Antibodies to the variant surface glycoprotein (VSG) are required for the control of African trypanosomes infecting the blood, whereas infections of the skin by low numbers of trypanosomes are controlled by innate resistance and do not require antibodies for their control. Low numbers of trypanosomes infecting the skin, although being killed by innate resistance, do not induce protection, but enhance susceptibility to re-infections due to suppression of innate resistance by adaptive immune responses.
We propose to pursue a vaccine strategy that overcomes the induction of immunosuppression but induces a Th1 imprint for protective immunity. We suggest that intradermal immunization with an optimally low dose of antigens of the whole parasite is necessary but not sufficient. We suggest that the immunization has to be accompanied by a treatment that inhibits the arginase pathway of antigen-presenting cells (APCs), but modestly enhances their inducible nitric oxide synthase (iNOS) pathway to induce Th1 memory cells specific for crucial common antigens, which enhance innate resistance.
Introduction
African trypanosomes are extracellular hemoprotozoa that cause disease in humans and livestock. Trypanosoma brucei gambiense and T. b. rhodesiense cause sleeping sickness in humans, also called human African trypanosomiasis (HAT), an emerging disease in East and Central Africa [1], [2]. Infections with T. congolense, T. vivax, or T. b. brucei cause disease in livestock [1]. Various species of tsetse flies (Glossina spp.) can harbor African trypanosomes and act as their intermediate hosts. Humans and animals become infected with trypanosomes by bites of infected tsetse flies. A temporary local inflammation, the so-called chancre, develops in the skin at the site of the bite [1]. The trypanosomes move from the skin into the blood via the lymph system (Figure 1).
Figure 1. Mode of natural infections by African trypanosomes.
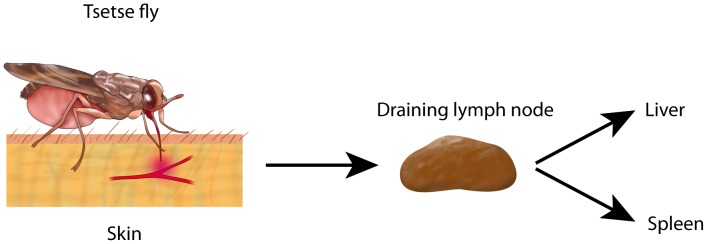
Infected tsetse flies bite the host by inserting the proboscis into the skin, inject saliva into the site, and puncture a small blood vessel, resulting in a small hemorrhage. The tsetse fly depicted here is sucking blood from the hemorrhage. During this process, trypanosomes are deposited into the skin. Trypanosomes enter the lymph system and then reach the draining lymph node and the bloodstream. Trypanosomes will circulate in the bloodstream. Whole trypanosomes or fractions thereof end up in macrophages of liver and spleen by antibody- and/or complement-mediated phagocytosis.
Mice are susceptible to infections by all African trypanosomes pathogenic for humans or livestock. Thus, infection of mice is a relevant model to study the immunobiology of infections by African trypanosomes.
Primary intradermal infections by low numbers of parasites in the skin are controlled by innate resistance mediated by induced nitric oxide (iNO) [3]. At this stage, adaptive immune responses are not protective but are immunosuppressive [3] (discussed below). At the blood stage of infection, antibodies are absolutely required for the control of parasitemia [4]–[6]. Antibodies to the VSG control parasitemia by mediating phagocytosis of the trypanosomes by macrophages of the liver and spleen.
Why Are There No Effective Vaccines?
African trypanosomes have developed a highly sophisticated and complex system of antigenic variation [7]. In the mammalian host, the whole parasite is covered with a coat of about 107 identical molecules of a glycoprotein, the VSG, which is anchored into the cell membrane via a glycolipid, glycosylphosphatidylinositol (GPI) [8], [9]. There is a widely held belief that the almost unlimited capacity for antigenic variation of the surface glycoproteins by the African trypanosomes is the major hurdle for producing a vaccine [5], [10]. In view of our recent experimental results on intradermal infections with low numbers of trypanosomes [3], [11], we do not share this belief.
Past research into the immunobiology of African trypanosomiasis has mostly been based on the immune responses of mice infected intraperitoneally, a route of infection that leads to development of parasitemia [3]–[5], [12]–[14]. Although these studies have provided great insight into the host–parasite relationship, they have neglected to investigate the very early immunological events triggered by the infecting parasites. Thus, we have developed a model for intradermal infections of mice, performed by syringe and needle [3], [11], [15].
Intraperitoneal infections of mice with either T. brucei or T. congolense lead to infections of the blood and definitely require antibodies to VSG for the control of parasitemia [4]–[6]. Mice are about 100-fold more susceptible to this route of infection than to intradermal infection [3]. Intradermal infections by low numbers (100–500) of African trypanosomes are controlled by innate resistance involving iNO and TNF-α, but require neither antibodies nor T cells for protection [3]. Relevant to these results, it was found that the average man required a minimal dose of 300–450 metacyclic T. b. rhodesiense to be infected by the bite of a tsetse fly [16]. Primary intradermal infections are better controlled in CD1d−/− or MHC class II−/− mice, indicating that the innate resistance to low numbers of trypanosomes in primary intradermal infections is suppressed by CD1d-restricted natural killer T cells and MHC class II–restricted T cells, of which the CD1d-restricted natural killer T cells appear to have the most suppressive effect [3].
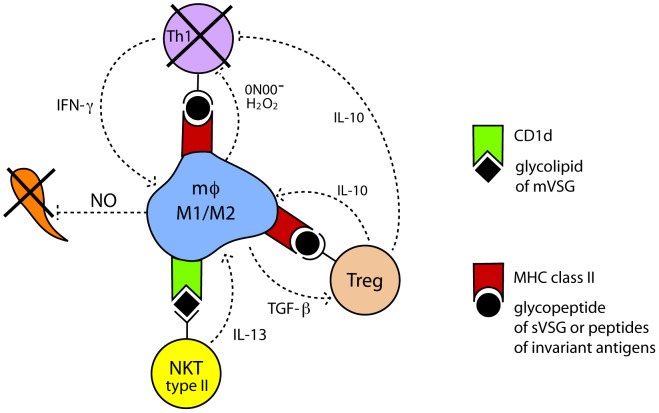
CD1d is an MHC class I–like molecule that presents glycolipid antigens, such as trypanosomal GPI, to a subset of T cells called natural killer T cells (NKT cells) [9], [17]. There are two subpopulations of NKT cells that vary in the programming of the T cell receptor (TCR): invariant NKT cells (iNKT), type I, and variant NKT cells, type II. Both types of NKT cells recognize, with their TCR, lipids presented by CD1d expressed on the surface of APCs [18]. Type I NKT cells, upon interacting with APCs, predominantly produce IFN-γ and activate the iNOS pathway in the APCs, whereas type II NKT cells produce IL-13 and activate the arginase 1 (Arg1) pathway in APCs [18]. We suspect that the type II NKT cells are predominantly mediating the immunosuppression at intradermal trypanosomal infections (Figure 2).
Figure 2. Minimal model: immunosuppression at primary intradermal infections by low numbers of trypanosomes.
Macrophages that have engulfed filopodia of trypanosomes [33] or whole killed trypanosomes will process trypanosome antigens and present them at their cell surface. Glycosylphosphatidylinositol (GPI) of membrane variant surface glycoprotein (mVSG) will be presented via CD1d to NKT cells [9], [17]. We argue that the NKT cells are predominantly type II NKT cells that release IL-13 which, in turn, skews the macrophages toward the M2 type. Thus, the antigen-presenting macrophages will predominantly be a mixed M1/M2 type (see text). MHC class II will present peptides to MHC class II–restricted T cells. The microenvironment will skew the naïve MHC class II–restricted T cells towards Tregs [3], [15], presumably via TGF-β produced by macrophages. Tregs, in turn, activate the Arg1 pathway of macrophages by production of IL-10. We propose that many of the naïve trypanosome-specific T cells that develop into Th1 effector cells are deleted by apoptosis, due to peroxynitrite (ONOO-) produced by macrophages under conditions of shortage of L-arginine supply [25], or are functionally impaired by down-regulation of CD3zeta [34].
Primary intradermal infections by 100–500 African trypanosomes that, in fact, are killed by innate resistance, not only fail to generate a long-term protective immunological state, but result in enhanced susceptibility to intradermal challenges [3]. Trypanosome-specific cells of draining lymph node and spleen are primed as early as 24 h after intradermal infection [3]. Surprisingly, intradermal injection of mice with a lysate (trypanosomes killed by sonication) of 102 T. brucei, strain Whatat 1.1, does not provide protection but makes such mice more susceptible to an intradermal challenge with 102 T. brucei strain 10–26 [3]. The enhanced susceptibility is unrelated to antigenic variation. We also found enhanced susceptibility in mice immunized into the back skin with a cloned and purified peptide of a T. congolense protein and challenged by infecting the foot pad [11]. Effector and memory lymphocytes preferentially home to non-lymphoid tissues such as skin [19], [20]. We suggest that intradermal infections with low numbers of trypanosomes or injections with mechanically killed trypanosomes prime the adaptive immune system to suppress protective immunity to an intradermal challenge.
All previous attempts to produce vaccines against African trypanosomes were only partially successful or failed entirely. A comprehensive review on previous vaccination attempts has been published recently [21].
We propose that in any attempt to produce an effective vaccine, it will be crucial to address the problem of induction of immunosuppression by the trypanosomes injected into the skin by infected tsetse flies.
Immunosuppression in Humans and Animals Infected by African Trypanosomes
Immunosuppression to Heterologous Antigens
Humans, cattle, and mice infected by African trypanosomes show lower immune responses to vaccines against various bacterial and viral diseases. In mice or cattle infected with T. brucei or T. congolense, there is reduced proliferation of T cells in response to stimulation by T cell mitogens, such as ConA or PHA, and a reduced antibody response to sheep red blood cells (SRBC) following immunization with SRBC [5], [12], [13], [22].
Immunosuppression to Trypanosomal Antigens
Sacks and Askonas [6] infected mice with T. brucei and tested the anti-VSG antibodies to the different variants after each of three waves of parasitemia. As the infections progressed, IgM and IgG anti-VSG antibody responses declined. IgG antibodies declined more rapidly. After the third parasitemia, only low levels of IgM anti-VSG antibodies were detectable.
Mechanisms of Immunosuppression
Roelants and Pinder [5] carried out an extensive review and concluded both suppressor macrophages and suppressor T cells are involved in the immunosuppression in mice infected with T. brucei or T. congolense. Askonas' lab has convincingly shown that macrophages become immunosuppressive after antibody-mediated phagocytosis of T. brucei [12].
Nitric oxide (NO) produced by macrophages is a mediator of immunosuppression in T. brucei infection of mice [13], [14], [23]. NO is a major mediator of immunosuppression only during the early phase of infection of the blood [13]. It is the stimulation of such macrophages by IFN-γ that, in synergy with TNF-α, induces the synthesis of high amounts of NO [13], [23].
M1 versus M2 Macrophages
The diverse biological activity of macrophages is mediated by phenotypically distinct subpopulations of cells that develop in response to inflammatory mediators in their microenvironment. Two major populations have been characterized: classically activated M1 macrophages and alternatively activated M2 macrophages [24]. The M1 type develops upon activation by IFN-α/β, IFN-γ, and/or TNF-α. The M2 type develops after activation by IL-10, IL-4, and/or IL-13 [24]. Activation of the inducible nitric oxide synthase (iNOS or NOS2) has been regarded as one of the most specific markers for M1 macrophages and activation of Arg1, the most specific marker of M2 macrophages [24], [25]. Both types of macrophages have been associated with immunosuppression. The L-arginine metabolism in macrophages controls T lymphocyte function [25]. Both the arginase pathway and the iNOS pathway use L-arginine as their substrate. Both pathways compete for the available L-arginine and cross-regulate each other [25]. Despite the distinct expression of iNOS and Arg1 in M1 and M2 macrophages, respectively, some macrophages have been shown to express both iNOS and Arg1 [24]. Thus, macrophages of mixed characteristic do exist.
BALB/c mice are more susceptible to T. congolense and T. brucei than relatively resistant C57BL/6 mice. In mice intraperitoneally infected with T. brucei, arginase mRNA is expressed higher in peritoneal macrophages of infected BALB/c than in those of infected C57BL/6 mice. In co-cultivation with macrophages, T. brucei directly induces increased Arg1 and Arg2 mRNA levels in macrophages as well as increases macrophage arginase activity [26]. From 2 days on after infection, arginase activity is increasingly up-regulated in peritoneal macrophages of Swiss mice subcutaneously infected with T. brucei. Under the same conditions, increasing iNOS activity is delayed by a couple of days [27].
Immunity to infections is mediated by memory T cells and B cells, which are generated from naïve precursor cells after exposure to the microbial antigens. Upon interaction of naïve T cells with the APC, naïve T cells rapidly proliferate and differentiate into effector T cells. This phase of proliferation lasts about 1 week and is followed by a contraction phase of about 14 days during which about 90% of the effector T cells die, whereas the remaining cells differentiate into memory T cells [28].
Th1 cells mediate resistance to African trypanosomes [22]. In natural infections, the tsetse fly injects the trypanosomes together with fly saliva. Initial injections of tsetse fly saliva induce Th2 responses [29]. The tsetse fly saliva will likely alter the microenvironment of the injection site of the skin, skewing APCs toward activating the Arg1 pathway by IL-4 [24] and thus, like the suppressor T cells, interfere with the innate resistance. We conclude that, in African trypanosomiasis, there is a lack of differentiation of trypanosome-specific Th1 cells into Th1 memory cells specific for variant and common parasite antigens.
We contend that, at the intradermal stage of infection, the immunosuppression is predominantly controlled by a mixed M1/M2 macrophage environment and by suppressor T cells [3], [24], [25] (Figure 2). Although tsetse saliva plays a role in the pathogenesis [29], the effect of saliva has to be bypassed in any vaccine strategy, as has been achieved in the highly successful vaccine against mosquito-transmitted yellow fever [30]. We propose that inhibiting the arginase pathway [27] and adequately supplying L-arginine [25], combined with intradermal immunization with low numbers of trypanosomes, will ameliorate or abolish the immunosuppressive environment, lead to induction of a trypanosome-specific Th1 imprint and, in turn, enhance innate resistance.
Our proposal to use a vaccination procedure that enhances Th1 cell differentiation appears to run counter to the observation that Th1 cell/IFN-γ-induced NO mediates profound immunopathology and immunosuppression in African trypanosomiasis [31]. NO, however, is a double-edged sword. Our reasoning is based on the observation that high concentrations of NO are immunosuppressive, whereas low concentrations of NO enhance Th1 cell differentiation [32].
Acknowledgments
We thank Juliane Deubner for drawing the figures shown in the manuscript.
Funding Statement
No specific funding was received for this work.
References
- 1.Mulligan HW, Potts WH, editors (1970) The African trypanosomiases. New York: Wiley-INTERSCIENCES.
- 2. Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, Jannin JG (2011) The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: the way forward. PLoS Negl Trop Dis 5: e1007 doi:10.1371/journal.pntd.0001007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei G, Bull H, Zhou X, Tabel H (2011) Intradermal infections of mice by low numbers of african trypanosomes are controlled by innate resistance but enhance susceptibility to reinfection. J Infect Dis 203: 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mansfield JM, Paulnock DM (2005) Regulation of innate and acquired immunity in African trypanosomiasis. Parasite Immunol 27: 361–371. [DOI] [PubMed] [Google Scholar]
- 5. Roelants GE, Pinder M (1984) Immunobiology of African trypanosomiasis. Contemp Top Immunobiol 12: 225–274. [DOI] [PubMed] [Google Scholar]
- 6. Sacks DL, Askonas BA (1980) Trypanosome-induced suppression of anti-parasite responses during experimental African trypanosomiasis. Eur J Immunol 10: 971–974. [DOI] [PubMed] [Google Scholar]
- 7. Barry JD, McCulloch R (2001) Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv Parasitol 49: 1–70. [DOI] [PubMed] [Google Scholar]
- 8. Ferguson MA (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci 112: 2799–2809. [DOI] [PubMed] [Google Scholar]
- 9. Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, et al. (1999) CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283: 225–229. [DOI] [PubMed] [Google Scholar]
- 10. Brun R, Blum J, Chappuis F, Burri C (2010) Human African trypanosomiasis. Lancet 375: 148–159. [DOI] [PubMed] [Google Scholar]
- 11. Marcoux V, Wei G, Tabel H, Bull HJ (2010) Characterization of major surface protease homologues of Trypanosoma congolense. J Biomed Biotechnol 2010: 418157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Askonas BA (1985) Macrophages as mediators of immunosuppression in murine African trypanosomiasis. Curr Top Microbiol Immunol 117: 119–127. [DOI] [PubMed] [Google Scholar]
- 13. Beschin A, Brys L, Magez S, Radwanska M, De Baetselier P (1998) Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. J Leukoc Biol 63: 429–439. [DOI] [PubMed] [Google Scholar]
- 14. Sternberg J, McGuigan F (1992) Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur J Immunol 22: 2741–2744. [DOI] [PubMed] [Google Scholar]
- 15. Wei G, Tabel H (2008) Regulatory T cells prevent control of experimental African trypanosomiasis. J Immunol 180: 2514–2521. [DOI] [PubMed] [Google Scholar]
- 16. Fairbairn H, Burtt E (1946) The infectivity to man of a strain of Trypanosoma rhodesiense transmitted cyclically by Glossina morsitans through sheep and antelope: Evidence that man requires a minimum infective dose of metacyclic trypanosomes. Annals of Tropical and Medical Parasitology 40: 270–313. [DOI] [PubMed] [Google Scholar]
- 17. Godfrey DI, Kronenberg M (2004) Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest 114: 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berzofsky JA, Terabe M (2008) A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol Immunother 57: 1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin YT, Wang CT, Chao PS, Lee JH, Wang LC, et al. (2011) Skin-homing CD4+ Foxp3+ T cells exert Th2-like function after staphylococcal superantigen stimulation in atopic dermatitis patients. Clin Exp Allergy 41: 516–525. [DOI] [PubMed] [Google Scholar]
- 20. Mackay CR (1993) Homing of naive, memory and effector lymphocytes. Curr Opin Immunol 5: 423–427. [DOI] [PubMed] [Google Scholar]
- 21. Magez S, Caljon G, Tran T, Stijlemans B, Radwanska M (2010) Current status of vaccination against African trypanosomiasis. Parasitology 137: 2017–2027. [DOI] [PubMed] [Google Scholar]
- 22. Hertz CJ, Filutowicz H, Mansfield JM (1998) Resistance to the African trypanosomes is IFN-gamma dependent. J Immunol 161: 6775–6783. [PubMed] [Google Scholar]
- 23. Schleifer KW, Mansfield JM (1993) Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol 151: 5492–5503. [PubMed] [Google Scholar]
- 24. Sica A, Bronte V (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 117: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5: 641–654. [DOI] [PubMed] [Google Scholar]
- 26. Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, et al. (2004) Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect. J Immunol 172: 6298–6303. [DOI] [PubMed] [Google Scholar]
- 27. Gobert AP, Daulouede S, Lepoivre M, Boucher JL, Bouteille B, et al. (2000) L-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect Immun 68: 4653–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pepper M, Jenkins MK (2011) Origins of CD4(+) effector and central memory T cells. Nat Immunol 12: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caljon G, Van Den Abbeele J, Sternberg JM, Coosemans M, De Baetselier P, et al. (2006) Tsetse fly saliva biases the immune response to Th2 and induces anti-vector antibodies that are a useful tool for exposure assessment. Int J Parasitol 36: 1025–1035. [DOI] [PubMed] [Google Scholar]
- 30. Norrby E (2007) Yellow fever and Max Theiler: the only Nobel Prize for a virus vaccine. J Exp Med 204: 2779–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tabel H, Wei G, Shi M (2008) T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol Rev 225: 128–139. [DOI] [PubMed] [Google Scholar]
- 32. Niedbala W, Wei XQ, Campbell C, Thomson D, Komai-Koma M, et al. (2002) Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor beta 2 expression via cGMP. Proc Natl Acad Sci U S A 99: 16186–16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shakibaei M, Frevert U (1992) Cell surface interactions between Trypanosoma congolense and macrophages during phagocytosis in vitro. J Protozool 39: 224–235. [DOI] [PubMed] [Google Scholar]
- 34. Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, et al. (2004) L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol 232: 21–31. [DOI] [PubMed] [Google Scholar]