Abstract
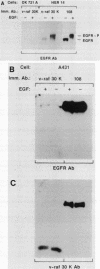
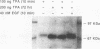
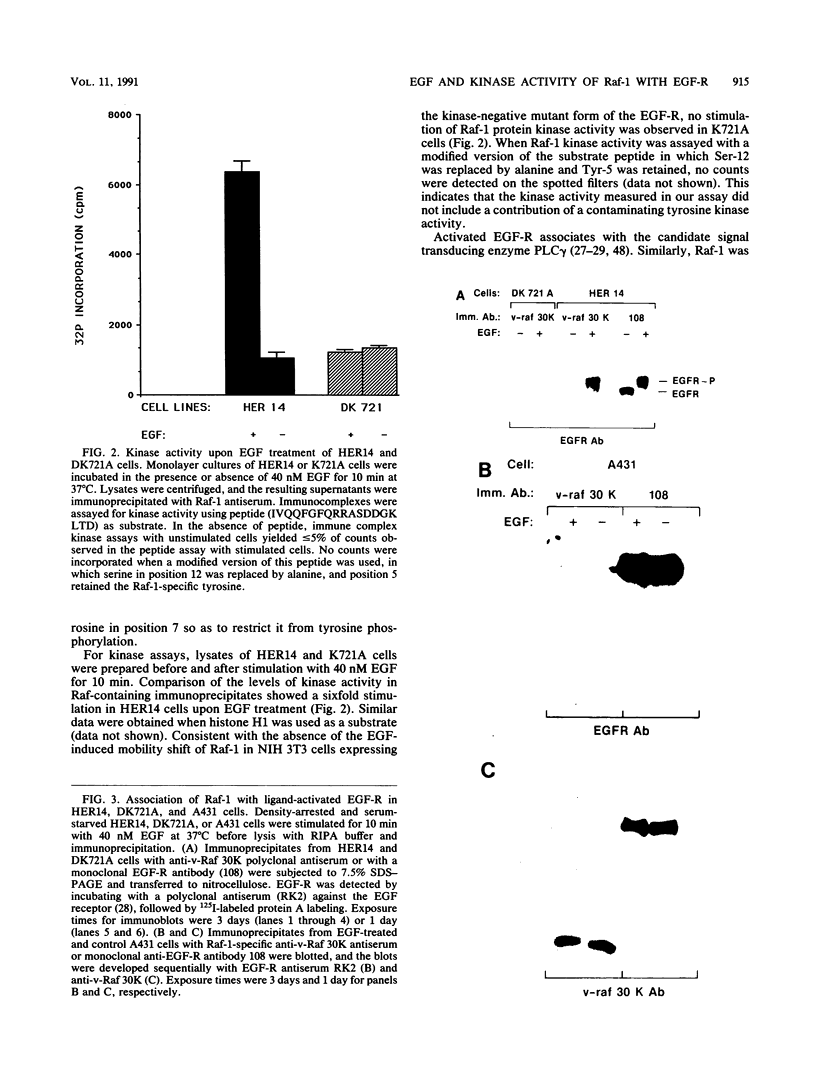
Raf-1 serine- and threonine-specific protein kinase is transiently activated in cells expressing the epidermal growth factor (EGF) receptor upon treatment with EGF. The stimulated EGF receptor coimmunoprecipitates with Raf-1 kinase and mediates protein kinase C-independent phosphorylation of Raf-1 on serine residues. Hyperphosphorylated Raf-1 has lower mobility on sodium dodecyl sulfate gels and has sixfold-increased activity in immunocomplex kinase assay with histone H1 or Raf-1 sequence-derived peptide as a substrate. Raf-1 activation requires kinase-active EGF receptor; a point mutant lacking tyrosine kinase activity in inactive in Raf-1 coupling and association. It is noteworthy that tyrosine phosphorylation of c-Raf-1 induced by EGF was not detected in these cells. These observations suggest that Raf-1 kinase may act as an important downstream effector of EGF signal transduction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccarini M., Sabatini D. M., App H., Rapp U. R., Stanley E. R. Colony stimulating factor-1 (CSF-1) stimulates temperature dependent phosphorylation and activation of the RAF-1 proto-oncogene product. EMBO J. 1990 Nov;9(11):3649–3657. doi: 10.1002/j.1460-2075.1990.tb07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Haupt D. M., App H., Rapp U. R. Insulin activates the Raf-1 protein kinase. J Biol Chem. 1990 Jul 25;265(21):12131–12134. [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Stimulation of ribosomal protein S6 kinase activity by pp60v-src or by serum: dissociation from phorbol ester-stimulated activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1733–1737. doi: 10.1073/pnas.83.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Oppermann H., Seeburg P., Kerby S. B., Gunnell M. A., Young A. C., Rapp U. R. The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gene. Nucleic Acids Res. 1986 Jan 24;14(2):1009–1015. doi: 10.1093/nar/14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll M. P., Clark-Lewis I., Rapp U. R., May W. S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of a protein kinase from Xenopus eggs highly specific for ribosomal protein S6. J Biol Chem. 1986 Jan 5;261(1):350–355. [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Huleihel M., Cleveland J. L., Kolch W., Beck T. W., Lloyd P., Pawson T., Rapp U. R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990 Jun;10(6):2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Szapary D., Schmidt A., Lyall R., Van Obberghen E., Dull T. J., Ullrich A., Schlessinger J. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol. 1987 Dec;7(12):4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Fukui M., Ueyama Y., Tamaoki N., Yamamoto T., Toyoshima K. B-raf, a new member of the raf family, is activated by DNA rearrangement. Mol Cell Biol. 1988 Jun;8(6):2651–2654. doi: 10.1128/mcb.8.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal S., Ziff E. Transactivation of c-fos and beta-actin genes by raf as a step in early response to transmembrane signals. Nature. 1990 Mar 29;344(6265):463–466. doi: 10.1038/344463a0. [DOI] [PubMed] [Google Scholar]
- Jenö P., Ballou L. M., Novak-Hofer I., Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):406–410. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenö P., Jäggi N., Luther H., Siegmann M., Thomas G. Purification and characterization of a 40 S ribosomal protein S6 kinase from vanadate-stimulated Swiss 3T3 cells. J Biol Chem. 1989 Jan 15;264(2):1293–1297. [PubMed] [Google Scholar]
- Kaibuchi K., Fukumoto Y., Oku N., Hori Y., Yamamoto T., Toyoshima K., Takai Y. Activation of the serum response element and 12-O-tetradecanoylphorbol-13-acetate response element by the activated c-raf-1 protein in a manner independent of protein kinase C. J Biol Chem. 1989 Dec 15;264(35):20855–20858. [PubMed] [Google Scholar]
- Kolch W., Schultz A. M., Oppermann H., Rapp U. R. Preparation of raf-oncogene-specific antiserum with raf protein produced in E. coli. Biochim Biophys Acta. 1988 Feb 28;949(2):233–239. doi: 10.1016/0167-4781(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Kovacina K. S., Yonezawa K., Brautigan D. L., Tonks N. K., Rapp U. R., Roth R. A. Insulin activates the kinase activity of the Raf-1 proto-oncogene by increasing its serine phosphorylation. J Biol Chem. 1990 Jul 25;265(21):12115–12118. [PubMed] [Google Scholar]
- Livneh E., Prywes R., Kashles O., Reiss N., Sasson I., Mory Y., Ullrich A., Schlessinger J. Reconstitution of human epidermal growth factor receptors and its deletion mutants in cultured hamster cells. J Biol Chem. 1986 Sep 25;261(27):12490–12497. [PubMed] [Google Scholar]
- Livneh E., Reiss N., Berent E., Ullrich A., Schlessinger J. An insertional mutant of epidermal growth factor receptor allows dissection of diverse receptor functions. EMBO J. 1987 Sep;6(9):2669–2676. doi: 10.1002/j.1460-2075.1987.tb02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Bellot F., Honegger A. M., Ullrich A., Schlessinger J., Zilberstein A. Tyrosine kinase activity is essential for the association of phospholipase C-gamma with the epidermal growth factor receptor. Mol Cell Biol. 1990 Feb;10(2):435–441. doi: 10.1128/mcb.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Heidecker G., Huleihel M., Cleveland J. L., Choi W. C., Pawson T., Ihle J. N., Anderson W. B. raf family serine/threonine protein kinases in mitogen signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):173–184. doi: 10.1101/sqb.1988.053.01.023. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. N., Klausner R. D., Rapp U. R., Samelson L. E. T cell antigen receptor engagement stimulates c-raf phosphorylation and induces c-raf-associated kinase activity via a protein kinase C-dependent pathway. J Biol Chem. 1990 Oct 25;265(30):18472–18480. [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Heidecker G., Rapp U. R., Kung H. F. Induction of transformation and DNA synthesis after microinjection of raf proteins. Mol Cell Biol. 1990 Jul;10(7):3828–3833. doi: 10.1128/mcb.10.7.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm S. M., Cleveland J. L., Rapp U. R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene. 1990 Mar;5(3):345–351. [PubMed] [Google Scholar]
- Tabarini D., Heinrich J., Rosen O. M. Activation of S6 kinase activity in 3T3-L1 cells by insulin and phorbol ester. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4369–4373. doi: 10.1073/pnas.82.13.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Suh P. G., Rhee S. G., Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B., Heidecker G., Huleihel M., Rapp U. R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989 May;9(5):2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]