Abstract
Background
Co-resistance against the first-line antibiotics ampicillin, chloramphenicol and trimethoprim/sulphamethoxazole or multidrug resistance (MDR) is common in non typhoid Salmonella (NTS). Use of alternative antibiotics, such as fluoroquinolones or third generation cephalosporins is threatened by increasing resistance, but remains poorly documented in Central-Africa.
Methodology/Principal findings
As part of a microbiological surveillance study in DR Congo, blood cultures were collected between 2007 and 2011. Isolated NTS were assessed for serotype and antimicrobial resistance including decreased ciprofloxacin susceptibility and extended-spectrum beta-lactamase (ESBL) production. In total, 233 NTS isolates (representing 23.6% of clinically significant organisms) were collected, mainly consisting of Salmonella Typhimurium (79%) and Salmonella Enteritidis (18%). The majority of NTS were isolated in the rainy season, and recovered from children ≤2 years old. MDR, decreased ciprofloxacin susceptibility, azithromycin and cefotaxime resistance were 80.7%, 4.3%, 3.0% and 2.1% respectively. ESBL production was noted in three (1.3%) isolates. Decreased ciprofloxacin susceptibility was associated with mutations in codon 87 of the gyrA gene, while ESBLs all belonged to the SHV-2a type.
Conclusions/Significance
Presence of almost full MDR among NTS isolates from blood cultures in Central Africa was confirmed. Resistance to fluoroquinolones, azithromycin and third generation cephalosporins is still low, but emerging. Increased microbiological surveillance in DR Congo is crucial for adapted antibiotic therapy and the development of treatment guidelines.
Author Summary
Invasive non typhoid Salmonella spp. (NTS) are an important cause of bloodstream infection in sub-Saharan Africa and associated with a high mortality. Levels of multidrug resistance have become alarmingly high. Treatment therefore increasingly relies on the oral fluoroquinolones such as ciprofloxacin, with third generation cephalosporins such as cefotaxime as alternatives for parenteral treatment. Azithromycin represents another alternative antimicrobial drug. Worldwide, increased use of these drugs is associated with spread of resistance as well, a phenomenon poorly documented in Central-Africa. In the present study, 233 NTS isolates were collected from blood cultures sampled between 2007 and 2011 in DR Congo, mainly from children ≤2 years of age. Most isolates were recovered during the rainy season. Widespread multidrug resistance was confirmed as well as decreased susceptibility to ciprofloxacin, resistance to azithromycin and resistance to third generation cephalosporins. Our findings demonstrate emergence of antibiotic resistance among NTS in DR Congo and underline the need for increased microbiological surveillance, being a prerequisite for rational antibiotic therapy and the development of standard treatment guidelines.
Introduction
Non typhoid Salmonella (NTS) are among the leading causes of bacterial bloodstream infections in sub-Saharan Africa [1], [2]. NTS bacteremia mainly affects immune compromised hosts and young children, in whom they are associated with high mortality rates up to 27% [3]. Usually, most cases of invasive non typhoid salmonellosis are due to either Salmonella enterica subsp. enterica serotype Typhimurium (further referred to as Salmonella Typhimurium), followed by Salmonella Enteritidis [2]. Resistance of NTS to the first line antibiotics ampicillin, chloramphenicol and trimethoprim/sulphamethoxazole (TMP-SMX) is usually high [4], [5], also in the Democratic Republic of the Congo (DR Congo) [6]–[9]. Treatment of NTS therefore increasingly relies on fluoroquinolones or third generation cephalosporins but these treatment options are threatened by decreased susceptibility to fluoroquinolones (referred to as decreased ciprofloxacin susceptibility) and extended-spectrum beta-lactamases (ESBLs) respectively. In Central Africa, decreased ciprofloxacin susceptibility in NTS is poorly documented [8], [10], [11] and NTS resistance to third generation cephalosporins in NTS has not yet been described [7], [10], in contrast to other regions in tropical Africa [12]–[15].
The present study describes the antimicrobial resistance profile of invasive NTS isolates recovered from bloodstream infections during a microbiological surveillance study in DR Congo over the years 2007–2011. The molecular mechanisms of decreased ciprofloxacin susceptibility and ESBLs were characterized and the genetic relationships of Salmonella Enteritidis and Salmonella Typhimurium isolates were assessed.
Materials and Methods
Ethics statement
Ethical approval was granted by the Ethical Committee of the University of Antwerp, Belgium and from the Ministry of Health in DR Congo. The present study complies with the World Health Organization and international guidelines (European Society of Clinical Microbiology and Infectious Diseases Study Group for Antimicrobial Resistance Surveillance and Clinical Laboratory Standards Institute) on antibiotic surveillance for which no recommendation for an informed consent has been issued. The diagnostic procedure – blood cultures – is part of the standard diagnostic work-up of patients with a suspicion of bacteremia. Clinical information -as presented- and information about use of antibiotics was the standard information present on the laboratory request form. Data have been reviewed and analysed anonymously.
Study setting
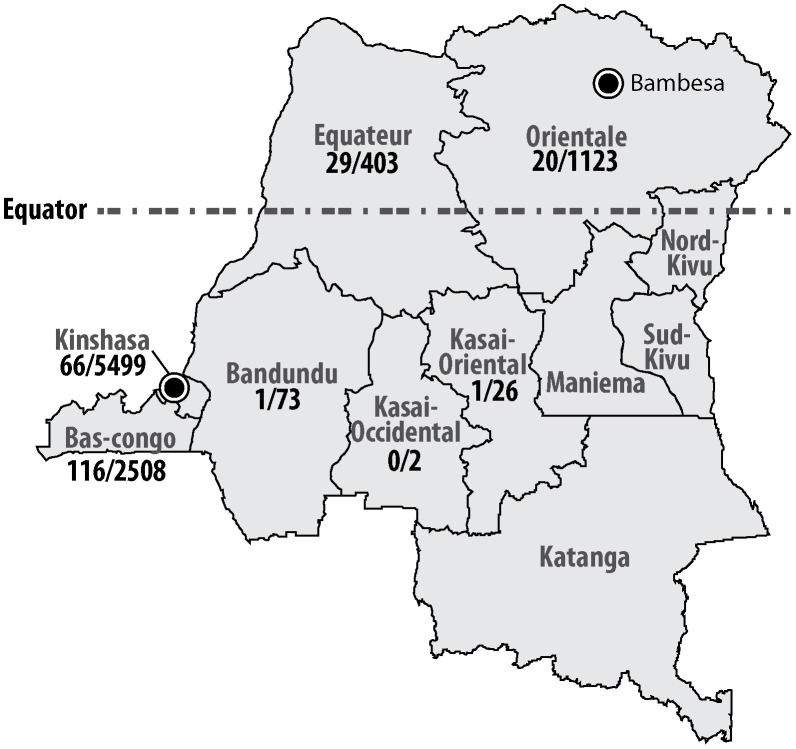
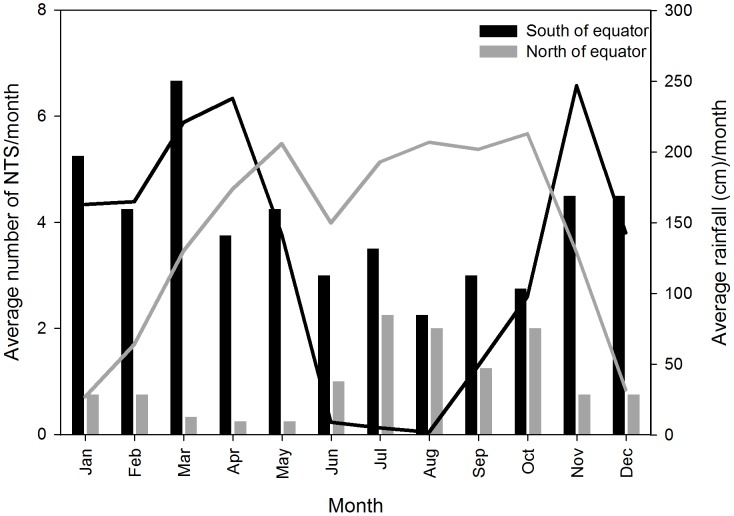
The study was carried out in seven out of eleven provinces of DR Congo (Figure 1). In Kinshasa, health care facilities involved in the detection and study of the epidemic increase of typhoid fever-associated peritonitis of 2004 were selected [16]. Health care facilities in other provinces were recruited based on existence of microbiological laboratories, professional contacts and the accessibility to reliable shipment facilities. South of the equator (provinces of Bas-Congo, Kinshasa, Bandundu, Kasai Oriental, Kasai Occidental), the wet season runs from November till May, north of the equator (provinces of Equateur and Orientale), the wet season runs from April till October. Rainfall data were retrieved from http://www.dr-congo.climatemps.com/, using the data from Kinshasa (4°23′S 15°26′E) and Bambesa (3°27′N 25°43′E) as representative for the rainfall respectively south and north of the Equator (Figure 2). Malaria is endemic in DR Congo and 97% of the population is living in areas of stable transmission [17]. In adults aged 15–49 years old, the HIV prevalence rate in 2009 in DR Congo was estimated 1.2–1.6% [18]. The expanded vaccination program for children included the Bacille Calmette-Guérin at birth and live oral polio, diphtheria, whole cell pertussis, tetanus, hepatitis B, monovalent measles and Haemophilus influenzae b vaccines. Typhoid vaccine is not routinely administered [19].
Figure 1. Origin of blood cultures and NTS in DR Congo (number of NTS grown/number of blood cultures received at INRB).
Approximate positions of Kinshasa (4°23′S 15°26′E), Bambesa (3°27′N 25°43′E) and the equator are indicated.
Figure 2. Average number of NTS per month south and north of the equator (black and grey bars respectively) and average monthly rainfall in Kinshasa (4°23′S 15°26′E, black line) and Bambesa (3°27′N 25°43′E, grey line).
Bacterial culture and identification
Criteria for blood culture sampling were clinical suspicion of bacteremia caused by a local (pneumonia, urinary tract infection, meningitis or other) or systemic (typhoid fever, endocarditis) infection diagnosed at consultation or admission. Typhoid fever was defined according to the case definitions of the Ministry of Health surveillance of communicable diseases [20]. At the start of the surveillance project, teams of clinicians and laboratory technicians were trained in indications and sampling of blood cultures. Blood cultures of 9,634 patients presenting to health care facilities with clinical suspicion of bacteremia were sampled as part of a microbiological surveillance program between April 2007 and January 2011. For children <14 years, 1–4 ml of blood was sampled into pediatric blood culture vials (BacT/ALERT FP; bioMérieux; Marcy L'Etoile; France). For adults, 2×10 ml of blood was inoculated into aerobic blood culture vials (BacT/ALERT FA; bioMérieux; Marcy L'Etoile; France). Age, gender, geographic origin, use of antibiotics prior to sampling of the blood culture and presumptive diagnosis with suspected focus of bacteremia were recorded as part of the standard information present on the laboratory request forms. Vials were shipped at room temperature to the Institut National de Recherche Biomédicale (INRB) in Kinshasa (maximum delay of 6 weeks), where they were incubated at 35°C and daily checked for growth by visual inspection of the indicator. If grown, cultures were Gram stained, subcultured and identified to the species level by standard biochemical methods. Skin or environmental bacteria (coagulase negative staphylococci, Corynebacterium spp., Propionibacterium acnes and Bacillus spp.) were categorized as contaminants, the other bacteria were considered as clinically significant organisms (CSO) [21].
Suspected colonies of Salmonella were identified as NTS using standard biochemical methods (characteristic aspect on Kligler Iron Agar (acid from glucose, gas, production of H2S), negative tests for urease, oxidase, β-galactosidase and indole production tests, positive tests for lysine decarboxylase and the serotype was determined with commercial antisera (Remel, Lenexa, Kansas). Identity of Salmonella species isolates was confirmed using the Vitek II system (Card GN21 341, bioMérieux). At the National Reference Laboratory for Salmonella and Shigella (Institute of Public Health, Brussels), the serotype of the Salmonella isolates was re-confirmed by slide agglutination with commercial monospecific antisera (Sifin, Berlin, Germany), following the Kauffmann-White scheme [22].
For analysis in the present study, only the first isolate per patient was considered.
Antimicrobial susceptibility
Susceptibility tests for ampicillin, cefotaxime and TMP-SMX and ESBL were performed using the Vitek II system (Card AB AST-N156, bioMérieux). For nalidixic acid, ciprofloxacin, chloramphenicol and azithromycin, minimal inhibitory concentration (MIC) values were determined using the E-test macromethod (bioMérieux). Breakpoints for resistance are summarized in table 1 and were according to Clinical Laboratory Standard Institute guidelines [23]. For ciprofloxacin and azithromycin, European Committee on Antimicrobial Susceptibility testing (EUCAST) V 2.0. guidelines [24] were followed. Strains co-resistant to ampicillin, chloramphenicol and TMP-SMX were considered as multidrug resistant (MDR) [25]. ESBLs flagged by the Vitek II system were phenotypically confirmed by the combined double-disk method using cefotaxime, ceftazidime and cefepime alone and in combination with clavulanic acid (Rosco Diagnostica, Taastrup, Denmark) according to CLSI guidelines and using Escherichia coli ATCC 25922 and Klebsiella pneumoniae ATCC 700603 as control strains [23]. Screening for mutations causing decreased ciprofloxacin susceptibility was performed by amplification and sequencing of the quinolone resistance-determining regions (QRDRs) of the gyrA, gyrB, and parC genes. The presence of the plasmid-mediated quinolone resistance qnr genes (qnrA, qnrB, and qnrS) was determined using PCR [26]. In confirmed ESBLs, the beta lactamase resistance genes were identified using a DNA microarray (Check-Points CT 101, Wageningen, the Netherlands) and PCR sequencing [27].
Table 1. Number blood cultures (BC) and number of Salmonella Typhimurium (T), Salmonella Enteritidis (E) and other NTS (O) by province and year of isolation.
| Province | 2007 | 2008 | 2009 | 2010+2011a | Total | |||||||||||
| BC | T | E | O | T | E | O | T | E | O | T | E | O | T | E | O | |
| Bas Congo | 2508 | 1 | 0 | 0 | 28 | 1 | 1 | 36 | 2 | 1 | 35 | 11b | 0 | 100 | 14 | 2 |
| Kinshasa | 5499 | 13 | 8 | 2 | 24 | 5 | 1 | 5 | 1 | 0 | 5 | 2 | 0 | 47 | 16 | 3 |
| Bandundu | 73 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Equateur | 403 | 0 | 0 | 0 | 14 | 4 | 0 | 5 | 1 | 0 | 5 | 0 | 0 | 24 | 5 | 0 |
| Kasai Occidental | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kasai Oriental | 26 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Orientale | 1123 | 0 | 0 | 0 | 3 | 0 | 0 | 6 | 3 | 0 | 3 | 3 | 1 | 12 | 6 | 2 |
| Total | 9634 | 14 | 8 | 2 | 69 | 12 | 2 | 52 | 7 | 2 | 49 | 15 | 1 | 184 | 42 | 7 |
January 2011 only.
Onset of an epidemic outbreak in Kisantu, province of Bas-Congo [11].
Pulsed field gel electrophoresis
Respectively 34 isolates of Salmonella Typhimurium and 16 of Salmonella Enteritidis were selected for pulsed field gel electrophoresis (PFGE). These isolates were representative according to geographic origin and year of isolation. PFGE was performed at the Institute of Public Health (IPH, Brussels) according to the Pulsenet protocol for Salmonella [28], using XbaI as restriction enzyme (New England Biolabs, Leusden, The Netherlands). Cluster analysis was performed with Bionumerics 5.1 (Applied Maths NV, Sint-Martens-Latem, Belgium), with as comparison settings the Dice similarity coefficient and UPMGA dendrogram type (optimization 0.50%, position tolerance 1.50%). The obtained PFGE profiles were compared to PFGE profiles of Salmonella Typhimurium or Salmonella Enteritidis originating from Belgium (n = 15 and n = 7 respectively) and Cambodia (n = 4 and n = 7 respectively), already stored in the IPH database.
Data analysis
All data were entered in an Excel database (Microsoft Corporation, Redmond, Washington, USA). Proportions were assessed for statistical significance using the Chi square test or Fisher exact test, considering p<0.05 as significant. If data were normally distributed, mean values of two groups were compared with the t-test, otherwise median values were compared with the Mann-Whitney Rank Sum Test (Stata 10, StatCorp, Texas, USA).
Results
Serotypes, demographic, clinical and epidemiological data
From 9,634 blood cultures performed, 989 clinically significant organisms (CSO) were grown (10.3%). The proportion of CSO/blood cultures ranged between a minimum of 7.2% in the age group of 30–39 and a maximum of 11.9% in the age group of 0–9 years old [29]. With 233 isolates (23.6%), NTS ranked first among the CSOs, followed by Salmonella Typhi (20.3%) [29]. Non typhoid Salmonella consisted of Salmonella Typhimurium (n = 184, 79.0%), Salmonella Enteritidis (n = 42, 18.0%) and other Salmonella serotypes [n = 7, 3.0%: Salmonella Kisangani (n = 2), Salmonella Virchow (n = 2), Salmonella 4,5 (n = 2) and a single isolate that could not be serotyped (Table 1)]. Most NTS (respectively 28.3 and 49.8%) originated from the provinces of Kinshasa and Bas Congo (Figure 1), in line with the higher number of blood cultures recovered from these provinces. Eleven out of a total of 42 Salmonella Enteritidis were isolated in Bas-Congo in 2010–2011, at the onset of an epidemic outbreak described in detail elsewhere [11].
Peaks in numbers of NTS fell in the rainy season (Figure 2). South of the equator, the rainy season from November till May, accounted for more NTS (mean of 4.7±1.0/month) than the dry season (mean of 2.9±0.5 NTS/month, p = 0.003). North of the equator, more NTS were observed from June till October (mean of 1.7±0.5 NTS/month), than in the rest of the year (mean of 0.57±0.3 NTS/month, p<0.001).
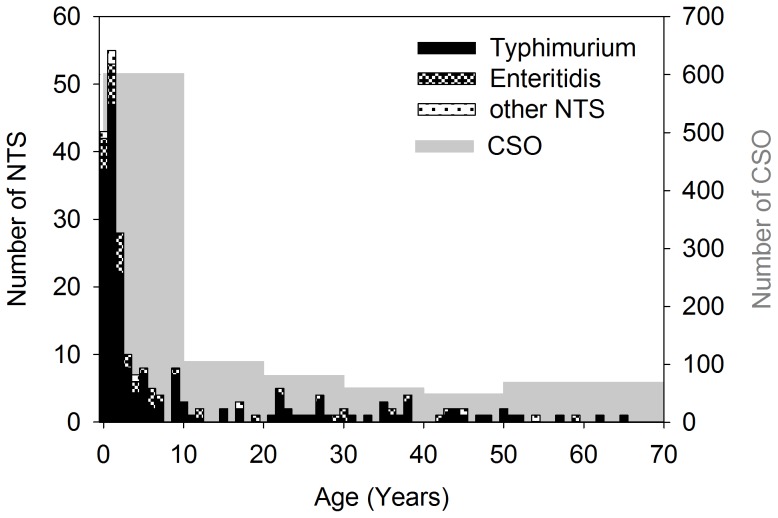
Patients' male/female ratio was 1.23 (125 male, 102 female, 6 no data). The age distribution of patients with NTS is illustrated in figure 3. The median patient age was 2 (interquartile range 1–11). There was no statistically significant difference between the median age of Salmonella Typhimurium and Salmonella Enteritidis patients (p = 0.1, Mann-Whitney Rank Sum Test). The presumptive diagnosis at the moment of sampling for the patients with NTS (in some patients more than one diagnosis was mentioned) was mainly typhoid fever (60.5%, 141/233), pneumonia (11.2%, 26/233), complicated urinary tract infection (7.7%, 18/233), meningitis (6.4%, 15/233), malaria (4.3%, 10/233), or non-specified other causes of bacteremia (14.6%, 34/233); for 8 patients (3.4%) no data were available.
Figure 3. Age distribution of NTS (black bars) and number of clinical significant organisms.
CSO<50: by age groups of 10 years, ≥50 was considered as one age group.
A total of 40.8% (95/233) of NTS positive patients had taken antibiotics less than 48 hours prior to blood sampling.
Antimicrobial susceptibility
The antimicrobial resistance profiles, MIC ranges, MIC50 and MIC90 for all NTS are shown in table 2, as well as the resistance profiles for Salmonella Typhimurium and Salmonella Enteritidis separately.
Table 2. Minimal inhibitory concentrations and antimicrobial resistance in 233 non-typhoidal Salmonella from DR Congo.
| Breakpoint MIC | NTS MIC range | NTS MIC50 | NTS MIC90 | % resistant NTS n = 233 | % resistant Salmonella Typhimurium n = 184 | % resistant Salmonella Enteritidis n = 42 | |
| Ampicillin | ≥32 | ≤2–≥32 | ≥32 | ≥32 | 88.0% | 94.0% | 64.3% |
| Chloramphenicol | ≥16a | 2–>256 | >256 | >256 | 83.7% | 90.2% | 61.9% |
| TMP-SMX | ≥4/76 | ≤1/19–≥16/304 | ≥16/304 | ≥16/304 | 88.0% | 94.0% | 64.3% |
| Nalidixic acid | ≥32 | 2–>256 | 3 | 6 | 4.3% | 4.9% | 0.0% |
| Ciprofloxacin | >0.064b | 0.006–0.19 | 0.012 | 0.023 | 4.3% | 4.9% | 0.0% |
| Azithromycin | >16b | 2–256 | 4 | 12 | 3.0% | 3.3% | 0.0% |
| Cefotaxime | ≥4 | ≤1–≥64 | ≤1 | ≤1 | 2.1% | 2.2% | 0.0% |
| ESBL | na | na | na | na | 1.3% | 1.1% | 0.0% |
| MDR | na | na | na | na | 80.7 | 87.0% | 59.5% |
| MDR+DCS | na | na | na | na | 3.9% | 4.3% | 0.0% |
| MDR+DCS+ESBL | na | na | na | na | 0.9% | 0.5% | 0.0% |
MIC: Minimal Inhibitory Concentration (mg/l); TMP-SMX: trimethoprim/sulphamethoxazole; MDR: multidrug resistant (Co-resistance against ampicillin, chloramphenicol and trimethoprim/sulphamethoxazole), DCS: decreased ciprofloxacin susceptibility; na not applicable.
intermediate susceptible isolates were considered together with the resistant isolates;
[24].
Respectively 88.0%, 83.7% and 88.0% (n = 205, 195 and 205) of all 233 NTS were resistant to ampicillin, chloramphenicol or TMP-SMX. The proportions of Salmonella Typhimurium resistant to ampicillin, chloramphenicol or TMP-SMX and MDR were significantly higher (p<0.001) compared to those among Salmonella Enteritidis. Decreased ciprofloxacin susceptibility was observed in 4.3% of isolates (Salmonella Typhimurium n = 9 and Salmonella 4,5 n = 1), all were nalidixic acid resistant and vice versa. In total 3.0% (7/233) of NTS were resistant to azithromycin: the isolates belonged to serotype Salmonella Typhimurium (n = 6) and Salmonella 4,5 (n = 1). Resistance to cefotaxime occurred in 2.1% of NTS (5/233, Salmonella Typhimurium n = 4 and Salmonella 4,5 n = 1). Vitek II labeled 9 strains as ESBL producers, only 3 (1.3%, Salmonella Typhimurium n = 2 and Salmonella 4,5 n = 1) were confirmed ESBL producers by double disk diffusion.
Multidrug resistance occurred in 80.7% of NTS (188/233). Combined MDR and decreased ciprofloxacin susceptibility was observed in nine strains. Two isolates (0.9%, one Salmonella Typhimurium and one Salmonella 4,5) combined MDR, decreased ciprofloxacin susceptibility, azithromycin and cefotaxime resistance and were ESBL producers. Only 10.3% of NTS isolates (8 Salmonella Typhimurium, 14 Salmonella Enteritidis, 1 Salmonella Kisangani and 1 Salmonella Virchow) were fully susceptible to all drugs tested.
In Salmonella Enteritidis isolated in Kinshasa (n = 16) significantly less MDR occurred (respectively 37.5% versus 73.1%, p = 0.02) than in Salmonella Enteritidis isolates recovered in the rest of DR Congo (n = 26). For Salmonella Typhimurium, this difference in MDR was not observed. However, Salmonella Typhimurium isolated from Kinshasa (n = 47) had a significantly higher proportion of azithromycin resistance (respectively 10.6% versus 0.7%, p = 0.005) than those isolated elsewhere (n = 137). For cefotaxime resistance in Salmonella Typhimurium, the difference between Kinshasa and the rest of DR Congo (respectively 6.4% versus 0.7%, p = 0.052) did not reach statistical significance. No difference in decreased ciprofloxacin susceptibility was seen in Salmonella Typhimurium between Kinshasa and other provinces (4.5 and 4.2%). All 3 ESBL producing isolates were recovered in Kinshasa.
Intake of antibiotics less than 48 hours prior to blood sampling was not associated with MDR, cefotaxime or azithromycin resistance (p>0.5), but was associated with decreased ciprofloxacin susceptibility (p = 0.03).
All 10 decreased ciprofloxacin susceptible isolates had mutations in the gyrA gene at position Asp87 that changed into Tyr (n = 8) or, in two isolates that were as well ESBLs, in Asn (n = 2). No mutations were observed in the gyrB or parC genes, nor were qnrA, qnrB, or qnrS genes detected in any of the 10 isolates. All 3 ESBL positives in double disk diffusion were identified by CheckPoints as SHV-2-like ESBLs and were confirmed as SHV-2a by PCR-sequencing.
Pulsed field gel electrophoresis
Among the 34 isolates of Salmonella Typhimurium and 16 of Salmonella Enteritidis analyzed in PFGE, respectively 19 and 10 different PFGE profiles were observed (Figure S1). For Salmonella Typhimurium, PFGE profile T4 was most prevalent occurring in 20.6% (7/34) of isolates from DR Congo. This profile also occurred in 40% (6/15) of Salmonella Typhimurium isolates from patients in Belgium stored in the IPH database. Typhimurium profile T3 (2/34) was also observed in a Salmonella Typhimurium isolate from Belgium. For Salmonella Enteritidis, profile E5 was most common (25.0%, 4/16). Profile E2, observed in 2 isolates from DR Congo, corresponded to one of the common Salmonella Enteritidis profiles also observed in Belgium. The 11 NTS isolates from DR Congo with PFGE profiles identical to Belgian profiles originated from Bas-Congo (n = 5), Kinshasa (n = 4), Bandundu (n = 1) and Equateur (n = 1) and were isolated in 2008 (n = 8), 2010 (n = 2) and 2011 (n = 1).
Discussion
The present study confirmed widespread MDR and low level decreased ciprofloxacin susceptibility and azithromycin resistance among NTS isolates from DR Congo, and demonstrated occurrence of ESBLs in Salmonella in Central Africa. The majority of isolated NTS consisted of Salmonella Typhimurium, it mainly affected infants and young children ≤2 years old and peaked in the wet season.
Some limitations are inherent to the study. The geographical origin of the isolates could have been biased by logistic difficulties such as limited road communications, especially during the rainy season. Although we cannot completely exclude that shipment delays of several days to weeks might have resulted in lower recovery rates, preliminary survival studies suggested that Gram negative bacteria remain viable for at least 8 weeks in inoculated and grown blood culture bottles in the environmental conditions encountered in DR Congo. Given antibiotic use prior to sampling, the inherent low sensitivity of blood culture [30], financial constraints preventing many patients from consulting, unfamiliarity of medical doctors with microbiological culture tools and possible variability in sampling criteria between different sites in function of time (e.g. incomplete sampling during evening and night shifts) and variability in training of clinicians, and in function of other diagnosis made (e.g. tendency not to take blood culture when thick blood film is Plasmodium positive in children is likely to under-represent NTS further), we believe that the presently described isolates represented only a fraction of the actual number of Salmonella infections and that calculations of NTS incidence rates is not possible based on our data. Although only an association for decreased ciprofloxacin susceptibility was observed with antibiotic intake (which consisted mainly of first line drugs), we cannot exclude some bias towards resistance. Besides qnrA, qnrB, and qnrS, the presence of other plasmid-mediated quinolone resistance genes was not determined nor was the parE gene sequenced, taking into account the low probability of mutations in the parE gene in parC and gyrB wild type Salmonella. Other mechanisms associated with decreased ciprofloxacin susceptibility can therefore not be completely excluded. Furthermore, we limited the number of PFGE analysis to a subset of representative strains in function of the moment and region of isolation, taking into account the relatively homogeneous PFGE profile observed earlier with Salmonella Typhi and Salmonella Typhimurium strains isolated in DR Congo [8], [29].
We were able to cover antimicrobial resistance patterns in a large surface of DR Congo (7 out of 11 provinces), where the NTS situation was hitherto poorly characterized. Indeed, previous data about NTS bacteremia in DR Congo comprise 6 studies only, performed over 35 years in 3 sites [6]–[9], [31], [32]. Comparison of these studies, although performed at sites almost 1000 km apart, illustrates the emergence of antimicrobial resistance in DR Congo. In the seventies, respectively 38 and 46% of Salmonella Typhimurium and Salmonella Enteritidis were reported as resistant [31]. In the eighties, antibiotic resistance was still only reported in 30% of NTS, but all tested isolates were sensitive to TMP-SMX [6]. In the early nineties, 100% resistance to ampicillin and chloramphenicol was already reported, and resistance to TMP-SMX started to emerge (7%), in the absence of resistance to fluoroquinolones or third generation cephalosporins [7]. The same report also mentions that in 1998–1999 resistance against TMP-SMX had increased to 90%, still in the absence of fluoroquinolone resistance. Also in the late nineties, over 50% of MDR was reported in Kinshasa [9]. Finally, in 2000–2006 in eastern DR Congo, 85–96% resistance to ampicillin, chloramphenicol, TMP-SMX and MDR, was observed and NTS resistance to fluoroquinolones was reported for the first time to be 3.8–6.1% [8]. These percentages, as predicted by the authors, are in line with those reported here for the whole country in 2007–2011, with the exception of resistance against third generation cephalosporin, which we observed for the first time. The antibiotic resistance profile we observed in DR Congo is very similar to the ones described in Togo [13], in Ghana, Kenya, Malawi and Mozambique [5], [33]–[35], although no 3rd generation cephalosporin resistance was observed in the later four studies. In Nigeria on the other hand, resistance in Salmonella Enteritidis appears to be more elevated [36]. We observed a difference in resistance between Salmonella Typhimurium and Salmonella Enteritidis to first generation antibiotics, confirming data from Malawi [34]. As suggested by these authors, acquisition of MDR and increased prevalence may suggest competition for an ecological transmission niche between these two serotypes. Decreased use of first line drugs after a MDR Salmonella Typhi outbreak in Kinshasa [16] may have caused a loss of MDR selection pressure, contrary to other parts of DR Congo where ampicillin, chloramphenicol and TMP-SMX are still frequently used [8]. Increased use of cefotaxime and particularly azithromycin as alternative treatments in Kinshasa may have selected for the observed higher resistance of Salmonella Typhimurium against these drugs. Furthermore, in the present study, decreased ciprofloxacin susceptibility was in all cases associated with nalidixic acid resistance and a mutation at codon 87 of the gyrA gene. Detection of nalidixic acid resistance therefore still remains a valuable test to screen for decreased ciprofloxacin susceptibility in DR Congo, contrary to the Asian situation [37].
The SHV-2 type ESBL, which was identified in all 3 ESBL producing isolates has been observed in a minority of South African ESBL positive Salmonella spp (5%) [14], and SHV-2-like ESBLs have been identified in Enterobacteriaceae isolated from drinking water in Kinshasa previously [38].
The observed seasonal peak of NTS isolation, coinciding with the rainy season, has been described before [6] and may be explained by contamination of the surface waters.
Host risk factors for NTS such as increased incidences of malaria and malnutrition [3] that peak at the same moment, have already been described as relatively common in NTS patients in DR Congo [8]. Prevalence of HIV, another host risk factor for NTS, is low in DR Congo [18], which is as well reflected by the age distribution of NTS in children ≤2 years, similar as 25 years ago [6] and its low occurrence in adults.
Appearance in DR Congo of PFGE profiles which are common to Belgian profiles, might be explained, by exchange of food products [3]. For example, it is well documented that Salmonella is a pathogen occurring particularly in poultry products, and that human Salmonella infections may be attributable to the consumption of contaminated chicken [39]. DR Congo represents the twelfth export destination of European Union poultry meat products, representing 2.8% of the total European poultry meat exports [40], [41]. Travelling of asymptomatic carriers [42] between the two countries, circulation of major worldwide clones [43], or the limited technical power of PFGE to discriminate the clonal relation of organisms might be alternative explanations for appearance of identical PFGE profiles in Belgium and DR Congo [34], [44]. The obtained PFGE profiles and representative strains are available for comparisons with profiles obtained with Salmonella from other countries.
As we did not determine the genotype of Salmonella Typhimurium isolated from DR Congo, we do not know if it belongs to multilocus sequence type (ST) 313, which is geographically confined to sub-Saharan Africa and represents the predominant ST-type among invasive Salmonella Typhimurium strains in Malawi and Kenya [45]. ST313 genetically differs from other STs within the serotype by a degraded genome capacity – a feature which is also noted among Salmonella Typhi and Salmonella Paratyphi and which suggest adaptation of ST313 to the human host [45], [46]. As one of the two ST313 lineages circulating in East-Africa is hypothesized to have originated in DR Congo [46], it would be of interest to determine if the ST313 lineages was present, or absent, in the Salmonella Typhimurium strain collection.
Due to the high prevalence of MDR, especially in Salmonella Typhimurium which appears the most prevalent NTS in DR Congo, the use of cheap first line drugs seems not indicated since they will no longer be active. Third generation cephalosporins and azithromycin may still be effective although resistance is emerging. Close surveillance of NTS and their microbial resistance patterns seems indicated to follow-up on emerging resistance, for which the actual surveillance study already provides a baseline, to prevent outbreaks, and to further rationalize therapy.
Furthermore, research into the possible NTS transmission pathways [1] and incidence rates remains indicated to take preventive measures and see their effect.
Supporting Information
Pulsed-field gel electrophoresis (PFGE) Xba I patterns of 34 S. Typhimurium (a) and 16 S. Enteritidis (b) isolates from DR Congo. Similarity between PFGE patterns was assessed by cluster analysis (Dice coefficient and UPGMA, tolerance and optimization of band position set at 1.5% and 0.5%). * PFGE profile also observed in Salmonella Typhimurium or Salmonella Enteritidis from Belgium.
(TIF)
Acknowledgments
We thank Anthony Smith (Centre for Enteric Diseases, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa) for helpful advice during preparation of the manuscript and Marleen Verlinden and Hilde De Boeck for technical support.
Funding Statement
This study was funded by Directorate General of Development Cooperation of the Belgian Government through Institutional Collaboration INRB-ITM (Project 2.01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA (2012) Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379: 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reddy EA, Shaw AV, Crump JA (2010) Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10: 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morpeth SC, Ramadhani HO, Crump JA (2009) Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, et al. (2006) Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol 6: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandomando I, Macete E, Sigauque B, Morais L, Quinto L, et al. (2009) Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 14: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 6. Green SD, Cheesbrough JS (1993) Salmonella bacteraemia among young children at a rural hospital in western Zaire. Ann Trop Paediatr 13: 45–53. [DOI] [PubMed] [Google Scholar]
- 7. Bahwere P, Levy J, Hennart P, Donnen P, Lomoyo W, et al. (2001) Community-acquired bacteremia among hospitalized children in rural central Africa. Int J Infect Dis 5: 180–188. [DOI] [PubMed] [Google Scholar]
- 8. Vandenberg O, Nyarukweba DZ, Ndeba PM, Hendriksen RS, Barzilay EJ, et al. (2010) Microbiologic and clinical features of Salmonella species isolated from bacteremic children in eastern Democratic Republic of Congo. Pediatr Infect Dis J 29: 504–510. [DOI] [PubMed] [Google Scholar]
- 9. Lunguya O, Asuni M, Mumba D, Mabwa L, Nsungu M, et al. (2005) Sérotypes et phamacoresistance des Salmonella et Shigella à Kinshasa (1994–1999). Congo Médical 4: 227–233. [Google Scholar]
- 10. Vlieghe E, Phoba MF, Tamfun JJ, Jacobs J (2009) Antibiotic resistance among bacterial pathogens in Central Africa: a review of the published literature between 1955 and 2008. Int J Antimicrob Agents 34: 295–303. [DOI] [PubMed] [Google Scholar]
- 11. Phoba MF, Lunguya O, Mayimon DV, Lewo di MP, Bertrand S, et al. (2012) Multidrug-resistant Salmonella enterica, Democratic Republic of the Congo. Emerg Infect Dis 18: 1692–1694 10.3201/eid1810.120525 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dougle ML, Hendriks ER, Sanders EJ, Dorigo-Zetsma JW (1997) Laboratory investigations in the diagnosis of septicaemia and malaria. East Afr Med J 74: 353–356. [PubMed] [Google Scholar]
- 13. Dagnra AY, Akolly K, Gbadoe A, Aho K, David M (2007) Emergence of multidrug resistant Salmonella strains in Lome (Togo). Med Mal Infect 37: 266–269. [DOI] [PubMed] [Google Scholar]
- 14. Usha G, Chunderika M, Prashini M, Willem SA, Yusuf ES (2008) Characterization of extended-spectrum beta-lactamases in Salmonella spp. at a tertiary hospital in Durban, South Africa. Diagn Microbiol Infect Dis 62: 86–91. [DOI] [PubMed] [Google Scholar]
- 15. Boisrame-Gastrin S, Tande D, Munck MR, Gouriou S, Nordmann P, et al. (2011) Salmonella carriage in adopted children from Mali: 2001–08. J Antimicrob Chemother 66: 2271–2276. [DOI] [PubMed] [Google Scholar]
- 16. Muyembe-Tamfum JJ, Veyi J, Kaswa M, Lunguya O, Verhaegen J, et al. (2009) An outbreak of peritonitis caused by multidrug-resistant Salmonella Typhi in Kinshasa, Democratic Republic of Congo. Travel Med Infect Dis 7: 40–43. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (2011) World Malaria Report: 2011. Geneva: WHO press. 248 p.
- 18.UNAIDS (2012) HIV and AIDS estimates (2009). Available: http://www.unaids.org/en/dataanalysis/tools/aidsinfo/.
- 19.World Health Organization (2012) Immunization Profile - Congo. Available: http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm?C=cog.
- 20. Lunguya O, Phoba MF, Mundeke SABE, Mukadi P, Muyembe-Tamfum JJ, et al. (2012) The diagnosis of typhoid fever in the Democratic Republic of the Congo. Transactions of the Royal Society of Tropical Medicine and Hygiene 106: 348–355. [DOI] [PubMed] [Google Scholar]
- 21.Garcia LS and Isenberg HD (2007). Clinical Microbiology Procedures Handbook. Washington: ASM Press.
- 22.Grimont PAD and Weill F-X (2007). Antigenic formulae of the Salmonella Serovars. Paris: World Health Organization, Institut Pasteur. 166 p.
- 23.CLSI (2012) Performance standards for antimicrobial susceptibility testing; Twenty-second informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute. 188 p.
- 24. EUCAST (2012) Breakpoint tables for interpretation of MICs and zone diameters. Version 2.0. Version 2.0: 1–73. [Google Scholar]
- 25.World Health Organization, Communicable Disease Surveillance and Response Vaccines and Biologicals (2003) The diagnosis, treatment and prevention of typhoid fever. Geneva: World Health Organization. 38 p.
- 26. Cavaco LM, Frimodt-Moller N, Hasman H, Guardabassi L, Nielsen L, et al. (2008) Prevalence of quinolone resistance mechanisms and associations to minimum inhibitory concentrations in quinolone-resistant Escherichia coli isolated from humans and swine in Denmark. Microb Drug Resist 14: 163–169 10.1089/mdr.2008.0821 [doi]. [DOI] [PubMed] [Google Scholar]
- 27. Bogaerts P, Hujer AM, Naas T, de Castro RR, Endimiani A, et al. (2011) Multicenter evaluation of a new DNA microarray for rapid detection of clinically relevant bla genes from beta-lactam-resistant gram-negative bacteria. Antimicrob Agents Chemother 55: 4457–4460 AAC.00353-11 [pii];10.1128/AAC.00353-11 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, et al. (2006) Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3: 59–67. [DOI] [PubMed] [Google Scholar]
- 29. Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, et al. (2012) Salmonella Typhi in the Democratic Republic of the Congo: multidrug resistance and fluoroquinolone decreased susceptibility on the rise. PLoS Negl Trop Dis 6: e1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mtove G, Amos B, von SL, Hendriksen I, Mwambuli A, et al. (2010) Invasive salmonellosis among children admitted to a rural Tanzanian hospital and a comparison with previous studies. PLoS One 5: e9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muyembe TL, Maes L, Makulu MU, Ghysels G, Vandeven J, et al. (1977) [Epidemiology and drug resistance of salmonella infections in Kinshasa 1974–1975]. Ann Soc Belg Med Trop 57: 545–556. [PubMed] [Google Scholar]
- 32. Cheesbrough JS, Taxman BC, Green SD, Mewa FI, Numbi A (1997) Clinical definition for invasive Salmonella infection in African children. Pediatr Infect Dis J 16: 277–283. [DOI] [PubMed] [Google Scholar]
- 33. Tabu C, Breiman RF, Ochieng B, Aura B, Cosmas L, et al. (2012) Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PLoS One 7: e31237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, et al. (2008) Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis 46: 963–969. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen MV, Sarpong N, Krumkamp R, Dekker D, Loag W, et al. (2012) Incidence and characteristics of bacteremia among children in Rural Ghana. PLoS One 7: e44063 10.1371/journal.pone.0044063 [doi];PONE-D-12-04543 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akinyemi KO, Bamiro BS, Coker AO (2007) Salmonellosis in Lagos, Nigeria: incidence of Plasmodium falciparum-associated co-infection, patterns of antimicrobial resistance, and emergence of reduced susceptibility to fluoroquinolones. J Health Popul Nutr 25: 351–358. [PMC free article] [PubMed] [Google Scholar]
- 37. Hakanen AJ, Lindgren M, Huovinen P, Jalava J, Siitonen A, et al. (2005) New quinolone resistance phenomenon in Salmonella enterica: nalidixic acid-susceptible isolates with reduced fluoroquinolone susceptibility. J Clin Microbiol 43: 5775–5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Boeck H, Miwanda B, Lunguya-Metila O, Muyembe-Tamfum JJ, Stobberingh E, et al. (2012) ESBL-positive enterobacteria isolates in drinking water. Emerg Infect Dis 18 Serial on the Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim SH, Kim S, Chun SG, Park MS, Park JH, et al. (2008) Phage types and pulsed-field gel electrophoresis patterns of Salmonella enterica serovar Enteritidis isolated from humans and chickens. J Microbiol 46: 209–213. [DOI] [PubMed] [Google Scholar]
- 40. Technical Centre for Agricultural and Rural Cooperation (2012) Executive brief: Poultry sector. Agritrade September 2011: 1–9. [Google Scholar]
- 41. USDA (2010) EU poultry meat and egg exports higher. International Egg and Poultry Review 13. [Google Scholar]
- 42. Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, et al. (2006) Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol 55: 585–591. [DOI] [PubMed] [Google Scholar]
- 43. Pang JC, Chiu TH, Helmuth R, Schroeter A, Guerra B, et al. (2007) A pulsed field gel electrophoresis (PFGE) study that suggests a major world-wide clone of Salmonella enterica serovar Enteritidis. Int J Food Microbiol 116: 305–312. [DOI] [PubMed] [Google Scholar]
- 44. Tien YY, Ushijima H, Mizuguchi M, Liang SY, Chiou CS (2012) Use of multilocus variable-number tandem repeat analysis in molecular subtyping of Salmonella enterica serovar Typhi isolates. J Med Microbiol 61: 223–232. [DOI] [PubMed] [Google Scholar]
- 45. Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, et al. (2009) Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19: 2279–2287 gr.091017.109 [pii];10.1101/gr.091017.109 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, et al. (2012) Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet ng.2423 [pii];10.1038/ng.2423 [doi].l. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pulsed-field gel electrophoresis (PFGE) Xba I patterns of 34 S. Typhimurium (a) and 16 S. Enteritidis (b) isolates from DR Congo. Similarity between PFGE patterns was assessed by cluster analysis (Dice coefficient and UPGMA, tolerance and optimization of band position set at 1.5% and 0.5%). * PFGE profile also observed in Salmonella Typhimurium or Salmonella Enteritidis from Belgium.
(TIF)