Abstract
Introduction
More than 80% of schistosomiasis patients in China live in the lake and marshland regions. The purpose of our study is to assess the effect of a comprehensive strategy to control transmission of Schistosoma japonicum in marshland regions.
Methodology/Principal Findings
In a cluster randomized controlled trial, we implemented an integrated control strategy in twelve villages from 2009 through 2011 in Gong'an County, Hubei Province. The routine interventions included praziquantel chemotherapy and controlling snails, and were implemented in all villages. New interventions, mainly consisting of building fences to limit the grazing area for bovines, building safe pastures for grazing, improving the residents' health conditions and facilities, were only implemented in six intervention villages. Results showed that the rate of S. japonicum infection in humans, bovines, snails, cow dung and mice in the intervention group decreased from 3.41% in 2008 to 0.81% in 2011, 3.3% to none, 11 of 6,219 to none, 3.9% to none and 31.7% to 1.7%, respectively (P<0.001 for all comparisons). In contrast, there were no statistically significant reductions of S. japonicum infection in humans, bovines and snails from 2008 to 2011 in the control group (P>0.05 for all comparisons). Moreover, a generalized linear model showed that there was a higher infection risk in humans in the control group than in the intervention group (OR = 1.250, P = 0.001) and an overall significant downward trend in infection risk during the study period.
Conclusions/Significance
The integrated control strategy, designed to reduce the role of bovines and humans as sources of S. japonicum infection, was highly effective in controlling the transmission of S. japonicum in marshland regions in China.
Trial Registration
Chinese Clinical Trial Registry ChiCTR-PRC-12002405.
Author Summary
More than 80% of schistosomiasis patients in China live in the lake and marshland regions. Hence, how to control transmission of Schistosoma japonicum in these regions is especially important. From 2009 through 2011, we implemented an integrated control strategy, designed to reduce the role of bovines and humans as sources of S. japonicum infection, in twelve villages Gong'an County of Hubei Province, which is located in typical marshland. After three years, the rate of S. japonicum infection in humans, bovines and snails significantly declined in the six intervention villages. In contrast, there was no significant decline in these indexes in the six control villages. Moreover, there was a higher infection risk in humans in the control group than the intervention group. Our study showed that the integrated control strategy was highly effective in controlling the transmission of S. japonicum in marshland regions of China.
Introduction
Schistosomiasis is an important public health issue in China where it continues to pose a serious threat to human well-being [1]–[3]. Since the People's Republic of China was established in 1949, the Chinese government has given high priority to the control of schistosomiasis, establishing a number of special bodies to manage control activities from the national to the township level [4]–[6]. These policies resulted in remarkable success in that the number of schistosomiasis patients were reduced by over 90%, from about 11.6 million cases in the 1950s to 726,000 cases in 2004 [7]. A further report in 2004 indicated that disease transmission had been interrupted or controlled in 42% of provinces, 40% of counties, and 53% of towns, previously endemic for schistosomiasis [8].
However, prospects for the control of schistosomiasis have been less optimistic in recent years, particularly since the termination of the World Bank Loan Project (WBLP) for schistosomiasis control at the end of 2001 [7]. Compared with the second national survey of S. japonicum in China in 1995, the third national survey conducted in 2004 showed that the prevalence of S. japonicum infection in humans had not substantially changed in the lake and marshlands and other areas of Southern China [7]. By the end of 2009, a total of 365,770 cases of S. japonicum were estimated in China, and 89 counties had not yet reached the criterion for transmission control which stipulated that the human prevalence should be less than 1% over a length of time [9], [10], i.e. the prevalence in these counties was >1%.
Over the past 2–3 decades, the strategies for S. japonicum control in southern China, including the vast lake and marshland regions, has involved chemical mollusciciding, alteration of the oncomelanid intermediate snails habitats, and synchronous chemotherapy with praziquantel for all villagers and their bovines [6], [11]. Historically, these strategies achieved some effect, but recent studies demonstrated there were some problems as the control options resulted in environmental pollution leading to ecological damage [11], [12]. Moreover, owing to the high rates of reinfection in both humans and bovines, frequent flooding, and the complex environment, more persons have been infected and the habitat of the Oncomelania snails has increased in the lake and marshlands [11], [13]. Consequently, a more effective strategy was needed urgently in these areas.
From 2005 through 2007, an important study of schistosomiasis japonica control was undertaken by Wang et al. in villages along the Poyang Lake in Jiangxi Province involving a comprehensive integrated approach aimed at reducing S. japonicum transmission to snails from cattle and humans, which play key roles as sources of S. japonicum [14]. The integrated strategy, which included removing bovines from snail-infested grasslands, providing farmers with mechanized farming equipment, building safe water systems, providing adequate sanitation, and implementing health education and synchronous chemotherapy with praziquantel for both villagers and bovines, was highly effective [14].
On the basis of identifying and controlling the main S. japonicum infection sources, we implemented a similar strategy (March 2009 through November 2011) to that employed by Wang et al. in the marshlands of Gong'an County [14], Hubei Province, another major endemic area for schistosomiasis japonica. The objective of the study was assessing the strategy's effect in marshland regions.
Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Protocol S1 and Checklist S1.
Ethics statement
Written ethical approval for this study was obtained from the Ethics Review Committee of Hubei Provincial Center for Disease Control and Prevention (no. 200803). Written informed consent was obtained from all adults and from parents or guardians of minors before participation in the study. The participants had the opportunity to withdraw from the study at any time.
Before beginning work on the study, the bovine owners provided consent to have their animals involved in the study. Moreover, permits for the bovines were obtained from Gong'an County Animal Husbandry Bureau. All animal work was carried out under the guidance of the Institute for Laboratory Animal Research (ILAR), and approved by Ethics Review Committee of Animal Experiments, Hubei Provincial Center for Disease Control and Prevention (no. 2008a05).
Both doses of praziquantel (i.e., single 40 mg/kg dose for humans identified as stool egg-positive or 25 mg/kg for infected bovines) were within Chinese Ministry of Health published guidelines [15].
Study area and participants
Gong'an County is located in typical marshland with a water area of 364 hectares (km2) along the mid-to-lower reaches of the Yangtze River in southwest Hubei Province, China. In 2009, there were 294 schistosomiasis-endemic villages (out of a total of 326), 539 cases of advanced schistosomiasis, and 36,612 cases of chronic schistosomiasis in Gong'an County; the prevalence of schistosomiasis in humans and bovines was 2.75% and 2.41%, respectively [16].
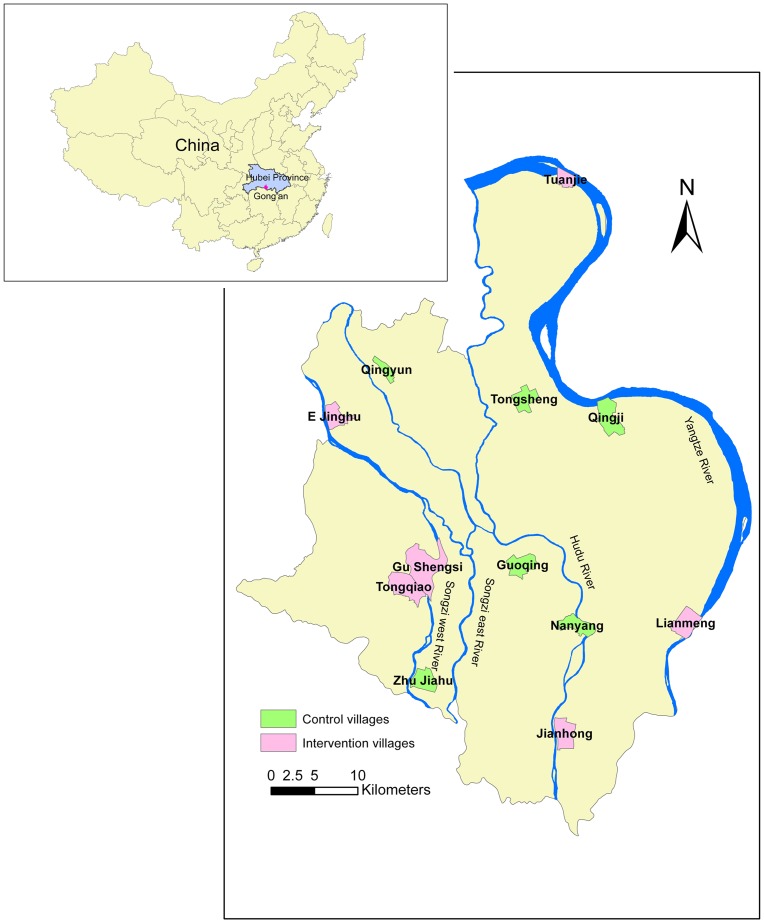
We carried out the study in 12 villages from 12 towns in Gong'an County which were selected by a two-stage random sampling procedure. The 12 towns were first randomly selected from 16 towns; then 12 schistosomiasis-endemic villages were selected from the 12 towns randomly (each town selected a village). Finally, these villages were randomly divided into intervention and control groups (Figure 1). E Jinghu, Gu Shengsi, Jianhong, Lianmeng, Tongqiao, Tuanjie were assigned to the intervention group and Guoqing, Nanyang, Qingyun, Qingji, Zhu Jiahu, Tongsheng were assigned to the control group (Figure 2). The villages had similar agriculture resources (rice, cotton and brassica napus) and the residents mainly were farmers (about 75%). The residents were exposed to contaminated water when they performed their agricultural or daily activities (i.e. cultivating crops, catching fish and washing clothes).
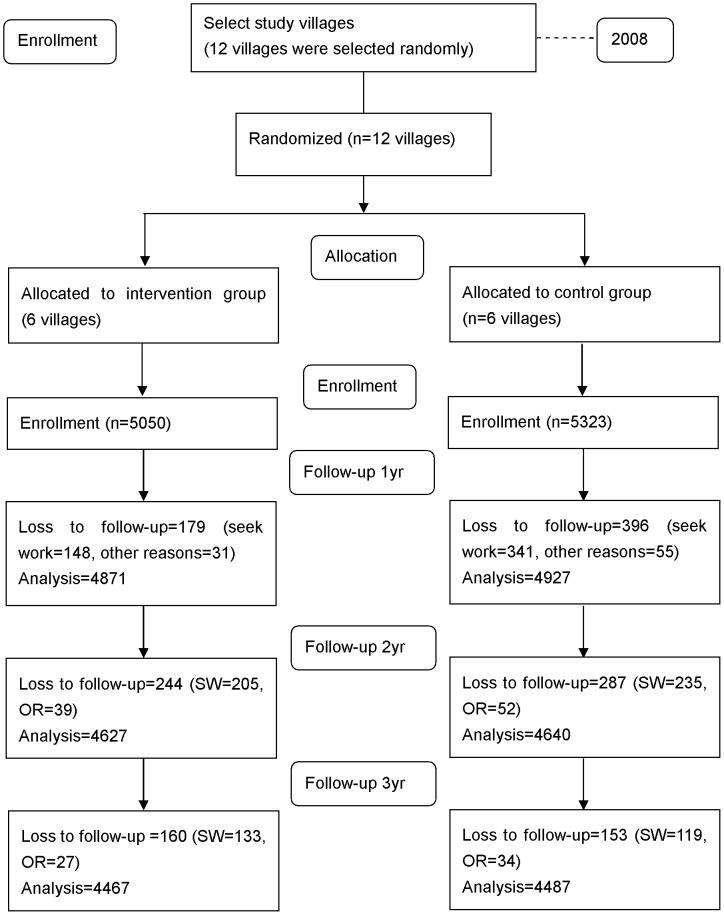
Figure 1. Trial flowchart.
Figure 2. Location of the study villages in the mid-to-lower reaches of the Yangtze River, Hubei province, China.
The participants met the following inclusion criteria: a) must have been a resident of the village for more than 12 months; b) should be aged 6–65 years; c) should continuously reside in the village for the study period; d) have no serious diseases, such as malignant cancer.
The comprehensive strategy to control transmission of S. japonicum
Prior to implementing the new control strategy, routine interventions had been used in Gong'an County to control S. japonicum infection, which comprised synchronous praziquantel chemotherapy of both villagers and bovines and mollusciciding of snails [17], [18]. These procedures were continued in all 12 study villages for the duration of the study but additional interventions were incorporated into the intervention group. These comprised of the building of fences to limit the grazing area for bovines, building safe pastures for grazing, improving the residents' health conditions and facilities, and strengthening specialized schistosomiasis clinics at the village level.
Fencing and safe pastures
Considering that bovines have been identified as the primary reservoir host of S. japonicum infection in China [6], [19]–[21], two measures were implemented (March to May, 2009) to reduce the influence of bovines on transmission. Firstly, 63 kilometres of fencing were installed to prevent animals from grazing in areas where Oncomelania snails were present. Warning signs were placed along the fenced areas, with the help of the local government authorities, and professional management staff were employed to ensure the intervention was effective.
Secondly, 7 safe grazing pasture areas were established totaling 190 hectares (1,900,000 m2). Each area was 20–30 hectares and was designed to contain about 1,100 animals. The pastures were carefully selected with no or very few snails, which, if present, were quickly eliminated by mollusciciding. Specialized managers were employed to manage each of the grazing areas and ponds were established to enable the bovines to drink uncontaminated water.
Fences comprised cement columns and barbed wire which ensured long-term usage. Each cement column was 160 centimeters (cm) in height and shaped as a triangular prism with 15 cm edges. Each column had three holes through which the barbed wire was fixed, and the distance between each was 2 meters.
Improving health and health care facilities
To reduce the potential of humans as a source of infection for snails, measures were undertaken (May to July, 2009) to improve the health and well-being of villagers and the existing health care facilities. Safe water was provided to 2,826 households by building 8 new public wells or improving household wells already in place. To deal with humans feces, 1,545 home lavatories were constructed or repaired with three-cell septic tanks and 618 households were provided with marsh-gas pool latrines. Furthermore, all fishermen were provided with fecal-matter containers to prevent them from excreting feces directly into river or lake freshwater.
Strengthening specialized schistosomiasis clinics
In order to implement the interventions in the intervention villages, specialized schistosomiasis clinics were strengthened at the village level (March to May, 2009). As well as being responsible for managing the fences and the safety of the pastures, the clinic staff were responsible for examining the residents and livestock for schistosome infection, treating infected people or livestock with praziquantel, and providing health education on preventing S. japonicum infections and, in particular, in explaining the significance of bovines and humans as sources of S. japonicum infection for snails.
Praziquantel chemotherapy
During the study period, all participants were screened annually for S. japonicum infection by indirect hemagglutination assay (IHA), followed by fecal examination of IHA-positives using the Kato-Katz technique [15] (three thick smears). Kato-Katz-positives were treated with praziquantel at a single oral dose of 40 mg/kg. As well, all bovines were checked annually by the miracidium hatching test in dung [15]. All infected bovines were treated with a single dose of praziquantel at a dosage of 25 mg/kg body weight.
Snail control
Before implementing the new control strategy, mollusciciding using niclosamide had been conducted in all schistosomiasis-endemic villages (included all 12 study villages) in Gong'an County as part of the routine procedure. During the study period, a comprehensive approach, comprising mollusciciding with niclosamide and environmental modification, which mainly included using cement to change ditches' original environment, was used for snail control in all 12 study villages. Snail surveys were carried out twice-yearly (spring and autumn) along the river banks, ditches and marshlands around the villages [15]. An average of 110 hectares of snail habitat in the study villages were treated annually with molluscicide or environmental modification (89 hectares in 2008, 141 hectares in 2009, 134 hectares in 2010 and 74 hectares in 2011).
Outcome measures
Five outcome measures, including the primary one (the prevalence of S. japonicum in humans) and four secondary ones (the rate of S. japonicum infection in bovines, cow dung, snails and mice), were determined annually and used to assess the strategy's effect.
Participants that were positive for both IHA and Kato-Katz were defined as infected and the prevalence of S. japonicum of participants in all 12 study villages was in October/November, after the second transmission season.
During the same period, the infection of S. japonicum of all bovines was checked in all 12 study villages by the miracidium hatching test in dung [15]. In addition, we gathered fresh cow dung from the grasslands which located in the 6 intervention villages (the sampled locations were the safe grazing pastures from 2009 to 2011), and used the miracidium hatching test to detect the infection of S. japonicum in cow dung.
In April and May, we systematically sampled Oncomelania hupensis snails along the river banks and in marshlands and ditches around all 12 study villages. In every village, we investigated at least 2000 sample units (0.11 m2 frame) and gathered all the live snails in the frame, crushed them, and examined them for S. japonicum infection using microscopy.
During the peak of the transmission season, July and August, we used exposure tests with mice to assess the infectivity of water in the 6 intervention villages [14]. Sentinel mice were exposed for 2 hours every day for 3 consecutive days. After 30 to 35 days, we sacrificed the mice and checked for adult worms of S. japonicum in their mesenteric veins.
Statistical analysis
All statistical analyses were performed with the use of Statistics Analysis System, version 9.1 (SAS Institute Inc., Cary, NC, USA). Confidence intervals (CIs) were calculated using standard formulae based on the binomial distribution (annual infection rate of humans). Chi-square test or Fisher exact probability test were used to examine the differences of proportions. A generalized linear model (GLM) with a logit link and a binomial error distribution was used to analyze the risk of S. japonicum infection in humans. Generalized estimating equations of parameters with an unstructured variance-covariance matrix were used to account for repeated measures on individuals during the study period. The SAS proc GENMOD was used to estimate the parameters. Two-sided P-values were calculated for all tests and P-values <0.05 were considered statistically significant.
Results
Table 1 showed the characteristics of the participants in 2008. The differences of age between intervention and control groups were statistically significant (P<0.001).
Table 1. Characteristics of the participants in intervention and control groups in 2008.
| Characteristics | Control group (n = 5323) | Intervention group (n = 5050) |
| Age, years | 38.4 (13.6) | 39.4 (13.4)** |
| Sex ratio (F/M), n | 2686/2637 | 2617/2433 |
Values are mean (SD) or number.
P<0.001 for control group vs. intervention group.
Figure 1 showed the flow diagram of the study design. In total, 10,373 participants were recruited for the study in 2008. The rates of loss to follow-up per year (2009–2011) for each village ranged from 1.1% to 18.5% in the intervention group and 2.1% to 25.2% in the control group (Table 2). The main reason for loss to follow-up was that the participants left the villages to seek work in urban areas.
Table 2. Schistosoma japonicum infection in humans in intervention and control groups*.
| Village | 2008 | 2009 | 2010 | 2011 | ||||||||
| N | Infection No. | Prevalence % (CI) | N | Infection No. | Prevalence % (CI) | N | Infection No. | Prevalence % (CI) | N | Infection No. | Prevalence % (CI) | |
| Intervention group | ||||||||||||
| E Jinghu | 535 | 19 | 3.55 (1.98, 5.12) | 508 | 16 | 3.15 (1.63, 4.67) | 452 | 10 | 2.21 (0.85, 3.57) | 436 | 5 | 1.15 (0.14, 2.15) |
| Gu Shengsi | 1102 | 38 | 3.45 (2.37, 4.53) | 1054 | 32 | 3.04 (2.00, 4.07) | 997 | 19 | 1.91 (1.06, 2.76) | 959 | 8 | 0.83 (0.26, 1.41) |
| Jianhong | 765 | 23 | 3.01 (1.79, 4.22) | 718 | 17 | 2.37 (1.25, 3.48) | 685 | 12 | 1.75 (0.77, 2.74) | 637 | 4 | 0.63 (0.01, 1.24) |
| Lianmeng | 994 | 38 | 3.82 (2.63, 5.02) | 964 | 31 | 3.22 (2.10, 4.33) | 892 | 19 | 2.13 (1.18, 3.08) | 885 | 8 | 0.90 (0.28, 1.53) |
| Tongqiao | 1021 | 33 | 3.23 (2.15, 4.32) | 1001 | 24 | 2.40 (1.45, 3.35) | 990 | 21 | 2.12 (1.22, 3.02) | 963 | 8 | 0.83 (0.26, 1.41) |
| Tuanjie | 633 | 21 | 3.32 (1.92, 4.72) | 626 | 18 | 2.88 (1.56, 4.19) | 611 | 12 | 1.96 (0.86, 3.07) | 587 | 3 | 0.51 (−0.07, 1.09) |
| Total § | 5050 | 172 | 3.41 (2.91 ,3.91) | 4871 | 138 | 2.83 (2.37 ,3.30) | 4627 | 93 | 2.01 (1.61 ,2.41) | 4467 | 36 | 0.81 (0.54 ,1.07) |
| Control group | ||||||||||||
| Guoqing | 788 | 26 | 3.30 (2.05, 4.55) | 755 | 21 | 2.78 (1.61, 3.96) | 737 | 22 | 2.99 (1.75, 4.22) | 716 | 16 | 2.23 (1.15, 3.32) |
| Nanyang | 1035 | 37 | 3.57 (2.44, 4.71) | 967 | 30 | 3.10 (2.01, 4.20) | 921 | 26 | 2.82 (1.75, 3.89) | 914 | 23 | 2.52 (1.50, 3.53) |
| Qingyun | 894 | 26 | 2.91 (1.80, 4.01) | 812 | 23 | 2.83 (1.69, 3.98) | 750 | 21 | 2.80 (1.62, 3.98) | 748 | 20 | 2.67 (1.52, 3.83) |
| Qingji | 963 | 32 | 3.32 (2.19, 4.46) | 889 | 28 | 3.15 (2.00, 4.30) | 822 | 25 | 3.04 (1.86, 4.22) | 818 | 23 | 2.81 (1.68, 3.95) |
| Zhu Jiahu | 712 | 22 | 3.09 (1.82, 4.36) | 697 | 17 | 2.44 (1.29, 3.59) | 683 | 16 | 2.34 (1.21, 3.48) | 595 | 16 | 2.69 (1.39, 3.99) |
| Tongsheng | 931 | 27 | 2.90 (1.82, 3.98) | 807 | 22 | 2.73 (1.60, 3.85) | 727 | 18 | 2.48 (1.34, 3.61) | 696 | 16 | 2.30 (1.18, 3.41) |
| Total # | 5323 | 170 | 3.19 (2.72, 3.67) | 4927 | 141 | 2.86 (2.40 ,3.33) | 4640 | 128 | 2.76 (2.29 ,3.23) | 4487 | 114 | 2.54 (2.08 ,3.00) |
CI denote 95% confidence intervals.
As compared with 2008 in the intervention group, there was a statistically significant change in the rate of infection in 2010 and 2011 (P<0.001), and there was no statistically significant change in 2009 (P = 0.101).
As compared with 2008 in the control group, there was no statistically significant change in the rate of infection from 2009 to 2011 (P = 0.328, P = 0.204, P = 0.055, respectively).
Infection in humans
The prevalence of S. japonicum in humans was 3.41%, 2.83%, 2.01%, and 0.81% from 2008 to 2011 in the intervention villages (comparing with 2008, P = 0.101, P<0.001 and P<0.001, respectively, Table 2). In the control villages, there was no significant statistically decline in the rate of infection from 2008 to 2011 (P>0.05 for all comparisons).
The generalized linear model (Table 3), yielding odds ratios (OR) adjusted for participants' age and gender, showed a higher infection risk in humans in the control villages than the intervention villages (OR = 1.250, P = 0.001); and an overall significant downward trend in infection risk during the study period.
Table 3. Analyzing the risk of Schistosoma japonicum infection in humans by generalized linear model*.
| Variables | OR (95% CI) | P-value |
| Intercept | 0.012 (0.009, 0.016) | <0.001 |
| Age | 1.004 (0.999, 1.009) | 0.091 |
| Gender | ||
| Female | 1.047 (0.919, 1.193) | 0.492 |
| Male | 1.000 | - |
| Study year | ||
| 2008 | 2.026 (1.670, 2.460) | <0.001 |
| 2009 | 1.734 (1.417, 2.123) | <0.001 |
| 2010 | 1.436 (1.166, 1.768) | <0.001 |
| 2011 | 1.000 | - |
| Group | ||
| Control group | 1.250 (1.095, 1.427) | 0.001 |
| Intervention group | 1.000 | - |
The model includes participants' age, gender, study year, group.
Infection in bovines
The S. japonicum infection rates in bovines were 3.3%, 2.9%, 1.3%, and 0.0% from 2008 to 2011 in the intervention villages (comparing with 2008, P = 0.820, P = 0.111 and P<0.001, respectively, Table 4). In contrast, there was no significant decline in the rate of infection during the 4-year period in the control villages (P>0.05 for all comparisons).
Table 4. Schistosoma japonicum infection in bovines in intervention and control groups*.
| Year | Intervention group | Control group | |||||
| N | Infection No. (%) | N | Infection No. (%) | P-valuea | P-valueb | P-valuec | |
| 2008 | 305 | 10 (3.3) | 345 | 11 (3.2) | - | - | 1.000 |
| 2009 | 309 | 9 (2.9) | 337 | 9 (2.7) | 0.820 | 0.821 | 1.000 |
| 2010 | 311 | 4 (1.3) | 325 | 8 (2.5) | 0.111 | 0.646 | 0.385 |
| 2011 | 318 | 0 (0.0) | 313 | 8 (2.6) | <0.001 | 0.650 | 0.004 |
Statistical method was the Fisher exactly probability test.
Compared with 2008 in the intervention group.
Compared with 2008 in the control group.
Compared between the intervention and control groups from 2008 to 2011.
Infection in snails
From 2008 to 2011, 11 of 6,219, 9 of 5,975, none of 25,010, and none of 15,490 snails were infected with S. japonicum in the intervention villages (comparing with 2008, P = 0.824, P<0.001 and P<0.001, respectively, Table 5). In the control villages, there was no significant statistically decline in the rate of infection from 2008 to 2011 (P>0.05 for all comparisons).
Table 5. Schistosoma japonicum infection in snails in intervention and control groups*.
| Year | Intervention group | Control group | ||||||
| N | Infection No. | N | Infection No. | P-valuea | P-valueb | P-valuec | ||
| 2008 | 6,219 | 11 | 4,574 | 9 | - | - | 0.824 | |
| 2009 | 5,975 | 9 | 8,664 | 6 | 0.824 | 0.054 | 0.187 | |
| 2010 | 25,010 | 0 | 11,889 | 13 | <0.001 | 0.231 | <0.001 | |
| 2011 | 15,490 | 0 | 9,493 | 8 | <0.001 | 0.116 | <0.001 | |
Statistical method was the Fisher exactly probability test.
Compared with 2008 in the intervention group.
Compared with 2008 in the control group.
Compared between the intervention and control groups from 2008 to 2011.
Infection in cow dung
In the intervention villages in 2008, 21 of 533 (3.9%) of the cow dung samples contained S. japonicum eggs. After the implementation of the new control strategy, 15 of 483 (3.1%), 5 of 476 (1.1%) and none of 356 cow dung were infected in 2009, 2010 and 2011, respectively (comparing with 2008, P = 0.501, P = 0.005 and P<0.001, respectively).
Infectivity of water
In 2008, before implementation of the new control strategy, 19 of 60 (31.7%) mice were infected with S. japonicum. In 2009, 2010 and 2011, 13 of 60 mice (21.7%), 7 of 120 (5.8%) and 2 of 120 (1.7%) mice were infected, respectively (comparing with 2008, P = 0.216, P<0.001 and P<0.001, respectively).
Adverse events
No serious adverse events were reported in the study.
Discussion
Over the past few years, investigations showed that more than 80% of S. japonicum patients in China were found in the lake and marshland areas of Hunan, Hubei, Jiangxi, Anhui, and Jiangsu provinces [22]. Therefore, schistosomiasis control in these regions was especially important.
Because of high rates of S. japonicum reinfection in both humans and bovines in marshland and lake regions [21], [23], [24], it was very difficult to reduce the rate of S. japonicum infection in humans to a relatively low level (such as less than 1%). The study of Wang et al. showed that a comprehensive control strategy, which was based on interventions to reduce the transmission of S. japonicum infection from cattle and humans to snails, can solve this problem [14]. However, the authors mentioned that their study included only a small number of villages and the villages were not selected in a random manner. So they were not sure whether the strategy was still highly effective in other endemic areas. A similar study was implemented in Jiangsu Province of China, but this study did not have parallel control groups [25]. In our study, we applied a similar strategy but strived to avoid these limitations. We adopted a random manner to select 12 villages in Gong'an County, Hubei Province and randomly assigned them to intervention or control groups.
Our study proved that the new comprehensive strategy to control transmission of S. japonicum was highly effective in the study areas. Firstly, the prevalence of schistosomiasis in humans declined to a relatively low level after implementing the new interventions. Compared with 2008, the rate of infection in humans in the intervention villages decreased from 3.41% in 2008 to 0.81% in 2011. The generalized linear model showed that a higher infection risk for humans in the control villages than in the intervention villages (OR = 1.250, P = 0.001). Secondly, comparing with 2008, the rate of infection in bovines, snails and cow dung in the intervention group decreased from 3.3% to none, 11 of 6,219 to none, and 3.9% to none in 2011, respectively. Thirdly, during the peak transmission period, after the implementation of the new control strategy, the rate of infection in sentinel mice decreased from 31.7% in 2008 to 1.7% in 2011. It should be noted that the randomized, controlled design pertains only to the human, bovine, and snail data while the environmental cow dung and infectious water data were only collected in the intervention villages.
Bovines are the main infection source of S. japonicum in the marshlands and lakes in China, and are responsible for an estimated 75%–90% of the egg contamination [20], [21]. Therefore, it was very important to reduce the role of bovines as an infection source. Recently, the main measures included providing farmers machines instead of bovines and prohibiting bovines in these regions. These measures were effective [14]. However, if these measures were used in some rural areas of Hubei Province, there would be some challenges. Firstly, the machines are not suitable due to the limitation of landform or farmers' knowledge. Secondly, the bovines can bring additional economic benefits for villagers, so the villagers were reluctant to give up cattle rearing.
For these reasons, in our study we adopted new interventions for bovines which included building safe pastures for grazing and preventing bovines from grazing on snail habitat beach areas. Our study proved that these measures were effective. Moreover, these measures were sustainable because the cost of fences and pastures was a one-time expense because the material is durable. We used a triangular cement fence post, which requires less cement than a square shape.
Our study showed that there was no statistically significant decline in the S. japonicum infection prevalence in humans, bovines and snails from 2008 to 2011 in the control villages. Past studies showed that the effect of chemotherapy with praziquantel for humans and cattle was limited in controlling schistosomiasis in lake and marshland regions [17]–[19], because it does not prevent reinfection [23]–[25]. In contrast, the integrated control strategy can reduce the transmission of S. japonicum infection from humans and cattle to snails, reducing contaminated water as a source of infection in humans [14]. However, this difference is of little practical consequence unless the integrated interventions lead to more sustainable control and a reduction of the basic reproductive number (R0). If R0 can be kept less than 1 over a length of time, the disease could be gradually eliminated [26]. This is probably the most important potential outcome of the intervention. However, a longer term follow-up study is needed to test this potential outcome. Taken together, we considered that the new interventions, especially the interventions for bovines and humans, were the most important components of the integrated control strategy.
A potential limitation of the study was that we did not investigate the status of S. japonicum infection in people who quit the study. Thus, we could not compare the difference of these people's infection between intervention and control groups.
Conclusions
An integrated control strategy, aiming to weaken the roles of bovines and humans as sources of S. japonicum infection, was effective in controlling the transmission of S. japonicum in marshland regions of China.
Supporting Information
CONSORT Checklist.
(DOC)
Trial protocol (Chinese language file).
(DOC)
Acknowledgments
We are very grateful to all staff at the Gong'an County Institute for Schistosomiasis Control and the villagers who participated in this study.
Funding Statement
This study was funded by the National Important Sci-tech Projects in China (2009ZX10004-906, 2012ZX10004-909); the National Science and Technology Support Program in China (2009BAI78B03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Trop 77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou XN (2008) Schistosomiasis progress and prospects in gobal: The schistosomiasis report of World Health Organization Scientific Working Group [in Chinese]. Beijing: People's Health Publishing House. 1p.
- 3. Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, et al. (2010) Schistosomiasis japonica control and research needs. Adv Parasitol 72: 145–178. [DOI] [PubMed] [Google Scholar]
- 4. Mao SB (1986) Recent progress in the control of schistosomiasis in China. Chin Med J (Engl) 99: 439–443. [PubMed] [Google Scholar]
- 5. Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, et al. (2005) The public health significance and control of schistosomiasis in China–then and now. Acta Trop 96: 97–105. [DOI] [PubMed] [Google Scholar]
- 6. McManus DP, Gray DJ, Ross AG, Williams GM, He HB, et al. (2011) Schistosomiasis research in the Dongting Lake region and its impact on local and national treatment and control in China. PLoS Negl Trop Dis 5: e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, et al. (2007) Epidemiology of schistosomiasis in the People's Republic of China, 2004. Emerg Infect Dis 13: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou XN, Wang TP, Wang LY, Guo JG, Yu Q, et al. (2004) The current status of schistosomiasis epidemics in China [in Chinese]. Chin J Epidemiol 25: 555–558. [PubMed] [Google Scholar]
- 9. Zhou XN, Jiang QW, Wu XH, Zhao GM, Lin DD, et al. (2007) The function and evolution of the criteria for control and elimination of schistosomiasis in China [in Chinese]. Chin J Schisto Control 19: 1–4. [Google Scholar]
- 10. Hao Y, Zheng H, Zhu R, Guo JG, Wang LY, et al. (2010) Schistosomiasisi situation in People's Republic of China in 2009 [in Chinese]. Chin J Schisto Control 22: 521–527. [Google Scholar]
- 11. Lin DD, Wu HW, Wu GL, Zhou XN (2007) Review and evaluation on optimal combined strategies for schistosomasis control in China [in Chinese]. Chin J Schisto Control 19: 234–236. [Google Scholar]
- 12. Spear RC, Seto E, Remais J, Carlton EJ, Davis G, et al. (2006) Fighting waterborne infectious diseases. Science 314: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 13. Zhou XN, Yang GJ, Yang K, Wang XH, Hong QB, et al. (2008) Potential impact of climate change on schistosomiasis transmission in China. Am J Trop Med Hyg 78: 188–194. [PubMed] [Google Scholar]
- 14. Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, et al. (2009) A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med 360: 121–128. [DOI] [PubMed] [Google Scholar]
- 15.Chinese Ministry of Health (2000) Handbook of schistosomiasis control. 3rd ed [in Chinese]. Shanghai: Shanghai Scientific and Technological Literature Publishing House. pp. 36–41, 72–80, 187, 233.
- 16. Tu ZW, Huang XB, Cai SX, Fan HP, Liu JB, et al. (2010) Evaluation of the endemic situation of schistosomiasis control in Hubei Province (2009) [in Chinese]. J of Pub Health and Prev Med 21: 14–17. [Google Scholar]
- 17. Xianyi C (2002) The challenges and strategies in schistosomiasis control program in China. Acta Trop 82: 279–282. [DOI] [PubMed] [Google Scholar]
- 18. Fenwick A, Rollinson D, Southgate V (2006) Implementation of human schistosomiasis control: Challenges and prospects. Adv Parasitol 61: 567–622. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Utzinger J, Zhou XN (2008) Schistosomiasis control: experiences and lessons from China. Lancet 372: 1793–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gray DJ, Williams GM, Li Y, Chen H, Forsyth SJ, et al. (2009) A cluster-randomised intervention trial against Schistosoma japonicum in the Peoples' Republic of China: bovine and human transmission. PLoS One 4: e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo J, Li Y, Gray D, Ning A, Hu G, et al. (2006) A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People's Republic of China. Am J Trop Med Hyg 74: 335–341. [PubMed] [Google Scholar]
- 22.Wang LD (2006) The epidemic status of schistosomiasis in China: results from the third nationwide sampling survey in 2004 [in Chinese]. Shanghai: Shanghai Scientific and Technological Literature Publishing House. 36p.
- 23. Engels D, Wang LY, Palmer KL (2005) Control of schistosomiasis in China. Acta Trop 96: 67–68. [DOI] [PubMed] [Google Scholar]
- 24. Balen J, Zhao ZY, Williams GM, McManus DP, Raso G, et al. (2007) Prevalence, intensity and associated morbidity of Schistosoma japonicum infection in the Dongting Lake region, China. Bull World Health Organ 85: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun LP, Wang W, Liang YS, Tian ZX, Hong QB, et al. (2011) Effect of an integrated control strategy for schistosomiasis japonica in the lower reaches of the Yangtze River, China: an evaluation from 2005 to 2008. Parasit Vectors 4: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma ZE, Zhou YC, Wang WD et al.. (2004) Mathematical model of the dynamics of infectious diseases [in Chinese]. Beijing: Science Press. 12p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Checklist.
(DOC)
Trial protocol (Chinese language file).
(DOC)