Abstract
Recent evidence of the occurrence of atypical Chlamydiaceae strains in pigeons, different from the established Chlamydiaceae, requires the development of a specific and rapid detection tool to investigate their prevalence and significance. Here is described a new real-time PCR assay that allows specific detection of atypical Chlamydiaceae from pigeons. The assay has been used to assess the dissemination of these strains in field samples collected from Parisian pigeon populations in 2009. The results suggest a limited dissemination compared to the usually higher prevalence of Chlamydia psittaci that is the main species associated with avian chlamydiosis.
Introduction
Avian chlamydiosis is a zoonotic disease caused by Chlamydia psittaci, an obligatory intracellular bacterium and member of the family Chlamydiaceae [1]. The infection is usually systemic and occasionally fatal in birds. The clinical signs vary greatly in severity and depend on the species and age of the birds as well as the causative strain [2]. Transmission of C. psittaci from birds to humans is reported regularly, particularly in high-risk individuals such as breeders or slaughterhouse workers [3]. Although the importance of C. psittaci as the causative agent of avian chlamydiosis in birds has been known for decades [4], several studies have recently provided evidence of the occurrence of other chlamydial species in birds such as C. abortus [5], [6], C. suis and C. muridarum [7] as well as C. pecorum and C. trachomatis [8]. Additionally, atypical strains of Chlamydiaceae were recently detected in chickens [9], [10], [11] and pigeons [8], [12], [13], [14].
A better characterisation of these atypical Chlamydiaceae and the availability of fast, sensitive and specific detection tools are needed in order to better understand their epidemiological importance. A specific 16S rDNA-based real-time PCR was developed recently for the specific detection of atypical Chlamydiaceae from chickens (hereafter named ACC) [14].
The work reported in this paper deals with the development and validation for routine diagnostic purposes of a real-time PCR based on the enolase A (enoA) gene target for the specific detection of atypical Chlamydiaceae from pigeons (hereafter named ACP). This assay has been used to investigate the dissemination of this agent in Parisian pigeon populations.
Methods
1. Microorganisms
Chlamydial strains and isolates used for determination of specificity, sensitivity and detection limit of the new ACP-specific real-time PCR assay are listed in Tables 1 and 2 . Chlamydiaceae were propagated in the yolk sac of 7 day-old embryonated chicken eggs and stored at −80°C. Non-chlamydial bacterial and fungal strains obtained from the University of Tours (France) and the Anses strain collection (Maisons-Alfort, France) were used for specificity testing. These included: Escherichia coli, Proteus mirabilis, Proteus vulgaris, Klebsiella pneumoniae, Salmonella enteritidis, Salmonella enteritidis serovar Gallinarum, Salmonella enteritidis serovar Typhi, Serratia marcescens, Morganella morganii, Citrobacter freundii, Yersinia enterocolitica, Yersinia pseudotuberculosis, Acinetobacter baumannii, Pasteurella haemolytica, Bordetella bronchiseptica, Staphylococcus aureus, Streptococcus pyogenes, Enterococcus faecalis, Mycobacterium avium subsp. avium, Aspergillus fumigatus, Aspergillus niger and Candida albicans.
Table 1. Characteristics of ACP isolates included in this study.
| ID | Country | Year of Isolation | Initial sample |
| 10–743/SC13 | France | 2010 | Cloacal swab |
| 10–881/SC42 | France | 2010 | Cloacal swab |
| DC96 | Germany | 2011 | Spleen |
| DC97 | Germany | 2012 | Faeces |
| PV_2806/51 | Italy | 1996 | Intestinal content |
| PV_2863/2 | Italy | 1996 | Intestinal content |
| PV_3515/3 | Italy | 1996 | Intestinal content |
| PV_3954/22 | Italy | 1996 | Intestinal content |
| PV_7341/13 | Italy | 1997 | Intestine |
| PV_7344/2 | Italy | 1997 | Intestine |
| PV_155757/2010 | Italy | 2010 | Pool of organs |
| PV_48558/2010 | Italy | 2010 | Pool of organs |
| PV_58394/2012 | Italy | 2012 | Pool of organs |
Table 2. Chlamydial strains and isolates used for determination of specificity of the ACP-specific real-time PCR assay.
| Species | Strains/isolates |
| Atypical Chlamydia from chicken (ACC) | 08–1274/3a, 08–1274/13a, 08–1274/19a, 08–1274/21a, 08–1274/22a, 08-1274/23a |
| C. psittaci | Lothb, VS1c, CP3c, GR9c, TT3c, NJ1c, Cal 10c, VS225c, 06–889a |
| C. abortus | AB7c, 1Bc, LLGd, S26/3c |
| C. pecorum | 824c, AB10c, iB3c, iB3c, LW679c, SBEc |
| C. felis | Dohycate |
| C. caviae | GPICc, 98–3196/1a |
Sources: a Anses Maisons-Alfort, France; b CHU Amiens, France; c INRA Tours, France; d Faculty of Veterinary Medicine – Aristotle University of Thessaloniki, Greece; e Vaccine strain.
2. Preparation of genomic DNA
Chlamydial strains cultivated onto vitellus membranes as well as biological samples were subjected to DNA extraction using the QIAamp DNA Mini Kit (Qiagen, Courteboeuf, France). DNA extracts were stored at −20°C before analysis.
DNA of non-chlamydial samples was extracted using the Instagen Matrix DNA Kit (BioRad, Marnes-la-Coquette, France) according to the manufacturer's instructions. DNA concentration and purity of non-chlamydial extracts were spectrophotometrically assessed by reading A260 and A280 and confirmed by visualization on 0.5% (w/v) agarose gels. Finally, DNAs with a mean concentration of 400 µg/ml were stored at −20°C until required for analysis.
3. Sequencing of the enoA gene locus
YP_enoA5 and YP_enoA6 used for amplification of an approximately 400-bp segment of the enoA gene were previously published by Pannekoek and colleagues [15]. PCR reactions were performed in a final volume of 25 µl. The mix contained 2 µl of DNA template, 2.5 µl of 10× PCR reaction buffer, 1.25 U of HotStarTaq DNA Polymerase (Qiagen, France), 100 µM of each deoxynucleotide triphosphate and 0.5 µM of each primer. The conditions used for amplification were 95°C for 1 min, 45 cycles of 94°C for 30 s, 48°C for 1 min, 72°C for 1 min and 30 s, 72°C for 10 min (final extension). PCR-amplified segments of enoA were sequenced by Eurofins MWG Operon (Nantes, France). Sequence alignments were conducted using the Bionumerics software package version 4.6 (Applied-Maths, Belgium). A dendrogram including one representative of each enoA sequence type for established Chlamydiaceae strains [15], [16], one representative for ACC [14] and one for ACP was constructed using the Unweighted Pair Group Method with Arithmetic Mean algorithm. Nucleotide sequences have been deposited under GenBank accession numbers HE983370 to HE983383.
4. ACP-specific real-time PCR
Specific primers ACP_Fw (5‘-CATGCAAGCTATTGAGAAAAGTGGT -3’) and ACP_Rv (5′- CCTTGATATGTACGTGTTTTCTCG -3′) as well as a specific probe ACP_Pr (5′-FAM- CACCCCTGGTGAAGATATTTCCTTAGCAT -TAMRA 3′) were designed based on enoA and synthesised by Eurofins MWG Operon. Amplification was performed using the 2× TaqMan Universal PCR Master Mix (Applied Biosystems, Courtaboeuf, France). The final volume of the reaction mixture amounted to 20 µl including 10 µl of master mix, 2 µl of DNA sample, 0.6 µM of each primer, 0.1 µM of the probe and sterile PCR water added to reach the final volume. Amplification was carried out at an ABI Prism 7000 thermocycler (Applied Biosystems) using the following cycling parameters: heating at 95 °C for 10 min, 50 cycles of 95°C for 15 s and 60°C for 1 min. For all PCR reactions, DNA of the isolate 10–743/SC13 arbitrarily defined as reference strain for ACP was used as a positive control while sterile water was used as negative control.
5. Performance evaluation of the ACP-specific real-time PCR
The detection limit of the real-time PCR was determined in triplicate (independent runs) using decimal serial dilutions of purified genomic DNA from 10–743/SC13 suspensions equivalent to 3×10−1 to 3×105 inclusion-forming units (IFUs) per PCR reaction. The concentration of this DNA was previously determined by comparison to a DNA purified from cell culture containing defined numbers of IFUs of C. psittaci. The standard curve and the number of IFUs were automatically determined by the software using manually entered IFU standard concentrations. The standard curve was generated by plotting Ct against the logarithm of IFU per PCR reaction mix. The Ct, at which the fluorescence crosses the threshold value, and the lower limit of detection of the assay were calculated automatically.
The specificity of the new real-time PCR was evaluated on 22 Chlamydiaceae DNA samples ( Table 2 ) yielding Ct values between 22 and 30 when tested with the 23S rRNA-based Chlamydiaceae-specific real-time PCR (Chlamydiaceae 23S-rtPCR) [17], and 22 non-chlamydial microorganisms.
DNA of strain 10–743/SC13 was amplified 30 times in the same run to determine intra-assay repeatability, and 11 times in separate runs on different days for inter-assay reproducibility. The respective CVs were then calculated.
6. Chlamydiaceae field samples
A panel of 125 DNAs extracted from cloacal swab samples collected from pigeons in a previous study [13] was used to evaluate the dissemination of the ACP. These samples, which contained an internal positive control (Exogenous internal positive control, TaqMan, Life technologies), were previously tested positive in the Chlamydiaceae 23S-rtPCR [17].
Results and Discussion
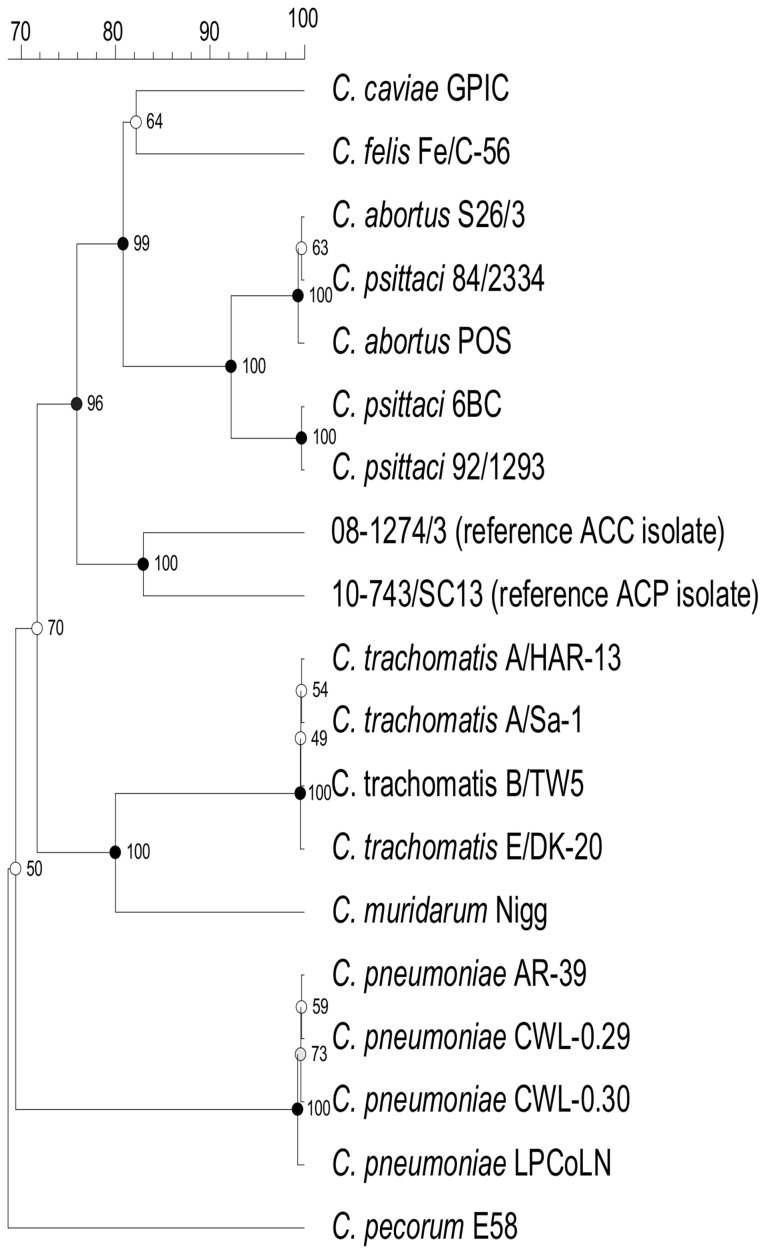
Recent evidence of the occurrence of atypical Chlamydiaceae strains in chickens and pigeons highlighted the necessity of specific and fast detection tools for the investigation of their epidemiological and clinical importance. Thirteen ACP strains have been isolated up to now in France, Germany and Italy ( Table 1 ). A similar approach to the one used for ACC [14] has been adopted in this study, except that the enolase A (enoA) gene has been selected as target. This gene locus had been previously included in a Multi-Locus Sequence Typing scheme for Chlamydia spp. [15], [16]. All enoA sequences of ACP strains in Table 1 proved identical. The consensus sequence was compared in silico to those of the nine established Chlamydiaceae species [15], [16] and also to ACC sequences [14]. Alignment (Figure S1) and phylogenetic analysis ( Figure 1 ) revealed that this sequence was specific to ACP and could be a convenient target for their unambiguous identification by real-time PCR.
Figure 1. Phylogenetic analysis of the enoA sequence for ACC (08–1274/3 reference), ACP (10–743/SC13 reference) and various established Chlamydiaceae strains (one representative of each enoA sequence type; [15] , [16]).
Bootstrap test was for 1000 repetitions. Horizontal distances correspond to genetic distances expressed in percentage of sequence similarity, vertical distances are arbitrary.
The specificity of the newly developed enoA-based real-time PCR assay (enoA-rtPCR) was tested with genomic DNA extracts of several bacterial and fungal species. Neither the DNA samples from non-chlamydial species (n = 22), nor the DNA samples from C. psittaci (n = 9), C. abortus (n = 4), C. pecorum (n = 6), C. felis (n = 1), C. caviae (n = 2) and newly described atypical chicken (ACC) isolates included in this study (n = 6) gave rise to a measurable signal. As expected, all DNA extracts from French (n = 2), German (n = 2) and Italian (n = 9) ACP isolates ( Table 1 ) were successfully amplified (data not shown).
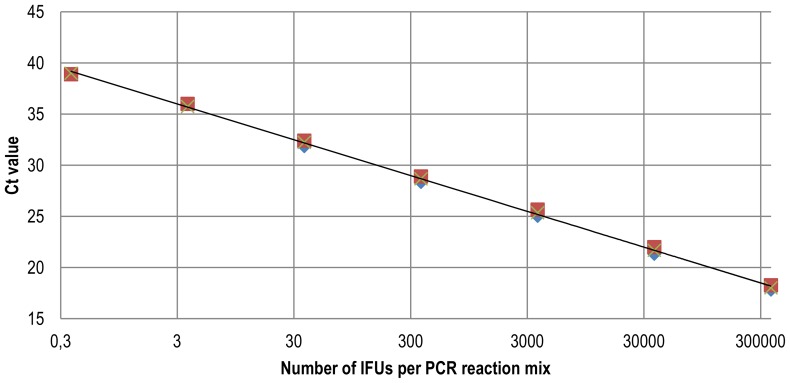
In order to determine the detection limit of the new real-time PCR, a decimal dilution series of the titrated 10–743/SC13 DNA was tested. The results showed that the assay was able to detect the DNA from 3 IFUs of the respective pathogen per PCR reaction with an efficiency of 93% ( Figure 2 ). The intra-assay and inter-assay CVs were 2.5% and 1.9% respectively. Therefore, the real-time PCR assay presented here has proved to be sensitive, specific and reproducible and offers the possibility of assessing the epidemiological importance of ACP infections in pigeon populations.
Figure 2. Real-time PCR sensitivity testing to detect genomic DNA of ACP isolate 10–743/SC13.
DNA was extracted and serially diluted. The concentration (IFU) was calculated from the Ct value based on the calibration curve from serial 10-fold dilutions of a DNA purified from cell culture containing defined numbers of IFUs of C. psittaci. Each dilution was subjected to real-time PCR analysis in triplicate. The r2 linearity value from the linear regression is 0.9987. y = −3.5111×log (x) +37,702, efficiency = 10(−1/slope)−1 = 93%.
To evaluate the suitability of the assay for this purpose, a retrospective examination of previously collected field samples in Parisian feral pigeon populations [13] has been carried out. Out of the 125 Chlamydiaceae-positive samples, 10 were positive (8%) with the new ACP-specific real-time PCR ( Table 3 ), including samples 09–489/LP23 and 09–589/S46 that had previously been identified as ACP by partial ompA and 16S rRNA sequencing [13]. These findings suggest a low dissemination of ACP in comparison with C. psittaci, which prevalence of 68% had been determined ( Table 3 ) using the incA-based C. psittaci-specific real-time PCR (incA-rtPCR C.psittaci) according to Menard and colleagues [18]. A mixed infection involving an ACP strain and C. psittaci was also found in two samples from Genevilliers ( Table 3 ).
Table 3. Summary of results from the study on urban pigeons from Paris.
| City (district number) | 23S-rtPCR Chlamydiaceae | incA-rtPCR C.psittaci | enoA-rtPCR ACP |
| No. of +ve samples* | No. of +ve samples* | No. of +ve samples | |
| Clamart_La Plaine (92) | 10/29 | 8/10 | 1/10 |
| Clamart_Maison Blanche (92) | 9/41 | 6/9 | 1/9 |
| Clamart_Trivaux (92) | 5/25 | 5/5 | 0/5 |
| Courbevoie (92) | 15/60 | 11/15 | 1/15 |
| Creil (60) | 16/66 | 13/16 | 1/16 |
| Fontenay sous bois_La Fontaine (93) | 7/77 | 6/7 | 0/7 |
| Genevilliers (92) | 23/142 | 12/23 | 6/12 |
| Pantin (93) | 4/71 | 4/4 | 0/4 |
| Paris_Jussieu (V) | 13/69 | 7/13 | 0/13 |
| Paris_La Roquette (XI) | 3/11 | 1/3 | 0/3 |
| Paris_Montreuil (XX) | 9/21 | 4/9 | 0/9 |
| Paris_Sacré Cœur (XVIII) | 5/19 | 3/5 | 0/5 |
| Paris_St Denis (X) | 2/36 | 1/2 | 0/2 |
| Paris_Vanves (XIV) | 4/27 | 4/4 | 0/4 |
| Total | 125/694 (18%) | 85/125 (68%) | 10/125 (8%) |
see reference [13]
Given the low number of samples tested and the limited geographical range covered, the results of the present study can only be regarded as preliminary. Atypical pigeon strains have been isolated in the laboratories of the authors from the intestinal content or organs of necropsied urban pigeons. Their pathogenicity for pigeons is unknown. Another open issue is the host range. A recently isolated atypical parrot strain from Germany (designated 10DC88) was found to possess an enoA sequence identical to those of ACP, so that it was positive with the ACP-specific real-time PCR assay. This indicates that ACP seem not to be restricted to pigeons and could possibly be circulating in other avian hosts.
All in all, as a rapid and specific diagnostic tool is now available, it seems straightforward to include ACP investigation to analyses of diagnostic samples from birds and to undertake systematic studies to characterize the epidemiology and etiology of ACP infections in avian populations.
Conclusions
A sensitive and specific real-time PCR assay for the detection of atypical Chlamydiaceae from pigeons has been developed and preliminary validated. The use of this new tool could contribute to a better understanding of the importance of these bacterial agents.
Supporting Information
Alignment of partial eno A sequence for ACC (08–1274/3 reference), ACP (10–743/SC13 reference) and various established Chlamydiaceae strains (one representative of each enoA sequence type; [15] , [16] ). Nucleotide homologies are represented by dots. The numbers refer to alignment positions. ACP-specific primer sequences are boxed and the probe sequence is underlined.
(TIF)
Acknowledgments
We are grateful to S. Thierry, D. Albert and G. Le Carrou (Anses Maisons-Alfort, France) for providing non-chlamydial materials and for their precious advice.
Funding Statement
This work was supported by grants from Region Ile-de-France (post-doctoral fellowship and Sustainable Development 145 Network R2DS, No. 2008-07). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Greub G (2010) International Committee on Systematics of Prokaryotes. Subcommittee on the taxonomy of the Chlamydiae: minutes of the closed meeting, 21 June 2010, Hof bei Salzburg, Austria. Int J Syst Evol Microbiol 60: 2694. [DOI] [PubMed] [Google Scholar]
- 2. Andersen AA (1997) Two new serovars of Chlamydia psittaci from North American birds. J Vet Diagn Invest 9: 159–164. [DOI] [PubMed] [Google Scholar]
- 3. Deschuyffeleer TP, Tyberghien LF, Dickx VL, Geens T, Saelen JM, et al. (2012) Risk assessment and management of Chlamydia psittaci in poultry processing plants. Ann Occup Hyg 56(3): 340–9 Review. [DOI] [PubMed] [Google Scholar]
- 4. Vanrompay D, Ducatelle R, Haesebrouck F (1995) Chlamydia psittaci infections: a review with emphasis on avian chlamydiosis. Vet Microbiol 45: 93–119. [DOI] [PubMed] [Google Scholar]
- 5. Herrmann B, Rahman R, Bergstrom S, Bonnedahl J, Olsen B (2000) Chlamydophila abortus in a Brown skua (Catharacta antarctica lonnbergi) from a subantarctic island. Appl Environ Microbiol 66: 3654–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pantchev A, Sting R, Bauerfeind R, Tyczka J, Sachse K (2009) New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. Vet J 181: 145–150. [DOI] [PubMed] [Google Scholar]
- 7.Lemus JA, Fargallo JA, Vergara P, Parejo D, Banda E (2010) Natural cross chlamydial infection between livestock and free-living bird species. PLoS One 5: e13512. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Sachse K, Kuehlewind S, Ruettger A, Schubert E, Rohde G (2012) More than classical Chlamydia psittaci in urban pigeons. Vet Microbiol 157: 476–480. [DOI] [PubMed] [Google Scholar]
- 9. Gaede W, Reckling KF, Dresenkamp B, Kenklies S, Schubert E, et al. (2008) Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health 55: 184–188. [DOI] [PubMed] [Google Scholar]
- 10. Laroucau K, Vorimore F, Aaziz R, Berndt A, Schubert E, et al. (2009) Isolation of a new chlamydial agent from infected domestic poultry coincided with cases of atypical pneumonia among slaughterhouse workers in France. Infect Genet Evol 9: 1240–1247. [DOI] [PubMed] [Google Scholar]
- 11. Robertson T, Bibby S, O'Rourke D, Belfiore T, Lambie H, et al. (2009) Characterization of Chlamydiaceae species using PCR and high resolution melt curve analysis of the 16S rRNA gene. J Applied Microbiol 107: 2017–2028. [DOI] [PubMed] [Google Scholar]
- 12.Vicari N, Laroucau K, Vorimore F, Barbieri I, Sachse K, et al. (2009) Molecular analysis of four chlamydial isolates from the intestine and cloacal swabs of feral pigeons sampled in Milan and Ferrara, Italy. (Oral communication). 1st European Meeting on Animal Chlamydioses and Zoonotic Aspects.Murcia, Spain. 14–16/06/09. [Google Scholar]
- 13. Gasparini J, Erin N, Bertin C, Jacquin L, Vorimore F, et al. (2011) Impact of urban environment and host phenotype on the epidemiology of Chlamydiaceae in feral pigeons (Columba livia). Environ Microbiol 13: 3186–3193. [DOI] [PubMed] [Google Scholar]
- 14. Zocevic A, Vorimore F, Marhold C, Horvatek D, Wang D, et al. (2012) Molecular characterization of atypical Chlamydia and evidence of their dissemination in different European and Asian chicken flocks by specific real-time PCR. Environ Microbiol 14(8): 2212–2222. [DOI] [PubMed] [Google Scholar]
- 15. Pannekoek Y, Morelli G, Kusecek B, Morre SA, Ossewaarde JM, et al. (2008) Multi locus sequence typing of Chlamydiales: clonal groupings within the obligate intracellular bacteria Chlamydia trachomatis . BMC Microbiol 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pannekoek Y, Dickx V, Beeckman DS, Jolley KA, Keijzers WC, et al. (2010) Multi locus sequence typing of Chlamydia reveals an association between Chlamydia psittaci genotypes and host species. PLoS ONE 5: e14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K (2006) Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes 20(1): 60–63. [DOI] [PubMed] [Google Scholar]
- 18. Ménard A, Clerc M, Subtil A, Mégraud F, Bébéar C, et al. (2006) Development of a real-time PCR for the detection of Chlamydia psittaci . J Med Microbiol 55: 471–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of partial eno A sequence for ACC (08–1274/3 reference), ACP (10–743/SC13 reference) and various established Chlamydiaceae strains (one representative of each enoA sequence type; [15] , [16] ). Nucleotide homologies are represented by dots. The numbers refer to alignment positions. ACP-specific primer sequences are boxed and the probe sequence is underlined.
(TIF)