Abstract
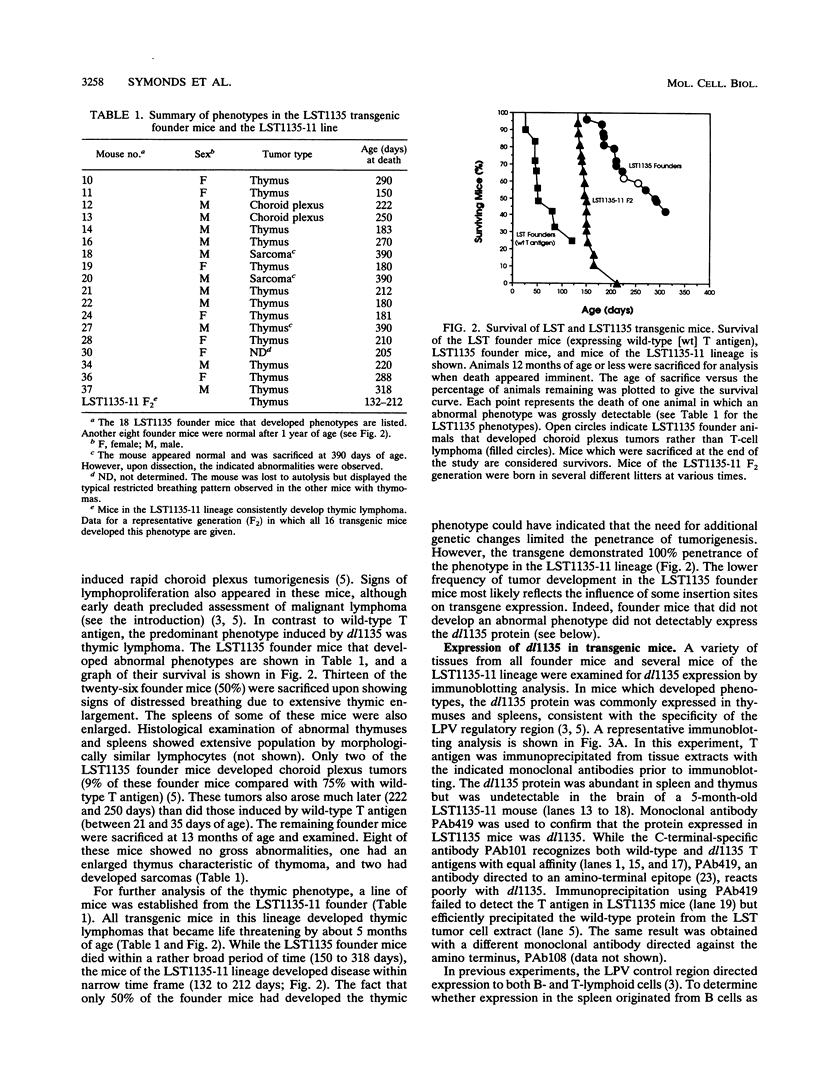
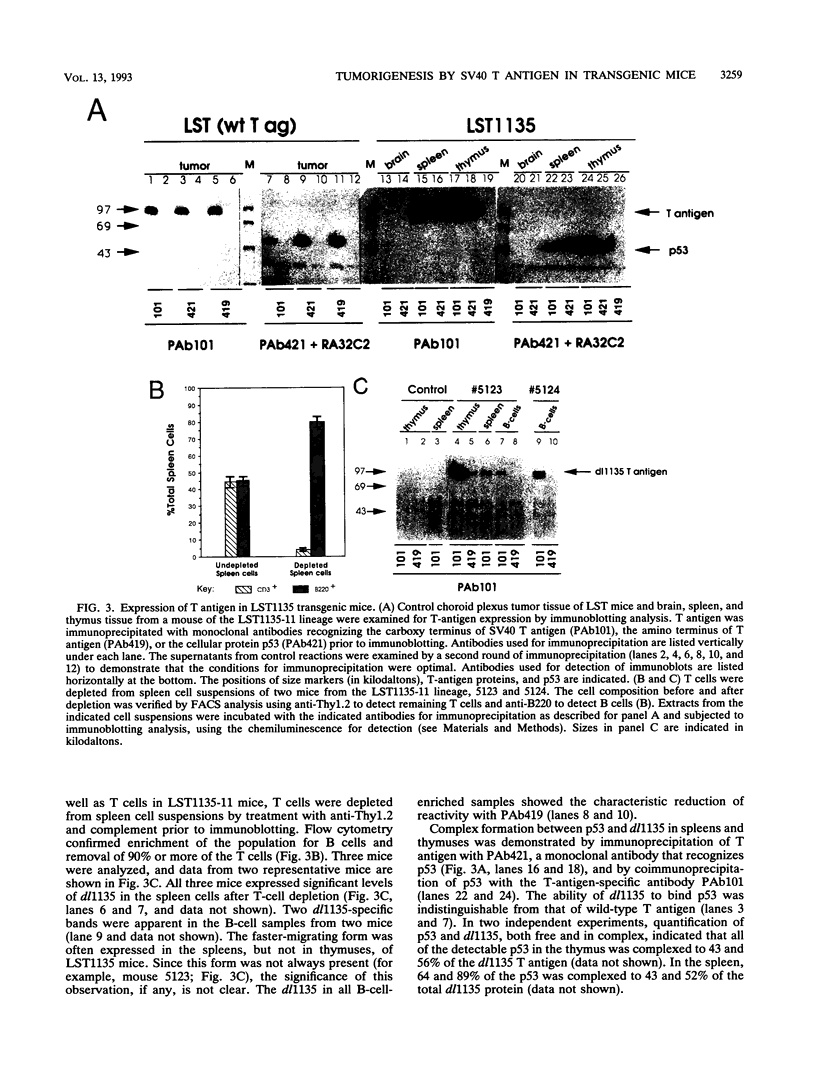
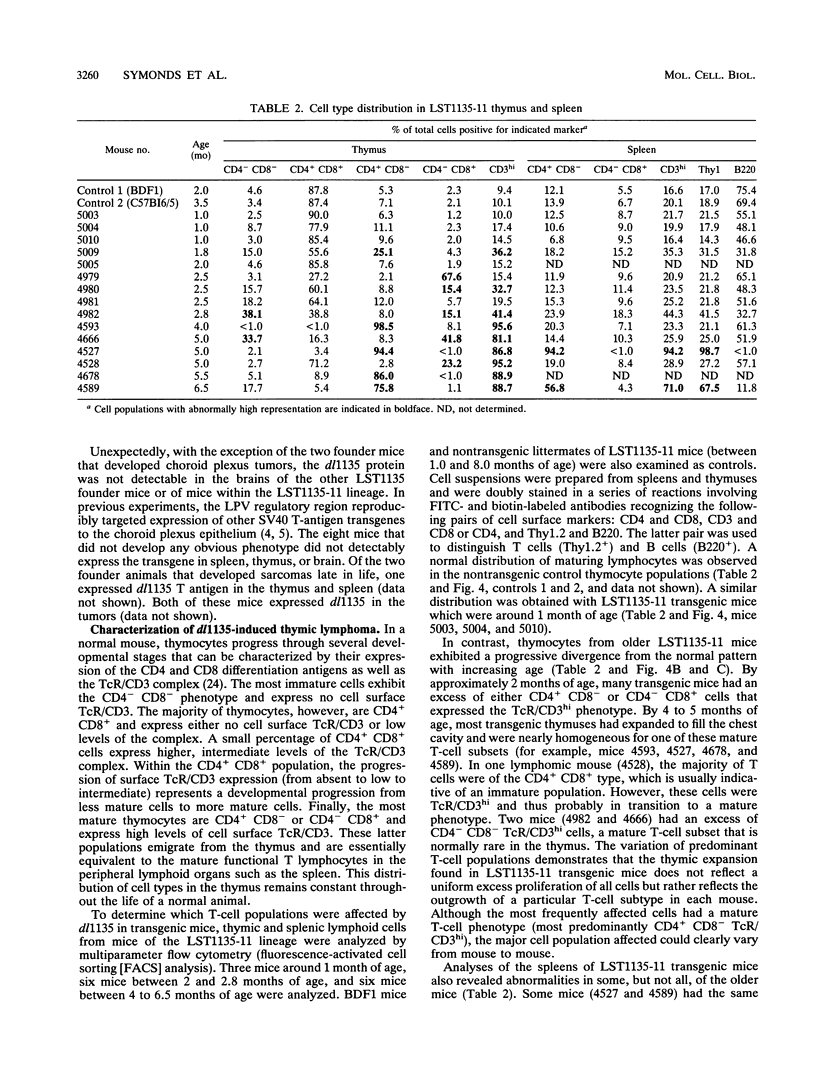
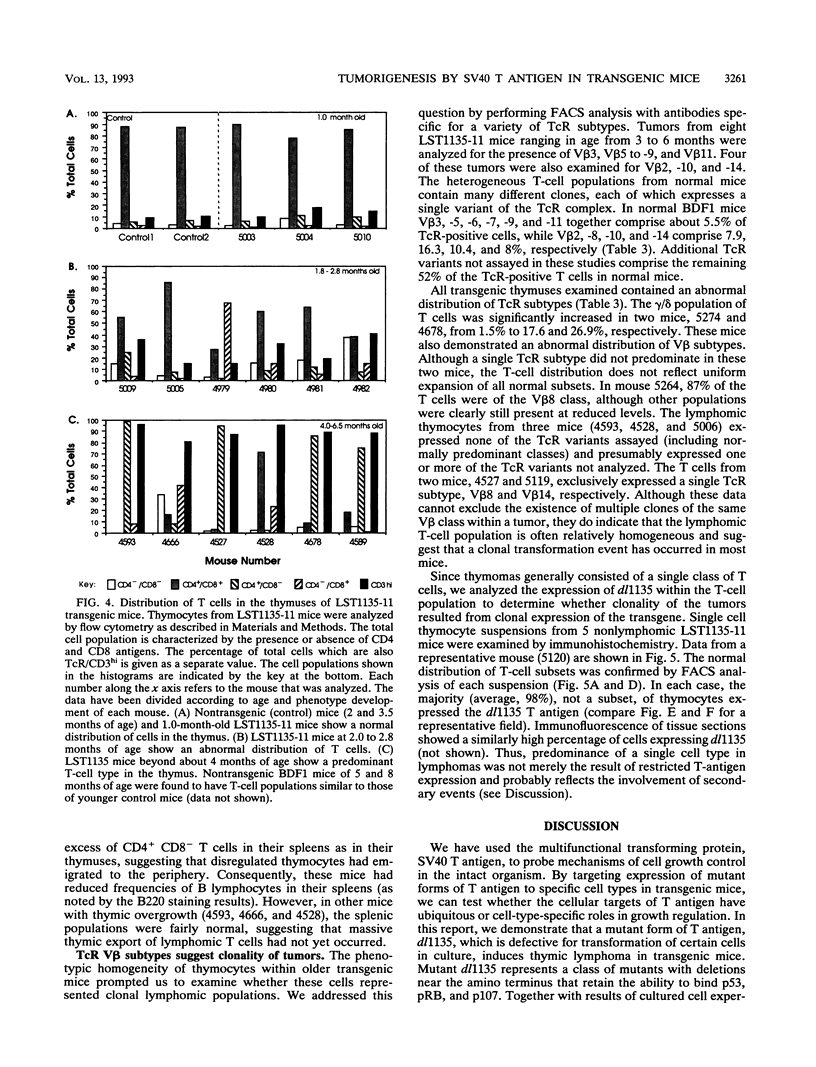
We have used the multifunctional transforming protein, simian virus 40 T antigen, as a probe to study the mechanisms of cell growth regulation in the intact organism. T antigen appears to perturb cell growth, at least in part, by stably interacting with specific cellular proteins that function to maintain normal cell growth properties. Experiments in cultured cells indicate that at least three distinct regions of simian virus 40 T antigen have roles in transformation. Two regions correlate with the binding of known cellular proteins, p53, pRB, and p107. A third activity, located near the amino terminus, has been defined genetically but not biochemically. By targeting expression of wild-type and mutant forms of T antigen to distinct cell types in transgenic mice, we have begun to systematically determine which activities play a role in tumorigenesis of each cell type. In this study, we sought to determine the role of the amino-terminal transformation function with such an analysis of the T-antigen mutant dl1135. This protein, which lacks amino acids 17 to 27, retains the p53-, pRB-, and p107-binding activities yet fails to transform cells in culture. To direct expression in transgenic mice, we used the lymphotropic papovavirus transcriptional signals that are specific for B and T lymphocytes and the choroid plexus epithelium of the brain. We show here that although defective in cell culture, dl1135 specifically induced the development of thymic lymphomas in the mouse. Expression of the protein was routinely observed in B- and T-lymphoid cells, although B-cell abnormalities were not observed. Choroid plexus tumors were observed only infrequently; however, dl1135 was not consistently expressed in this tissue. Within a given transgenic line, the penetrance of T-cell tumorigenesis was 100% but appeared to require secondary events, as judged from the clonal nature of the tumors. These experiments suggest that the amino-terminal region of T antigen has a role in the transformation of certain cell types (such as fibroblasts in culture and B lymphocytes) but is dispensable for the transformation of T lymphocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Transgenic models for haemopoietic malignancies. Biochim Biophys Acta. 1991 Apr 16;1072(1):9–31. doi: 10.1016/0304-419x(91)90004-5. [DOI] [PubMed] [Google Scholar]
- Adams J. M., Cory S. Transgenic models of tumor development. Science. 1991 Nov 22;254(5035):1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Neilson K., Van Dyke T. Lymphotropic papovavirus early region is specifically regulated transgenic mice and efficiently induces neoplasia. J Virol. 1989 May;63(5):2204–2214. doi: 10.1128/jvi.63.5.2204-2214.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Van Dyke T. Uniform cell-autonomous tumorigenesis of the choroid plexus by papovavirus large T antigens. Mol Cell Biol. 1991 Dec;11(12):5968–5976. doi: 10.1128/mcb.11.12.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Tobin G. J., Pipas J. M., Van Dyke T. T-antigen mutant activities in vivo: roles of p53 and pRB binding in tumorigenesis of the choroid plexus. Oncogene. 1992 Jun;7(6):1167–1175. [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. A monoclonal antibody that recognizes B cells and B cell precursors in mice. J Exp Med. 1981 Feb 1;153(2):269–279. doi: 10.1084/jem.153.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby W. W., Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dobbelstein M., Arthur A. K., Dehde S., van Zee K., Dickmanns A., Fanning E. Intracistronic complementation reveals a new function of SV40 T antigen that co-operates with Rb and p53 binding to stimulate DNA synthesis in quiescent cells. Oncogene. 1992 May;7(5):837–847. [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992 Mar;66(3):1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin A. M., Abraham K. M., Forbush K. A., Farr A. G., Davison B. L., Perlmutter R. M. Disruption of thymocyte development and lymphomagenesis induced by SV40 T-antigen. Int Immunol. 1990;2(2):173–180. doi: 10.1093/intimm/2.2.173. [DOI] [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Transgenic mice as probes into complex systems. Science. 1989 Dec 8;246(4935):1265–1275. doi: 10.1126/science.2686032. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Uchida N., Friedman J., Weissman I. L. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Levine A. J., Momand J. Tumor suppressor genes: the p53 and retinoblastoma sensitivity genes and gene products. Biochim Biophys Acta. 1990 Jun 1;1032(1):119–136. doi: 10.1016/0304-419x(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990 Aug;177(2):419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Manfredi J. J., Prives C. Binding of p53 and p105-RB is not sufficient for oncogenic transformation by a hybrid polyomavirus-simian virus 40 large T antigen. J Virol. 1990 Nov;64(11):5250–5259. doi: 10.1128/jvi.64.11.5250-5259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares V., Lodin Z. An autoradiographic study of DNA synthesis in adolescent and adult mouse forebrain. Brain Res. 1974 Aug 23;76(3):557–561. doi: 10.1016/0006-8993(74)90835-x. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Marsilio E., Cheng S. H., Schaffhausen B., Paucha E., Livingston D. M. The T/t common region of simian virus 40 large T antigen contains a distinct transformation-governing sequence. J Virol. 1991 Oct;65(10):5647–5652. doi: 10.1128/jvi.65.10.5647-5652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Fischer-Fantuzzi L., Vesco C., Pipas J. M., Oren M. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J Virol. 1987 Aug;61(8):2648–2654. doi: 10.1128/jvi.61.8.2648-2654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Srinivasan A., Farber J. M., Pipas J. M. Mutants with changes within or near a hydrophobic region of simian virus 40 large tumor antigen are defective for binding cellular protein p53. Virology. 1989 Jan;168(1):13–21. doi: 10.1016/0042-6822(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L., Danna K. J. The amino-terminal 147 amino acids of SV40 large T antigen transform secondary rat embryo fibroblasts. Virology. 1991 Mar;181(1):412–415. doi: 10.1016/0042-6822(91)90516-e. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Peden K. W., Pipas J. M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989 Dec;63(12):5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y., Aizawa S., Furuta Y., Yagi T., Ikawa Y., Saitoh K., Yamada Y., Toyoshima K., Yamamoto T. Induction of a variety of tumors by c-erbB2 and clonal nature of lymphomas even with the mutated gene (Val659----Glu659). EMBO J. 1990 Jan;9(1):181–190. doi: 10.1002/j.1460-2075.1990.tb08094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds H., Chen J. D., Van Dyke T. Complex formation between the lymphotropic papovavirus large tumor antigen and the tumor suppressor protein p53. J Virol. 1991 Oct;65(10):5417–5424. doi: 10.1128/jvi.65.10.5417-5424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Cartwright C. A., Wright J. H., Eckhart W., Peden K. W., Srinivasan A., Pipas J. M. Properties of a simian virus 40 mutant T antigen substituted in the hydrophobic region: defective ATPase and oligomerization activities and altered phosphorylation accompany an inability to complex with cellular p53. J Virol. 1989 Aug;63(8):3362–3367. doi: 10.1128/jvi.63.8.3362-3367.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Pipas J. M., Kierstead T., Cole C. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3' end of SV40 gene A. Virology. 1988 Jan;162(1):76–89. doi: 10.1016/0042-6822(88)90396-0. [DOI] [PubMed] [Google Scholar]
- Thompson D. L., Kalderon D., Smith A. E., Tevethia M. J. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology. 1990 Sep;178(1):15–34. doi: 10.1016/0042-6822(90)90375-2. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yaciuk P., Carter M. C., Pipas J. M., Moran E. Simian virus 40 large-T antigen expresses a biological activity complementary to the p300-associated transforming function of the adenovirus E1A gene products. Mol Cell Biol. 1991 Apr;11(4):2116–2124. doi: 10.1128/mcb.11.4.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Costa R. H., Darnell J. E., Jr, Chen J. D., Van Dyke T. A. Distinct positive and negative elements control the limited hepatocyte and choroid plexus expression of transthyretin in transgenic mice. EMBO J. 1990 Mar;9(3):869–878. doi: 10.1002/j.1460-2075.1990.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Zhu J., Rice P. W., Gorsch L., Abate M., Cole C. N. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992 May;66(5):2780–2791. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]





