Abstract
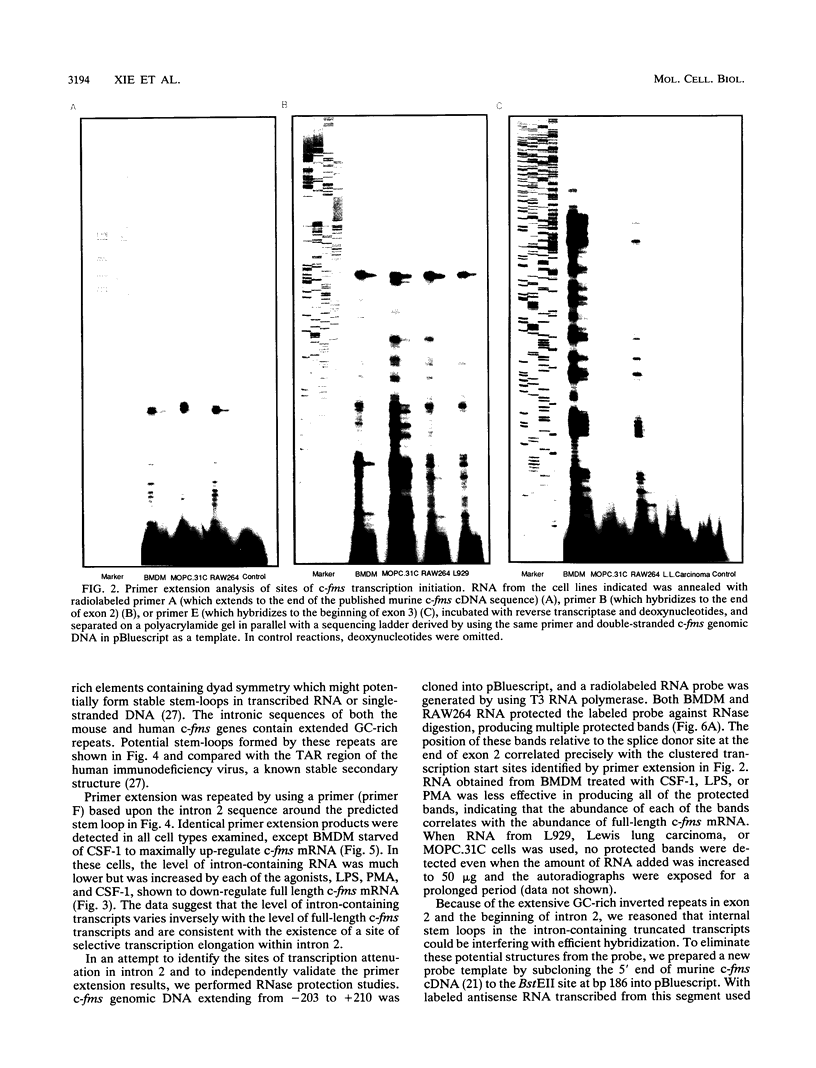
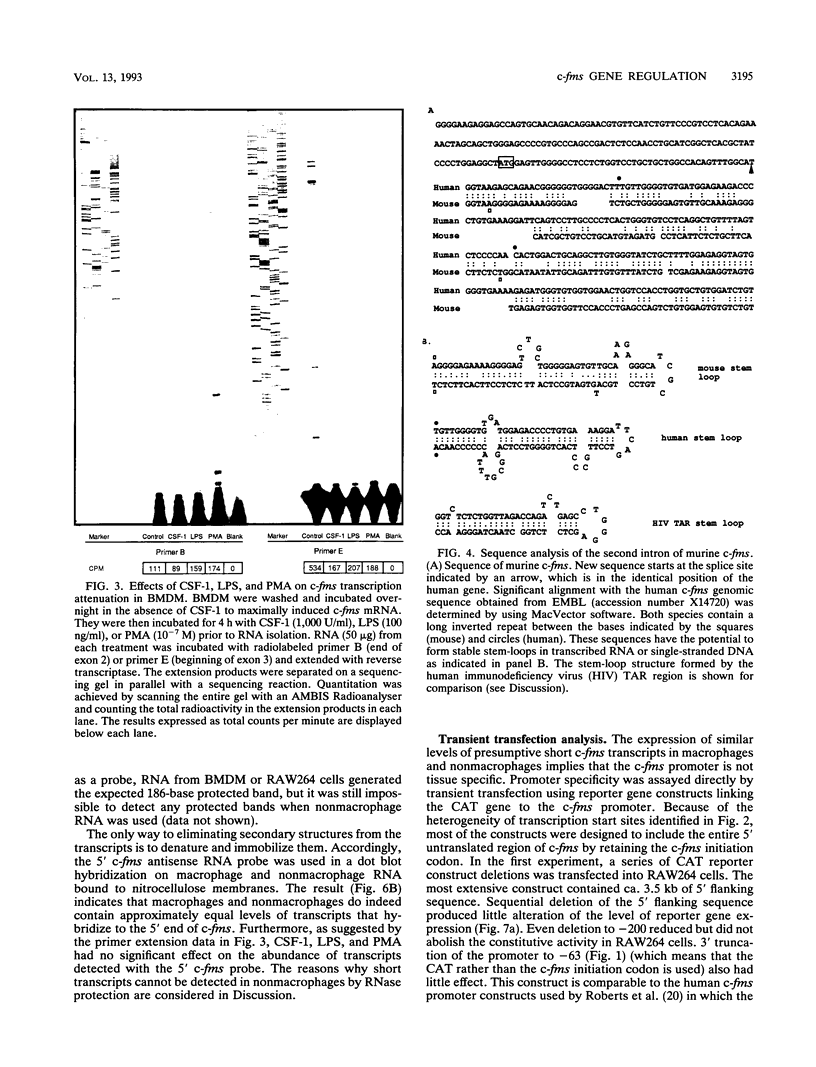
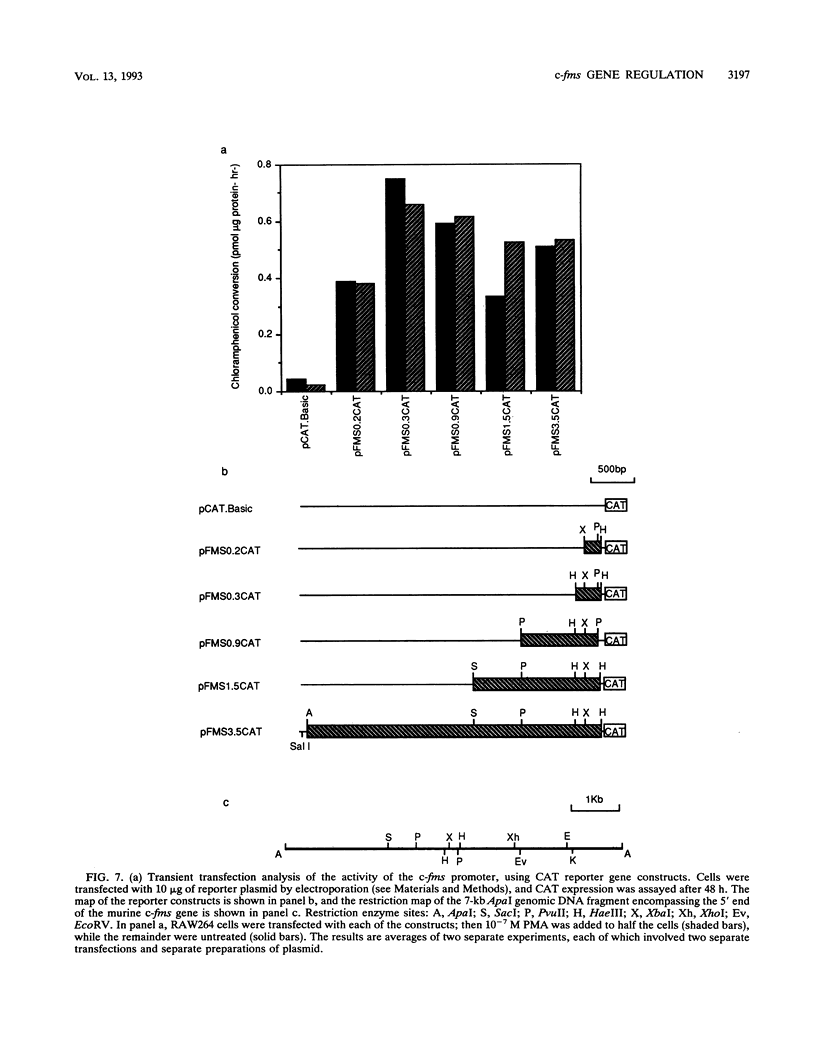
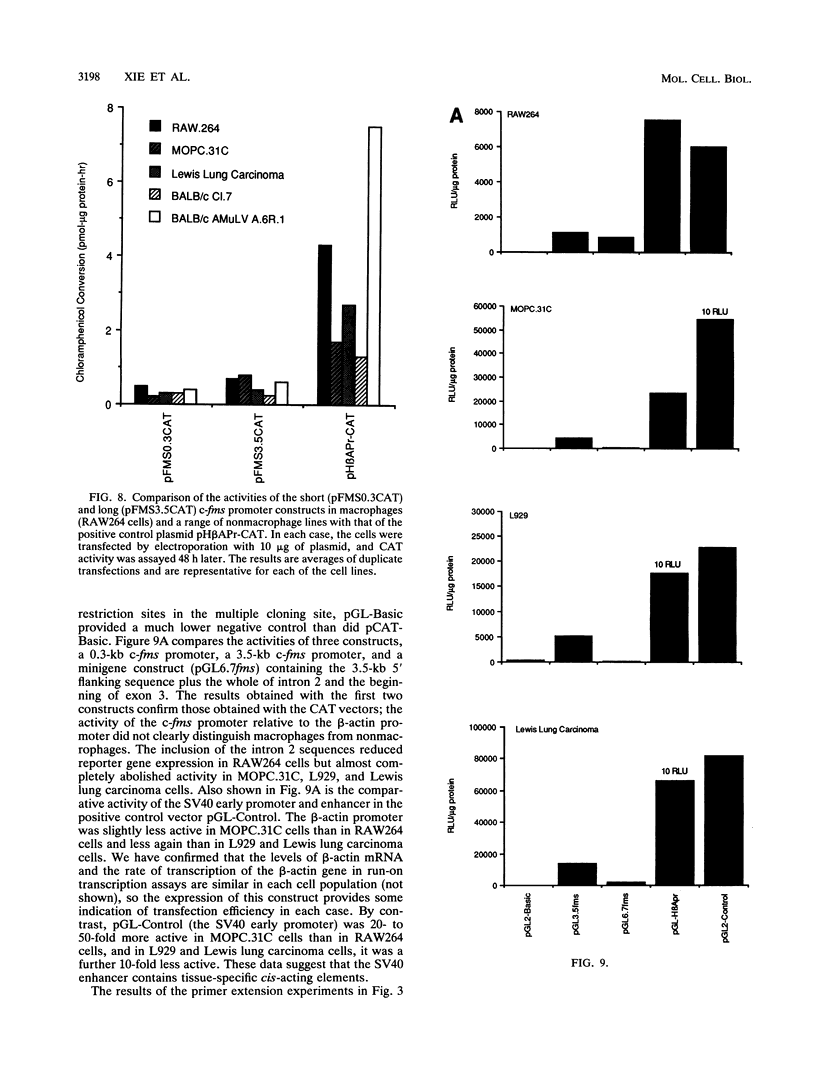
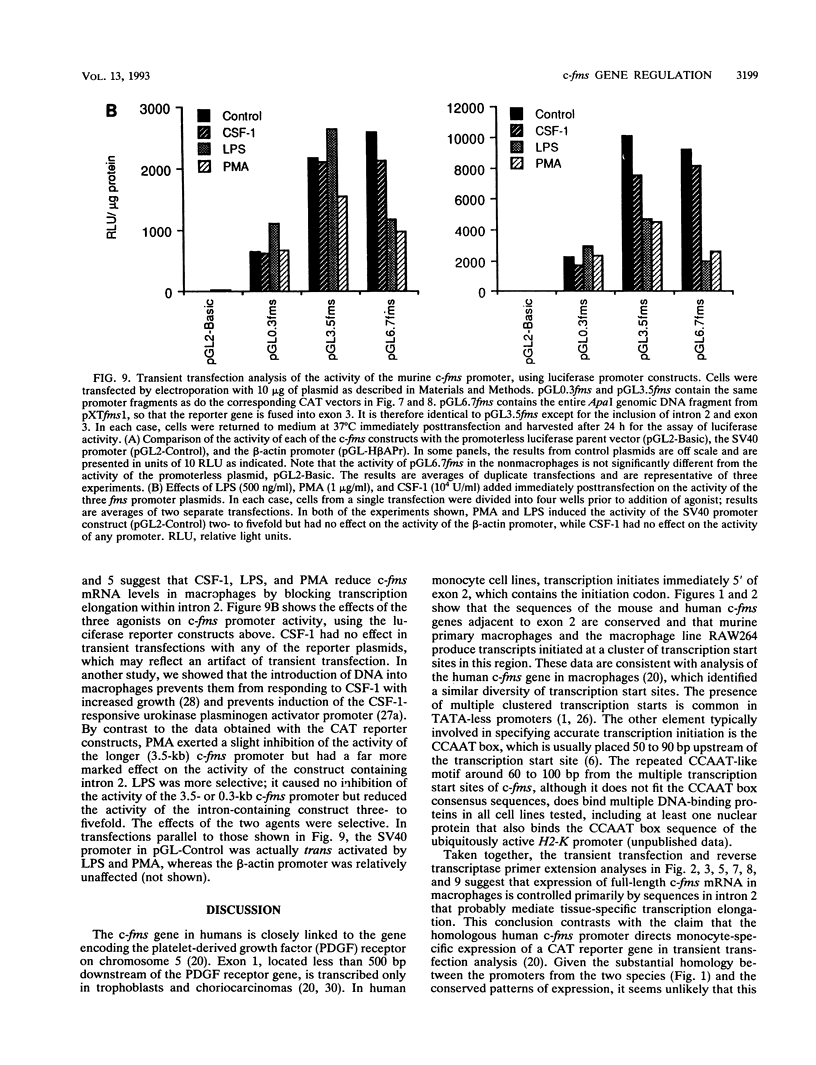
The gene encoding the receptor for macrophage colony-stimulating factor 1 (CSF-1), the c-fms protooncogene, is selectively expressed in immature and mature mononuclear phagocytes and trophoblasts. Exon 1 is expressed only in trophoblasts. Isolation and sequencing of genomic DNA flanking exon 2 of the murine c-fms gene revealed a TATA-less promoter with significant homology to human c-fms. Reverse transcriptase primer extension analysis using exon 2 primers identified multiple clustered transcription initiation sites. Their position was confirmed by RNase protection. The same primer extension products were detected in equal abundance from macrophage or nonmacrophage sources of RNA. c-fms mRNA is acutely down-regulated in primary macrophages by CSF-1, bacterial lipopolysaccharide (LPS), and phorbol myristate acetate (PMA). Each of these agents reduced the abundance of c-fms RNA detectable by primer extension using an exon 3 primer without altering the abundance of presumptive short c-fms transcripts detected with exon 2 primers. Primer extension analysis with an intron 2 primer detected products at greater abundance in nonmacrophages. Templates detected with the intronic primer were induced in macrophages by LPS, PMA, and CSF-1, suggesting that each of the agents caused a shift from full-length c-fms mRNA production to production of unspliced, truncated transcripts. The c-fms promoter functioned constitutively in the RAW264 macrophage cell line, the B-cell line MOPC.31C, and several nonhematopoietic cell lines. Macrophage-specific expression and responsiveness to selective repression by LPS and PMA was achieved by the incorporation of intron 2 into the c-fms promoter-reporter construct. The results suggest that expression of the c-fms gene in macrophages is controlled by sequences in intron 2 that act by regulating transcription elongation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. C., Jambou R. C., Swick A. G., Kahn J. W., Azizkhan J. C. Transcriptional initiation is controlled by upstream GC-box interactions in a TATAA-less promoter. Mol Cell Biol. 1990 Dec;10(12):6632–6641. doi: 10.1128/mcb.10.12.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady A. I., Stacey K. J., Nimmo K. A., Murphy K. M., von der Ahe D., Pearson D., Botteri F. M., Nagamine Y., Hume D. A. Constitutive expression of the urokinase plasminogen activator gene in murine RAW264 macrophages involves distal and 5' non-coding sequences that are conserved between mouse and pig. Nucleic Acids Res. 1991 Dec 25;19(24):6839–6847. doi: 10.1093/nar/19.24.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Innis J. W., Sun M. H., Wright D. A., Kellems R. E. Sequence requirements for transcriptional arrest in exon 1 of the human adenosine deaminase gene. Mol Cell Biol. 1991 Dec;11(12):6248–6256. doi: 10.1128/mcb.11.12.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Tourkine N., Belin D., Vassalli P., Jeanteur P., Blanchard J. M. c-fos gene transcription in murine macrophages is modulated by a calcium-dependent block to elongation in intron 1. Mol Cell Biol. 1991 May;11(5):2826–2831. doi: 10.1128/mcb.11.5.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Kamdar S. J., Duffy T. M. Tumor-derived products induce Il-1 a, Il-1 b, Tnf a, and Il-6 gene expression in murine macrophages: distinctions between tumor- and bacterial endotoxin-induced gene expression. J Leukoc Biol. 1991 May;49(5):474–482. doi: 10.1002/jlb.49.5.474. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S., Fichelson S., Sola B., Bordereaux D., Hampe A., André C., Galibert F., Tambourin P. Frequent c-fms activation by proviral insertion in mouse myeloblastic leukaemias. Nature. 1987 Sep 17;329(6136):259–261. doi: 10.1038/329259a0. [DOI] [PubMed] [Google Scholar]
- Grewal T., Theisen M., Borgmeyer U., Grussenmeyer T., Rupp R. A., Stief A., Qian F., Hecht A., Sippel A. E. The -6.1-kilobase chicken lysozyme enhancer is a multifactorial complex containing several cell-type-specific elements. Mol Cell Biol. 1992 May;12(5):2339–2350. doi: 10.1128/mcb.12.5.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella G. L., Ayroldi E., Espinoza-Delgado I., Varesio L. Lipopolysaccharide, but not IFN-gamma, down-regulates c-fms mRNA proto-oncogene expression in murine macrophages. J Immunol. 1990 May 1;144(9):3574–3580. [PubMed] [Google Scholar]
- Haley J. D., Waterfield M. D. Contributory effects of de novo transcription and premature transcript termination in the regulation of human epidermal growth factor receptor proto-oncogene RNA synthesis. J Biol Chem. 1991 Jan 25;266(3):1746–1753. [PubMed] [Google Scholar]
- Hirao I., Nishimura Y., Tagawa Y., Watanabe K., Miura K. Extraordinarily stable mini-hairpins: electrophoretical and thermal properties of the various sequence variants of d(GCGAAAGC) and their effect on DNA sequencing. Nucleic Acids Res. 1992 Aug 11;20(15):3891–3896. doi: 10.1093/nar/20.15.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Denkins Y. M. Activation of macrophages to express cytocidal activity correlates with inhibition of their responsiveness to macrophage colony-stimulating factor (CSF-1): involvement of a pertussis toxin-sensitive reaction. Immunol Cell Biol. 1989 Aug;67(Pt 4):243–249. doi: 10.1038/icb.1989.37. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Nayar R. Encapsulation is not involved in the activities of recombinant gamma interferon associated with multilamellar phospholipid liposomes on murine bone marrow-derived macrophages. Lymphokine Res. 1989 Winter;8(4):415–425. [PubMed] [Google Scholar]
- Innis J. W., Kellems R. E. A heat-labile factor promotes premature 3' end formation in exon 1 of the murine adenosine deaminase gene in a cell-free transcription system. Mol Cell Biol. 1991 Nov;11(11):5398–5409. doi: 10.1128/mcb.11.11.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Ko M. S., Nakauchi H., Takahashi N. The dose dependence of glucocorticoid-inducible gene expression results from changes in the number of transcriptionally active templates. EMBO J. 1990 Sep;9(9):2835–2842. doi: 10.1002/j.1460-2075.1990.tb07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Tourkine N., Jeanteur P., Blanchard J. M. Demonstration in living cells of an intragenic negative regulatory element within the rodent c-fos gene. Cell. 1990 May 4;61(3):485–496. doi: 10.1016/0092-8674(90)90530-r. [DOI] [PubMed] [Google Scholar]
- Reddy C. D., Reddy E. P. Differential binding of nuclear factors to the intron 1 sequences containing the transcriptional pause site correlates with c-myb expression. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7326–7330. doi: 10.1073/pnas.86.19.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Shapiro L. H., Ashmun R. A., Look A. T. Transcription of the human colony-stimulating factor-1 receptor gene is regulated by separate tissue-specific promoters. Blood. 1992 Feb 1;79(3):586–593. [PubMed] [Google Scholar]
- Rothwell V. M., Rohrschneider L. R. Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res. 1987 Sep-Oct;1(4):311–324. [PubMed] [Google Scholar]
- Sariban E., Imamura K., Sherman M., Rothwell V., Pantazis P., Kufe D. Downregulation of c-fms gene expression in human monocytes treated with phorbol esters and colony-stimulating factor 1. Blood. 1989 Jul;74(1):123–129. [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Skalnik D. G., Dorfman D. M., Perkins A. S., Jenkins N. A., Copeland N. G., Orkin S. H. Targeting of transgene expression to monocyte/macrophages by the gp91-phox promoter and consequent histiocytic malignancies. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8505–8509. doi: 10.1073/pnas.88.19.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Transcription elongation and eukaryotic gene regulation. Oncogene. 1990 Jun;5(6):777–785. [PubMed] [Google Scholar]
- Stanley E. R. Action of the colony-stimulating factor, CSF-1. Ciba Found Symp. 1986;118:29–41. doi: 10.1002/9780470720998.ch3. [DOI] [PubMed] [Google Scholar]
- Visvader J., Verma I. M. Differential transcription of exon 1 of the human c-fms gene in placental trophoblasts and monocytes. Mol Cell Biol. 1989 Mar;9(3):1336–1341. doi: 10.1128/mcb.9.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Weber B., Horiguchi J., Luebbers R., Sherman M., Kufe D. Posttranscriptional stabilization of c-fms mRNA by a labile protein during human monocytic differentiation. Mol Cell Biol. 1989 Feb;9(2):769–775. doi: 10.1128/mcb.9.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]