Abstract
Trans-lesion DNA synthesis (TLS) is a DNA damage-tolerance mechanism that uses low-fidelity DNA polymerases to replicate damaged DNA. The inherited cancer-propensity syndrome xeroderma pigmentosum variant (XPV) results from error-prone TLS of UV-damaged DNA. TLS is initiated when the Rad6/Rad18 complex monoubiquitinates proliferating cell nuclear antigen (PCNA), but the basis for recruitment of Rad18 to PCNA is not completely understood. Here, we show that Rad18 is targeted to PCNA by DNA polymerase eta (Polη), the XPV gene product that is mutated in XPV patients. The C-terminal domain of Polη binds to both Rad18 and PCNA and promotes PCNA monoubiquitination, a function unique to Polη among Y-family TLS polymerases and dissociable from its catalytic activity. Importantly, XPV cells expressing full-length catalytically-inactive Polη exhibit increased recruitment of other error-prone TLS polymerases (Polκ and Polι) after UV irradiation. These results define a novel non-catalytic role for Polη in promoting PCNA monoubiquitination and provide a new potential mechanism for mutagenesis and genome instability in XPV individuals.
INTRODUCTION
Living organisms are constantly exposed to ubiquitous genotoxins from endogenous and external sources (1). However, cells have evolved numerous DNA damage response (DDR) pathways that protect genomic DNA and prevent genetic instability (2). Trans-lesion synthesis (TLS) is a DDR mechanism involving specialized DNA polymerases that can replicate damaged DNA templates (3).
TLS relies on inherently error-prone DNA polymerases of the Y family to replicate damaged DNA (4). TLS by Y-family polymerases (Polη, Polι, Polκ and Rev1) (5) maintains replication in cells harbouring damaged DNA, albeit at the cost of reduced fidelity. Each TLS polymerase performs relatively error-free replication past a preferred cognate lesion; in the absence of the appropriate TLS polymerase for its preferred lesion, mutagenic replication by error-prone polymerases predisposes to genetic instability (2).
Polη is unique among Y-family polymerases in its ability to perform accurate replication past UV-damaged DNA (6,7). Lack of Polη in the inherited cancer-propensity syndrome xeroderma pigmentosum variant (XPV) (8) results in error-prone replication by other Y-family polymerases in sunlight-exposed cells (9,10). Thus, UV-induced mutagenesis due to Polη deficiency compromises genetic integrity to manifest as exquisite sunlight sensitivity and early skin cancer propensity.
A prerequisite for error-prone replication in TLS is the Rad6/Rad18-mediated monoubiquitination of proliferating cell nuclear antigen (PCNA) at the highly conserved lysine K164 (11,12). Y-family polymerases contain ubiquitin-binding (UBZ) domains that confer affinity to monoubiquitinated PCNA (13,14). Failure to monoubiquitinate PCNA at K164 phenocopies XPV by compromising TLS and sensitizing cells to UV light and other ubiquitous genotoxins (15–18). Several other DDR pathways also depend on PCNA monoubiquitination, including SHPRH/HTLF-mediated template switching (19), ZRANB3-dependent replication fork restart (20), SNM1A-dependent intrastrand cross-link repair (21) and the Fanconi Anaemia pathway activation (22).
Despite its pivotal role in the DDR, the molecular mechanisms regulating Rad18-mediated PCNA monoubiquitination are incompletely understood. The Rad18–Rad6 complex is thought to be recruited to the vicinity of damaged DNA via direct interactions with RPA-coated ssDNA (23,24). However, Rad18 lacks PCNA-binding motifs, and it is unclear how Rad18 is targeted specifically to PCNA at stalled forks (or other sites of post-replication repair). A recent report by Zou and colleagues (25) identified Spartan as a binding partner of both Rad18 and PCNA and proposed that Spartan acts as a scaffold for recruiting Rad18 to PCNA. Consistent with a role for Spartan in targeting Rad18 to PCNA, those workers found DNA damage-induced PCNA monoubiquitination was modestly attenuated in Spartan-depleted cells. However, several other more recent publications have reported alternative roles for Spartan in DNA damage signalling (26–29), and it is unclear whether Spartan or alternative putative mediators exist to facilitate recruitment of Rad18 to PCNA.
In mammalian cells, Rad18 exists in complex with Polη (30,31), and association of Rad18 with Polη is necessary for normal DNA damage tolerance (30–32). Assembly of the Rad18–Polη complex is stringently controlled by Cdc7 and Chk1 kinases, which serve to integrate TLS with S-phase progression and the S-phase checkpoint, respectively (30,32). Here we report that the Polη–Rad18 interaction plays a key role in targeting Rad18 to PCNA and facilitating efficient PCNA monoubiquitination. Interestingly, the novel role of Polη in stimulation of PCNA monoubiquitination is fully dissociable from its activity as a DNA polymerase. We show that the Polη–Rad18 interaction provides the basis for coupling PCNA monoubiquitination with DNA damage-inducible checkpoint pathways mediated by p53 and Chk1. Our results also provide a potential explanation for numerous reports that Polη confers tolerance of non-cognate lesions (33,34) and that catalytically inactive Polη can partially rescue the DNA damage-sensitivity phenotypes of XPV cells (35,36). Moreover, because some XPV cells express a catalytically inactive Polη that retains the ability to promote PCNA monoubiquitination, our results also indicate a new molecular mechanism for the mutagenesis and cancer propensity of XPV patients.
MATERIALS AND METHODS
Cell culture and transfection
H1299, HDF, XP115LO [GM02359(37,38)] and HCT-116 WT and Rad18−/− cells (39) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin–streptomycin. SiRNA and pcDNA, pACCMV and pCAGGS plasmid transfections were done using Lipofectamine 2000 (Invitrogen) as previously described (30).
Materials, siRNA, plasmid and adenovirus construction
siRNA oligonucleotide sequences were as follows: non-targeting Control, 5′–UAGCGACUAAACACAUCAAUU–3′; Polη, 5′–GCAGAAAGGCAGAAAGUUA–3′; Polη-3′ UTR, 5′–CCAUUUAGGUGCUGAGUUA–3′; Polη-5′ UTR, 5′–GAAUAAAUCUCGCUCGAAA–3′; Chk1, 5′–GCGUGCCGUAGACUGUCCA–3′; USP1, 5′–TCGGCAATACTTGCTATCTTA–3′; Polκ, 5′–GUAAAGAGGUUAAGGAAA–3′; Rad18 3′ UTR, 5′–UUAUAAAUGCCCAAGGAAAUU–3′; Spartan 5′–ACCGGACUUGCAGGCACUGUUUGUU–3′. CFP was cloned onto the C-terminus of Rad18 in pACCMV using BamH1 and Xba1 restriction sites. Rad18 and CFP were separated by a linker of the sequence 5′–ACCTCTTCCGGTTCCAGTCCCTGTTCCGGGTCCTGCTCCTATGCGTATGGCTCC–5′. Rad18-Δ(402–445) and Rad18-C28F were generated as described previously (31) and cloned into pACCMV using EcoRI and BamHI restriction sites. Polη-ΔPCNA-interacting peptide (PIP) was cloned into pACCMV using EcoRI and BamHI restriction sites and a C-terminal primer containing phenylalanine to alanine mutations at AA 705 and 707. Catalytically inactive Polη was generated by mutating codons D13, E22, D115 and E116 to alanine in the N-terminal catalytic active site to disrupt coordination of Mg2+ ions between dNTP, primer and active site moieties and block nucleotide incorporation(40); this construct was then cloned into pACCMV using EcoRI and BamHI restriction sites. N-terminal Polη truncations were generated with 5′ and 3′ primers containing EcoRI and BamHI restriction sites, respectively, and cloned into pACCMV. The Rad18–Polη fusion was constructed by PCR amplification of Polη with primers containing 3′ BamHI and 5′ XbaI restriction sites, followed by ligation into pACCMV-Rad18. Polη-ΔPLTH and Polκ+PLTH were generated with C-terminal primers omitting or adding, respectively, codons for the PLTH domain, followed by a BamHI restriction site for ligation into pACCMV. pDEST-SFB-Spartan was obtained from Lee Zou (MGH Cancer Center). Adenovirus constructions were performed by recombination of pACCMV constructs with pJM17 as described previously (41).
Adenoviral expression and titration
Adenoviral infection was performed as described previously by adding to cultured cells CsCl-purified adenovirus (41). Infections in H1299 cells were typically done at 0.1–1.0 × 109 pfu/ml and in XPV/HDF cells at 0.1–5.0 × 109 pfu/ml. Titration to expression levels approximately equal to endogenous was done by serial infections followed by immunoblotting of extracts with antibodies against the endogenous protein.
Fluorescence microscopy
H1299 or XPV cells were grown to ∼60% confluency on glass-bottom plates (Mat-tek) and then infected with adenovirus (CFP-Rad18-WT, YFP-Polη, GFP-Polκ and respective mutants) to achieve expression approximately equal to endogenous as determined by Western blot. For co-expression and knockdown experiments, co-infection or transfection was performed 6 h before adenoviral infection. Twenty hours after infection, cells were exposed to genotoxins and then prepared for live or fixed-cell imaging on a Zeiss 710 confocal microscope. For high-magnification representative images, Z-stacks at 0.5-µm intervals were collected throughout the entire cell volume using a 63× oil-immersion objective and 2.3× optical zoom. 3D projections of Z-stacks were performed using Grouped Zprojector on ImageJ. For cells expressing multiple chromophores (YFP and CFP-tagged proteins), appropriate excitation lasers, laser intensities and emission filter bandwidths were selected to eliminate bleedthrough. For live-cell imaging, cells were kept out of the incubator for no more than 10 min. For fixed-cell imaging, H1299 cells were washed 3× with cold phosphate-buffered saline (PBS), then extracted for 60 s in cold CSK buffer, washed 3× with PBS, then fixed for 10 min in 2% PFA in PBS; XPV cells were washed 3× in cold PBS and then fixed for 15 min in methanol at −20°C. Post-fixation, all cells were covered with Vectashield Solution (Vector Laboratories) and imaged within 24 h. For foci quantification, five representative images containing ∼60 cells were captured using 0.5 µm Z-stacks with a 40× using oil-immersion lens. After 3D projection, the number of cells clearly containing >100 nuclear foci were counted as a fraction of total chromophore-expressing cells.
Triton extraction, immunoprecipitation and immunoblotting
Extracts containing soluble and chromatin-associated proteins were prepared as previously described (30) by lysing cultured cells into cold cytoskeleton buffer (CSK buffer; 10 mM Pipes, pH 6.8, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 1 mM DTT, 0.1 mM ATP, 1 mM Na3VO4, 10 mM NaF and 0.1% Triton X-100) supplemented with phosphatase and protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). For immunoprecipitation of whole cell lysate (WCL) or chromatin-bound proteins, Triton-insoluble proteins were released by sonication on ice for three 10-s intervals followed by centrifugation at 15 k g for 10 min. After normalizing to a protein concentration of 1 µg/µl, immunoprecipitation was conducted at 4°C by rotating overnight with HA-coupled or primary antibody-bound sepharose beads (Roche Diagnostics, Indianapolis, IN, USA). After immunoprecipitation, the beads were washed five times for 15 min in CSK buffer and then resuspended in minimum volume of Laemmli buffer. For immunoblot experiments, cell extracts or immunoprecipitates were separated by SDS-PAGE, followed by incubation overnight with the following primary antibodies: PCNA (sc-56), Chk1 (sc-7898), β-Actin (sc-130656), all from Santa Cruz Biotech (Santa Cruz, CA, USA); Polη (A301–231 A), Polκ (A301–975 A), Polι (A301–304 A) and R18 (A301–340 A), all from Bethyl Laboratories (Montgomery, TX, USA); and p53 (Ab-6) from Lab Vision (Fremont, CA, USA).
Genotoxin treatments
UV irradiation and benzo(a)pyrene diolepoxide (BPDE) treatment were performed as previously described (30), and BPDE (National Cancer Institute Carcinogen Repository) was dissolved in anhydrous Me2SO and added directly to the growth medium as a 1000× stock to give various final concentrations, as indicated in the figure legends. For UVC treatment, the growth medium was removed from the cells, reserved and replaced with PBS. The plates were transferred to a UV cross-linker (Stratagene, Santa Clara, CA, USA) and then irradiated. The UVC dose delivered to the cells was confirmed with a UV radiometer (UVP BioImaging Systems, Upland, CA, USA). The reserved medium from the cells was replaced, and cells were returned to the incubator.
In vitro binding and ubiquitination assays
C-His6-PCNA-expressing Top10 Escherichia coli (acquired from Marila Cordiero-Stone, UNC-CH) were collected and lysed in pH 8 buffer containing 50 mM NaPO4, 300 mM NaCl, 20 mM imidazole and 0.1% Triton-X. After sonication and clarification, His6-PCNA was purified over Ni-NTA beads. For His6-PCNA pulldown experiments, HA-Rad18 was adenovirally expressed in H1299 cells alone or together with YFP-Polη and lysed in NaPO4/NaCl/imidazole/Triton-X buffer. After sonication, clarification and normalization to a protein concentration of 1 µg/µl, cell lysates were rotated overnight at 4°C with His6-PCNA on Ni-NTA beads. The beads were then washed five times in the same buffer before addition of Laemmli buffer, boiling and analysis by SDS-PAGE/Western Blot. For in vitro ubiquitination assays, H1299 cells expressing HA-Rad18 alone or together with YFP-Polη were lysed in NaPO4/NaCl/imidazole/Triton-X buffer and immunoprecipitated with HA-sepharose beads. After washing the beads extensively, the beads were resuspended in 50 µl buffer and the following were added: His6-PCNA (eluted from Ni-NTA beads with the same buffer plus 200 mM imidazole), 500 µM FLAG-ubiquitin, 10× Energy Regeneration Solution and 100 nM Ubiquitin Activating Enzyme (UBE1), all from Boston Biochem (Cambridge, MA, USA). After incubation for 16 h at 4°C, the mixture was mixed with Laemmli buffer, boiled and analysed by SDS-PAGE and Western Blot.
UV cytotoxicity assay
XPV or HDF cells were split into 24-well plates to a density of ∼25%. Twelve hours later, the cells were infected with empty control adenovirus or adenovirus expressing YFP-Polη. Twenty-four hours after infection, the cells were exposed to UV light in the presence or absence of 1 mM caffeine. After 48 h, 50 mg/ml Thiazolyl Blue Tetrazolium Bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) was added to each well and allowed to incubate at 37°C for 2 h. The cells were then rinsed with PBS and dissolved in 0.5 ml DMSO. The absorbance at 570 nm was then measured for each well and normalized to the sham-treated samples. The minimum dose of YFP-Polη that conferred resistance to UV light in XPV cells (∼0.5 × 109 pfu/ml, Supplementary Figure S2) was determined by this method and used for survival assays.
Statistics
P values for statistical significance were determined by the unpaired Student’s t-test with a two-tailed 95% confidence interval.
RESULTS
Polη promotes efficient Rad18-mediated PCNA monoubiquitination
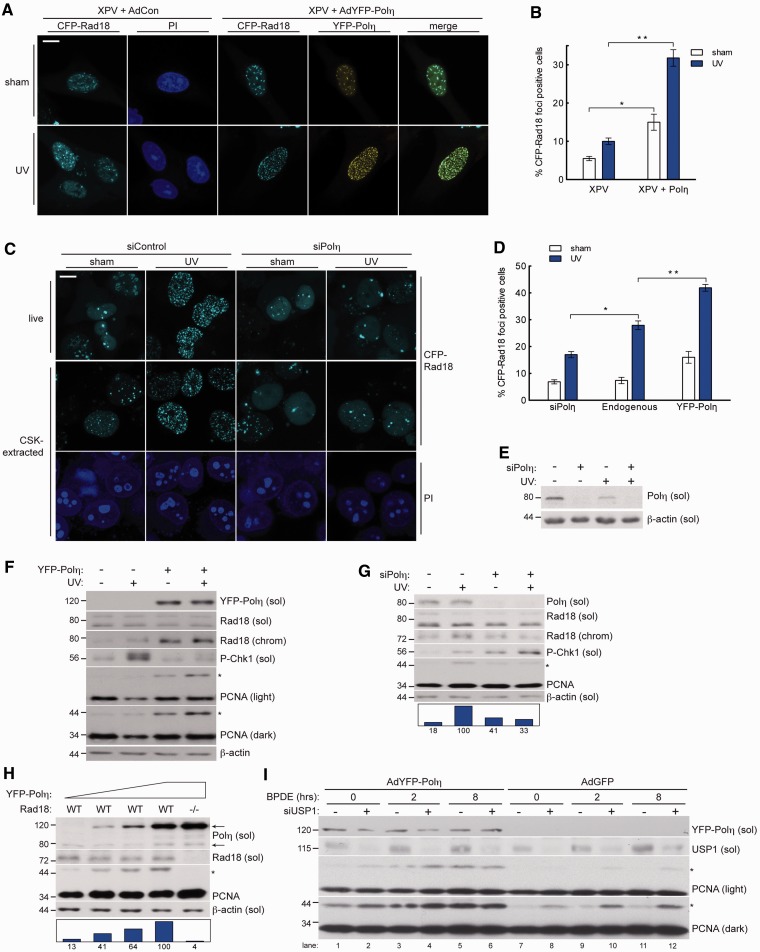
Current models suggest that Rad18 plays proximal roles in TLS, chaperoning Polη to damaged chromatin and monoubiquitinating PCNA to stably engage Y family polymerases at sites of PRR (12,13). Unexpectedly, we observed deficient redistribution of Rad18 to nuclear foci (representing sites of replication stalling) in patient-derived XPV cells (XP115LO) following UV irradiation (Figure 1A and B, Supplementary Figure S1). In Polη-corrected XP115LO cells, Rad18 redistributed to nuclear foci in a UV-inducible manner and co-localized with Polη, indicating that Rad18 redistribution to repair foci is Polη dependent in XP115LO cells. Similar to results obtained with XPV cells, redistribution of Rad18 was defective in Polη-depleted H1299 cells (Figure 1C and E), which have intact TLS and rely on Polη for UV tolerance (30). As shown in Figure 1D, basal and UV-induced formation of Rad18 foci were dependent on Polη, indicating a general role for Polη in impacting Rad18 redistribution.
Figure 1.
Polη promotes damage-induced Rad18 redistribution and PCNA monoubiquitination. (A) Representative images of CSK-extracted nuclei from CFP-Rad18-expressing XP115LO XPV cells co-infected with empty control adenovirus or adenovirus expressing YFP-Polη (at levels that restore UV tolerance—see Supplementary Figure S6) and exposed to UV (10 J/m2) or sham irradiated. Scalebar = 10 μm. (B) Quantification of CFP-Rad18 foci-positive H1299 nuclei as a percentage of CFP-Rad18-expressing cells as shown in (A); *P = 0.0001; **P = 0.001. Error bars = SEM. (C) Representative images of live (top) and CSK-extracted (bottom) nuclei from H1299 cells treated with non-targeting control siRNA (left) or siRNA targeting Polη (right) and imaged 2 h after sham or UV (10 J/m2) irradiation. Scalebar = 10 microns. (D) Quantification of CFP-Rad18 foci-positive nuclei as a percentage of CFP-Rad18-expressing cells as shown in (D). *P = 0.001; **P = 0.0003. (E) Immunoblot of fractionated lysates from H1299 cells expressing CFP-tagged Rad18 as shown in (D) and treated with non-targeting control siRNA or siRNA against Polη and then lysed 2 h after treatment with 10 J/m2 UV or sham treated. (F) Immunoblot of fractionated lysates from XP115LO XPV cells treated with empty control adenovirus or adenovirus expressing YFP-Polη at levels shown in (A) and lysed 2 h after treatment with 10 J/m2 UV. (G) Immunoblot of fractionated lysates from HDF cells treated with non-targeting control siRNA or siRNA against Polη and then lysed 2 h after treatment with 10 J/m2 UV or sham irradiation. (H) Immunoblot of fractionated lysates from HCT-116 WT cells (lanes 1–4) or RAD18−/− cells (lane 5) treated with increasing titers of YFP-Polη adenovirus and lysed 24 h post-infection. Upper and lower arrows denote YFP-tagged and endogenous Polη, respectively. (I) Immunoblot of fractionated lysates from H1299 cells expressing empty control adenovirus or adenovirus expressing YFP-Polη and treated with non-targeting control siRNA or siRNA against USP1 and then lysed at indicated times after treatment with 200 nM BPDE. On all Western blots, asterisk denotes monoubiquitinated PCNA and bar graphs represent intensity of monoubiquitinated PCNA band relative to the maximum band on each film.
We next asked whether Polη status also affected PCNA-directed Rad18 E3 ligase activity. Polη-complemented XPV cells exhibited higher basal and damage-induced PCNA monoubiquitination compared with parental XPV cells, and Polη expression was associated with increased chromatin binding of Rad18 (Figure 1F). Conversely, UV-induced PCNA monoubiquitination was compromised in Polη-depleted normal human diploid fibroblasts (HDF) relative to Polη-replete controls (Figure 1G). Polη depletion thus partially phenocopies the expected effect of depleting RPA (Supplemental Figure S2), which is thought to initiate TLS by coating ssDNA and triggering ATR/Chk1 signalling and subsequent PCNA monoubiquitination (42,43). Rad18 redistribution and PCNA monoubiquitination were also attenuated in Polη-depleted cells after BPDE treatment (Supplementary Figure S3), indicating that the effect of Polη on Rad18 activity is not genotoxin specific.
Next, we determined whether increased Polη expression affects Rad18 and PCNA monoubiquitination. When expressed in HCT-116 cells at levels ranging from ∼2 - to 25-fold higher than endogenous, PCNA monoubiquitination increased in a dose-dependent fashion with Polη (Figure 1H). Importantly, PCNA monoubiquitination was not induced by Polη in isogenic Rad18-null HCT-116 cells, indicating that the effect of Polη on PCNA modification is Rad18 mediated (Figure 1H, right lane).
Potentially, the stimulatory effect of Polη expression on PCNA monoubiquitination could result (at least in part) from reduced PCNA de-ubiquitylation activity. Ubiquitin-Specific Protease 1 (USP1) is the only known PCNA-directed de-ubiquitylating (DUB) enzyme (44). To determine whether inhibition of USP1 activity contributes to Polη-dependent PCNA monoubiquitination, we determined the effects of Polη expression on PCNA modification in USP1-depleted cells. As expected, basal levels of PCNA monoubiquitination were increased by USP1 depletion (Figure 1H). However, Polη expression further increased PCNA monoubiquitination in cells lacking USP1 (compare lanes 2 and 8), both basally and 2 and 8 h after DNA damage. We conclude that that Polη stimulates PCNA monoubiquitination by Rad18 via USP1-independent mechanisms. We cannot exclude the formal possibility that Polη-dependent PCNA monoubiquitination is not mediated by reduced activity of putative alternative PCNA-directed DUBs. However, the results of Figure 1 and data presented below indicate that Polη facilitates redistribution of Rad18 to sites of DNA damage and promotes efficient PCNA monoubiquitination.
Rad18–Polη interaction is necessary for efficient PCNA monoubiquitination
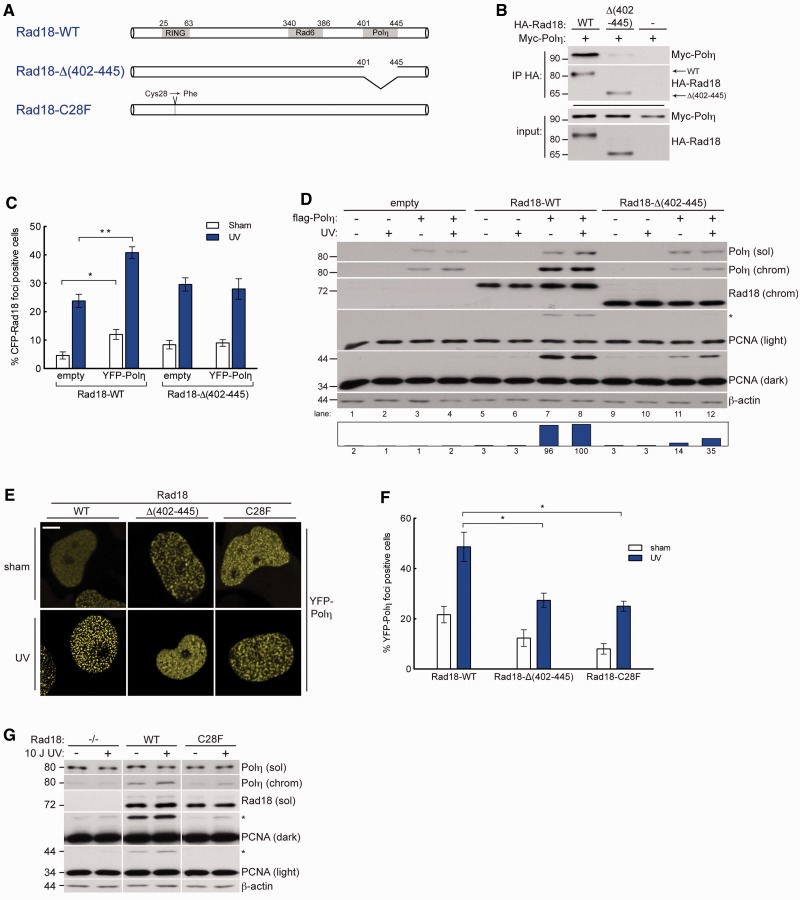
Because Rad18 and Polη form a complex after DNA damage (31), we next asked whether Polη-dependent redistribution of Rad18 and PCNA monoubiquitination required Rad18–Polη interactions. The Polη-binding region of Rad18 has been mapped to amino acid (AA) residues 402–445 (Figure 2A) (31). Therefore, we determined the effect of Polη status on the activity of a Polη-interaction defective Rad18 mutant, Rad18-Δ(402–445), that retains E3 Ub-ligase activity and other DNA repair functions (31).
Figure 2.
Physical interaction between Rad18 and Polη drives efficient damage-induced PCNA ubiquitination. (A) Schematic of Rad18-WT (top); Rad18-Δ(402–445), a Polη-binding deficient mutant lacking the Polη binding domain (AA 402–445); Rad18-C28F, an E3 ligase-inactive mutant in which the RING-finger cysteine has been substituted with phenylalanine. (B) Immunoblot analysis of anti-HA immunoprecipitates from H1299 cells co-expressing HA-Rad18-WT or HA-Rad18-Δ(402–445) with Myc-Polη. (C) Quantification of CFP-Rad18 foci-positive nuclei as a percentage of H1299 cells expressing CFP-Rad18-WT or CFP-Rad18-Δ(402–445) and treated with empty adenovirus or Myc-Polη-expressing adenovirus followed by UV (10 J/m2) treatment or sham irradiation. *P = 0.095; **P = 0.0006. Error bars = SEM. (D) Immunoblots of fractionated lysates from Rad18-depleted H1299 cells that were reconstituted with siRNA-resistant Rad18-WT, Rad18-Δ(402–445), or empty vector (for control) alone or together with FLAG-Polη, followed by treatment with UV (10 J/m2) or sham-irradiation. (E) Representative images of CSK-extracted nuclei from YPF-Polη expressing H1299 cells that were depleted of endogenous Rad18 and then reconstituted with siRNA-resistant Rad18-WT, or Rad18-Δ(402–445), or Rad18-C28F and treated with UV (10 J/m2) or sham irradiated. Scalebar = 10 um. (F) Quantification of YFP-Polη foci-positive nuclei as a percentage of YFP-Polη-expressing cells in cultures complemented with Rad18-WT, Rad18-Δ(402–445), or Rad18-C28F as shown in (E). *upper P = 0.026, lower P = 0.0238. (G) Immunoblots of fractionated lysates from Rad18-depleted H1299 cells that were reconstituted with siRNA-resistant WT-Rad18, C28F-Rad18, or empty vector, and then treated with UV (10 J/m2) or sham irradiated.
Consistent with in vitro binding studies (31), Rad18-Δ(402–445) failed to co-immunoprecipitate Polη from cell lysates (Figure 2B). To test how Polη–Rad18 binding affected subcellular Rad18 distribution, we depleted H1299 cultures of endogenous Rad18 and reconstituted with near-physiological levels of siRNA-resistant CFP-Rad18-WT or CFP-Rad18-Δ(402–445). As shown in Figure 2C and Supplementary Figure S4, co-expression of Polη significantly increased basal and damage-induced redistribution of Rad18-WT to nuclear foci but had no effect on the redistribution of Rad18-Δ(402–445). In replicate cultures of Rad18-complemented cells, Polη-induced PCNA monoubiquitination was severely compromised in cells complemented with Rad18-Δ(402–445) when compared with cells expressing Rad18-WT (Figure 2D, compare lanes 1 and 8 with 11 and 12). Therefore, Polη–Rad18 interactions are necessary for Polη-dependent PCNA monoubiquitination.
The stable engagement of TLS polymerases with stalled replication forks depends on their UBZ/UBM-mediated interactions with monoubiquitinated PCNA (12–14). As expected, the reduced PCNA monoubiquitination in cells complemented with Rad18-Δ(402–445) was associated with decreased Polη chromatin binding (Figure 2D) and reduced formation of Polη nuclear foci (Figure 2E and F), when compared with Rad18-WT-expressing cells. Thus, Rad18-Δ(402–445) partially recapitulates phenotypes conferred by the E3 ubiquitin ligase-deficient Rad18-C28F mutant (Figure 2A), including defective PCNA monoubiquitination (Figure 2G) and reduced recruitment of Polη to chromatin (Figure 2E and F).
Polη–PCNA interactions drive Rad18-mediated PCNA monoubiquitination
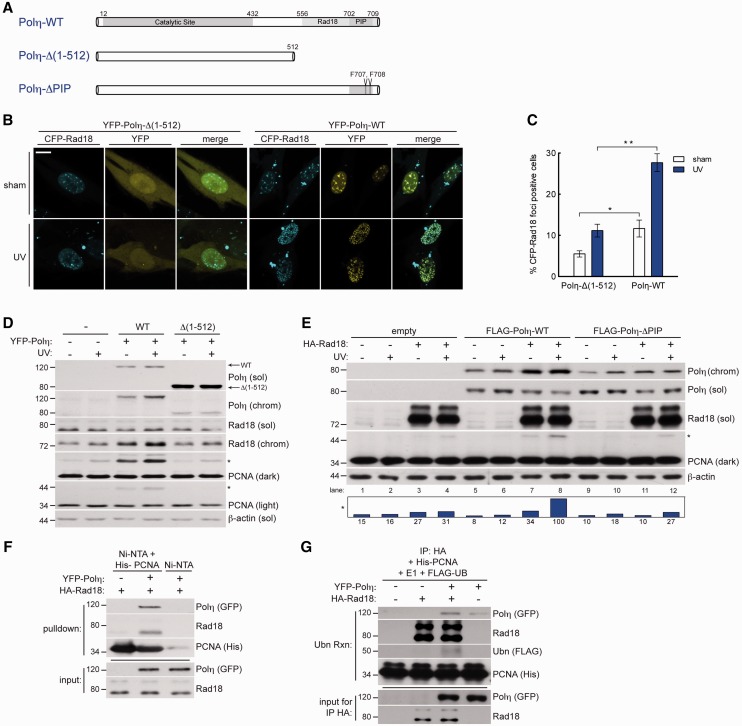
C-terminal Polη truncations are the most common defect in XPV (8,45,46), in which both the PIP box (47) and the Rad18-binding domains (31) are deleted. To test whether Polη XPV C-terminal truncation mutants exhibit defects in Rad18 regulation, we complemented XPV cells with WT-Polη or similar levels of a common XPV Polη mutant that retains full catalytic activity (48) but fails to confer UV resistance [Polη-Δ(1–512), lacking residues 513–713, Figure 3A]. As expected, complementation of XPV cells with Polη-WT conferred normal Rad18 redistribution (Figure 3B and C) and resulted in increased PCNA monoubiquitination (Figure 3D). However, complementation of XPV cells with Polη-Δ(1–512) failed to restore Rad18 redistribution or PCNA monoubiquitination to the same extent as those complemented with Polη-WT (Figure 2B–D), indicating that the C-terminal domain of Polη is important not only for Polη chromatin binding, but also for Rad18 nuclear redistribution and Rad18-mediated PCNA monoubiquitination.
Figure 3.
Polη physically bridges Rad18 and PCNA to promote efficient PCNA monoubiquitination after DNA damage. (A) Schematic of Polη-WT (top); Polη-Δ(1–512), a C-terminal truncation lacking AA 513–713 (middle); and Polη-ΔPIP, full length Polη with two PIP box phenylalanines mutated to alanine (bottom). (B) Representative images of CSK-extracted nuclei from XPV cells that were co-infected with CFP-Rad18 and YFP-Polη-Δ(1–512) adenovirus (left) or YFP-Polη-WT (right) and treated with UV (10 J/m2) or sham irradiated. Scalebar = 10 um. (C) Quantification of CFP-Rad18 foci-positive nuclei as a percentage of CFP-Rad18-expressing XPV cells expressing YFP-Polη-Δ(1–512) or YFP-Polη-WT adenovirus. *upper P = 0.018; **P = 0.0001; Error bars = SEM. (D) Immunoblots of fractionated lysates from XPV cells complemented with Polη-WT or Polη-Δ(1–512) and treated with 10 J/m2 UV. (E) Immunoblots of fractionated lysates from Rad18-depleted H1299 cells that were reconstituted with siRNA-resistant Rad18-WT together with FLAG-tagged Polη-WT or Polη-ΔPIP and treated with sham or 10 J/m2 UV. (F) In vitro pulldown assay. His6-PCNA-loaded Nickel beads (or unloaded beads) were incubated with lysates from UV-irradiated H1299 cells expressing HA-Rad18 or both HA-Rad18 and YFP-Polη. (G) In vitro ubiquitination assay. HA-Rad18 complexes immunoprecipitated from UV-irradiated H1299 cells expressing HA-Rad18 alone or in combination with YFP-Polη were mixed with recombinant His6-PCNA, E1, FLAG-ubiquitin, and an ATP-regenerating system and conjugated FLAG-Ub was detected by immunoblotting with anti-FLAG antibodies.
To test whether loss of PCNA binding contributes to defective PCNA monoubiquitination in Polη-Δ(1–512)-complemented XPV cells, we generated point mutations in the PIP box that abrogate PCNA binding (47) (Figure 3A). In Rad18-depleted cells complemented with physiological levels of Rad18-WT, Polη-WT, but not Polη-ΔPIP, promoted PCNA monoubiquitination by Rad18 (Figure 3E, compare lanes 7 and 8 with 11 and 12). Therefore, Polη–PCNA association via the PIP box of Polη contributes to maximal Rad18-mediated PCNA monoubiquitination.
Polη scaffolding mediates Rad18–PCNA association
Although Polη possesses a PIP box (47,49) and interacts directly with PCNA (47), no PCNA-interacting domain has been identified for Rad18, and the mechanism for association of Rad18 with PCNA is unknown. The Polη dependence of Rad18-mediated PCNA monoubiquitination (Figures 1–3) suggested that Polη may serve as a ‘molecular bridge’ or scaffold to facilitate Rad18–PCNA interactions. To test this hypothesis, we developed a cell-free system to determine the Polη dependence of PCNA–Rad18 interactions (if any). Recombinant PCNA was immobilized on Ni-NTA beads (or unloaded beads for controls) and incubated with extracts from cells expressing Rad18 alone or in combination with Polη. When extracts from Rad18-expressing cells were incubated with PCNA-Ni beads, we were unable to detect association between Rad18 and PCNA (Figure 3F, lane 1). However, we readily detected Rad18 association with immobilized PCNA incubated with lysates from Rad18 and Polη co-expressing cultures (Figure 3F, lane 2). Therefore, we conclude that Polη promotes Rad18–PCNA interactions or stabilizes Rad18–PCNA complexes.
We modified this cell-free assay to test whether the presence of Polη influenced PCNA monoubiquitination by Rad18. HA-Rad18 was expressed in UV-irradiated H1299 cells individually or in combination with Polη and then immunoprecipitated using anti-HA antibodies. The resulting immune complexes were then mixed with recombinant PCNA, E1, FLAG-ubiquitin and an ATP-regenerating system. As shown in Figure 3G, Rad18 immune complexes conjugated FLAG-ubiquitin to PCNA in a manner that was stimulated by Polη (compare lanes 2 and 3).
Taken together, the results of Figure 3 suggest that Polη promotes efficient PCNA monoubiquitination via a bridging mechanism that facilitates physical interaction between Rad18 and PCNA.
Polη-induced PCNA monoubiquitination is dissociable from catalytic activity
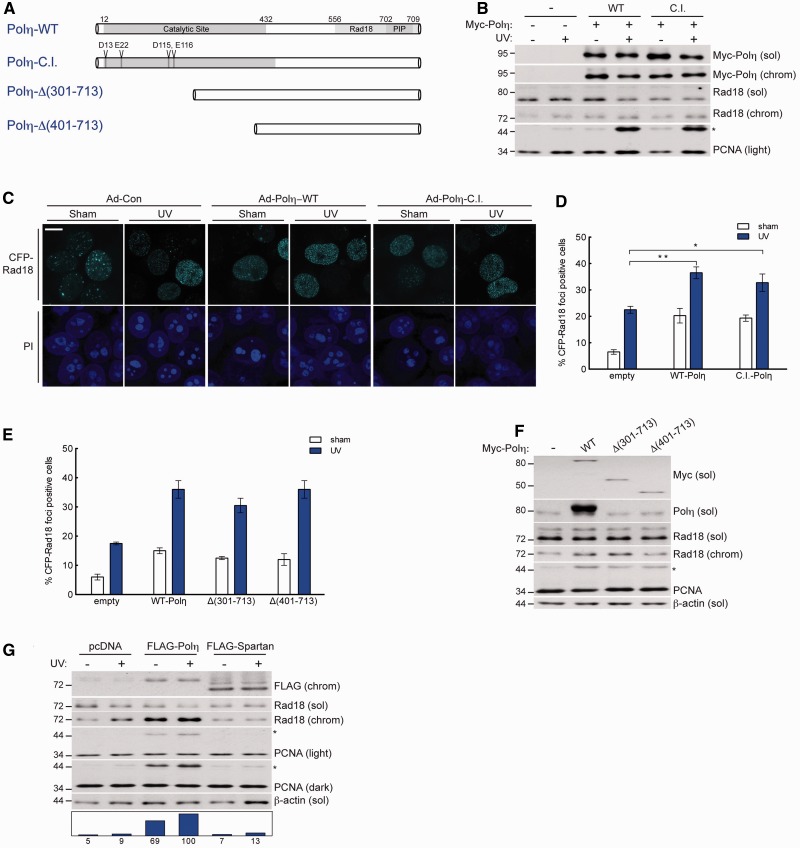
To test whether DNA polymerase activity was required for Polη to promote PCNA monoubiquitination, we generated a Polη mutant harbouring four inactivating point substitutions in conserved residues necessary for catalytic activity (40) (Figure 4A). Catalytically inactive mutant Polη (Polη-C.I.) and wild-type Polη both stimulated PCNA monoubiquitination to similar levels (Figure 4B). Additionally, Polη-C.I. caused Rad18 redistribution to nuclear foci in a manner nearly identical to Polη-WT (Figure 4C and D). Thus, the function of Polη in promoting Rad18-mediated PCNA monoubiquitination is dissociable from its catalytic role as a DNA polymerase.
Figure 4.
Physical bridging of Rad18 and PCNA by Polη is dissociable from its DNA polymerase activity. (A) Schematic of Polη-WT (top); full-length catalytically inactive Polη, Polη-C.I, in which amino acids D13, E22, D115 and E116 are mutated to alanine; and N-terminal Polη truncation mutants, Polη-Δ(301–713) and Polη-Δ(401–713). (B) Immunoblots of fractionated lysates from Myc-Polη-WT or Myc-Polη-C.I.-expressing H1299 cells that were treated with UV (10 J/m2) or sham irradiated. (C) Representative images of CSK-extracted nuclei from H1299 cells that were co-infected with CFP-Rad18 and empty control adenovirus (left), Myc-Polη-WT (middle) or Myc-Polη-C.I. (right) and treated with UV (10 J/m2) or sham irradiated. Scalebar = 10 um. (D) Quantification of CFP-Rad18 foci-positive nuclei as a percentage of CFP-Rad18-expressing H1299 cells expressing empty control adenovirus, Myc-Polη-WT or Myc-Polη-C.I. **P = 0.0016; *P = 0.0287; Error bars = SEM. (E) Quantification of CFP-Rad18 foci-positive nuclei as a percentage of CFP-Rad18-expressing H1299 cells expressing empty control adenovirus, Myc-Polη-WT, Myc-Polη-Δ(301–713) or Myc-Polη-Δ(401–713). Error bars = SEM. (F) Immunoblot of fractionated lysates from H1299 cells expressing empty control adenovirus, Myc-Polη-WT, Myc-Polη-Δ(301–713) or Myc-Polη-Δ(401–713). (G) Immunoblot of fractionated lysates from H1299 cells expressing empty vector control, FLAG-Polη or FLAG-Spartan and lysed 2 h after treatment with 10 J/m2 UV or sham treatment.
To probe the molecular determinants of PCNA monoubiquitination induced by Polη, we performed structure–function studies using Polη truncation mutants that progressively eliminated AAs 1–400 spanning the catalytic domain (Figure 4A). Interestingly, when expressed at equal levels, the Polη-truncation mutants mobilized Rad18 to nuclear foci in a manner similar to Polη-WT (Figure 4E). A Polη truncation constituting only 300 C-terminal amino acids induced a level of PCNA monoubiquitination comparable with WT-Polη (Figure 4F). Therefore, the Rad18- and PCNA-binding C-terminus of Polη represents the minimal domain that is necessary and sufficient to regulate Rad18 activity and promote PCNA monoubiquitination.
Recent work identified a novel PIP box and UBZ-containing protein termed ‘Spartan’ that promotes PCNA monoubiquitination via a bridging mechanism between PCNA and Rad18, similar to that which we have defined for Polη (25). To compare the relative contribution of Spartan and Polη to Rad18-mediated PCNA monoubiquitination, we expressed FLAG-Polη or FLAG-Spartan in H1299 cells. When expressed at comparable levels, Polη induced an increase in PCNA monoubiquitination that was nearly 10-fold higher than that conferred by Spartan (Figure 4G) and siRNA-mediated knockdown of Polη decreased UV-induced PCNA monoubiquitination significantly more than Spartan knockdown (Supplementary Figure S5, compare lanes 4 and 6). Together, these data indicate that scaffolding of PCNA and Rad18 by Polη plays an important role in the regulation of PCNA monoubiquitination.
Y family polymerase specificity of Polη-dependent PCNA monoubiquitination
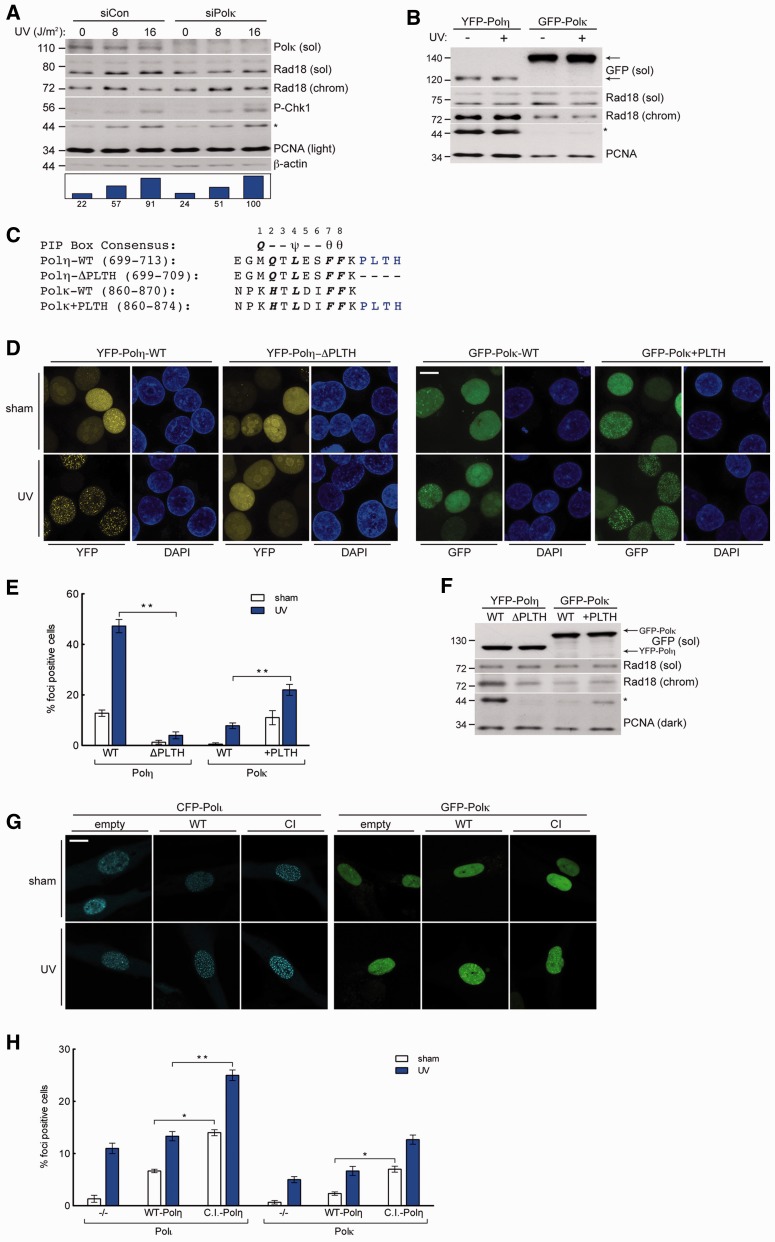
We next asked whether the stimulatory effect of Polη on Rad18 activity was shared by other Y-family TLS polymerases. Similar to Polη, Polκ associates with Rad18 (41), redistributes to form nuclear foci in response to DNA damage, and associates with PCNA via C-terminal PIP box. Therefore, for the purpose of comparison with Polη, we determined the effects of manipulating Polκ expression levels on PCNA monoubiquitination. In contrast with Polη knockdown, Polκ depletion did not attenuate PCNA monoubiquitination basally or after genotoxin treatment (Figure 5A). Even when expressed at levels ∼15-fold higher than Polη, Polκ did not induce the robust PCNA monoubiquitination response elicited by Polη (Figure 5B). Polκ also induced far less Rad18 redistribution to chromatin. Hence, the role of Polη in promoting genotoxin-induced PCNA monoubiquitination is not shared by all Y-family TLS polymerases.
Figure 5.
High-affinity interaction with PCNA drives Polη-specific induction of PCNA monoubiquitination. (A) Immunoblot of fractionated lysates from control or Polκ-depleted H1299 cells that were lysed 2 h after treatment with UV (10 J/m2) or sham irradiation. (B) Immunoblot of fractionated lysates from H1299 cells expressing YFP-Polη or GFP-Polκ and lysed 2 h after treatment with UV (10 J/m2) or sham irradiated. (C) Sequence of the C-terminus of Polη and Polκ and the mutants used in domain-swap experiments: Polη-ΔPLTH and Polκ+PLTH. PIP-box consensus amino acids are in bold, where ψ = I/L/M; θ = Y/F. (D) Representative images of CSK-extracted nuclei from H1299 cells that were infected with GFP-Polκ-WT, GFP-Polκ+PLTH, YFP-Polη-WT or YFP-Polη-ΔPLTH and treated with 10 J/m2 UV or sham irradiated. Scalebar = 10 μm. (E) Quantification of foci-positive nuclei as a percentage of H1299 cells expressing in YFP-Polη-WT, YFP-Polη-ΔPLTH, GFP-Polκ-WT or GFP-Polκ+PLTH. *left P = 0.0001; **P = 0.0004; Error bars = SEM. (F) Immunoblot of fractionated lysates from H1299 expressing YFP-Polη-WT, YFP-Polη-ΔPLTH, GFP-Polκ-WT or GFP-Polκ+PLTH. (G) Representative images of CSK-extracted nuclei from XPV cells that were co-infected with CFP-Polι or GFP-Polκ and empty control adenovirus (left), Myc-Polη-WT (middle) or Myc-Polη-C.I. and treated with UV (10 J/m2) or sham irradiated. Scalebar = 10 µm. (H) Quantification of CFP-Polι foci-positive nuclei as a percentage of CFP-Polι-expressing XPV cells (left) and GFP-Polκ foci-positive nuclei as a percentage of GFP-Polκ-expressing XPV cells (right), after co-infection with empty control adenovirus, Myc-Polη-WT or Myc-Polη-C.I. and treatment with UV (10 J/m2) or sham irradiation. *left P = 0.0009; **P = 0.0004, *right P = 0.0022; Error bars = SEM.
Because Polη and Polκ both associate with Rad18 after DNA damage (30,41), differences in Rad18 binding do not explain the inability of Polκ to promote PCNA monoubiquitination. We therefore hypothesized that differences in TLS polymerase–PCNA binding account for the differential contributions of Polη and Polκ to PCNA monoubiquitination. Domains flanking the PIP boxes in the various Y family members confer dramatically different PCNA-binding affinities (49); specifically, the high PCNA-binding affinity of Polη relative to other TLS polymerases is attributed in large part to the ‘PLTH’ sequence immediately C-terminal to its PIP box (Figure 5C).
To test whether PCNA-binding affinity influences relative PCNA monoubiquitination activity, we performed domain-swap experiments in which we removed the PLTH motif from Polη (generating Polη-ΔPLTH) or added it to Polκ (generating Polκ+PLTH) (Figure 5C). We then compared the subcellular distribution of wild-type and mutant forms of Polη and Polκ. As expected, Polη-ΔPLTH showed reduced nuclear focus formation and was also compromised for PCNA monoubiquitination activity relative to Polη-WT (Figure 5D and F). Conversely, whereas Polκ-WT was localized diffusely throughout the nucleus, Polκ+PLTH showed a focal distribution pattern more similar to that of Polη-WT. Interestingly, Polκ+PLTH induced more robust PCNA monoubiquitination than Polκ-WT (Figure 5F), demonstrating that addition of the PLTH (from the Polη PIP) to the Polκ PIP increases its ability to induce PCNA monoubiquitination. Therefore, high affinity binding of Polη to PCNA confers the unique ability among Y-family polymerases to promote PCNA monoubiquitination.
DNA damage-induced PCNA monoubiquitination contributes to the PCNA binding of all Y-family TLS polymerases (11–13). We hypothesized that Polη would influence other Y-family TLS polymerases by facilitating PCNA monoubiquitination, independently of its catalytic activity. Therefore, we compared the UV-inducible redistribution of Polι and Polκ in parental XPV cells or XPV cells reconstituted with Polη-WT or Polη-C.I. Consistent with prior studies (50,51), we found that basal and UV-induced Polι and Polκ redistribution to nuclear foci was higher in Polη-WT-reconstituted XPV cells compared with the Polη-defective parental cell line (Figure 5G and H). Importantly, we found that Polη-C.I. dramatically increased both basal and UV-induced redistribution of Polι and Polκ to nuclear foci. We conclude that cells expressing full-length catalytically inactive Polη retain Rad18-stimulatory activity, which in turn promotes recruitment of alternative error-prone polymerases to stalled replication forks.
p53 promotes PCNA monoubiquitination via transcriptional induction of Polη
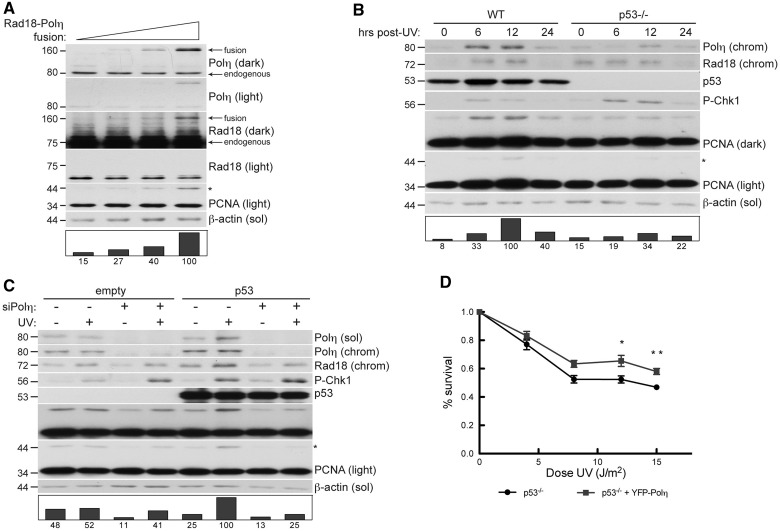
Because Rad18-mediated PCNA monoubiquitination was sensitive to Polη expression, it was of interest to determine relative levels of Rad18 and Polη within cells. Therefore, we expressed an in-frame fusion of full-length Polη and full-length Rad18 in cultured cells, which allowed us to perform quantitative comparison of each endogenous protein relative to the Rad18–Polη fusion (Figure 6A) using appropriate antibodies.
Figure 6.
Rad18–Polη interaction is checkpoint sensitive and p53 regulated in response to DNA damage. (A) Immunoblots of fractionated lysates from H1299 cells transfected with increasing quantities of pACCMV-Rad18-Polη fusion construct. (B) Immunoblot of fractionated lysates from HCT-116 WT or HCT-116 p53−/− cells that were UV treated (30 J/m2) and lysed at indicated times after irradiation. (C) Immunoblots of fractionated lysates of H1299 cells that were transfected with empty pcDNA as control or pcDNA-p53, followed by non-targeting control siRNA or siRNA against Polη. Cells were lysed 6 h after 10 J/m2 UV. (D) UV sensitivity of WT or p53−/− HDF incubated in 1 mM caffeine and exposed to increasing doses of UV. Cells were infected with YFP-Polη adenovirus at a dose that confers UV survival in XP115LO cells (see Supplementary Figure S6). **P = 0.0305 at 12 J/m2. **P = 0.0036 at 15 J/m2.
At expression levels comparable with endogenous Polη, the Rad18–Polη fusion protein was nearly undetectable in immunoblots with anti-Rad18 antibody (Rad18 light), and prolonged exposures revealed that expression of the fusion at these levels was substantially lower than endogenous Rad18 (Rad18 dark). This surprising result demonstrated that cellular Rad18 protein expression is several orders of magnitude higher than Polη in human cells; we estimate that Rad18 expression exceeds Polη by ∼75-fold. Importantly, expression of the Rad18–Polη fusion protein to a level double that of endogenous Polη (and negligible compared with endogenous Rad18) induced a 6-fold increase in PCNA monoubiquitination (right lane), showing that Rad18-mediated PCNA monoubiquitination is highly sensitive to Polη levels. These findings prompted us to determine whether physiologically relevant changes in Polη expression influence PCNA monoubiquitination.
POLH is a transcriptional target of activated p53, and DNA damage stimulates p53-dependent increases in Polη protein expression (52). Because endogenous Polη levels are limiting for Rad18 activity, we hypothesized that p53-induced Polη expression contributes to PCNA monoubiquitination. Therefore, we compared UV-induced PCNA monoubiquitination in WT HCT-116 cells and an isogenic p53-null HCT-116 line (Figure 6B). As expected, Polη protein levels were lower in p53-null cells (compared with WT) after UV. Interestingly, PCNA was monoubiquitinated after UV treatment in a manner that was temporally co-incident with Polη expression in p53-expressing HCT-116 cells, but not in p53−/− cells.
To test whether the p53-induced PCNA monoubiquitination was Polη dependent, we depleted Polη in p53-null H1299 cells that were transfected with empty vector or pcDNAp53. As shown in Figure 6C, transient expression of p53 led to concomitant increases in Polη expression and UV-induced PCNA monoubiquitination (compare lanes 1 and 2 with 5 and 6). Importantly, Polη depletion severely impaired PCNA monoubiquitination in p53-expressing cells compared to controls. Therefore, the p53-dependent component of PCNA monoubiquitination is Polη-mediated.
Because loss of p53 sensitizes normal fibroblasts, but not XPV cells, to UV (53,54), we hypothesized that the UV protection conferred by Polη is mediated by p53. To test this hypothesis, we compared UV survival in p53-depleted HDF cells infected with ‘empty’ control adenovirus or adenovirus expressing Polη at levels that confer UV survival in XPV fibroblasts (Supplementary Figure S6). We found that Polη expression modestly, but significantly, increased UV survival in p53−/− cells (Figure 6D). Therefore, loss of p53-mediated Polη regulation indeed contributed to the UV sensitivity of p53 null fibroblasts. Together, these results suggest that Polη facilitates PCNA monoubiquitination in a p53-dependent manner, thereby revealing a novel link between the p53 pathway and TLS (Figure 7).
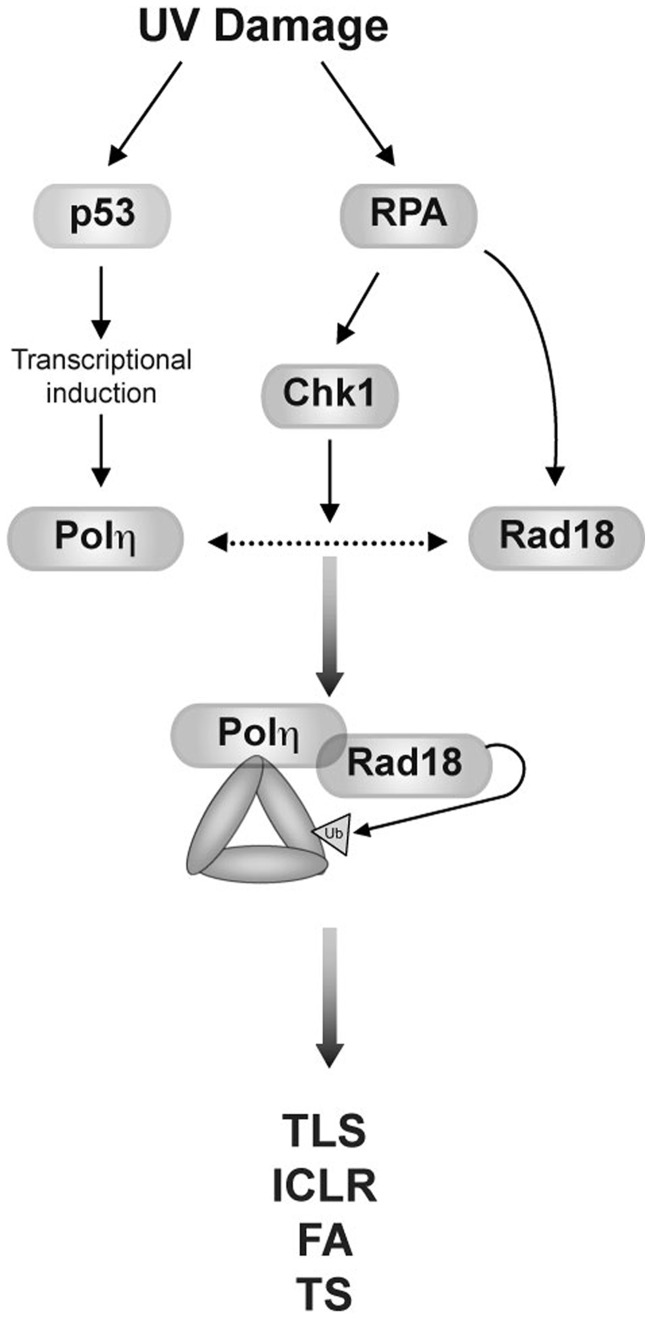
Figure 7.
Contributions of p53 and Chk1 signalling to Polη-facilitated PCNA monoubiquitination. UV-induced p53 activity leads to transcriptional induction of Polη expression (left). RPA-coated ssDNA generated through helicase-polymerase uncoupling directly recruits Rad18 and promotes Polη-Rad18 association via Chk1 signalling (right), thereby stimulating PCNA monoubiquitination and dependent DDR pathways.
DISCUSSION
The results described here are consistent with existing models of TLS pathway activation involving an initial redistribution of the Rad18–Polη complex to the vicinity of damaged DNA (most likely via association of Rad18 with RPA-coated ssDNA) (23,55). However, our results extend current models in that we propose Rad18 is in turn targeted to PCNA, its relevant substrate at the stalled replication fork, by Polη (Figure 7). Specifically, the extreme C-terminus of Polη physically bridges Rad18 and PCNA to stimulate PCNA monoubiquitination (Figure 3), a function unique to Polη among TLS polymerases and fully dissociable from its TLS polymerase activity. The results of this study challenge the notion that TLS constitutes a simple linear pathway in which Rad18 acts upstream of Polη to promote TLS. Instead, we propose that Rad18 and Polη play mutually dependent roles in TLS pathway activation.
Non-catalytic effector functions have been identified for other participants of the DDR, including Rev1 (56), Rad18 (31), NBS1 (57) and Chk1 (58), but this is the first demonstration of a DNA polymerase-independent activity for Polη. A non-catalytic role for Polη in stimulating PCNA monoubiquitination helps explain results of recent studies by other labs. For example, XPV cells are hypersensitive to BPDE and other genotoxins whose DNA lesions are not bypassed by Polη (33,34); clearly, a polymerase-independent function of Polη that promotes PCNA monoubiquitination and activation of Polκ (the TLS polymerase that mediates bypass of BPDE adducts) explains the BPDE sensitivity of XPV cells. In other studies, catalytically dead Polη mutants conferred DNA damage tolerance (36,59) and mutagenesis (35,36). Because PCNA monoubiquitination at K164 is necessary for tolerance of UV and other genotoxins (15–17), restoration of UV survival by catalytically dead Polη (36) is explained by its scaffold function that promotes PCNA monoubiquitination, thus recruiting other TLS polymerases that facilitate tolerance, albeit at a cost of increased mutagenesis.
The Polη scaffolding function identified here has important implications for the molecular basis of genetic instability in XPV patients. Mutagenesis in XPV cells is widely believed to result solely from deficient Polη polymerase activity (60), leading to error-prone TLS of UV-damaged DNA by alternative and inappropriate TLS polymerases (10). Many XPV mutations encode C-terminally-truncated forms of Polη that lack Rad18- and PCNA-binding domains (45). However, in XPV cells in which Polη catalytic activity is perturbed while Rad18-PCNA bridging activity remains intact, high rates of UV-induced mutation frequencies may be conferred not only by loss of thymine dimer bypass activity by Polη, but also by stimulation of Rad18-mediated PCNA monoubiquitination and recruitment of alternative error-prone DNA polymerases.
Our finding that cellular Rad18 expression vastly exceeds Polη was unexpected, yet fully explains why PCNA monoubiquitination is exquisitely sensitive to slight alterations in Polη levels (Figure 6). Potentially, any process that affects Polη expression (52), stability (61–63), or nuclear localization (64) or its association with Rad18 is likely to affect PCNA monoubiquitination and in turn influence TLS. Indeed, we show here that transcriptional induction of POLH by p53 contributes to PCNA monoubiquitination. The Polη-Rad18 interaction is dependent on checkpoint signalling via Chk1 (30). Therefore, the results of this study may explain the long-standing observation that Chk1 signalling is required for efficient PCNA monoubiquitination (41,58). In fact, the Rad18-Δ(402–445) mutant in this study that failed to monoubiquitinate PCNA inducibly in response to Polη expression lacks the Chk1-dependent phosphorylation sites required for Polη binding (30). Therefore, the Polη-dependent mechanism for PCNA monoubiquitination described here may provide the basis for crosstalk between TLS and multiple processes including p53 signalling and the S-phase checkpoint.
Several DNA damage-tolerance pathways depend on PCNA monoubiquitination, including replication fork restart (20), template switching (19), intrastrand cross-link repair (21) and the Fanconi Anaemia pathway (22). Hence, Polη contributes to cross-talk between multiple DDR pathways via PCNA monoubiquitination; loss of this element of the DDR in XPV underscores the importance of their orchestrated convergence to preserve genetic stability.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures 1–6.
FUNDING
National Institutes of Health (NIH) [E509558 and E5016280 to C.V. and 1F30ES019449 to M.D.]. Funding for open access charge: NIH [E509558, E5016280].
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr. Hauro Ohmori, Dr. Yuichi Machida and Dr. James Wohlschlegel for helpful discussions. We thank Dr. Marila Cordiero Stone (UNC-CH, USA) for the C-His6-PCNA bacterial expression plasmid and XP115LO (XPV) cells. We thank Dr. Bill Kaufmann (UNC-CH, USA) for the WT and p53-depleted HDF. We thank Dr. Lee Zou and Dr. Richard Centore (MGH, USA) for providing Spartan constructs. We are grateful to Dr. Bronwyn M. Gunn for critical reading of the manuscript.
REFERENCES
- 1.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 2.Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science. 2002;296:1627–1630. doi: 10.1126/science.1070236. [DOI] [PubMed] [Google Scholar]
- 3.Friedberg EC, Fischhaber PL, Kisker C. Error-prone DNA polymerases: novel structures and the benefits of infidelity. Cell. 2001;107:9–12. doi: 10.1016/s0092-8674(01)00509-8. [DOI] [PubMed] [Google Scholar]
- 4.Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu. Rev. Biochem. 2002;71:17–50. doi: 10.1146/annurev.biochem.71.083101.124707. [DOI] [PubMed] [Google Scholar]
- 5.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, et al. The Y-family of DNA polymerases. Mol. Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann AR, Kirk-Bell S, Arlett CF, Paterson MC, Lohman PH, de Weerd-Kastelein EA, Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc. Natl Acad. Sci. USA. 1975;72:219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi E, Ogi T, Kusumoto R, Iwai S, Masutani C, Hanaoka F, Ohmori H. Error-prone bypass of certain DNA lesions by the human DNA polymerase kappa. Genes Dev. 2000;14:1589–1594. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Woodgate R, McManus TP, Mead S, McCormick JJ, Maher VM. Evidence that in xeroderma pigmentosum variant cells, which lack DNA polymerase eta, DNA polymerase iota causes the very high frequency and unique spectrum of UV-induced mutations. Cancer Res. 2007;67:3018–3026. doi: 10.1158/0008-5472.CAN-06-3073. [DOI] [PubMed] [Google Scholar]
- 11.Freudenthal BD, Gakhar L, Ramaswamy S, Washington MT. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nat. Struct. Mol. Biol. 2010;17:479–484. doi: 10.1038/nsmb.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 13.Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science. 2005;310:1821–1824. doi: 10.1126/science.1120615. [DOI] [PubMed] [Google Scholar]
- 14.Plosky BS, Vidal AE, Fernandez de Henestrosa AR, McLenigan MP, McDonald JP, Mead S, Woodgate R. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 2006;25:2847–2855. doi: 10.1038/sj.emboj.7601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown S, Niimi A, Lehmann AR. Ubiquitination and deubiquitination of PCNA in response to stalling of the replication fork. Cell Cycle. 2009;8:689–692. doi: 10.4161/cc.8.5.7707. [DOI] [PubMed] [Google Scholar]
- 16.Hendel A, Krijger PH, Diamant N, Goren Z, Langerak P, Kim J, Reissner T, Lee KY, Geacintov NE, Carell T, et al. PCNA ubiquitination is important, but not essential for translesion DNA synthesis in mammalian cells. PLoS Genet. 2011;7:e1002262. doi: 10.1371/journal.pgen.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krijger PH, van den Berk PC, Wit N, Langerak P, Jansen JG, Reynaud CA, de Wind N, Jacobs H. PCNA ubiquitination-independent activation of polymerase eta during somatic hypermutation and DNA damage tolerance. DNA Repair (Amst) 2011;10:1051–1059. doi: 10.1016/j.dnarep.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Niimi A, Brown S, Sabbioneda S, Kannouche PL, Scott A, Yasui A, Green CM, Lehmann AR. Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc. Natl Acad. Sci. USA. 2008;105:16125–16130. doi: 10.1073/pnas.0802727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JR, Zeman MK, Chen JY, Yee MC, Cimprich KA. SHPRH and HLTF act in a damage-specific manner to coordinate different forms of postreplication repair and prevent mutagenesis. Mol. Cell. 2011;42:237–249. doi: 10.1016/j.molcel.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciccia A, Nimonkar AV, Hu Y, Hajdu I, Achar YJ, Izhar L, Petit SA, Adamson B, Yoon JC, Kowalczykowski SC, et al. Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress. Mol. Cell. 2012;47:396–409. doi: 10.1016/j.molcel.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K, Moldovan GL, D'Andrea AD. RAD18-dependent recruitment of SNM1A to DNA repair complexes by a ubiquitin-binding zinc finger. J. Biol. Chem. 2010;285:19085–19091. doi: 10.1074/jbc.M109.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng L, Huntoon CJ, Karnitz LM. RAD18-mediated ubiquitination of PCNA activates the Fanconi anemia DNA repair network. J. Cell Biol. 2010;191:249–257. doi: 10.1083/jcb.201005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies AA, Huttner D, Daigaku Y, Chen S, Ulrich HD. Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein a. Mol. Cell. 2008;29:625–636. doi: 10.1016/j.molcel.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huttner D, Ulrich HD. Cooperation of replication protein A with the ubiquitin ligase Rad18 in DNA damage bypass. Cell Cycle. 2008;7:3629–3633. doi: 10.4161/cc.7.23.7166. [DOI] [PubMed] [Google Scholar]
- 25.Centore RC, Yazinski SA, Tse A, Zou L. Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV-induced DNA damage response. Mol. Cell. 2012;46:625–635. doi: 10.1016/j.molcel.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis EJ, Lachaud C, Appleton P, Macartney TJ, Nathke I, Rouse J. DVC1 (C1orf124) recruits the p97 protein segregase to sites of DNA damage. Nat. Struct. Mol. Biol. 2012;19:1093–1100. doi: 10.1038/nsmb.2394. [DOI] [PubMed] [Google Scholar]
- 27.Juhasz S, Balogh D, Hajdu I, Burkovics P, Villamil MA, Zhuang Z, Haracska L. Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance. Nucleic Acids Res. 2012;40:10795–10808. doi: 10.1093/nar/gks850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machida Y, Kim MS, Machida YJ. Spartan/C1orf124 is important to prevent UV-induced mutagenesis. Cell Cycle. 2012;11:3395–3402. doi: 10.4161/cc.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosbech A, Gibbs-Seymour I, Kagias K, Thorslund T, Beli P, Povlsen L, Nielsen SV, Smedegaard S, Sedgwick G, Lukas C, et al. DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat. Struct. Mol. Biol. 2012;19:1084–1092. doi: 10.1038/nsmb.2395. [DOI] [PubMed] [Google Scholar]
- 30.Day TA, Palle K, Barkley LR, Kakusho N, Zou Y, Tateishi S, Verreault A, Masai H, Vaziri C. Phosphorylated Rad18 directs DNA polymerase eta to sites of stalled replication. J. Cell Biol. 2010;191:953–966. doi: 10.1083/jcb.201006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe K, Tateishi S, Kawasuji M, Tsurimoto T, Inoue H, Yamaizumi M. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 2004;23:3886–3896. doi: 10.1038/sj.emboj.7600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkley LR, Palle K, Durando M, Day TA, Gurkar A, Kakusho N, Li J, Masai H, Vaziri C. c-Jun N-terminal kinase-mediated Rad18 phosphorylation facilitates Poleta recruitment to stalled replication forks. Mol. Biol. Cell. 2012;23:1943–1954. doi: 10.1091/mbc.E11-10-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher VM, McCormick JJ, Grover PL, Sims P. Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat. Res. 1977;43:117–138. doi: 10.1016/0027-5107(77)90137-3. [DOI] [PubMed] [Google Scholar]
- 34.Maher VM, Ouellette LM, Mittlestat M, McCormick JJ. Synergistic effect of caffeine on the cytotoxicity of ultraviolet irradiation and of hydrocarbon epoxides in strains of Xeroderma pigmentosum. Nature. 1975;258:760–763. doi: 10.1038/258760a0. [DOI] [PubMed] [Google Scholar]
- 35.Pavlov YI, Nguyen D, Kunkel TA. Mutator effects of overproducing DNA polymerase eta (Rad30) and its catalytically inactive variant in yeast. Mutat. Res. 2001;478:129–139. doi: 10.1016/s0027-5107(01)00131-2. [DOI] [PubMed] [Google Scholar]
- 36.Ito W, Yokoi M, Sakayoshi N, Sakurai Y, Akagi JI, Mitani H, Hanaoka F. Stalled Polη at its cognate substrate initiates an alternative translesion synthesis pathway via interaction with REV1. Genes Cells. 2012;17:98–108. doi: 10.1111/j.1365-2443.2011.01576.x. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 38.Pawsey SA, Magnus IA, Ramsay CA, Benson PF, Giannelli F. Clinical, genetic and DNA repair studies on a consecutive series of patients with xeroderma pigmentosum. Q. J. Med. 1979;48:179–210. [PubMed] [Google Scholar]
- 39.Shiomi N, Mori M, Tsuji H, Imai T, Inoue H, Tateishi S, Yamaizumi M, Shiomi T. Human RAD18 is involved in S phase-specific single-strand break repair without PCNA monoubiquitination. Nucleic Acids Res. 2007;35:e9. doi: 10.1093/nar/gkl979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biertumpfel C, Zhao Y, Kondo Y, Ramon-Maiques S, Gregory M, Lee JY, Masutani C, Lehmann AR, Hanaoka F, Yang W. Structure and mechanism of human DNA polymerase eta. Nature. 2010;465:1044–1048. doi: 10.1038/nature09196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bi X, Barkley LR, Slater DM, Tateishi S, Yamaizumi M, Ohmori H, Vaziri C. Rad18 regulates DNA polymerase kappa and is required for recovery from S-phase checkpoint-mediated arrest. Mol. Cell. Biol. 2006;26:3527–3540. doi: 10.1128/MCB.26.9.3527-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 44.Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D'Andrea AD. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 2006;8:339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 45.Broughton BC, Cordonnier A, Kleijer WJ, Jaspers NG, Fawcett H, Raams A, Garritsen VH, Stary A, Avril MF, Boudsocq F, et al. Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients. Proc. Natl Acad. Sci. USA. 2002;99:815–820. doi: 10.1073/pnas.022473899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanioka M, Masaki T, Ono R, Nagano T, Otoshi-Honda E, Matsumura Y, Takigawa M, Inui H, Miyachi Y, Moriwaki S, et al. Molecular analysis of DNA polymerase eta gene in Japanese patients diagnosed as xeroderma pigmentosum variant type. J. Invest. Dermatol. 2007;127:1745–1751. doi: 10.1038/sj.jid.5700759. [DOI] [PubMed] [Google Scholar]
- 47.Haracska L, Johnson RE, Unk I, Phillips B, Hurwitz J, Prakash L, Prakash S. Physical and functional interactions of human DNA polymerase eta with PCNA. Mol. Cell Biol. 2001;21:7199–7206. doi: 10.1128/MCB.21.21.7199-7206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hishiki A, Hashimoto H, Hanafusa T, Kamei K, Ohashi E, Shimizu T, Ohmori H, Sato M. Structural basis for novel interactions between human translesion synthesis polymerases and proliferating cell nuclear antigen. J. Biol. Chem. 2009;284:10552–10560. doi: 10.1074/jbc.M809745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannouche P, Fernandez de Henestrosa AR, Coull B, Vidal AE, Gray C, Zicha D, Woodgate R, Lehmann AR. Localization of DNA polymerases eta and iota to the replication machinery is tightly co-ordinated in human cells. EMBO J. 2002;21:6246–6256. doi: 10.1093/emboj/cdf618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabbioneda S, Gourdin AM, Green CM, Zotter A, Giglia-Mari G, Houtsmuller A, Vermeulen W, Lehmann AR. Effect of proliferating cell nuclear antigen ubiquitination and chromatin structure on the dynamic properties of the Y-family DNA polymerases. Mol. Biol. Cell. 2008;19:5193–5202. doi: 10.1091/mbc.E08-07-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu G, Chen X. DNA polymerase eta, the product of the xeroderma pigmentosum variant gene and a target of p53, modulates the DNA damage checkpoint and p53 activation. Mol. Cell Biol. 2006;26:1398–1413. doi: 10.1128/MCB.26.4.1398-1413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wani MA, Zhu QZ, El-Mahdy M, Wani AA. Influence of p53 tumor suppressor protein on bias of DNA repair and apoptotic response in human cells. Carcinogenesis. 1999;20:765–772. doi: 10.1093/carcin/20.5.765. [DOI] [PubMed] [Google Scholar]
- 54.Laposa RR, Feeney L, Crowley E, de Feraudy S, Cleaver JE. p53 suppression overwhelms DNA polymerase eta deficiency in determining the cellular UV DNA damage response. DNA Repair (Amst) 2007;6:1794–1804. doi: 10.1016/j.dnarep.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomida J, Masuda Y, Hiroaki H, Ishikawa T, Song I, Tsurimoto T, Tateishi S, Shiomi T, Kamei Y, Kim J, et al. DNA damage-induced ubiquitylation of RFC2 subunit of replication factor C complex. J. Biol. Chem. 2008;283:9071–9079. doi: 10.1074/jbc.M709835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tissier A, Kannouche P, Reck MP, Lehmann AR, Fuchs RP, Cordonnier A. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amst) 2004;3:1503–1514. doi: 10.1016/j.dnarep.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Yanagihara H, Kobayashi J, Tateishi S, Kato A, Matsuura S, Tauchi H, Yamada K, Takezawa J, Sugasawa K, Masutani C, et al. NBS1 recruits RAD18 via a RAD6-like domain and regulates Pol eta-dependent translesion DNA synthesis. Mol. Cell. 2011;43:788–797. doi: 10.1016/j.molcel.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 58.Yang XH, Shiotani B, Classon M, Zou L. Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev. 2008;22:1147–1152. doi: 10.1101/gad.1632808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temviriyanukul P, Meijers M, van Hees-Stuivenberg S, Boei JJ, Delbos F, Ohmori H, de Wind N, Jansen JG. Different sets of translesion synthesis DNA polymerases protect from genome instability induced by distinct food-derived genotoxins. Toxicol. Sci. 2012;127:130–138. doi: 10.1093/toxsci/kfs074. [DOI] [PubMed] [Google Scholar]
- 60.Lange SS, Takata K, Wood RD. DNA polymerases and cancer. Nat. Rev. Cancer. 2011;11:96–110. doi: 10.1038/nrc2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jung YS, Liu G, Chen X. Pirh2 E3 ubiquitin ligase targets DNA polymerase eta for 20S proteasomal degradation. Mol. Cell Biol. 2010;30:1041–1048. doi: 10.1128/MCB.01198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung YS, Qian Y, Chen X. DNA polymerase eta is targeted by Mdm2 for polyubiquitination and proteasomal degradation in response to ultraviolet irradiation. DNA Repair (Amst) 2012;11:177–184. doi: 10.1016/j.dnarep.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skoneczna A, McIntyre J, Skoneczny M, Policinska Z, Sledziewska-Gojska E. Polymerase eta is a short-lived, proteasomally degraded protein that is temporarily stabilized following UV irradiation in Saccharomyces cerevisiae. J. Mol. Biol. 2007;366:1074–1086. doi: 10.1016/j.jmb.2006.11.093. [DOI] [PubMed] [Google Scholar]
- 64.Jung YS, Hakem A, Hakem R, Chen X. Pirh2 E3 ubiquitin ligase monoubiquitinates DNA polymerase eta to suppress translesion DNA synthesis. Mol. Cell Biol. 2011;31:3997–4006. doi: 10.1128/MCB.05808-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.