Abstract
Background and Aims
Cystic Fibrosis associated liver disease (CFLD) develops in approximately 30% of CF patients. However, routine sensitive diagnostic tools for CFLD are lacking. Within this study, we aimed to identify new experimental biomarkers for the detection of CFLD.
Methods
45 CF patients were included in the study and received transient elastography. Differential regulation of 220 different serum proteins was assessed in a subgroup of patients with and without CFLD. Most interesting candidate proteins were further quantified and validated by ELISA in the whole patient cohort. To assess a potential relation of biomarker expression to the degree of hepatic fibrosis, serum biomarkers were further determined in 18 HCV patients where liver histology was available.
Results
43 serum proteins differed at least 2-fold in patients with CFLD compared to those without liver disease as identified in proteome profiling. In ELISA quantifications, TIMP-4 and Endoglin were significantly up-regulated in patients with CFLD as diagnosed by clinical guidelines or increased liver stiffness. Pentraxin-3 was significantly decreased in patients with CFLD. Serum TIMP-4 and Endoglin showed highest values in HCV patients with liver cirrhosis compared to those with fibrosis but without cirrhosis. At a cut-off value of 6.3 kPa, transient elastography compassed a very high diagnostic accuracy and specificity for the detection of CFLD. Among the biomarkers, TIMP-4 and Endoglin exhibited a high diagnostic accuracy for CFLD. Diagnostic sensitivities and negative predictive values were increased when elastography and TIMP-4 and Endoglin were combined for the detection of CFLD.
Conclusions
Serum TIMP-4 and Endoglin are increased in CFLD and their expression correlates with hepatic staging. Determination of TIMP-4 and Endoglin together with transient elastography can increase the sensitivity for the non-invasive diagnosis of CFLD.
Introduction
Cystic fibrosis associated liver disease (CFLD) has a cumulative incidence of approximately 30% [1], [2] and accounts for 2.5% of the overall mortality of CF patients, thereby representing the third most common cause of death in CF patients [3].
Due to the high prevalence, the early onset and the often progressive course of CFLD, the reliable recognition of CF patients at risk of developing CFLD is of urgent clinical necessity [4]. To date, liver histology is still the most commonly used gold standard for the assessment of chronic liver diseases. However, as only 1/50000 of the liver volume is evaluated with liver biopsy, it is controversially discussed in focally distributed liver disease and thereby not generally recommended for the assessment of CFLD [4], [5]. Further, liver biopsy exhibits a significant intra-/interobserver variability [4], [6], [7], [8] and, due to its invasive nature, is limited in its acceptance and repetition, especially in children [9], [10], [11]. Current guideline criteria recommend a combination of physical examination, liver biochemistry and ultrasound to diagnose CFLD [4]; however, the reliable identification of CF patients at risk of developing CFLD remains a major clinical challenge [4].
Consequently, research efforts have been focused on the development of non-invasive methods for the diagnosis of CFLD. First studies have successfully evaluated liver stiffness measured by transient elastography (TE) for the assessment of CFLD [12], [13], [14]. Additionally, a growing understanding of the pathogenesis of hepatic fibrosis identified non-invasive quantitative serum biomarkers of hepatic fibrogenesis, which are pathophysiologically derived from extracellular matrix (ECM) turnover and might directly translate the molecular pathogenesis of fibrosis into clinical application. Thereby, these so-called class I fibrosis markers, can act as potentially powerful serum biomarkers of hepatic fibrosis [14], [15], [16].
Within this study, we followed a dual intent: First, we aimed to identify novel serum biomarkers of liver disease in CF patients using a proteome profiling approach. Based on these results, we secondly verified increased expression of selected promising candidate proteins in CFLD by ELISA and further analysed their diagnostic value for the assessment of CFLD in comparison to that of TE. Using this approach, our results identify TIMP-4 and Endoglin as novel and promising serum markers of liver disease in CF patients that hold the potential to facilitate the non-invasive assessment of CFLD.
Materials and Methods
Patients
This study has been conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all participating patients or their parents. The study was approved by the ethics committee of the medical faculty of the Justus-Liebig-University Giessen (Gaffkystrasse 11c, 35392 Giessen, Germany) with the approval no. 75/09. The diagnosis of CF was established by sweat test and later confirmed by genetic tests in all subjects. All patients were treated according to European and U.S. guidelines [17], [18]. After exclusion of other causes for chronic liver disease, the diagnosis of CFLD was established according to recent guidelines [4] if least two of the following conditions on at least two consecutive examinations spanning a one-year period were present: (i) Hepatomegaly (liver span >2 cm below the costal margin on the medioclavicular line) confirmed by ultrasound, (ii) two abnormal serum liver enzyme levels (ALT, AST, γGT > ULN), (iii) ultrasound abnormalities other than hepatomegaly (increased, heterogeneous echogenicity, nodularity, irregular margins).
To correlate the expression of experimental biomarkers to the histologic degree of hepatic fibrosis deposition, a cohort of 18 patients with chronic Hepatitis C Virus (HCV) infection was studied. In HCV patients, liver biopsy samples were taken via right intercostal space from the right liver lobe after desinfection and local anesthesia of the skin with an 18 gauge needle (Menghini needle, Braun-Melsungen, outer diameter 1.2 mm). An experienced pathologist who was blinded to the patients’ clinical results analyzed the biopsy specimens. Liver fibrosis stages were evaluated semi-quantitatively according to the Desmet/Scheuer [16] scoring system.
Transient elastography (TE)
Liver stiffness by TE was evaluated using the same FibroScan® (Echosens, Paris, France) device in all patients. Non-invasive measurements were performed by a single experienced investigator blinded to the clinical status of the patients on the right lobe of the liver through the intercostal space at a depth of 25 and 65 mm from skin surface. In children below 15 kg of weight the FibroScan® S probe, developed for liver stiffness measurements in children, was used. For each patient, the stiffness value was calculated as the median of ten successful measurements. TE was considered valid if 10 successful measurements with a success rate ≥ 60% and an interquartile range ≤ 30% of the median were obtained. Results are expressed in kilopascal (kPa). Total examination time was approximately 5 minutes per patient.
Routine laboratory tests and determination of biomarkers of hepatic fibrosis
All patients underwent routine haematological and biochemical investigations on the day of TE. The following proteome profiling arrays were used (all R&D Systems, Minneapolis, USA): human chemokine array kit (Cat. No. ARY017), human angiogenesis array kit (Cat. No. ARY007), human cytokine array panel A (Cat. No. ARY005), human soluble receptor array kit, non-hematopoietic panel (ARY012). Serum proteome profiler analyses for each array were at least repeated twice in two independent experiments and the volume of serum used ranged from 50 μl to 400 μl. TIMP-4, Endoglin, HGF and Pentraxin-3 were further quantified in undiluted serum (TIMP-4, Pentraxin-3) or in 1∶2 and 1∶10 diluted serum (HGF and Endoglin) with using commercially available ELISA Kits (Pentraxin-3: human Pentraxin-3 ELISA, BlueGene Biotech CO, Shanghai, China; TIMP-4: human TIMP-4 Quantikine ELISA Kit, Cat. No. DTM400; Endoglin: human Endoglin/CD105 Quantikine ELISA Kit, Cat. No. DNDG00; HGF: human HGF Quantikine ELISA Kit, Cat. No. DHG00, all R&D Systems, Minneapolis, USA).
Statistical analysis
Statistical analysis was performed with SPSS 17.0 (SPSS Inc, Chicago, Ill). Normal distribution of the data was tested using the Kolmogorov–Smirnov test and visualization of histograms. Failing to meet criteria for normal distribution, differences in TE values and serum markers between patients with and without CFLD were assessed using the Mann-Whitney U test for unpaired samples. Liver stiffness and expression of serum markers are shown in Box-and-Whisker Plots. The upper and lower hinges of the box represent the 75th and 25th percentile, respectively. The line indicates the median value; error bars represent the minimum and maximum. Values deviating from the box by 1.5- to 3-fold interquartile range were defined as outliers (o). Significant differences are pointed out (*p<0.05, **p<0.01). Measurement agreement between clinical markers of CFLD and the novel diagnostic modalities assessed in the this studies has been performed in Bland-Altman-Analyses [19]. To directly compare the concordance of the differently scaled clinical and experimental markers every measured value “a” of the respective test was transferred into a commensurable variable “a′” using the following equation: a′ = (a- ā)/sa.
The diagnostic performances of TE and fibrosis markers were assessed by receiver operating characteristic (ROC) curves. The ROC curve is a plot of sensitivity versus (1 – specificity) for all possible cut-off values for the prediction of different fibrosis stages. The most commonly used index of accuracy is the area under the ROC (AUROC) curve with values close to 1.0 indicating a high diagnostic accuracy.
Results
Patient characteristics and proteome profile screening in patients with CFLD
From 45 CF patients that were consecutively enrolled between June 2008 and December 2010, 17 ( = 38%) were diagnosed with CFLD according to recent guidelines [4]. CF patients with CFLD had significantly elevated levels of liver transaminases and γ-GT whereas albumin levels and platelet counts were decreased in patients with CFLD compared to those without CFLD. Demographic and clinical characteristics of the CF study populations are shown in Table 1.
Table 1. Demographic and clinical data of the CF patient cohort.
| CF patients (n = 45) | ||
| Characteristics | no CFLD (n = 28) | CFLD (n = 17) |
| Demographic and clinical data | ||
| Male (n/%) | 17/61% | 9/53% |
| Female (n/%) | 11/39% | 8/47% |
| Age (y) | ||
| mean (median) ± SD | 21.4 (20.5) ± 11.8 | 29 (28) ± 10.8 |
| range | 5 – 50 | 14 – 47 |
| BMI | ||
| mean (median) ± SD | 19 (18.9) ± 3.6 | 20.3 (20.3) ± 2.1 |
| Pancreas insufficiency (n) | ||
| no pancreatic insufficiency | 5 | 0 |
| pancreatic insufficiency | 23 | 17 |
| Treatment with UDCA (n) | 9 | 15 |
| Biochemistry | ||
| Alanine aminotransferase (U/L) | ||
| mean (median) ± SD | 23 (22) ± 7.4 | 35 (33) ± 18 * |
| range | 12 – 50 | 12 – 79 |
| Aspartate aminotransferase (U/L) | ||
| mean (median) ± SD | 21 (20) ± 6.3 | 33 (27) ± 20.3 ** |
| range | 10 – 36 | 11 – 98 |
| γ-glutamyl transpeptidase (U/L) | ||
| mean (median) ± SD | 14 (14) ± 6.5 | 86 (26) ± 110 ** |
| range | 6 – 34 | 9 – 321 |
| Alkaline Phosphatase (U/L) | ||
| mean (median) ± SD | 179 (126) ± 106 | 248 (168) ± 185 |
| range | 62 – 452 | 84 – 668 |
| Bilirubin (mg/dL) | ||
| mean (median) ± SD | 0.42 (0.4) ± 0.22 | 0.94 (0.68) ± 0.76 |
| range | 0.1 – 0.9 | 0.2 – 2.18 |
| Albumin (g/dL) | ||
| mean (median) ± SD | 4.5 (4.5) ± 0.23 | 4.2 (4.1) ± 0.47 ** |
| range | 4.0 – 4.9 | 3.1 – 4.8 |
| Prothrombin time (%) | ||
| mean (median) ± SD | 91 (95) ± 11 | 81 (84) ± 20.6 |
| range | 71 – 112 | 45 – 108 |
| Platelet count (G/L) | ||
| mean (median) ± SD | 306 (316) ± 66 | 226 (277) ± 120 * |
| range | 131 – 464 | 18 – 362 |
significantly different, P <0.05
significantly different, P <0.01
UDCA: Ursodeoxycholic Acid
To identify potential candidate proteins which might act as biomarkers of hepatic fibrosis we initially screened a broad range of differentially regulated serum proteins in patients with CFLD and those without. For this purpose, serum of 4 patients with CFLD and of 4 patients without CFLD was applied to 4 different serum proteome profilers, thereby assessing key proteins and enzymes involved in angiogenesis (Figure 1), soluble receptors and related proteins released by non-hematopoietic cells and including key proteins involved in the metabolism of the extracellular matrix (Figure 2 and 3) and chemokines regulating the migration of monocytes, neutrophils, and lymphocytes (Figure 4). Serum proteome profiler analyses for each array were at least repeated twice in two independent experiments. Using this approach, we comparatively assessed the expression of a total of serum 220 proteins in patients without CFLD and those with proven CFLD. Based on optical densitometry analyses of the proteome profilers, 36 serum proteins were at least 2-fold increased in patients with CFLD compared to those without CFLD. Many of the proteins found up-regulated in CFLD have previously been described in fibrotic processes of different organs and/or liver fibrosis such as GM-CSF, ADAMTS-1, IP-10, PDGF-AB/-BB, TGF-ß1 or Activin A. Another 9 serum proteins were found to be at least 2-fold decreased in our serum proteome analysis.
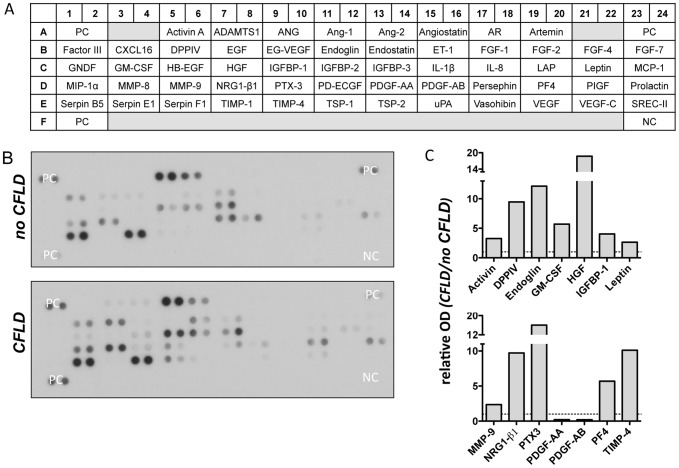
Figure 1. Assessment of angiogenesis related proteins in patients with CFLD.
Relative expression of 55 different angiogenesis related proteins (A) were determined from pooled serum from patients with established CFLD (n = 4) and those without liver disease (n = 4). All proteins were determined in duplicate and based on optical densitometry of the corresponding bands (B), angiogenesis related proteins that were at least 2-fold differentially regulated in patients with CFLD compared to those without were identified. A total of 12 angiogenesis proteins were at least 2-fold increased in CFLD with highest relative expression observed for DDPIV, Endoglin, HGF, NRG1-β1, Pentraxin-3 and TIMP-4 (C). PDGF-AA and PDGF-BB were at least 2-fold decreased in patients with CFLD compared to those without (C). PC: positive control, NC: negative control, OD: optical density
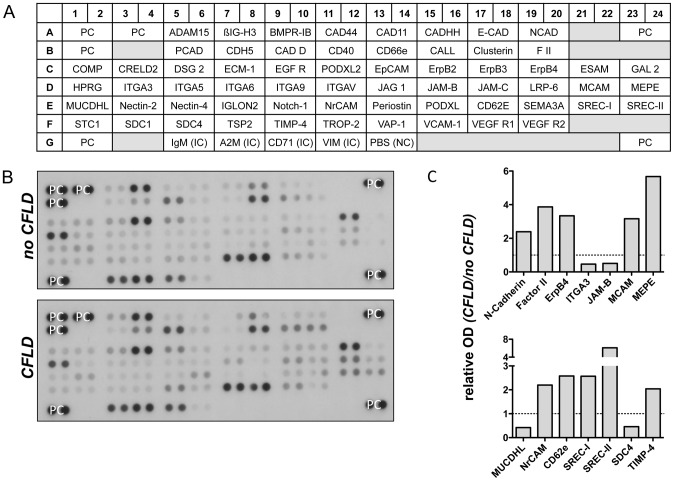
Figure 2. Assessment of soluble receptors and related proteins from non-hematopoietic cells in patients with CFLD (Array A).
Relative expression of 62 different soluble receptors and related proteins from non-hematopoietic proteins (A) were determined from pooled sera from patients with established CFLD (n = 4) and those without liver disease (n = 4). All proteins were determined in duplicate and based on optical densitometry of the corresponding bands (B), proteins were identified that were at least 2-fold differentially regulated in patients with CFLD compared to those without. A total of 10 soluble receptors and related proteins from non-hematopoietic cells were at least 2-fold increased in CFLD with highest relative expression observed for MEPE and SREC-II (C). ITGA3, JAM-B, MUCDHL and SDC4 were at least 2-fold decreased in patients with CFLD compared to those without (C). PC: positive control, NC: negative control, IC: internal control, OD: optical density
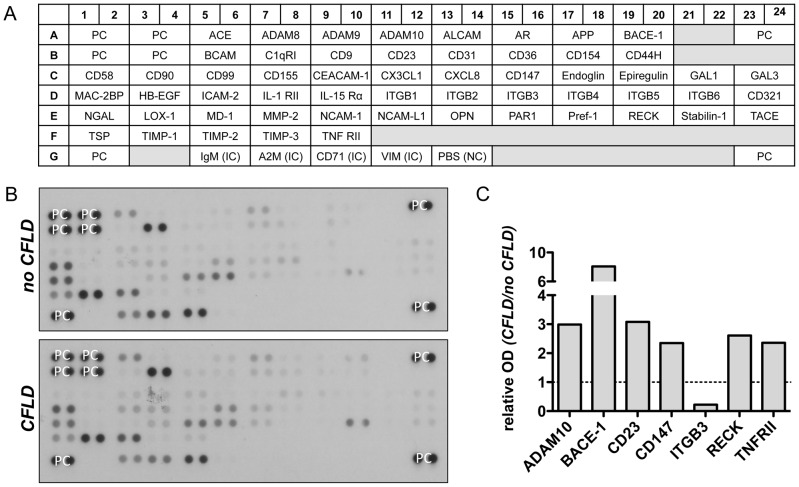
Figure 3. Assessment of soluble receptors and related proteins from non-hematopoietic cells in patients with CFLD (Array B).
Relative expression of 57 different soluble receptors and related proteins from non-hematopoietic proteins (A) were determined from pooled serum from patients with established CFLD (n = 4) and those without liver disease (n = 4). All proteins were determined in duplicate and based on optical densitometry of the corresponding bands (B), proteins were identified that were at least 2-fold differentially regulated in patients with CFLD compared to those without. ADAM10, BACE-1, CD23, CD147, RECK, TNFRII were at least 2-fold increased in CFLD whereas ITGB3 was at least 2-fold decreased in patients with CFLD compared to those without (C). PC: positive control, NC: negative control, IC: internal control, OD: optical density
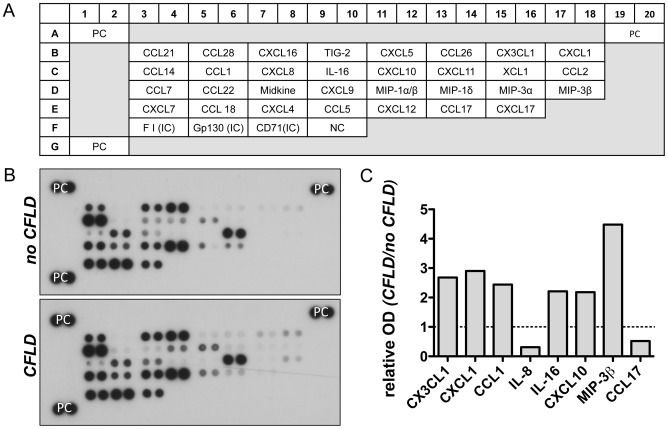
Figure 4. Assessment of chemokines in patients with CFLD.
Relative expression of 31 different chemokines regulating the migration of monocytes, neutrophils, and lymphocytes (A) were determined from pooled serum from patients with established CFLD (n = 4) and those without liver disease (n = 4). All proteins were determined in duplicate and based on optical densitometry of the corresponding bands (B), proteins were identified that were at least 2-fold differentially regulated in patients with CFLD compared to those without. CX3CL1, CXCL1, CCL1, IL-16, CXCL10 and MIP-3β were at least 2-fold increased in CFLD whereas IL-8 and CCL17 was at least 2-fold decreased in patients with CFLD compared to those without (C). ). PC: positive control, NC: negative control, IC: internal control, OD: optical density
Due to their relatively high abundance in CFLD patients and their pathophysiologic relation to the metabolism of extracellular matrix and/or angiogenesis as key events for the development of hepatic matrix deposition, we chose to further validate and quantify the serum levels of TIMP-4, Endoglin, Hepatocyte growth factor (HGF), and Pentraxin-3 (PTX3) in ELISA measurements in the whole CF cohort of 45 patients.
Expression of serum fibrosis biomarkers in patients with CFLD
Serum was available from all 45 CF patients for quantification of the above-mentioned proteins by ELISA. All measurements were performed in duplicate.
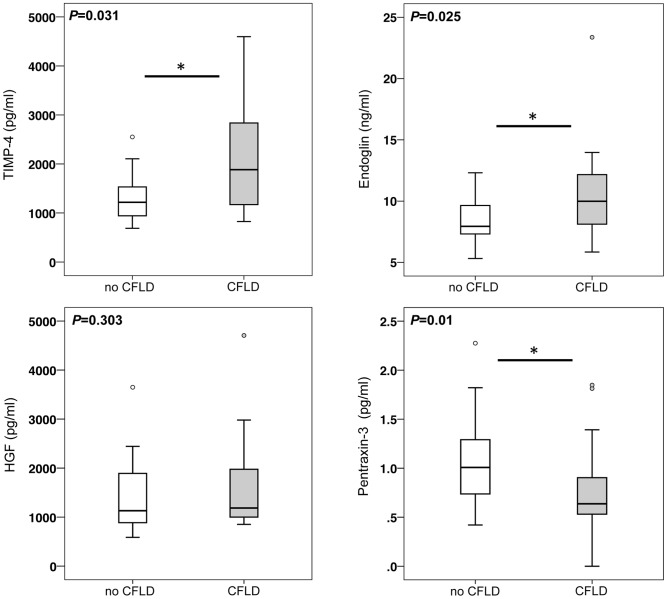
CF Patients with CFLD diagnosed according to recent guidelines exhibited significantly increased serum levels of TIMP-4 and Endoglin compared to those without liver disease. In contrast, serum PTX3 was significantly decreased in CF patients with hepatopathy. Serum levels of HGF did not significantly differ between CF patients with liver disease and those without (Figure 5).
Figure 5. Increased serum concentrations of TIMP-4 and Endoglin and decreased levels of pentraxin-3 in CF patients with liver disease.
In the whole CF cohort, patients with CFLD (n = 17) exhibited significantly higher serum levels of TIMP-4 and Endoglin than patients without liver disease (n = 28). Pentraxin-3 serum levels were significantly decreased in patients with CFLD whereas serum concentration of hepatocyte growth factor (HGF) was unchanged between CF patients with and without CFLD.
As a further means for the assessment of the expression of serum fibrosis markers in CFLD, we measured liver stiffness values by transient elastography (TE) in our patient cohort, which has been proven to accurately diagnose liver disease and hepatic fibrosis of various etiologies, including CFLD [13], [14], [20], [21]. For this purpose we initially assessed the diagnostic value of TE in our CF patient cohort. As shown in Figure 6, with a median of 9.95 kPa, patients with CFLD exhibited a significantly higher liver stiffness compared to patients without liver disease, in which a median liver stiffness of 4.3 kPa was measured. Further, with an AUROC of 0.906, TE exhibited an excellent diagnostic accuracy in receiver operating characteristic (ROC) analysis and the sum of diagnostic sensitivity and specificity for the detection of CFLD was calculated to be maximal at a cut-off of 6.3 kPa based on ROC analysis (Table 2 and 3).
Figure 6. Increased liver stiffness in patients with CFLD.
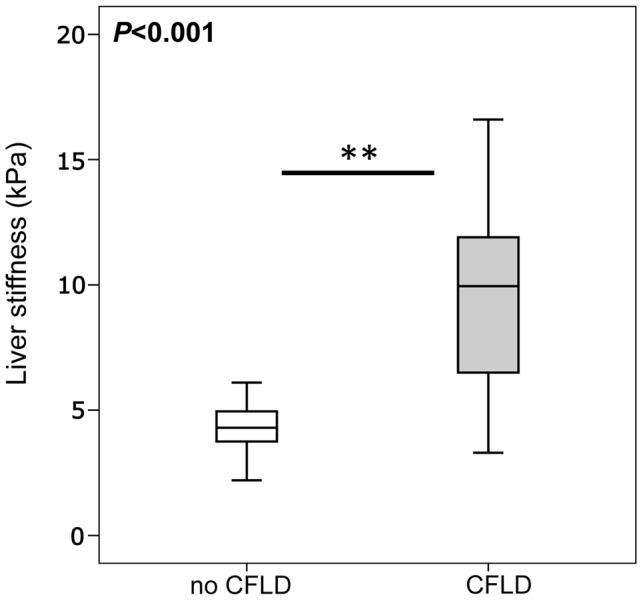
Median liver stiffness measured by transient elastography, was significantly increased in patients with CFLD (n = 17) compared to patients without liver disease (n = 28) (CFLD: 9.95 kPa vs no CFLD: 4.3 kPa). CFLD was diagnosed according to recent best practice guidelines [4].
Table 2. Diagnostic accuracy of novel diagnostic markers and clinical markers for the detection of CFLD.
| ROC analysis | |||
| AUC | 95% CI | p-value | |
| Novel diagnostic markers | |||
| Transient elastography | 0.906 | 0.779 – 1.000 | <0.001 |
| TIMP-4 | 0.693 | 0.520 – 0.866 | 0.031 |
| Endoglin | 0.702 | 0.533 – 0.871 | 0.025 |
| Clinical markers | |||
| APRI | 0.748 | 0.584 – 0.912 | 0.008 |
| Alkaline phosphatase (ALP) | 0.611 | 0.437 – 0.785 | 0.215 |
ROC: receiver operating characteristic; AUC: area under the curve; CI: confidence interval; APRI: AST/Platelets-Ratio-Index
Table 3. Diagnostic performances of novel diagnostic markers and clinical markers for the detection of CFLD.
| Diagnostic performances | ||||||
| Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| Clinical markers | ||||||
| APRI | 0.133 | 46.7 | 96.4 | 87.5 | 77.1 | |
| (22.3 – 72.6) | (79.8 – 99.8) | (46.7 – 99.3) | (59.4 – 89) | |||
| ALP | # | 70.6 | 81.5 | 70.6 | 81.5 | |
| (44 – 88.6) | (61.3 – 93) | (44 – 88.6) | (61.3 – 93) | |||
| Novel markers | ||||||
| - alone | ||||||
| TE | 6.3 kPa | 82 | 100 | 100 | 90.33 | |
| (55.8 – 95.3) | (85 – 100) | (73.2 – 100) | (73.1 – 97.5) | |||
| TIMP-4 | 1603 pg/ml | 64.7 | 82.1 | 68.8 | 79.3 | |
| (38.6 – 84.7) | (62.4 – 93.2) | (41.5 – 87.9) | (59.7 – 91.3) | |||
| Endoglin | 8.6 ng/ml | 70.6 | 71.4 | 60 | 80 | |
| (44 – 80.6) | (51.1 – 86) | (36.4 – 80) | (58.7 – 92.4) | |||
| - in combination | ||||||
| TIMP-4 + | 1603 pg/ml | 88.2 | 53.6 | 53.6 | 88.2 | |
| Endoglin | 8.6 ng/ml | (62.3 – 98) | (34.2 – 72) | (34.2 – 72) | (62.3 – 98) | |
| TE + | 6.3 kPa | 88.2 | 71.4 | 65.2 | 90.9 | |
| Endoglin | 8.6 ng/ml | (62.3 – 98) | (51.1 – 86) | (42.8 – 82.8) | (69.4 – 98.4) | |
| TE + | 6.3 kPa | 100 | 82.1 | 77.3 | 100 | |
| TIMP-4 | 1603 pg/ml | (77.1 – 100) | (62.4 – 93.2) | (54.2 – 91.3) | (82.2 – 100) | |
age and gender specific cut-off, values determined by the Department for Laboratory Medicine and Clinical Chemistry of the University Hospital Giessen according to the International Federation of Clinical Chemistry
PPV: positive predictive value; NPV: negative predictive value; CI: confidence interval
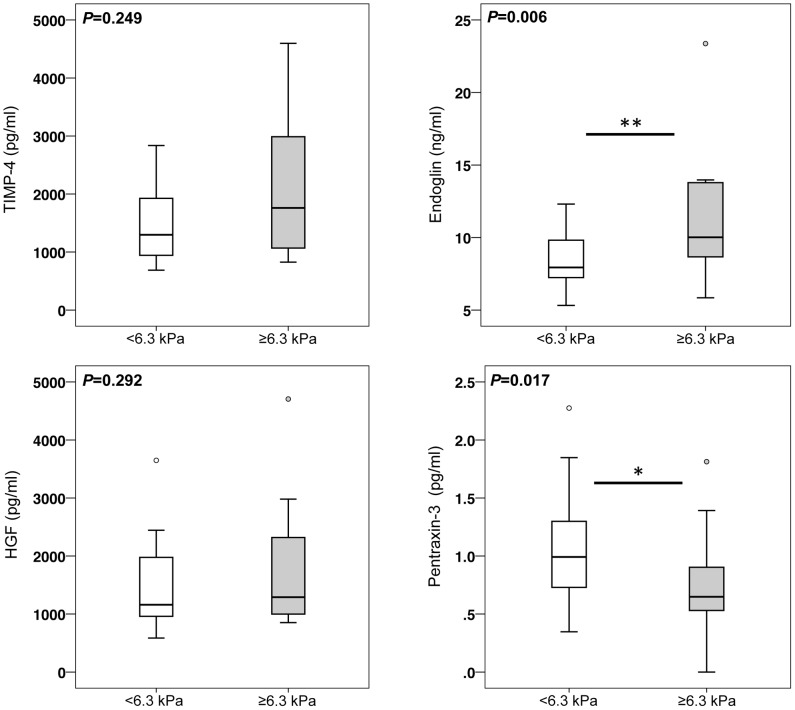
When serum levels of hepatic fibrosis markers were then compared in patients with TE values below or above this threshold, we observed similar results to those obtained from the comparison of patients with and without CFLD as defined by recent guidelines: Endoglin was significantly increased in patients with liver stiffness values above 6.3 kPa whereas PTX3 was again significantly decreased in patients with TE values above 6.3 kPa (Figure 7). As seen in patients with CFLD as diagnosed by guideline criteria, TIMP-4 serum levels were also increased in patients with stiffness values >6.3 kPa compared to those with values below 6.3 kPa, although these differences did not reach statistical significance. Serum expression of HGF was unchanged between patient with and without CFLD, as diagnosed by TE with a cut-off of 6.3 kPa (Figure 7).
Figure 7. Increased serum levels of Endoglin and decreased concentration of pentraxin-3 in CF patients with increased liver stiffness. CF patients with liver stiffness values above a threshold value of 6.3 kPa, indicative for CFLD, exhibited significantly higher serum levels of Endoglin and significantly lower serum levels of pentraxin-3 compared to CF patients with liver stiffness values below 6.3 kPa.
TIMP-4 was also increased in patients with a liver stiffness above the threshold of 6.3 kPa, although these elevations did not reach statistical significance. The cut-off value of 6.3 kPa for the identification of patients with CFLD was derived from ROC analyses as value at which the sum of diagnostic sensitivity and specificity for the detection of CFLD was maximal (Table 3).
To address CF lung manifestation and pulmonary fibrosis as a potential confounder of the above results of a significant regulation of TIMP-4, Endoglin and PTX3 in the presence of liver disease, we assessed their serum expression in CF patients with a forced expiratory volume in one second (FEV1) below and above 70%, with a vital capacity (VC) of below and above 80%, and with a ratio between FEV1 and VC below and above 70% (FEV1/VC), which serve as established indicators of CF lung disease and have been the primary outcome in many clinical trials [17], [22], [23], [24]. Of note, none of the above serum markers was different in patients with and without impairment of lung function as assessed by FEV1, VC and FEV1/VC (Figure S1). Further, serum marker levels were unaltered between patients without pancreatic insufficiency (PI, Figure S2), indicating that the increased expression of TIMP-4 and Endoglin and the decreased expression of PTX3 occur indeed relatively specific for the existence of liver disease without being affected by pancreas and lung disease as other major manifestations of CF.
As treatment with Ursodeoxycholic acid (UDCA) improves liver function tests and biliary drainage and thus represents the guideline recommended therapy for CFLD [4], we further assessed whether liver stiffness and biomarker expression was influenced by the treatment with UDCA. While TE values were slightly increased in patients that received UDCA, the biomarkers TIMP-4, Endoglin and PTX3 were not significantly altered between CF patients with and without UDCA treatment (Figure S3) and there was no association between the duration of UDCA therapy and liver stiffness and biomarker expression (data not shown).
Expression of serum markers of hepatic fibrosis correlates with hepatic staging
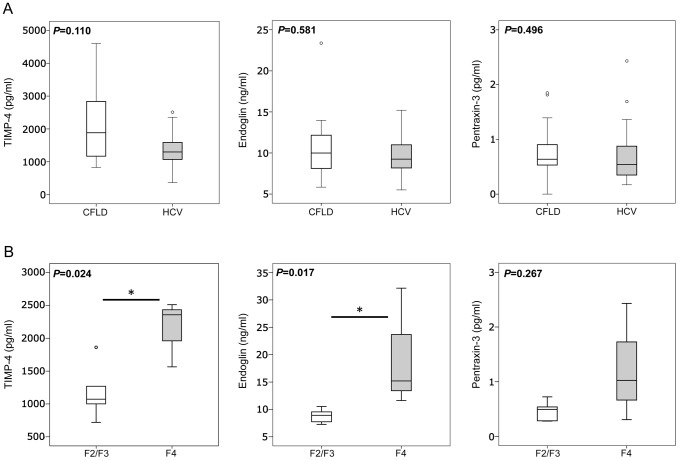
Based on the above results we hypothesized that serological expression of TIMP-4, Endoglin and Pentraxin-3 might correlate with hepatic staging as determined by liver histology. However, due to the high percentage of children (35.6%) in our CF patient cohort and as liver staging by histology has further been controversially discussed or even to be unreliable in focally distributed liver disease as seen in CFLD, we did not assess liver histology in our CF patients. We instead assessed serum expression of TIMP-4, Endoglin and Pentraxin-3 in 18 patients with HCV that underwent liver histology during routine clinical care. Interestingly, serum levels of all three biomarkers were comparable and without significant differences between patients with CFLD and patients with HCV liver disease (Figure 8A). When serum levels of TIMP-4, Endoglin and Pentraxin-3 were then compared between HCV patients with complete fibrosis/cirrhosis (F = 4) and those HCV with portal fibrosis with few or numerous fibrotic septae but without cirrhosis (F = 2/3), significantly increased levels of TIMP-4 and Endoglin in HCV patients with complete fibrosis/cirrhosis were found. Pentraxin-3 remained unchanged in the different HCV fibrosis stages (Figure 8B). Taken together, these results suggest that increased expression of TIMP-4 and Endoglin represents a mechanism specific for hepatic fibrosis that correlates with hepatic staging but is irrespective of the underlying etiology of liver disease.
Figure 8. Increased serum levels of TIMP-4 and Endoglin in HCV patients with complete liver cirrhosis.
Serum levels of TIMP-4, Endoglin and Pentraxin-3 were comparable and without significant differences between patients with CFLD and those with HCV liver disease (top row, A). In the cohort of patients with HCV liver disease, patients with complete fibrosis/cirrhosis (F = 4) exhibited significantly increased serum levels of TIMP-4 and Endoglin compared those HCV patients with portal fibrosis with few or numerous fibrotic septae but without cirrhosis (F = 2/3), whereas Pentraxin-3 remained unchanged in the different HCV fibrosis stages (bottom row, B).
Diagnostic performances of biomarkers of hepatic fibrosis and transient elastography for the detection of CFLD
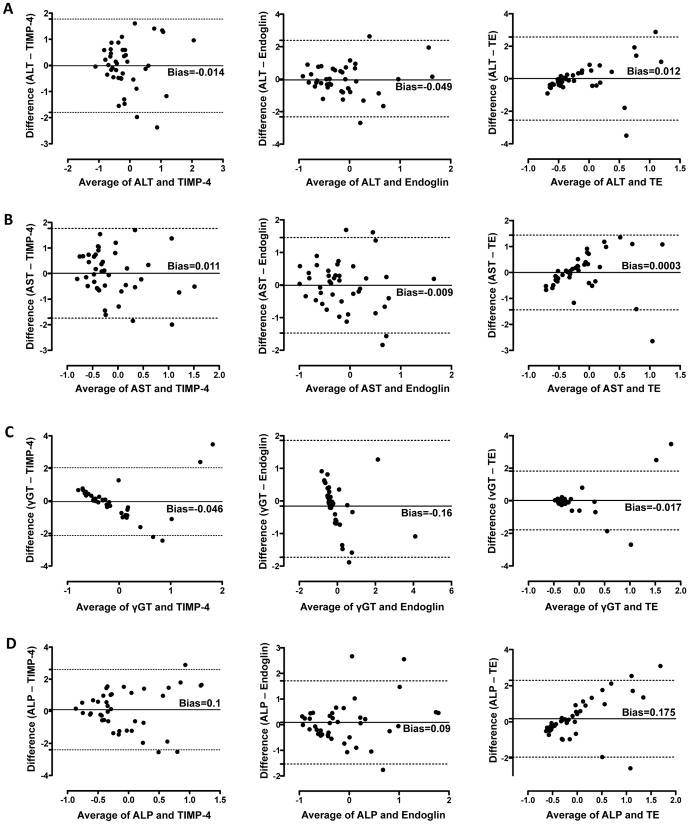
Based on these results, we hypothesized that the observed increased expression of TIMP-4 and Endoglin in CFLD might well be usable in diagnostic approaches. To provide an outlook on a potential diagnostic utility of TIMP-4, Endoglin and TE for CFLD, we first compared the agreement of TIMP-4, Endoglin and TE measurements to levels of ALT, AST, γGT and ALP as commonly used and established parameters for CFLD diagnostic in Bland-Altman Analyses. As shown in Figure 9, TIMP-4 and Endoglin showed very good agreement with ALT (Figure 9A) and AST (Figure 9B) levels. Comparison of the concordance of TE and levels of ALT (Figure 9A), AST (Figure 9B) and γGT (Figure 9C) showed good agreement of the different methods likewise; however, a greater bias was observed with higher average values. Further, when clinical cholestasis markers were compared with the novel modalities for CFLD diagnosis assessed in this report, Bland-Altman Analyses revealed the presence of a proportional error in the agreement of γGT and the biomarkers TIMP-4 and Endoglin (Figure 9C) and in the agreement of ALP and TE (Figure 9D).
Figure 9. Concordance between clinical markers and novel diagnostic markers for CFLD.
Measurement agreement between AST, ALT, γGT and ALP as commonly used clinical markers of CFLD and TIMP-4, Endoglin and transient elastography as novel diagnostic modalities was assessed in Bland-Altman Analyses. TIMP-4 and Endoglin showed very good agreement with ALT (A) and AST (B) levels. Comparison of the concordance of TE and levels of ALT (A), AST (B) and γGT (C) showed good agreement of the different methods although a greater bias was observed with higher average values. A proportional error was observed in the agreement of γGT and the biomarkers TIMP-4 and Endoglin (C) and in the agreement of ALP and TE (D). The dashed line shows 95% limits of agreement, the solid line indicates the systemic error (bias).
We then aimed to directly compare the diagnostic performance of TIMP-4, Endoglin and TE as novel diagnostic markers for CFLD to clinical markers of hepatopathy. Importantly, the liver enzymes AST, ALT and γGT were part of the diagnostic algorithm used for identification of patients with CFLD in this study, thereby making it impossible to assess their diagnostic performance accurately and objectively within this study. To counteract this potential selection bias of AST, ALT and γGT in their assessment of the diagnostic value of common clinical markers, we instead calculated the diagnostic performances of the AST/Platelets-Ratio-Index (APRI) as a non-invasive fibrosis index [25], [26] and that of alkaline phosphatase, which also has not been part of the diagnostic algorithm to diagnose CFLD. As assessed in ROC analyses, with and AUROC of 0.908 TE exhibited an excellent accuracy for the detection of CFLD. Further, with an AUROC of 0.702 and 0.693, Endoglin and TIMP-4 exhibited a good accuracy for CFLD diagnosis (Table 2). Importantly, TE exhibited a by far superior diagnostic accuracy for CLFD compared to APRI and ALP whereas the biomarkers Endoglin and TIMP-4 exhibited a better (AP) or almost equal diagnostic accuracy (APRI) when compared to the clinical markers (Table 2).
At a cut-off of 8.6 ng/ml for Endoglin and 1603 pg/ml for TIMP-4, the sum of sensitivity and specificity were calculated to be maximal for the detection of CFLD (Table 3).
Importantly, when Endoglin or TIMP-4 at these cut-off values were then combined with TE for the diagnosis of CFLD, the diagnostic sensitivity of TE improved considerably: for TE alone, a diagnostic sensitivity of 82% was calculated; when TE was combined with TIMP-4 or Endoglin, the sensitivity improved to 100% and 88%, respectively. As a result, the negative predicative value was also considerably improved when TE was combined with TIMP-4 and Endoglin compared to TE alone. The diagnostic performances of TE, Endoglin, and TIMP-4 alone or combined and in comparison to the clinical markers ALP and APRI are shown in Table 3.
Discussion
Currently established approaches used for diagnosis and follow-up of CFLD exhibit certain limitations such low sensitivity (liver enzymes, ultrasound), invasiveness and sampling error (biopsy), exposure to radiation (CT scan) or high costs (MRI) [27].
However, as CFLD occurs in approximately 30% of all CF patients [1], [2], [28] and in 5 to 10% of CF patients progression towards multilobular cirrhosis occurs early in life [1], [2], reliable non-invasive diagnostic tests for CFLD are urgently needed. Regarding the special requirements in children, non-invasive tools should also enable a fast but still accurate CFLD screening in daily practice and allow for cost-effective longitudinal follow-up.
With the intention to identify novel candidate protein acting as serum markers of liver disease, we initially screened a broad range of serum protein in patients with and without CFLD using a proteome profiling approach. Based on these results we then quantified expression of TIMP-4, Endoglin, HGF and Pentraxin-3 as promising and novel serum markers in a larger cohort of CF patients and comparatively analysed their diagnostic value for the detection of CFLD compared to that of TE. Our results provide evidence (i) that serum TIMP-4 and Endoglin are significantly increased in patients with CFLD compared to those without while serum Pentraxin-3 levels are significantly decreased in CFLD patients; (ii) that TIMP-4 and Endoglin show highest expression in patients with complete liver fibrosis/cirrhosis as exemplified in HCV patients (iii) that TIMP-4 and Endoglin exhibit a good diagnostic accuracy for the detection of CFLD; (iv) that the determination of serum TIMP-4 and Endoglin together with transient elastography can increase the diagnostic sensitivity for the non-invasive detection of CFLD.
Our results of elevated liver stiffness in patients with CFLD corroborate the results of previous studies. In a cohort of pediatric patients with liver disease of various etiologies, among them 20 patients with CF, Breton and co-workers showed that patients with liver disease had significantly increased liver stiffness values [29]. Specifically, in CFLD, Witters and co-workers recently demonstrated that adults and children with CFLD had a significantly elevated mean liver stiffness of 8.8 kPa compared to 5 kPa in individuals without CFLD [13]. In a very recent report, it was further shown that CF patients with CFLD had increased TE values compared to patients without CFLD, with highest liver stiffness measurements seen in patients with cirrhosis [21]. Interestingly, with a mean liver stiffness of 9.95 kPa in patients with CFLD compared to 4.3 kPa in those without CFLD, we found very similar data in our total patient cohort compared to those described by Witters and colleagues. Furthermore, a cut-off of 6.3 kDa was calculated to be optimal for the detection of CFLD within the present study, thereby corroborating the cut-off defined in previous studies on the detection of CFLD by TE [14], [21].
Apart from TE, research has identified potentially powerful quantitative serum biomarkers of hepatic fibrosis [14], [15], [16]. Above all, so called class I markers which are pathophysiological derived from the metabolism of the extracellular matrix, directly translate the molecular pathogenesis of fibrosis into clinical application and therefore represent promising fibrosis markers [15]. Generally spoken, an ideal marker for liver fibrosis should have a high sensitivity and specificity, and should also be easily assessable inexpensive, accurate, and reproducible for follow-up [30]. Serum fibrosis markers are easily obtained and, unlike special diagnostic procedures such as transient elastography that is mainly limited to larger clinics and centers, are widely available and therefore represent and an appealing possibility to fulfill these outlined criteria. Given these considerations, we chose to screen a broad spectrum of candidate proteins that might serve as serum markers of liver disease and hepatic fibrosis in CFLD patients using an unbiased proteome profiling approach. We simultaneously determined the relative expression of a total of 220 serum proteins in patients with CFLD and those without. Our results show that 36 serum proteins are at least 2-fold increased in patients with CFLD. Among these up-regulated proteins, we found several proteins that have been well described in the context of hepatic fibrosis, e.g. such as LAP/TGF-ß1 [31], [32], members of the activin family [33], the plasminogen activator system such as uPA [34], or members of the CX3C chemokine family such as fractalkine [35]. Apart from that, our study identified further proteins previously not been described in the context of liver disease and fibrosis. Based on their relatively high abundance in the proteome screen and there close pathophysiologic relation to the metabolism of extracellular matrix and/or angiogenesis as key events for the development of hepatic matrix deposition, we chose to further validate and quantify the serum levels of TIMP-4, Endoglin, Hepatocyte growth factor (HGF), and Pentraxin-3 (PTX3) in ELISA measurements in the whole CF cohort of 45 patients.
Tissue inhibitors of metalloproteinases (TIMPs) have been shown to be critically involved in hepatic fibrogenesis. Their fibrogenetic potential is believed to be mediated by the inhibition of ECM degradation and the subsequent accumulation of fibrotic tissue [36]. Enhanced expression of TIMP-1 and TIMP-2 occurs in rat models of liver injury [37] and in various human liver diseases [38]. In patients with chronic HCV infection, TIMP-1 mRNA levels correlate with the grade of liver fibrosis [39] and HCV itself is able to stimulate TIMP-1 mRNA expression [40]. Serum TIMP-2 and liver TIMP-2 mRNA are also increased in HCV induced liver disease [40], [41]. TIMP-4 mRNA has been shown to be increased in a model of experimental biliary atresia [42], but apart from that data on the role of TIMP-4 for liver disease and hepatic fibrosis are scarce. Due to their prominent role in hepatic fibrogenesis, members of the TIMP-family have been utilized in diagnostic panels and indices [43], [44]. In this report, we now show that TIMP-4 as a previously rather unrecognized member of the TIMP-family is significantly increased in cystic fibrosis associated liver disease. As a proof of principle in HCV patients, we also provide evidence that TIMP-4 serum levels show highest values in patients with complete liver cirrhosis. Further, in the CF patient cohort TIMP-4 exhibited a high accuracy for the detection of liver disease and when used in combination with transient elastography for CFLD diagnosis, the diagnostic sensitivity and negative prediction was considerably improved. Thereby, TIMP-4 holds the potential to be a complimentary serum marker that facilitates the accurate non-invasive assessment of CFLD.
Endoglin is a TGF-β co-receptor that is involved in the regulation of the activity of TGF-β signaling, a key cytokine for hepatic fibrogenesis. Apart from endothelial cells, Endoglin is expressed on a variety of profibrogenic cell populations such as mesangial cells, scleroderma and cardiac fibroblasts and hepatic stellate cells (HSC) [45], [46], [47], [48]. A recent study has shown that Endoglin expression is increased in transdifferentiating hepatic stellate cells in vitro and in models of liver fibrosis in vivo [49]. As mechanism of action, Endoglin interacts with and is phosphorylated by TβRII and it has been shown in vitro that overexpression of Endoglin leads to TGF-β1-driven Smad1/5 phosphorylation and α-smooth muscle actin expression in a HSC, thereby promoting HSC activation [49]. First reports evaluated circulating Endoglin levels in patients with liver disease of diverse etiologies [50], [51]. Here, high Endoglin levels showed a significant association with the severity of chronic liver disease [50], [51], suggesting an active role for Endoglin in the fibrotic process.
In this report, we observed a significantly increased Endoglin expression in cystic fibrosis associated liver disease. Similar to our observations for TIMP-4, Endoglin serum levels were highest in HCV patients with complete liver cirrhosis, suggesting a close relation to hepatic staging. Likewise, Endoglin exhibited a high accuracy for the detection of CFLD and increased the diagnostic sensitivity and negative prediction considerably when used in combination with transient elastography. Thereby, our study identifies Endoglin as a further promising serum marker with the potential to improve the accurate non-invasive assessment of CFLD.
Hepatocyte growth factor is a mesenchyme-derived growth factor mainly produced by monocytes and hepatocytes. Although originally believed to be a liver specific mitogen [52], HGF is now recognized not only as a potent mitogenic growth factor on hepatocytes, but rather as a pleiotropic factor that exhibits mitogenic, motogenic, morphogenic, and antiapoptotic activities on various cell types. In the liver, HGF is produced by nonparenchymal cells, such as Kupffer cells, sinusoidal endothelial cells (SECs), and HSCs and the degree of liver HGF expression correlates with serum HGF levels [53]. The clinical significance of serum HGF levels has been assessed in acute hepatic failure and chronic liver diseases. In this respect it has been shown that HGF serum levels correlate with hepatic fibrosis and might serve as a prognostic maker in acute liver failure [54], [55], [56], [57]. Although the proteome screening within this report showed a relatively high abundance in patients with CFLD compared to those without, we were not able to verify this result in ELISA quantifications within the whole CF cohort. It might be speculated that one of the reasons for this might be due to the pleiotropic functions and lack of liver specificity of HGF as discussed above.
Pentraxins comprise a superfamily of evolutionary well-conserved proteins that are characterized by the pentraxin domains as a characteristic structural motif [58]. The short PTXs CrP and serum amyloid P are produced in the liver in response to inflammatory cytokines and therefore are classical acute-phase proteins. PTX3, as a prototypic member of the long pentraxin family, is induced primary upon inflammatory stimuli in a variety of cells, including monocytes/macrophages, dendritic cells, endothelial cells, vascular smooth muscle cells, fibroblasts, and adipocytes [59]. Due to the prominent role in the crossroad between innate immunity, ECM deposition and vascular biology [59], PTX3 serum levels have been assessed in a variety of diseases including vasculitis [60], myocardial infarction [61], [62] and systemic inflammation [63]. Importantly, serum PTX3 levels have been shown to be increased in patients with non-alcoholic steatohepatitis (NASH) and also to correlate with the histological staging in NASH [64]. As a rather unexpected result, we observed a significantly decreased expression of PTX3 in patients with CFLD rather an increase in our study. Similarly to HGF, it might well be that these discrepancies are due to the multiple functions of PTX3 on the one hand and also the different etiologies of liver disease, i.e. non-focally distributed NASH compared to focally distributed CFLD, on the other hand.
In conclusion, we used a proteome profiling based approach to identify serum markers of CFLD within the current study. Our results are the first to identify TIMP-4 and Endoglin as novel serum markers that can accurately diagnose CFLD. Further, their determination may confirm and enhance the diagnostic accuracy of TE for the detection of CFLD.
Supporting Information
Concentrations of serum biomarkers in relation to the severity of CF lung disease. CF patients were stratified into those with a forced expiratory volume in one second (FEV1) below and above 70%, with a vital capacity (VC) of below and above 80%, and with a ratio between FEV1 and VC below and above 70% (FEV1/VC), all of which serve as established indicators of the severity CF lung disease. Neither TIMP-4 nor Endoglin or Pentraxin-3 differed in patients with and without impairment of lung function as assessed by FEV1, VC and FEV1/VC.
(TIF)
Concentrations of serum biomarkers in CF patients with and without pancreatic insufficiency. CF patients were stratified into those with (PI) and without pancreatic insufficiency (no PI). Neither TIMP-4 nor Endoglin or Pentraxin-3 differed in patients with and without pancreatic insufficiency.
(TIF)
Liver stiffness and concentrations of serum biomarkers in CF patients with and without treatment of UDCA. CF patients were stratified into those with (UDC) and without existing therapy with Ursodeoxycholic acid (no UDCA). While patients with UDCA exhibited a slightly increased liver stiffness, neither TIMP-4 nor Endolgin or Pentraxin-3 differed in patients with and without existing UDCA therapy.
(TIF)
Acknowledgments
We thank the Mukoviszidose Förderverein Giessen, especially Prof. H. Lindemann, for the grateful support of our work. The authors acknowledge excellent technical assistance from Annette Tschuschner. The authors are grateful for the help from the staff of the GI division and CF outpatient clinic for their help in conducting this study.
Funding Statement
This work was supported by grants from the Deutsche Forschungsgemeinschaft (RO 957/7-1 and RO 957/8-1), a research Grant of the University Medical Center Giessen and Marburg (UKGM 10/2010 GI), and from ZooMAP (01KI1003E, Bundesministerium für Bildung und Forschung, BMBF). Dr. Timo Rath has received grants for young researchers (“Anschubfinanzierung”) from the Justus-Liebig-University Giessen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, et al. (2002) Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology 36: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 2. Lindblad A, Glaumann H, Strandvik B (1999) Natural history of liver disease in cystic fibrosis. Hepatology 30: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 3. Moyer K, Balistreri W (2009) Hepatobiliary disease in patients with cystic fibrosis. Curr Opin Gastroenterol 25: 272–278. [DOI] [PubMed] [Google Scholar]
- 4. Debray D, Kelly D, Houwen R, Strandvik B, Colombo C (2011) Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 10 Suppl 2S29–36. [DOI] [PubMed] [Google Scholar]
- 5. Sokol RJ, Durie PR (1999) Recommendations for management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr 28 Suppl 1S1–13. [DOI] [PubMed] [Google Scholar]
- 6. Bedossa P, Dargere D, Paradis V (2003) Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 38: 1449–1457. [DOI] [PubMed] [Google Scholar]
- 7. Colloredo G, Guido M, Sonzogni A, Leandro G (2003) Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 39: 239–244. [DOI] [PubMed] [Google Scholar]
- 8. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, et al. (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 97: 2614–2618. [DOI] [PubMed] [Google Scholar]
- 9. Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344: 495–500. [DOI] [PubMed] [Google Scholar]
- 10. Cadranel JF, Rufat P, Degos F (2000) Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32: 477–481. [DOI] [PubMed] [Google Scholar]
- 11. Castera L, Negre I, Samii K, Buffet C (1999) Pain experienced during percutaneous liver biopsy. Hepatology 30: 1529–1530. [DOI] [PubMed] [Google Scholar]
- 12.Malbrunot-Wagner AC, Bridoux L, Nousbaum JB, Riou C, Dirou A, et al.. (2011) Transient elastography and portal hypertension in pediatric patients with cystic fibrosis Transient elastography and cystic fibrosis. J Cyst Fibros. [DOI] [PubMed]
- 13. Witters P, De Boeck K, Dupont L, Proesmans M, Vermeulen F, et al. (2009) Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. J Cyst Fibros 8: 392–399. [DOI] [PubMed] [Google Scholar]
- 14. Rath T, Menendez KM, Kugler M, Hage L, Wenzel C, et al. (2012) TIMP-1/-2 and transient elastography allow non invasive diagnosis of cystic fibrosis associated liver disease. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 44: 780–787. [DOI] [PubMed] [Google Scholar]
- 15. Gressner AM, Gao CF, Gressner OA (2009) Non-invasive biomarkers for monitoring the fibrogenic process in liver: a short survey. World J Gastroenterol 15: 2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rath T, Roderfeld M, Guler C, Wenzel C, Graf J, et al. (2011) YKL-40 and transient elastography, a powerful team to assess hepatic fibrosis. Scandinavian Journal of Gastroenterology 46: 1369–1380. [DOI] [PubMed] [Google Scholar]
- 17. Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr, et al. (2007) Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 176: 957–969. [DOI] [PubMed] [Google Scholar]
- 18. Kerem E, Conway S, Elborn S, Heijerman H (2005) Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros 4: 7–26. [DOI] [PubMed] [Google Scholar]
- 19. Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310. [PubMed] [Google Scholar]
- 20. Menten R, Leonard A, Clapuyt P, Vincke P, Nicolae AC, et al. (2010) Transient elastography in patients with cystic fibrosis. Pediatric radiology 40: 1231–1235. [DOI] [PubMed] [Google Scholar]
- 21. Karlas T, Neuschulz M, Oltmanns A, Guttler A, Petroff D, et al. (2012) Non-invasive evaluation of cystic fibrosis related liver disease in adults with ARFI, transient elastography and different fibrosis scores. PloS one 7: e42139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramsey BW, Boat TF (1994) Outcome measures for clinical trials in cystic fibrosis. Summary of a Cystic Fibrosis Foundation consensus conference. The Journal of pediatrics 124: 177–192. [DOI] [PubMed] [Google Scholar]
- 23. Kerem E, Conway S, Elborn S, Heijerman H (2005) Standards of care for patients with cystic fibrosis: a European consensus. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 4: 7–26. [DOI] [PubMed] [Google Scholar]
- 24. Gault R (2002) Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association 14: 157–158. [DOI] [PubMed] [Google Scholar]
- 25. Baranova A, Lal P, Birerdinc A, Younossi ZM (2011) Non-invasive markers for hepatic fibrosis. BMC gastroenterology 11: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castera L (2011) Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best practice & research Clinical gastroenterology 25: 291–303. [DOI] [PubMed] [Google Scholar]
- 27. Colombo C, Russo MC, Zazzeron L, Romano G (2006) Liver disease in cystic fibrosis. J Pediatr Gastroenterol Nutr 43 Suppl 1S49–55. [DOI] [PubMed] [Google Scholar]
- 28. Herrmann U, Dockter G, Lammert F (2010) Cystic fibrosis-associated liver disease. Best Pract Res Clin Gastroenterol 24: 585–592. [DOI] [PubMed] [Google Scholar]
- 29. Breton E, Bridoux-Henno L, Guyader D, Danielou H, Jouan H, et al. (2009) [Value of transient elastography in noninvasive assessment in children's hepatic fibrosis]. Arch Pediatr 16: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 30. Schuppan D, Afdhal NH (2008) Liver cirrhosis. Lancet 371: 838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bissell DM, Roulot D, George J (2001) Transforming growth factor beta and the liver. Hepatology 34: 859–867. [DOI] [PubMed] [Google Scholar]
- 32. Gressner AM, Weiskirchen R, Breitkopf K, Dooley S (2002) Roles of TGF-beta in hepatic fibrosis. Frontiers in bioscience : a journal and virtual library 7: d793–807. [DOI] [PubMed] [Google Scholar]
- 33. Rodgarkia-Dara C, Vejda S, Erlach N, Losert A, Bursch W, et al. (2006) The activin axis in liver biology and disease. Mutation research 613: 123–137. [DOI] [PubMed] [Google Scholar]
- 34. Zhang LP, Takahara T, Yata Y, Furui K, Jin B, et al. (1999) Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. Journal of hepatology 31: 703–711. [DOI] [PubMed] [Google Scholar]
- 35. Garcia-Alvarez M, Berenguer J, Guzman-Fulgencio M, Micheloud D, Catalan P, et al. (2011) High plasma fractalkine (CX3CL1) levels are associated with severe liver disease in HIV/HCV co-infected patients with HCV genotype 1. Cytokine 54: 244–248. [DOI] [PubMed] [Google Scholar]
- 36. Hemmann S, Graf J, Roderfeld M, Roeb E (2007) Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. Journal of hepatology 46: 955–975. [DOI] [PubMed] [Google Scholar]
- 37. Roeb E, Purucker E, Breuer B, Nguyen H, Heinrich PC, et al. (1997) TIMP expression in toxic and cholestatic liver injury in rat. J Hepatol 27: 535–544. [DOI] [PubMed] [Google Scholar]
- 38. Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ (1996) Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 110: 821–831. [DOI] [PubMed] [Google Scholar]
- 39. Yata Y, Takahara T, Furui K, Zhang LP, Jin B, et al. (1999) Spatial distribution of tissue inhibitor of metalloproteinase-1 mRNA in chronic liver disease. J Hepatol 30: 425–432. [DOI] [PubMed] [Google Scholar]
- 40. Lichtinghagen R, Bahr MJ, Wehmeier M, Michels D, Haberkorn CI, et al. (2003) Expression and coordinated regulation of matrix metalloproteinases in chronic hepatitis C and hepatitis C virus-induced liver cirrhosis. Clin Sci (Lond) 105: 373–382. [DOI] [PubMed] [Google Scholar]
- 41. Boker KH, Pehle B, Steinmetz C, Breitenstein K, Bahr M, et al. (2000) Tissue inhibitors of metalloproteinases in liver and serum/plasma in chronic active hepatitis C and HCV-induced cirrhosis. Hepatogastroenterology 47: 812–819. [PubMed] [Google Scholar]
- 42. Nadler EP, Li X, Onyedika E, Greco MA (2010) Differential expression of hepatic fibrosis mediators in sick and spontaneously recovered mice with experimental biliary atresia. The Journal of surgical research 159: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, et al. (2004) Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127: 1704–1713. [DOI] [PubMed] [Google Scholar]
- 44. Patel K, Gordon SC, Jacobson I, Hezode C, Oh E, et al. (2004) Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. Journal of hepatology 41: 935–942. [DOI] [PubMed] [Google Scholar]
- 45. Rodriguez-Barbero A, Obreo J, Eleno N, Rodriguez-Pena A, Duwel A, et al. (2001) Endoglin expression in human and rat mesangial cells and its upregulation by TGF-beta1. Biochemical and biophysical research communications 282: 142–147. [DOI] [PubMed] [Google Scholar]
- 46. Meurer SK, Tihaa L, Lahme B, Gressner AM, Weiskirchen R (2005) Identification of endoglin in rat hepatic stellate cells: new insights into transforming growth factor beta receptor signaling. The Journal of biological chemistry 280: 3078–3087. [DOI] [PubMed] [Google Scholar]
- 47. Leask A, Abraham DJ, Finlay DR, Holmes A, Pennington D, et al. (2002) Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis and rheumatism 46: 1857–1865. [DOI] [PubMed] [Google Scholar]
- 48. Chen K, Mehta JL, Li D, Joseph L, Joseph J (2004) Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circulation research 95: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 49. Meurer SK, Tihaa L, Borkham-Kamphorst E, Weiskirchen R (2011) Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cellular signalling 23: 683–699. [DOI] [PubMed] [Google Scholar]
- 50. Yagmur E, Rizk M, Stanzel S, Hellerbrand C, Lammert F, et al. (2007) Elevation of endoglin (CD105) concentrations in serum of patients with liver cirrhosis and carcinoma. European journal of gastroenterology & hepatology 19: 755–761. [DOI] [PubMed] [Google Scholar]
- 51. Clemente M, Nunez O, Lorente R, Rincon D, Matilla A, et al. (2006) Increased intrahepatic and circulating levels of endoglin, a TGF-beta1 co-receptor, in patients with chronic hepatitis C virus infection: relationship to histological and serum markers of hepatic fibrosis. Journal of viral hepatitis 13: 625–632. [DOI] [PubMed] [Google Scholar]
- 52. Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, et al. (1989) Molecular cloning and expression of human hepatocyte growth factor. Nature 342: 440–443. [DOI] [PubMed] [Google Scholar]
- 53. Moriyama M, Matsumura H, Watanabe A, Oshiro S, Aoki H, et al. (2005) Evaluation of serum concentrations of human hepatocyte growth factor during interferon therapy for chronic hepatitis C. Intervirology. 48: 223–229. [DOI] [PubMed] [Google Scholar]
- 54. Yamagami H, Moriyama M, Tanaka N, Arakawa Y (2001) Detection of serum and intrahepatic human hepatocyte growth factor in patients with type C liver diseases. Intervirology 44: 36–42. [DOI] [PubMed] [Google Scholar]
- 55. Shiota G, Okano J, Kawasaki H, Kawamoto T, Nakamura T (1995) Serum hepatocyte growth factor levels in liver diseases: clinical implications. Hepatology 21: 106–112. [PubMed] [Google Scholar]
- 56. Tsubouchi H, Kawakami S, Hirono S, Miyazaki H, Kimoto M, et al. (1992) Prediction of outcome in fulminant hepatic failure by serum human hepatocyte growth factor. Lancet 340: 307. [DOI] [PubMed] [Google Scholar]
- 57. Andersen ES, Ruhwald M, Moessner B, Christensen PB, Andersen O, et al. (2011) Twelve potential fibrosis markers to differentiate mild liver fibrosis from cirrhosis in patients infected with chronic hepatitis C genotype 1. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 30: 761–766. [DOI] [PubMed] [Google Scholar]
- 58. Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, et al. (2006) Pentraxins as a key component of innate immunity. Current opinion in immunology 18: 10–15. [DOI] [PubMed] [Google Scholar]
- 59. Garlanda C, Bottazzi B, Bastone A, Mantovani A (2005) Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annual review of immunology 23: 337–366. [DOI] [PubMed] [Google Scholar]
- 60. Fazzini F, Peri G, Doni A, Dell'Antonio G, Dal Cin E, et al. (2001) PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis and rheumatism 44: 2841–2850. [DOI] [PubMed] [Google Scholar]
- 61. Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, et al. (2000) PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 102: 636–641. [DOI] [PubMed] [Google Scholar]
- 62. Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, et al. (2004) Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 110: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 63. Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, et al. (2001) Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. The Journal of infectious diseases 184: 355–358. [DOI] [PubMed] [Google Scholar]
- 64. Yoneda M, Uchiyama T, Kato S, Endo H, Fujita K, et al. (2008) Plasma Pentraxin3 is a novel marker for nonalcoholic steatohepatitis (NASH). BMC gastroenterology 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Concentrations of serum biomarkers in relation to the severity of CF lung disease. CF patients were stratified into those with a forced expiratory volume in one second (FEV1) below and above 70%, with a vital capacity (VC) of below and above 80%, and with a ratio between FEV1 and VC below and above 70% (FEV1/VC), all of which serve as established indicators of the severity CF lung disease. Neither TIMP-4 nor Endoglin or Pentraxin-3 differed in patients with and without impairment of lung function as assessed by FEV1, VC and FEV1/VC.
(TIF)
Concentrations of serum biomarkers in CF patients with and without pancreatic insufficiency. CF patients were stratified into those with (PI) and without pancreatic insufficiency (no PI). Neither TIMP-4 nor Endoglin or Pentraxin-3 differed in patients with and without pancreatic insufficiency.
(TIF)
Liver stiffness and concentrations of serum biomarkers in CF patients with and without treatment of UDCA. CF patients were stratified into those with (UDC) and without existing therapy with Ursodeoxycholic acid (no UDCA). While patients with UDCA exhibited a slightly increased liver stiffness, neither TIMP-4 nor Endolgin or Pentraxin-3 differed in patients with and without existing UDCA therapy.
(TIF)