female rhesus monkeys (Macaca Mulatta) is a well-characterized ethologically relevant, translational animal model used to study the adverse health effects of chronic psychosocial stress exposure in women (Adams et al., 1985; Cohen, 1999; Gust et al., 1991; Jarrell et al., 2008; Kaplan et al., 1996; Michopoulos et al., 2009; Morgan et al., 2002; Paiardini et al., 2009; Sapolsky, 2005; Shively, 1998; Wilson et al., 2008). We tested the hypothesis that socially subordinate females would increase overall caloric intake in a dietary environment mimicking a typical western situation wherein both low fat, high fiber diet and a HFSD were made available. In addition, we tested the hypothesis that a history of HFSD consumption would interact psychosocial stress promote excess calorie consumption even in a healthier dietary environment.
2. Methods and materials
2.1. Animals
Previously ovariectomized adult female rhesus monkeys (n = 39) living in indoor–outdoor enclosures at the Yerkes National Primate Research Center (YNPRC) Field Station (in groups of 4 and 5 females and 1 male) were used as subjects. No hormone replacement was used during the study. All animals had access to experimental diets ad libitum via previously validated automated feeders that allow for constitutive quantification of individual caloric intake (Arce et al., 2010; Wilson et al., 2008). Briefly, activation of a radio-frequency (RF) antenna via a RF identification chip within each animal's wrists signaled a computer to dispense a single pellet of food via a pellet. Each group of animals had access to two different feeder systems and a computer recorded each feeding event in a log. Dominant females rarely (<1%) take the pellet from subordinate animals and pellets are never discarded (Wilson et al., 2008). The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
Social stability in macaque groups, regardless of size, is maintained by a dominance hierarchy (Bernstein, 1976). Lower-ranking individuals in a social group receive a greater frequency of aggression from higher-ranking group mates and emit higher levels of submissive behaviors towards these more dominant individuals. A direct consequence of low social status in female rhesus monkeys is reduced control over both social and physical environments (Sapolsky, 2005) that result in disruption of LHPA function including diminished glucocorticoid negative feedback (Jarrell et al., 2008; Shively, 1998; Wilson et al., 2008). Therefore, social subordination in female rhesus monkeys is a well-characterized model with which to study the negative effects of chronic psychosocial stress exposure on behavior and physiology, including reproductive dysfunction (Adams et al., 1985; Michopoulos et al., 2009), immune compromise (Gust et al., 1991; Paiardini et al., 2009), addictive behavior (Morgan et al., 2002), and cardiovascular disease (Kaplan et al., 1996). In the current study, the outcome of dyadic interactions between females obtained from four 30-min observations throughout each study phase using an established ethogram (Jarrell et al., 2008), was used to establish group dominance ranks. As previously described (Kaplan et al., 1984), females ranked one and two were classified as dominant (n = 15) and females ranked 3–5 were considered as subordinate (n = 24). Social groups had been formed and dominance ranks stable for 108 months prior to the initiation of this study.
2.2. Experimental design
Females were studied for two two-week diet condition phases separated by a three-week washout period. Each phase consisted of either a dietary choice between a low calorie monkey chow (LCD; 3.45 kcal/g, Purina 5038) and a high fat and sugar diet (HFSD; 3.73 kcal/g Purina Typical American Diet #5038) or access to only the LCD (no choice condition). The order of diet condition presentation was counterbalanced so that half the females received the choice condition first and the other half the no choice condition first. The caloric composition of the LCD was 12% fat, 18% protein, and 4.14% sugar carbohydrate and 65.9% fiber carbohydrate. The calories of the HFSD were distributed as 36% fat, 18% protein, 16.4% sugar carbohydrate and 29.6% fiber–starch carbohydrate.
Body weights and non-fasted blood samples for the analysis of serum leptin, insulin, and glucose were taken at the beginning and end of each phase of the study at 0800 h to assess changes due to diet availability. To assess the effects of social status on glucocorticoid negative feedback, a dexamethasone suppression test (DST) was administered the week prior to the start of the study. Serum samples were collected at 0800, 1100, and 1730 h. Immediately following the sample at 1730 h, females received the dexamethasone injection (0.25 mg/kg, IM). Samples were collected the following morning at 0800 and 1100 h for cortisol analysis.
To assess the effects of diet availability and intake on cortisol response to an acute stressor, all females were exposed to a social separation stressor (SSS) paradigm on the second week of each diet phase. Isolation of a female rhesus monkey from her social group and confinement for 30 min results in a significant increase in serum cortisol levels (Collura et al., 2009). Blood samples for cortisol analysis were obtained at time 0 (1300) and 40 min later, after which animals were returned to their home caging.
2.3. Blood sampling and assay methods
All subjects were habituated to being removed from their group for conscious venipuncture using previously described procedures in place in the lab for over 30 years (Walker et al., 1982). Blood samples were obtained within 10 min from entering the animal area to minimize arousal (Blank et al., 1983). Serum levels of cortisol were measured by radioimmunoassay with a commercially available kit (Beckman-Coulter/DSL, Webster, TX). Using 25 μl, the assay has a range from 0.5 to 60 μg/dl with an inter- and intra-assay CV of 4.9% and 8.7%, respectively (Michopoulos et al., 2009). Serum leptin was measured by a RIA using a commercially available kit (Millipore, St. Louis, MO). Assaying 100 μl, the assay has a range of 0.5–100 ng/ml. Intra-assay CVs were 6.84% and inter-assay were 7.24% (Wilson et al., 2003). Serum insulin was assayed with a RIA kit from Siemens having a sensitivity of 3–372 IU/L and an inter-and intra-assay CV of 9.02%and 5.87%, respectively (Jarrell et al., 2008). Serum glucose was determined by a commercially available colorimetric enzyme assay (Stanbio Laboratory, Boerne, TX), having a range from 0 to 27 mmol/L and inter-and intra-assay CVs of 2.12% and 4.21%, respectively (Jarrell et al., 2008). All assays were done at the YNPRC Biomarkers Core Lab.
2.4. Statistical analyses
Data were summarized as mean ± standard error of the mean (SEM). The main and interaction effects of status (dominant vs. subordinate), order of diet presentation (choice first vs. no choice first), diet condition (choice vs. no choice), weeks, days, and time of day on caloric intake, metabolic measures, and cortisol response to an acute stressor were evaluated with analysis of variance for repeated measures (RM-ANOVA) using SPSS 19 for Mac (IBM). The feeding data were transformed with a log 10 to correct for the lack of homogeneity of variance. The change in cortisol levels due to the SSS was analyzed in this manner to assess the effects of diet availability and history on LHPA response to an acute stressor. Finally, Pearson product moment correlations were used to assess the association between selected variables and caloric intake. Test results with a p < 0.05 were considered significant and post hoc analysis conducted when necessary.
3. Results
3.1. Social status categorization and DST
As shown in Fig. 1A, rates of aggression received and submissive behavior emitted for monkeys at each social dominance rank position. Social rank significantly affected the amount of aggression received (F4, 29 = 8.15, p < 0.001) and submission emitted by females (F4, 29 = 5.13, p = 0.003). Categorizing females ranked 1 and 2 as dominant and those ranked 3 through 5 as subordinate results in a significant main effect of status on submissive behaviors emitted (F1,35 = 13.6, p = 0.001) and increased levels of aggression received (F1, 35 = 10.0, p = 0.003).
Figure 1.
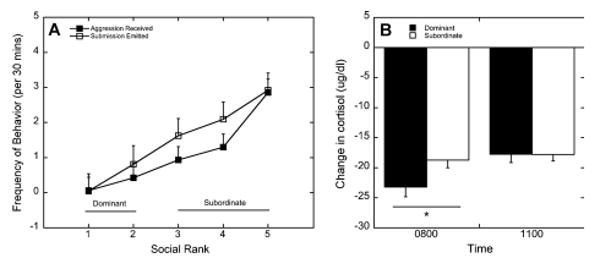
(A) Mean ± SEM rates (per 30 min) of aggressive behavior received and submission behavior emitted by females at each social dominance rank. Rates of aggression received and submission emitted were higher in animals categorized as subordinate females (ranks 3–5) compared with those categorized as dominant (ranks 1 and 2). (B) Mean ± SEM suppression in cortisol levels following dexamethasone administration in dominant (closed bars) and subordinate (open bars) females. Reduced suppression at 0800 by subordinates (*p < 0.05) reflects diminished glucocorticoid negative feedback.
Glucocorticoid negative feedback, assessed by suppression of cortisol following dexamethasone administration, was affected significantly by a time–status interaction (F1, 35 = 4.41, p = 0.049). Comparing cortisol concentrations following dexamethasone to those obtained at baseline the previous day showed that the change in cortisol at 0800 due to dexamethasone suppression was significantly greater in dominant females than in subordinate females (p = 0.034; Fig. 1B). At 1100, dominant animals had similar levels of cortisol as subordinate females (p > 0.05).
3.2. Effects of diet availability on calorie intake
Irrespective of the initial diet condition (choice or no-choice), total caloric intake was significantly affected by the interaction of social status and diet condition (F1, 35 = 4.67, p = 0.034; Fig. 2). Dominant females ate a similar number of total calories during the no choice and choice diet conditions (F1, 28 = 2.28, p = 0.142; Fig. 2). Subordinate females, however, consumed significantly more calories during the choice condition compared to the no choice diet condition (F1, 35 = 4.19, p = 0.048; Fig. 2). During the choice condition, subordinate females ate more calories from the LCD than did dominant females (p = 0.05; Fig. 2). Both dominant and subordinate females preferred the HFSD to the LCD during this choice condition (F1, 37 = 7.10, p = 0.011; Fig. 2) and, while it appeared subordinate females consumed more calories from the HFSD than did dominant animals, the difference was not significant (p = 0.121). Nonetheless, during the choice phase, total caloric intake was greater in subordinates compared with dominant monkeys (p = 0.01). This preference for the HFSD over the LCD was most evident during the daytime (26.0 ± 4.35 kcal/h vs. 14.3 ± 1.57 kcal/h) than during nighttime feeding (10.1 ± 2.44 kcal/hvs. 4.8 ± 1.42 kcal/h). Finally, the intake of the HFSD during the choice condition was significantly predicted from both baseline (r37 = 0.33, p = 0.04) and post dexamethasone serum concentrations of cortisol (r37 = 0.39, p = 0.02). Furthermore, these parameters of cortisol secretion also significantly predicted total caloric intake during the choice condition (baseline: r37 = 0.33, p = 0.04; post-dexamethasone: r37 = 0.44, p = 0.01) but not LCD during the choice condition (baseline: r37 = 0.05, p = 0.77; post-dexamethasone: r37 = 0.15, p = 0.37). Similarly, neither parameter of cortisol secretion was associated with intake of LCD during the no choice condition (baseline: r37 = 0.27, p = 0.09; post-dexamethasone: r37 = 0.26, p = 0.12).
Figure 2.
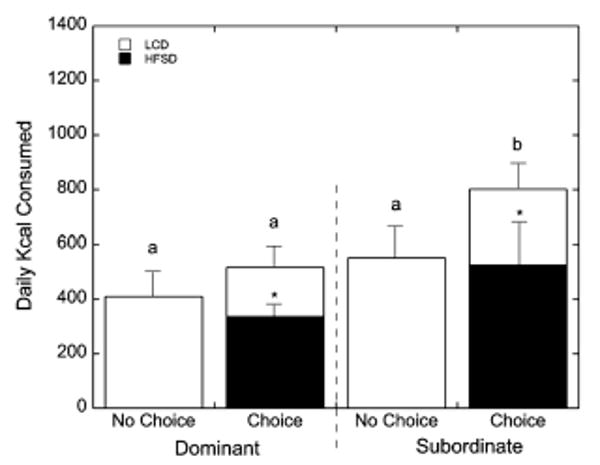
Mean ± SEM daily (24-h) caloric intake in dominant and subordinate females by diet condition (choice vs. no choice). Open bars represent intake of the low calorie diet (LCD) and closed bars indicate caloric intake of the high fat and sugar diet (HFSD) diet. Asterisks (*) denote overall preference of the HFSD over the LCD during the choice condition. Different letters (a and b) above each of the four group – diet conditions indicate caloric intake was significantly different (p < 0.05) based on post hoc tests. Overall caloric intake and intake of the LCD was significantly increased during the choice condition in subordinate compared to dominant females (p < 0.05).
Importantly, the order of diet phase, whether animals received the no choice or choice condition first, had a significant effect on caloric intake (F1, 35 = 7.12, p = 0.011), and this effect was modified by social status (F1, 35 = 4.07, p = 0.05, Table 1, Fig. 3). The total number of calories consumed in the two diet conditions did not vary significantly by order of diet presentation in dominant females (F1, 13 = 0.002, p = 0.966). Similarly, when the no choice preceded the choice phase, subordinate females consumed a similar number of calories during this LCD-only condition compared with that of the dominant animals (p = 0.479). In contrast, if the choice condition came first, subordinate females consumed significantly more calories during the subsequent no choice phase than did dominant females (749 ± 87 kcal/day vs. 480 ± 112 kcal/day, p = 0.030; Fig. 3) and significantly more than subordinate counterparts who had no history of HFSD intake (749 ± 87 kcal/day vs. 356 ± 83 kcal/day) (p = 0.006; Fig. 3), suggesting exposure to a HFSD diet changed appetite regulation for a healthier LCD in subordinates but not dominant females.
Table 1.
Mean ± SEM kcal per day consumed for each two-week diet phase during the daytime (0600–1800 h) and nighttime (1800–0600 h) in dominant (n = 15) and subordinate (n = 24) females.
| Diet order | Diet | Time | No choice | Choice | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Dominant | Subordinate | Dominant | Subordinate | |||
| LCD only first | LCD | Daytime | 251 ± 80 | 226 ± 61 | 123 ± 43 | 162 ± 33 |
| Nighttime | 96 ± 42 | 130 ± 32 | 28 ± 22 | 65 ± 17 | ||
| Total | 348 ± 53 | 356 ± 40 | 151 ± 55 | 227 ± 42 | ||
| HFSD | Daytime | – | – | 210 ± 122 | 359 ± 93 | |
| Nighttime | – | – | 110 ± 19 | 108 ± 51 | ||
| Total | – | – | 320 ± 82 | 467 ± 63 | ||
| Total | Daytime | 251 ± 80 | 226 ± 61 | 333 ± 82 | 521 ± 63 | |
| Nighttime | 96 ± 42 | 130 ± 32 | 138 ± 47 | 173 ± 34 | ||
| Total | 348 ± 53 | 356 ± 40 | 470 ± 64 | 694 ± 48 | ||
| Choice firsta | LCD | Daytime | 374 ± 80 | 556 ± 61 | 172 ± 40 | 226 ± 33 |
| Nighttime | 106 ± 42 | 193 ± 32 | 37 ± 21 | 103 ± 17 | ||
| Total | 480 ± 53 | 749 ± 40 | 210 ± 52 | 329 ± 42 | ||
| HFSD | Daytime | – | – | 272 ± 114 | 406 ± 93 | |
| Nighttime | – | – | 80 ± 63 | 185 ± 51 | ||
| Total | – | – | 352 ± 72 | 591 ± 63 | ||
| Total | Daytime | 374 ± 80 | 556 ± 61 | 445 ± 71 | 632 ± 63 | |
| Nighttime | 106 ± 42 | 193 ± 32 | 118 ± 42 | 288 ± 34 | ||
| Total | 480 ± 53 | 749 ± 40 | 563 ± 59 | 920 ± 48 | ||
A main effect of diet order presentation.
Figure 3.
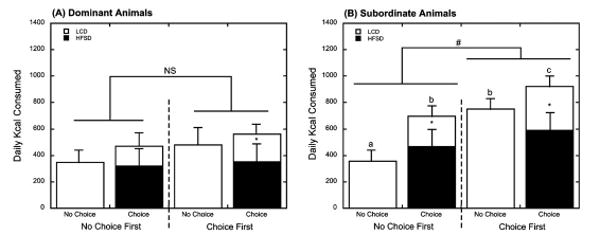
Mean ± SEM daily (24-h) caloric intake in dominant (A) and subordinate (B) females by diet condition and order of diet condition. Open bars represent intake of the low calorie diet (LCD) and closed bars indicate caloric intake of the high fat and sugar diet (HFSD). Asterisks (*) denote overall preference of the HFSD over the LCD during the choice condition (p < 0.05). Subordinate animals consumed more overall calories when the choice condition preceded the no choice condition (#) whereas order of diet condition did not affect caloric intake in dominant animals. Letters denote significant (p < 0.05) differences in intake during each diet condition in subordinate females due to order of diet presentation.
3.3. Effects of diet availability on cortisol response to an acute stressor
The increase in cortisol following the social separation was significantly greater during the diet choice phase compared to the no choice condition (F1, 35 = 20.5, p < 0.001; Fig. 4). This effect of diet condition on cortisol responsivity was not affected by social status (F1, 35 = 0.339, p = 0.564) or diet order (data not shown; F1, 35 = 0.013, p = 0.909). However, during the choice dietary condition, the change in serum cortisol following the social separation significantly predicted caloric intake of the HFSD (r35 = 0.44, p = 0.007) but not the LCD (r35 = 0.01, p = 0.976; Fig. 5) in all females. In contrast, the change in serum cortisol following the social separation did not predict subsequent intake of the LCD during the no choice phase (r35 = 0.06, p = 0.70).
Figure 4.
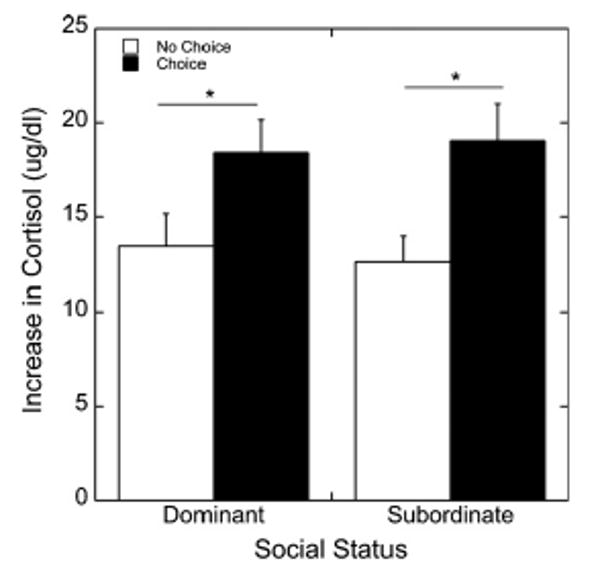
Mean ± SEM increase in cortisol levels due to social separation stressor test during the no choice (open bars) and choice (closed bars) diet conditions broken down by social status (dominant vs. subordinate) and order of diet presentation. *p < 0.01.
Figure 5.
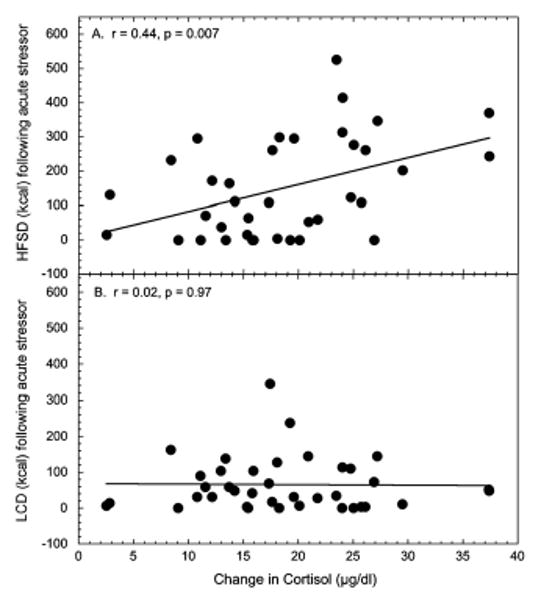
Simple linear regression using the increase in cortisol following the social separation stressor to predict consumption of high fat and sugar diet (HFSD; panel A) and low calorie diet (LCD; panel B) in the 9-h period following the stressor in all animals. Two animals whose intake of the HFSD exceeded 2 standard deviations from the group mean were excluded. Not shown is the non-significant regression of the change in cortisol following the social separation predicting caloric intake of the LCD during the no choice condition (r35 = 0.06, p = 0.70).
3.4. Effects of diet availability on body weight and serum hormones
Prior to the start of the study and while females were maintained on the LCD, dominant females (9.26 ± 0.40 kg) weighed significantly more than subordinates (7.60 ± 0.29 kg; F1, 35 = 11.1, p = 0.002), a phenotype consistent with earlier reports of socially housed female rhesus monkeys (Jarrell et al., 2008). As illustrated in Table 2, the change in body weight was significantly different during the no choice (−0.18 ± 0.04 kg) compared with the choice diet phases (−0.01 ± 0.04; F1, 35 = 11.1, p = 0.002). The diet phase dependent change in body weight was not affected by social status (F1, 35 = 1.32, p = 0.259) or diet order (F1, 35 = 2.66, p = 0.112).
Table 2.
Mean ± SEM measures of body weight and serum leptin, insulin, and glucose concentrations at the end of each two-week diet condition (no choice vs. choice) for both dominant and. subordinate females. Data are presented by the order of diet condition, whether the choice or the no choice phase came first. Also, shown is the change in the measure from the beginning of each condition to the end of the two-week condition. Letters depict main effects of diet and numbers illustrate differences due to a significant diet condition by order of diet presentation interaction. See text for details.
| Endpoint | Measure | Initial diet | Dominant | Subordinate | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No choice | Choice | No choice | Choice | |||
| Weight (kg) | Final | LCD only | 8.74 ± 0.56 | 8.44 ± 0.54 | 7.93 ± 0.52 | 7.62 ± 0.41 |
| Choice | 9.41 ± 0.52 | 9.68 ± 0.50 | 7.15 ± 0.43 | 7.24 ± 0.41 | ||
| Change | LCD only | −0.17 ± 0.09 a | 0.03 ± 0.09 b | −0.21 ± 0.07 a | −0.23 ± 0.07 b | |
| Choice | −0.21 ± 0.09 a | 0.04 ± 0.09 b | −0.13 ± 0.07 a | 0.11 ± 0.07 b | ||
| Leptin (ng/ml) | Final | LCD only | 17.6 ± 2.65 | 15.3 ± 3.22 | 7.59 ± 2.02 | 10.1 ± 2.46 |
| Choice | 13.5 ± 2.47 | 11.9 ± 3.01 | 11.9 ± 2.02 | 15.4 ± 2.46 | ||
| Change | LCD only | 2.06 ± 2.78 | -2.13 ± 3.40 | 0.91 ± 2.13 | 6.84 ± 2.59* | |
| Choice | 5.52 ± 2.60 | 4.71 ± 3.18 | 0.51 ± 2.13 | 9.41 ± 2.59* | ||
| Insulin (μU/ml) | Final | LCD only | 93.1 ± 24.3 | 50.6 ± 24.7 | 76.4 ± 18.6 | 71.3 ± 18.8 |
| Choice | 77.7 ± 22.8 | 128 ± 23.1 | 43.6 ± 18.6 | 72.5 ± 18.6 | ||
| Change | LCD only | −28.0 ± 13.31 | 25.3 ± 19.02 | −11.3 ± 10.21 | 41.1 ± 14.52 | |
| Choice | 19.3 ± 12.5 | 4.10 ± 17.8 | 6.13 ± 10.2 | 13.7 ± 14.5 | ||
| Glucose (mg/dl) | Final | LCD only | 96.7 ± 5.63 a | 95.2 ± 5.73 b | 89.6 ± 3.98 a | 93.1 ± 4.05 b |
| Choice | 90.8 ± 4.88 a | 108 ± 4.96 b | 88.5 ± 3.98 a | 95.8 ± 4.05 b | ||
| Change | LCD only | −7.78 ± 6.12 | 2.83 ± 12.8 | −3.74 ± 4.71 | 11.1 ± 9.75 | |
| Choice | 9.34 ± 5.772 | −17.4 ± 11.91 | 14.2 ± 4.71 2 | −7.09 ± 9.75 1 | ||
The significantly greater increase in leptin levels only in subordinate females during the choice condition.
At the start of the study and while females had been maintained on the LCD, dominant females (15.61 ± 2.01 ng/ml) had significantly higher serum levels of leptin more than subordinates (8.53 ± 1.51 ng/ml; t1, 37 = 2.81, p = 0.01), a phenotype again consistent with earlier reports of socially housed female rhesus monkeys (Jarrell et al., 2008). During the course of the study, social status and diet condition interacted to significantly affect the change in serum leptin over the two weeks of the choice vs. the no choice phase (F1, 35 = 8.04, p = 0.008; Table 2), as serum leptin increased significantly during the choice condition in subordinates (p = 0.005), but not dominant females (p = 0.196; Table 2) regardless of order of diet condition.
At the start of the study and while females had been maintained on the LCD, serum insulin was higher in dominant compared to subordinates but the difference was not significant (103.2 ± 23.0 IU/ml vs. 73.8 ± 14.5 IU/ml, t37 = 1.25, p = 0.22). Serum concentrations of insulin and glucose at the end of the LCD-only or choice diet condition, regardless of diet order, did not vary significantly by status (F1, 35 = 1.13, p = 0.296; F1, 35 = 2.74, p = 0.107, respectively; Table 2). However, the change in insulin levels was significantly affected by a diet condition–order of diet availability interaction (F1, 35 = 6.67, p = 0.014; Table 2). When the LCD-only condition was first, serum insulin increased significantly during the choice condition relative to the LCD-only condition (p = 0.005). However, when the choice condition was first, the increase in serum insulin during the choice phase was similar to the subsequent LCD only phase (p = 0.906; Table 2). Although the increase in serum insulin was greater in subordinates during the choice condition, the difference was not significant (p > 0.05).
A similar pattern prior to any diet intervention was observed for serum glucose, with non-significantly higher levels in dominant females (105.3 ± 4.9 mg/dl vs. 95.47 ± 2.6 mg/dl, t37 = 1.77, p = 0.09). Serum glucose levels were higher in all females at the end of the choice compared to the no choice condition (98.0 ± 2.37 vs. 91.4 ± 2.33; F1, 35 = 4.60, p = 0.039). A diet condition–order of diet presentation effect was also seen in glucose levels (F1, 35 = 7.63, p = 0.009). Glucose levels were not different between the two diet conditions when the LCD-only condition was first (p = 0.262). However, when the choice condition was first, decreased during the choice condition but increased during the no choice condition (p = 0.001; Table 2).
4. Discussion
Results here, summarized in Table 3, suggest that the chronic psychosocial stress experienced by subordinate females increases calorie intake only when these females are exposed to a dietary environment similar to human beings, where both a LCD and HFSD are available. These data are in agreement with a number of studies in male rodents (Dallman et al., 2007; Foster et al., 2006; Warne, 2009) and people (Epel et al., 2004; Torres and Nowson, 2007; Wallis and Hetherington, 2009) that show that stressor exposure increases intake of calorically dense diets. However, our data extend these findings to show that a history of HFSD consumption sustains excess calorie intake of the healthier LCD following removal of the HFSD choice in females experiencing the chronic stress of social subordination but not dominant females whose LHPA axis is more tightly regulated. Indeed, dominant females consume a similar number of calories regardless of diet availability or history. This pattern of emotional feeding in subordinate females is associated with a dysregulation of feedback inhibition of the LHPA axis, a phenotype similar to many stress-induced disorders in people (Chrousos, 2009).
Table 3.
A summary of major outcome differences between dominant (Dom) and subordinate (Sub) females during the two diet conditions. If a difference is stated between groups or dietary conditions, it was significant (p < 0.05).
| Outcome measure | Result |
|---|---|
| Aggression received | Sub receive more aggression |
| Submission emitted | Sub emit more submissive behaviors |
| Dex suppression test | Sub have reduced glucocorticoid negative feedback |
| Post-stressor increase in serum cortisol | Dom and Sub respond similarly and the response is greater during the choice compared to LCD-only condition |
| Average caloric intake |
|
| Body weight |
|
| Serum leptin | Serum leptin increased significantly during choice diet condition in Sub but not Dom females |
| Serum insulin |
|
| Serum glucose | Serum glucose was higher at the end of the choice condition |
The significantly greater food intake by subordinates in a calorically rich dietary environment is consistent with other data showing exposure to chronic stress increases food intake (Arce et al., 2010; Tamashiro et al., 2006; Warne, 2009). However, we did not observe a significant increase in body weight in subordinates despite the substantial increase in caloric intake. It is possible that the increased energy intake was obviated by increased activity and energy expenditure in subordinates (Solomon et al., 2011). However, it could also be that that the duration of access to the HFSD was too short, as we have previously shown that three week access significantly increases body weight in subordinates (Arce et al., 2010). Novel, however, is the finding that the consuming a HFSD also increases consumption of low fat, low sugar foods but only in subordinate females. This was not the case prior to the availability of the HFSD because groups that started initially with the no-choice LCD condition did not show any difference in caloric intake between subordinate and dominant females (347 ± 112 kcal/day vs. 356 ± 87kcal/day). These data imply that in an environment where a choice of diets with different palatable qualities is available, females exposed to continual psychosocial stressors increase total overall caloric intake, not just excess calories from highly palatable food.
In addition, exposure to a high fat, high sugar diet interacted with social subordination to produce changes in subsequent caloric intake after the highly palatable diet had been removed. Dominant animals consumed a similar number of calories during both diet conditions regardless of order of diet presentation, indicating diet history had no lasting effect on food intake in these animals. However, when the diet choice condition preceded the no choice phase, subordinates ingested more calories than did dominant females during the subsequent no-choice diet condition and significantly more calories than subordinates who had the no choice condition first and no previous history of HFSD consumption (Table 2, Fig. 3). These data support our previous preliminary observations (Arce et al., 2010) and suggest that exposure to psychosocial stress interacts with diet history to promote additional caloric intake even in a healthy dietary environment. The lasting effect of stress and experience of comfort food ingestion may account for the high probability of failure observed in human beings when attempting to lose weight by adopting a healthier dietary environment after years of eating calorically dense foods (Kassirer and Angell, 1998).
Presentation of the diet choice first resulted in an increase in both insulin and glucose levels in all females that remained high during the following no choice diet condition. Because total calorie consumption was not different in any of the conditions for dominant females, these data raise the possibility that the lasting effect of HFSD exposure on caloric intake in subordinate females may be due to a synergistic stress- and HFSD-induced loss of sensitivity to satiety signals, like insulin and leptin that are critical for maintaining homeostatic energy balance (Lustig, 2008). In rats, the combination of chronic stress and a high-fat diet exacerbates insulin resistance compared to the effect of either a high fat diet or chronic stress alone (Fu et al., 2009). As evidence of this, serum leptin levels were increased in subordinate but not dominant females during the choice diet condition, yet subordinates maintained high caloric intake in the following no-choice phase. In humans and in rats (Mietus-Snyder and Lustig, 2008), increased levels of glucocorticoids produce overeating and weight gain despite increased leptin levels, suggesting that glucocorticoids may lead to leptin insensitivity. Although serum glucose was not elevated during the choice phase in subordinates, continued access to this rich dietary environment may have significantly reduced insulin sensitivity, increased serum glucose and worsened leptin sensitivity (Lustig, 2008).
Based on studies in rodents, a possible explanation for increased consumption of calories by subordinate female monkeys when a HFSD is available is that intake of a palatable diet acts to reduce LHPA reactivity, most typically shown by an attenuation of the LHPA response to an acute stressor in previously stressed animals given access to a dietary environment that includes a choice of macronutrients (Dallman et al., 2005; la Fleur et al., 2005; Pecoraro et al., 2004; Ulrich-Lai et al., 2010). These observations in male rats are supported by data showing obese women who report a high degree of emotional feeding have a reduced cortisol response to an acute stressor (Tomiyama et al., 2011). Furthermore, the data from rodents argue that availability of a choice between a LCD and a sugar or fat diet is important to show this effect of stress hormone attenuation by diet (Warne, 2009). We chose to present females with a choice between a LCD and HFSD rather than just a HFSD condition as we felt it more closely mimicked the dietary environment available to people. Nonetheless, we observed that the increase in serum cortisol following the social separation stressor was significantly higher during the choice diet condition compared to the no choice diet condition regardless of social status or order of the diet regimen. This result is consistent with our previous data showing that intake of a calorically dense diet augments the cortisol response to an acute stressor in both dominant and subordinate animals (Arce et al., 2010). Our data are consistent with those showing intake of calorically dense foods increases LHPA activity in rodents (Kamara et al., 1998; Tannenbaum et al., 1997) and in humans (Pasquali et al., 2002). The most parsimonious explanation for this is that when more energy resources are available, due to increased caloric intake, more cortisol is released to mobilize these increased energy resources. Furthermore, data showing that glucocorticoids are key signals changing food salience and increasing caloric intake (Warne, 2009) are consistent with the notion that emotional feeding is sustained by an activation and not a diminution of the LHPA axis. Indeed, data from the present study show that post dexamethasone and post acute stressor serum levels of cortisol significantly predict intake of a HFSD but not a LCD. These data suggest that acute stress superimposed on chronic stress may promote continued intake of high caloric diets. Nonetheless, additional prospective studies are needed to better understand the neuroadaptations that sustain emotional feeding in the face of chronic stress.
An important consideration in understanding the factors the initiate and sustain emotional feeding are possible sex differences. Human data indicate that women (Zellner et al., 2006) and not men (Zellner et al., 2007) most often show emotional feeding in response to socio-emotional stressors and are twice as likely as men to suffer from eating and affective disorders (Barry et al., 2008; Weissman and Olfson, 1995; Wurtman and Wurtman, 1995). One could argue that the well-established sex difference in stress responsivity (Dalla et al., 2005; Handa et al., 1994; Kaplan et al., 1996; Kirschbaum et al., 1992; Young, 1998) may translate to differences in stress-induced ingestion of calorically dense diets. Nonetheless, data from male rodents clearly show males that experience restraint (Dallman et al., 2007; Warne, 2009) or social stress (Foster et al., 2006; Tamashiro et al., 2006) become hyperphagic in a high caloric dietary environment. A smaller number of studies in female rodents also indicate that stressor exposure promotes food intake and visceral obesity (Bartness, 1996; Solomon et al., 2011). Although ovarian estradiol is known to reduce food intake (Wade and Schneider, 1992) by limiting the size of a meal (Asarian and Geary, 2006), its effect on stressed-induced consumption of calorically dense diets is unknown. For example, a recent study in premenopausal women reported that subjects expressing high levels of stress engage in more emotional feeding and show a blunted response in cortisol to an acute stressor (Tomiyama et al., 2011). However, it is unclear how ovarian cycle stage affected this pattern. Our model using ovariectomized rhesus monkeys holds the promise of determining how replacement therapy with estradiol may alter the social status differences in consumption of calorically dense diets. Because estradiol exacerbates the loss of glucocorticoid negative feedback in subordinate females (Wilson et al., 2005) and increased exposure to glucocorticoid is a key signal in the emergence of stress eating (Dallman et al., 2007), one might predict that the effects of estradiol limiting food intake will be lost in the face of a chronic stressor.
Although we did not see a reduction in LHPA activity following acute stress in subordinate or dominant females during the choice diet condition, we did observe that the cortisol response to the social separation predicts the subsequent intake of the HFSD and not LCD independent of social status. These data presented in the current study parallel findings in women indicating that responses to acute stressors may influence subsequent eating behavior by altering food intake and preference for calorically dense food (Adam and Epel, 2007; Appelhans et al., 2010; Epel et al., 2001, 2004). What our data show is that the chronic stress of subordination promotes persistent, increased caloric intake in a rich dietary environment but that the cortisol response to an acute stressor, regardless of a chronic stress background, predicts the subsequent short term intake of a calorically dense but not a lower caloric diet.
Evidence suggests that changes in dopamine (DA) activity is a potential mechanism linking stress to comfort food ingestion (Bassareo and Di Chiara, 1999; Martel and Fantino, 1996a,b; Pelchat, 2002; Rada et al., 2005) as chronic psychosocial stress exposure reduces DA function, characterized by reduced DA D2 receptor (D2R) availability that is associated with anhedonia and increased susceptibility to addiction (Anisman and Matheson, 2005; Harfstrand et al., 1986; Izzo et al., 2005; Koob and Kreek, 2007; Lucas et al., 2004; Macey et al., 2000; Sauvage and Steckler, 2001; Swanson et al., 1983). Indeed, the imposition of social subordination in macaques leads to a reduction of D2R binding potential assessed by PET in striatal regions (Grant et al., 1998; Morgan et al., 2002) and increased cocaine self-administration (Morgan et al., 2002). This “reward deficiency syndrome”, characterized by reduced DA activity (Blum et al., 1996), is both predictive of an addictive phenotype (Volkow et al., 2003; Volkow and Wise, 2005) and observed in obesity (Wang et al., 2001). Therefore, it is hypothesized that a biobehavioral strategy to activate DA pathways compromised by stress is to consume a HFSD, thereby increasing levels of DA in the nucleus accumbens (Bassareo and Di Chiara, 1999), a finding not seen when consuming a LCD or palatable food devoid of calories such as saccharin (Bassareo and Di Chiara, 1999; Blackburn et al., 1986; Marinelli et al., 2006; Small et al., 2003). What is compelling, however, are data showing that consuming a HFSD, independent of stress, produces deficits in mesolimbic D2R availability (Geiger et al., 2009; Johnson and Kenny, 2010; Lee et al., 2009). Because both insulin and leptin can reduce the rewarding value of food (Berthoud et al., 2011; Lustig, 2006; Shizgal et al., 2001), possibly by attenuating DA release (Krugel et al.,2003) and/or increasing D2R binding in these reward pathways (Pfaffly et al., 2010), the insulin and leptin insensitivity that the emergences as obesity develops could account for this diet-induced suppression of mesolimbic D2R availability. Together, these data suggest that psychosocial stress exacerbates diet-induced reductions in D2R, further sustaining the drive for unhealthy eating and creating a downward spiral often observed in addiction. Indeed, the persistence of increased caloric intake by subordinate females following the removal of the HFSD and re-establishment of a healthier dietary environment may be a compulsive food-seeking behavior driven by the motivation to engage a hypoactive reward system.
In summary, these data are consistent with previous data showing exposure to psychosocial stress increases consumption of overall calories. In addition, these data suggest that chronic psychosocial stress increases eating of low caloric food choices in the presence of a high caloric food choice. Moreover, results show that stress interacts with a history of HFSD consumption to increase food intake even when high caloric food is no longer available. Although it is well established that exposure to social stressors can act as an environmental trigger to induce alterations in appetite regulation, caloric intake, and body weight gain (Bjorntorp, 2001; Dallman et al., 2005; Rosmond, 2004; Schwartz, 2009; Scott et al., 2008), the present study indicates that stressor exposure is a key explanatory factor for determining how an individual's eating can be modulated immediately and for the long-term by the ingestion of calorically dense diets.
Acknowledgments
The study was conducted with the expert technical assistance of Jennifer Whitley, Shannon Bounar, Jodi Godfrey, Christine Marsteller, Jonathon Lowe, Rebecca Herman, Robert Johnston and Gregory Henry. This study would not have been possible without the dedication of the animal husbandry staff at the YNPRC. The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Role of funding source: Funding support for this study was provided by NIH grants HD46501 (MW), MH081816 (DT), RR00165, and F31MH085445 (VM). Further support was provided by the Center for Behavioral Neuroscience through the STC Program of the National Science Foundation IBN-9876754. The NIH and the CBN had no role in the study design, the collection, analysis, and interpretation of the data, nor in writing the manuscript or the decision on where to submit the manuscript for publication.
Footnotes
Conflicts of interest: All authors declare that they have no conflicts of interest.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Pagoto SL, Peters EN, Spring BJ. HPA axis response to stress predicts short-term snack intake in obese women. Appetite. 2010;54:217–220. doi: 10.1016/j.appet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav. 2010;101:446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D, Pietrzak RH, Petry NM. Gender differences in associations between body mass index and DSM-IV mood and anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions. Ann Epidemiol. 2008 doi: 10.1016/j.annepidem.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ. Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiol Behav. 1996;60:517–529. doi: 10.1016/s0031-9384(96)80027-8. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60(2):459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1266–R1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Increased dopamine metabolism in the nucleus accumbens and striatum following consumption of a nutritive meal but not a palatable non-nutritive saccharin solution. Pharmacol Biochem Behav. 1986;25:1095–1100. doi: 10.1016/0091-3057(86)90091-2. [DOI] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta Endocrinol (Copenh) 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Vital signs: state-specific obesity prevalence among adults–United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:951–955. [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social status and susceptibility to respiratory infections. Ann N Y Acad Sci. 1999;896:246–253. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Warne JP, Foster MT, Pecoraro NC. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J Physiol. 2007;583:431–436. doi: 10.1113/jphysiol.2007.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86:599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Font JC, Fabbri D, Gil J. Decomposing cross-country differences in levels of obesity and overweight: does the social environment matter? Soc Sci Med. 2010;70:1185–1193. doi: 10.1016/j.socscimed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–R1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- Fu JH, Xie SR, Kong SJ, Wang Y, Wei W, Shan Y, Luo YM. The combination of a high-fat diet and chronic stress aggravates insulin resistance in Wistar male rats. Exp Clin Endocrinol Diabetes. 2009;117:354–360. doi: 10.1055/s-0028-1119406. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotrans-mission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo–pituitary–adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity (Silver Spring) 2008;16:52–58. doi: 10.1038/oby.2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- Hinney A, Vogel CI, Hebebrand J. From monogenic to polygenic obesity: recent advances. Eur Child Adolesc Psychiatry. 2010;19:297–310. doi: 10.1007/s00787-010-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacol Biochem Behav. 2005;81:701–708. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LE, Carney CP. Increased risk for metabolic syndrome in persons seeking care for mental disorders. Ann Clin Psychiatry. 2006;18:149–155. doi: 10.1080/10401230600801085. [DOI] [PubMed] [Google Scholar]
- Kamara K, Eskay R, Castonguay T. High-fat diets and stress responsivity. Physiol Behav. 1998;64:1–6. doi: 10.1016/s0031-9384(97)00534-9. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female ‘protection’ among cynomolgus macaques. Atherosclerosis. 1984;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kassirer JP, Angell M. Losing weight–an ill-fated New Year's resolution. N Engl J Med. 1998;338:52–54. doi: 10.1056/NEJM199801013380109. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- Lee AK, Mojtahed-Jaberi M, Kyriakou T, Aldecoa-Otalora Astarloa E, Arno M, Marshall NJ, Brain SD, O'Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2009 doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lustig RH. Childhood obesity: behavioral aberration or biochemical drive?Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2:447–458. doi: 10.1038/ncpendmet0220. [DOI] [PubMed] [Google Scholar]
- Lustig RH. Which comes first?The obesity or the insulin? The behavior or the biochemistry? J Pediatr. 2008;152:601–602. doi: 10.1016/j.jpeds.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a micro-dialysis study. Pharmacol Biochem Behav. 1996a;55:297–302. doi: 10.1016/s0091-3057(96)00087-1. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996b;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81:1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietus-Snyder ML, Lustig RH. Childhood obesity: adrift in the “limbic triangle”. Annu Rev Med. 2008;59:147–162. doi: 10.1146/annurev.med.59.103106.105628. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23:286–293. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, de Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose–response study. J Clin Endocrinol Metab. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76:347–352. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- Pfaffly J, Michaelides M, Wang GJ, Pessin JE, Volkow ND, Thanos PK. Leptin increases striatal dopamine D2 receptor binding in leptin-deficient obese (ob/ob) mice. Synapse. 2010;64:503–510. doi: 10.1002/syn.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Obesity and depression: same disease, different names? Med Hypotheses. 2004;62:976–979. doi: 10.1016/j.mehy.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei–potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ. Your brain on fat: dietary-induced obesity impairs central nutrient sensing. Am J Physiol Endocrinol Metab. 2009;296:E967–E968. doi: 10.1152/ajpendo.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res. 2008;64:97–105. doi: 10.1016/j.jpsychores.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shizgal P, Fulton S, Woodside B. Brain reward circuitry and the regulation of energy balance. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S17–S21. doi: 10.1038/sj.ijo.0801906. [DOI] [PubMed] [Google Scholar]
- Simon GE, Arterburn DE. Does comorbid psychiatric disorder argue for or against surgical treatment of obesity? Gen Hosp Psychiatry. 2009;31:401–402. doi: 10.1016/j.genhosppsych.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jankord R, Flak JN, Herman JP. Chronic stress, energy balance and adiposity in female rats. Physiol Behav. 2011;102:84–90. doi: 10.1016/j.physbeh.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–542. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic–pituitary–adrenal activity in the rat. Am J Physiol. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16:235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Emotions and eating self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52:355–362. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- Werrij MQ, Mulkens S, Hospers HJ, Jansen A. Overweight and obesity: the significance of a depressed mood. Patient Educ Couns. 2006;62:126–131. doi: 10.1016/j.pec.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, Gould KG. Leptin administration increases nocturnal concentrations of luteinizing hormone and growth hormone in juvenile female rhesus monkeys. J Clin Endocrinol Metab. 2003;88:4874–4883. doi: 10.1210/jc.2003-030782. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol Behav. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic–hypothalamic–pituitary–adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26 doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Wurtman JJ. Depression and weight gain: the serotonin connection. J Affect Disord. 1993;29:183–192. doi: 10.1016/0165-0327(93)90032-f. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3(Suppl 4):477S–480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1:21–27. [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiol Behav. 2006;87:789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Saito S, Gonzalez J. The effect of stress on men's food selection. Appetite. 2007;49:696–699. doi: 10.1016/j.appet.2007.06.013. [DOI] [PubMed] [Google Scholar]


