Abstract
BACKGROUND
Cardiac allograft vasculopathy (CAV), the major cause of late allograft loss after cardiac transplantation, results from donor-directed cellular and humoral alloimmune responses. Graft vascular endothelial cells (EC) are primary targets of these destructive responses, suggesting that factors associated with endothelial injury and repair could serve as biomarkers of CAV.
METHODS
Using a protein profiler array platform, we measured the levels of 55 angiogenesis-related proteins in sera from 33 adult heart transplant recipients, including 17 with angiographically documented CAV and 16 age- and gender-matched controls without CAV. All patients were >2 years after heart transplant.
RESULTS
The study population was 75% male with a mean age of 62 ± 11 years. On average, patients were 12 ± 5 years after heart transplantation. We found that vascular endothelial growth factor (VEGF)-C, VEGF-A, angiopoietin-2, artemin, urokinase-type plasminogen activator and vasohibin were strongly associated with established CAV (all p < 0.01). Multivariable modeling identified VEGF-C, VEGF-A and platelet factor-4 (PF-4) as significant independent biomarkers of CAV. Furthermore, receiver-operating characteristic curve analysis demonstrated that the combination of all 3 molecules provided outstanding performance for the diagnosis of CAV (area under the curve [AUC] = 0.98; p < 0.001).
CONCLUSIONS
Serum levels of VEGF-C, VEGF-A and PF-4 demonstrate strong associations with established CAV and, together with related angiogenesis factors, may serve as a reliable, non-invasive diagnostic test for CAV in cardiac transplant recipients.
Keywords: heart transplantation, coronary artery disease, angiogenic proteins, biologic markers
Cardiac transplantation remains an important life-saving therapy for patients with end-stage heart failure. Advances in the diagnosis, prevention and treatment of acute cardiac allograft rejection have significantly improved short-term graft survival rates.1 However, these same strategies have failed to interrupt the development and progression of cardiac allograft vasculopathy (CAV), the leading cause of long-term graft failure after heart transplantation.1,2 Once CAV is detectable by coronary angiography or intravascular ultrasound (IVUS), newer therapies, such as mammalian target-of-rapamycin (mTOR) inhibitors, only attenuate the rate of disease progression.3,4 Thus, it is apparent that long-term success after transplantation will only become a reality with the design of therapeutic strategies that prevent the development and/or the progression of the disease process. This goal will require the development of clinically reliable biomarkers that identify CAV at its earliest stage of development, and can be used to individualize and monitor treatment.
CAV is a multi-factorial process that is characteristically associated with donor-directed alloimmune responses, resulting in interstitial inflammation, ongoing graft injury and cellular/intramyocardial apoptosis.5,6 Several studies have indicated that intragraft microvascular endothelial cell activation is an early finding in CAV development.7,8 Graft vascular endothelial cells (EC) respond to cytokines and growth factors produced by infiltrating mononuclear cells that are associated with early reperfusion injury, acute rejection or chronic rejection9–17; it has been reported that both microvascular and large-vessel EC respond similarly to such stimuli.7,18 The induced expression of adhesion molecules and chemokines by EC result in the recruitment of leukocytes and the expression of MHC Class I and II molecules on donor graft EC enables the presentation of alloantigen to infiltrating lymphocytes.14,19–21 In addition, it is increasingly appreciated that alloantibody-mediated injury to the graft primarily involves targeting of the EC, thus making EC injury and repair an important common pathway for analysis.11,15,17,22
In this study, we aimed to test the hypothesis that blood levels of proteins involved in vascular injury and repair could serve as sensitive biomarkers of CAV. Therefore, we profiled serum levels of proteins involved in angiogenesis in cohorts of heart transplant recipients with and without established CAV in order to identify molecules that might serve as candidate biomarkers for further development into a clinical assay.
Methods
Patient population
Adult heart transplant recipients followed in the ambulatory clinic at the Brigham and Women’s Hospital were prospectively enrolled in this study. All patients were at least 2 years removed from orthotopic heart transplantation. The cross-sectional design maximized our ability to enroll patients with CAV. Exclusion of patients in the early post-transplant period minimized the chance of identifying confounders, such as biomarkers of acute cellular rejection. Patients who had undergone heart retransplantation or multi-organ transplantation were excluded. A total of 17 patients with CAV (cases) and 16 age- and gender-matched control patients without CAV (controls) were enrolled over a 12-month period. Serum was obtained from each patient and baseline demographic characteristics, indication for transplant, rejection history, current immunosuppression and cytomegalovirus (CMV) serologic status were collected. Serum was frozen at minus 80°C on the same day the samples were drawn and then stored until use. The protocol was approved by the Committee on Clinical Investigation at Boston Children’s Hospital and written informed consent was obtained from all study subjects.
Clinical management
The majority of patients received triple-drug immunosuppression. In this older cohort, cyclosporine was the primary immunosuppressive medication used in the majority of patients (Table 1); goal trough levels were 200 to 250 µg/liter early post-transplant and 50 to 100 µg/liter late post-transplant. Coronary angiography was performed annually as part of a clinical protocol, starting 1 year post-transplant. Beyond 8 to 10 years post-transplant, the decision to perform coronary angiography was individualized based on patient-specific factors.
Table 1.
Patient, Donor, and Graft Characteristics
| CAV (n = 17) | No CAV (n = 16) | p | |
|---|---|---|---|
| Patient characteristics | |||
| Male gender | 11 (69%) | 14 (82%) | 0.44 |
| Ethnicity | 1 | ||
| Caucasian | 14 (88%) | 15 (88%) | |
| African American | 0 (0%) | 1 (6%) | |
| Hispanic | 1 (6%) | 1 (6%) | |
| Asian/Pacific Islander | 1 (6%) | 0 (0%) | |
| Age at time of transplant (years) | 51.0 ± 10.8 | 48.2 ± 12.1 | 0.48 |
| Indication for transplant (n = 32) | 0.86 | ||
| Coronary artery disease | 4 (25%) | 5 (31%) | |
| Dilated cardiomyopathy: ischemic | 1 (6%) | 1 (6%) | |
| Dilated cardiomyopathy: other | 7 (44%) | 4 (25%) | |
| Hypertrophic cardiomyopathy | 1 (6%) | 2 (13%) | |
| Restrictive cardiomyopathy | 0 (0%) | 1 (6%) | |
| Congenital or valvular heart disease | 3 (19%) | 3 (19%) | |
| CAD-related indication for transplant (n = 32) | 5 (29%) | 7 (44%) | 0.48 |
| Recipient CMV status IgG positive (n = 20) | 8 (67%) | 5 (63%) | 1 |
| Donor and graft characteristics (n = 31) | |||
| Male gender | 7 (44%) | 9 (60%) | 0.48 |
| Donor age (years) | 41.4 ± 12.2 | 30.9 ± 11.6 | 0.02 |
| Donor CMV status IgG-positive | 5 (33%) | 4 (25%) | 0.70 |
| Graft ischemic time (minutes) | 175 ± 54 | 152 ± 48 | 0.23 |
| Patient characteristics at time of study sample | |||
| Time since transplant (years) | 11.3 ± 3.9 | 12.4 ± 5.2 | 0.51 |
| Time from CAV diagnosis to sample (years) | 5.7 ± 3.5 | N/A | |
| Number of episodes of acute rejection in first year post-transplant (ISHLT Grade 3A or higher) | 1 [0, 1] | 1 [0, 5] | 0.19 |
| Immunosuppression at time of study | |||
| Prednisone | 17 (100%) | 15 (94%) | 0.49 |
| Tacrolimus | 3 (18%) | 0 (0%) | 0.23 |
| Cyclosporine | 12 (71%) | 14 (88%) | 0.40 |
| Sirolimus | 5 (29%) | 1 (6%) | 0.18 |
| Mycophenolate mofetil | 5 (29%) | 6 (38%) | 0.72 |
| Azathioprine | 7 (41%) | 8 (50%) | 0.73 |
CAD, coronary artery disease; CAV, cardiac allograft vasculopathy; CMV, cytomegalovirus; ISHLT, International Society for Heart and Lung Transplantation.
In our program, angiography is only performed prior to 1 year post-transplant for urgent clinical indications such as acute allograft dysfunction or arrhythmia. Any donor with significant coronary artery disease risk factors, a history of cardiac arrest or age >40 years is screened with coronary angiography prior to accepting the donor heart for transplant. Intravascular ultrasound (IVUS) is not performed as part of the clinical protocol, and therefore IVUS data were not available. Endomyocardial biopsy is performed as follows: weekly for 4 weeks; every other week for 8 weeks; monthly until prednisone has been tapered to 5 to 6 mg/day; and then every 6 to 12 months, depending upon rejection history. Biopsies are graded for acute cellular rejection using the prevailing ISHLT criteria.23,24
Classification of CAV
The most recent coronary angiogram, hemodynamics and echocardiogram were interpreted by the clinicians caring for each patient at the Brigham and Women’s Hospital and were used to identify patients for referral and enrollment. A single cardiologist investigator (K.P.D.) also reviewed cardiac testing results to confirm classification of study subjects. The angiograms were graded according to the published consensus guidelines from the International Society of Heart and Lung Transplantation.25 The reviewer (K.P.D.) was blinded to the results of other clinical diagnostic tests performed on each patient.
Profiling levels of angiogenesis-related proteins
Concentrations of 55 angiogenesis-related proteins were determined using a human angiogenesis protein array kit (ARY007; R&D Systems, Minneapolis, MN). Serum samples were assayed using standard techniques by the R&D Biomarker Testing Service, blinded to patient data. Briefly, 0.5 ml of patient serum was added to a biotinylated detection antibody cocktail and was incubated with a nitrocellulose membrane spotted (in duplicate) with specific capture antibodies. The membranes were washed, incubated with streptavidin–horseradish peroxidase and, after an additional wash step, were developed by chemiluminescence. Each blot was scanned using a transmission mode scanner and expression of each molecule was determined by densitometry using automated image analysis software. Densitometric values of duplicate samples were averaged and subtracted from the average densitometric value for each negative control to compensate for background. Values are reported in densitometric units (DU). Molecules that were present at levels below that of the negative control were assigned a value of 0 DU. This assay allows for quantitative comparisons and ranking of patients based on levels of individual molecules.
VEGF-A serum concentration was analyzed using a sandwich enzyme-linked immunoassay (ELISA) according to the protocol provided by the manufacturer (DVE00; R&D Systems, Minneapolis, MN). In addition, VEGF-A serum concentration was analyzed using a magnetic bead–based quantitative multiplex assay, according to the manufacturer’s protocol (HAGP1MAG-12K; Millipore, Billerica, MA), using a Luminex 200 device. Data were analyzed using XPONENT software (version 3.1; Millipore).
Statistical methods
Patients’ characteristics were compared between the cases and controls using Student’s t-test for normally distributed continuous variables, Wilcoxon’s rank-sum test for continuous variables with a skewed distribution and Fisher’s exact test for proportions. Median levels of each serum biomarker were compared between cases and controls by the Mann–Whitney U-test and displayed using box- and-whisker plots. Candidate biomarkers with p <0.05 in univariable analysis were entered into a backward stepwise multivariable logistic regression model to identify which candidate biomarkers were independently associated with CAV using the likelihood ratio test to assess significance. Receiver-operating characteristic (ROC) curve analysis was applied to determine diagnostic accuracy based on area under the curve (AUC) for each significant multivariate predictor and composite of predictive biomarkers together. Statistical analysis was performed using SPSS software (SPSS, Inc./IBM, Chicago, IL) and all reported p-values are 2-tailed.
Results
Patient characteristics
Within our cohort of 33 heart transplant recipients, 17 patients had angiographically documented CAV. Among the patients with CAV, 69% were male, 88% were Caucasian, the average age at transplant was 51 ± 11 years, and the average time from transplant to study enrollment was 11 ± 4 years (Table 1). All patients with CAV had established disease with an average time from diagnosis of CAV to study enrollment of 5.7 ± 3.5 years. All but 1 of the CAV patients were at least 1.5 years removed from the original diagnosis of CAV at the time of study enrollment. Forty-four percent of patients with CAV were transplanted for non-ischemic dilated cardiomyopathy. Coronary artery disease (25%) and congenital or valvular heart disease (19%) were the next most common indications. There were no significant differences between cases and controls with regard to gender, ethnicity, age at time of transplant, indication for transplant, time since transplant and CMV status (Table 1). Donor age was significantly greater in patients with CAV (41 ± 12 years vs 31 ± 12; p = 0.02), but notably most donors were <50 years of age, thus excluding the highest risk strata.26 Graft ischemic time and the number of episodes of acute rejection in the first posttransplant year were not significantly different between groups, although the study was not powered for risk factor analysis. The majority of patients in both groups were on triple-drug immunosuppression, with prednisone, cyclosporine and azathioprine being the most frequently used combination. Among the patients with CAV, 9 (53%) had ISHLT Grade 1 disease, 3 (18%) had ISHLT Grade 2 disease, and 5 (29%) had ISHLT Grade 3 disease.
CAV and proteins involved in angiogenesis and endothelial proliferation
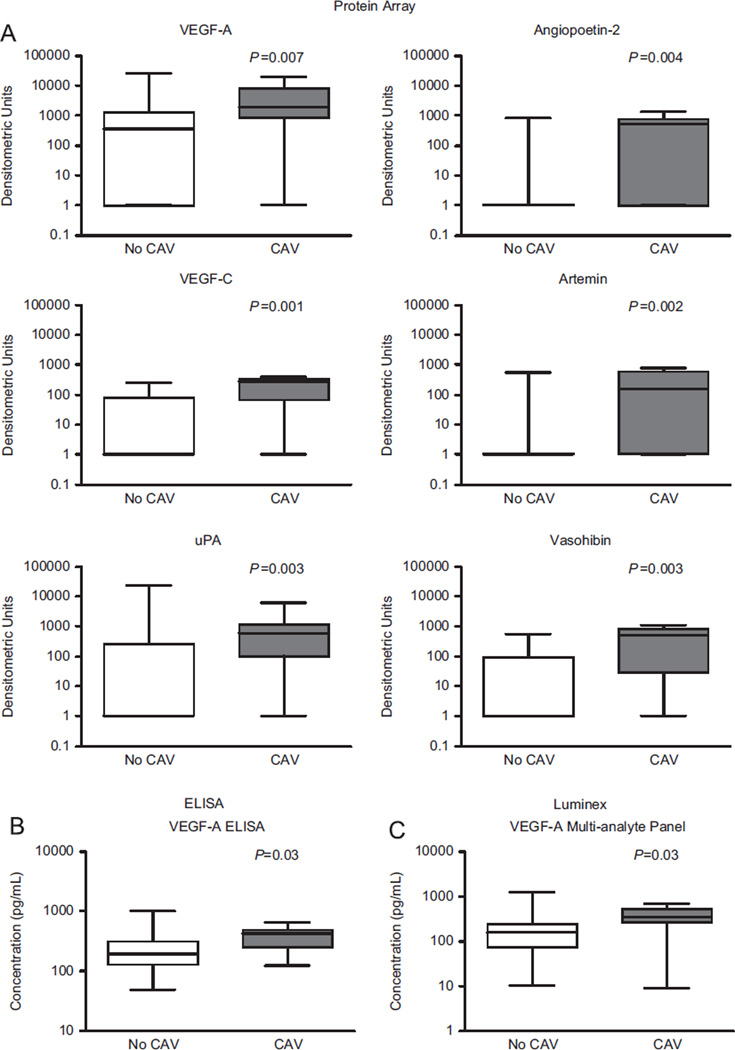
There were significant differences in the levels of 21 of the 55 angiogenesis-related factors between patients with and without CAV (Table 2). Of these 21 proteins, vascular endothelial growth factor (VEGF)-C, artemin, urokinase plasminogen activator, vasohibin, angiopoietin-2 and VEGF-A showed the greatest differences between cases and controls (Figure 1). The 34 proteins that failed to show statistically significant differences are listed in Table S1 (supplementary data associated with this article can be found, in the online version, at www.jhltonline.org).
Table 2.
Levels of Angiogenesis-related Proteins Showing Statistically Significant Differences in Univariable Analysis
| Protein | CAV (n = 17) | No CAV (n = 16) | p |
|---|---|---|---|
| VEGF-C | 274 [63, 328] | 0 [0, 99] | 0.001 |
| Artemin | 157 [0, 579] | 0 [0, 0] | 0.002 |
| uPA | 590 [103, 1,192] | 0 [0, 272] | 0.003 |
| Vasohibin | 500 [28, 851] | 0 [0, 96] | 0.003 |
| Angiopoietin-2 | 520 [0, 799] | 0 [0, 0] | 0.004 |
| VEGF-A | 1,951 [806, 7,902] | 372 [0, 1,423] | 0.007 |
| Endothelin-1 | 434 [0, 908] | 0 [0, 116] | 0.01 |
| Thrombospondin-2 | 459 [10, 983] | 0 [0, 237] | 0.01 |
| Amphiregulin | 353 [0, 752] | 0 [0, 136] | 0.02 |
| TGF-β1 | 492 [67, 719] | 46 [0, 138] | 0.02 |
| CCL3 | 757 [37, 1,393] | 18 [0, 470] | 0.02 |
| Persephin | 328 [0, 737] | 0 [0, 33] | 0.02 |
| PF-4 | 1,066 [484, 8,840] | 84 [0, 2,548] | 0.02 |
| SerpinB5 | 704 [43, 1,358] | 0 [0, 421] | 0.02 |
| Thrombospondin-1 | 718 [0, 1,200] | 0 [0, 170] | 0.02 |
| Epidermal growth factor | 943 [0, 1,540] | 0 [0, 566] | 0.03 |
| FGF1 (acidic) | 433 [0, 661] | 0 [0, 252] | 0.04 |
| FGF2 (basic) | 290 [26, 531] | 0 [0, 117] | 0.04 |
| HBEGF | 830 [109, 1,842] | 209 [0, 816] | 0.04 |
| TYMP | 683 [44, 1,099] | 54 [0, 328] | 0.04 |
| SerpinF1 | 1,115 [188, 1,639] | 287 [0, 769] | 0.04 |
The densitometric value for each molecule is presented as the median [25th percentile, 75th percentile]. Comparisons were made using the Mann–Whitney U-test. CAV, cardiac allograft vasculopathy; FGF1, fibroblast growth factor 1; FGF2, fibroblast growth factor 2; HBEGF, heparin-binding EGF-like growth factor; TGF-β1, transforming growth factor-beta1; CCL3, chemokine (C-C motif) ligand 3; TYMP, thymidine phosphorylase; serpinB5, serpin peptidase inhibitor, clade B (ovalbumin), member 5; PF-4, platelet factor-4; serpinF1, serpin peptidase inhibitor, clade F (alpha-2 anti-plasmin, pigment epithelium-derived factor), member 1; uPA, plasminogen activator, urokinase; VEGF-A, vascular endothelial growth factor-A; VEGF-C, vascular endothelial growth factor-C.
Figure 1.
Proteins involved in vascular injury and repair responses are associated with cardiac allograft vasculopathy (CAV). (A) Proteins were quantitated using chemiluminescence after capture by membrane-bound antibody. These six proteins were selected as they had the strongest association with CAV in univariable analysis (p < 0.01). Data are displayed using box-and-whisker plots with medians displayed on a logarithmic scale. Measurements below the limit of detection were assigned a value of 1 densitometric unit for the purpose of display. (B) Box-and-whisker plots showing the concentration of VEGF-A (pg/ml) as determined by ELISA. (C) Box-and-whisker plots showing the concentration of VEGF-A (pg/ml), as determined by quantitative multiplex assay.
The difference in VEGF-A serum concentration, which has been reported to be associated with CAV risk,27,28 was further validated using a VEGF-A ELISA assay and VEGF-A multiplex assay. By ELISA, we found that VEGF-A serum concentrations were significantly higher in patients with established CAV (421 pg/ml [interquartile range 242] vs 195 pg/ml [IQR 187]; p = 0.03). The magnetic bead–based multiplex assay also revealed higher VEGF-A concentrations in patients with CAV (348 pg/ml [IQR 157] vs 158 pg/ml [IQR 127]; p = 0.03). There were no significant differences in levels of VEGF-A, VEGF-C and platelet factor-4 (PF-4) among patients with CAV based on whether or not sirolimus was used as part of the immunosuppressive regimen.
VEGF-C, VEGF-A and PF4 as sensitive and specific biomarkers for established CAV
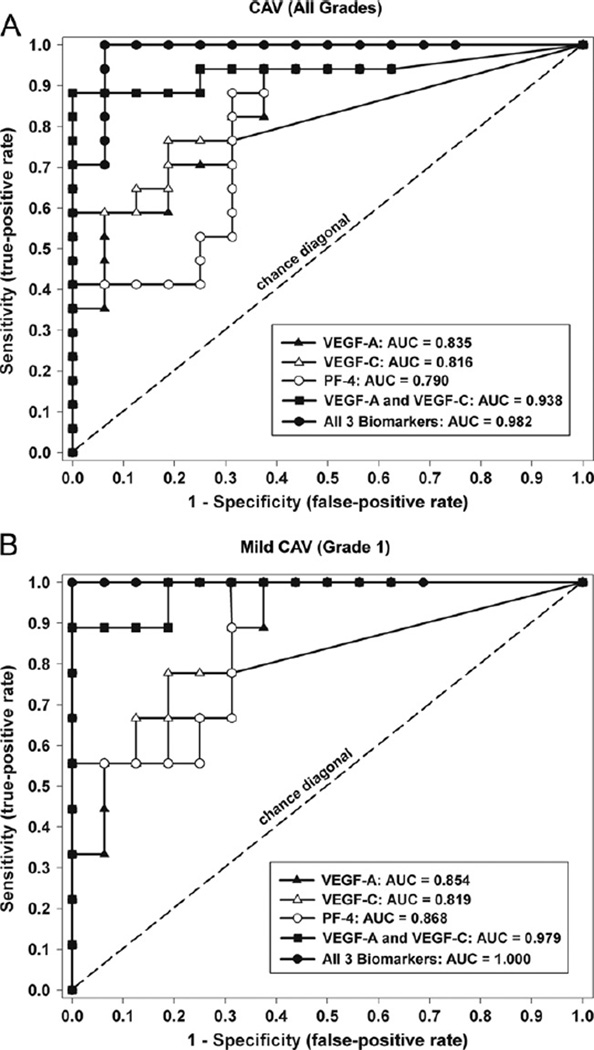
Multivariate logistic regression modeling identified VEGF-C, VEGF-A and PF-4 as the strongest independent biomarkers associated with established CAV. ROC analysis was used to determine the diagnostic test characteristics of these biomarkers, both alone and in combination. These biomarkers provide excellent diagnostic separation of patients with CAV from patients without CAV (Figure 2A and Table 3). In addition, we found that these biomarkers accurately separate the subset of patients with mild CAV (Grade 1) from patients without CAV (Figure 2B and Table 3).
Figure 2.
Receiver-operating characteristic (ROC) curve analysis for vascular endothelial growth factor (VEGF)-A, VEGF-C and platelet factor (PF)-4. These biomarkers were identified as significant independent predictors in the multivariable logistic regression model. (A) Test performance characteristics for those patients with CAV (all grades) vs controls without CAV. (B) Test performance characteristics for separating patients with mild CAV (Grade 1) from controls without CAV.
Table 3.
Receiver-Operating Characteristic (ROC) Curve Analysis for Biomarkers with the Strongest Independent Predictive Value in the Multivariable Logistic Model
| CAV (all grades) vs controls | CAV (Grade 1) vs controls | |||||
|---|---|---|---|---|---|---|
| Biomarker | AUC | 95% CI | p | AUC | 95% CI | p |
| VEGF-A | 0.835 | 0.700–0.973 | <0.001 | 0.854 | 0.708–1.000 | 0.003 |
| VEGF-C | 0.816 | 0.665–0.967 | 0.002 | 0.819 | 0.623–1.000 | 0.008 |
| PF-4 | 0.790 | 0.632–0.949 | 0.004 | 0.868 | 0.726–1.000 | 0.002 |
| VEGF-A and VEGF-C | 0.938 | 0.840–0.999 | <0.001 | 0.979 | 0.931–1.000 | <0.001 |
| All 3 combineda | 0.982 | 0.942–1.000 | <0.001 | 1.000 | 0.999–1.000 | <0.001 |
AUC, area under the curve; CI, confidence interval; PF-4, platelet factor-4; VEGF-A, vascular endothelial growth factor-A; VEGF-C, vascular endothelial growth factor-C.
Based on multivariable logistic regression analysis (combined AUC value is equivalent to the c-index).
When evaluating the entire CAV population (all grades), each individual biomarker had good test performance characteristics (range of AUC = 0.790 to 0.835; p < 0.005 for all). However, when VEGF-A and VEGF-C were modeled together, the performance characteristics improved substantially (AUC = 0.938; 95% CI 0.850 to 0.999; p < 0.001). When PF-4 was added to the model, the diagnostic performance characteristics were optimized for the identification of patients with CAV (AUC = 0.982; 95% CI 0.940 to 1.000; p < 0.001). By considering VEGF-A, VEGF-C and PF-4 in the model and selecting an optimal diagnostic cut-off, the current data suggest that these biomarkers are 100% sensitive and 94% specific for the diagnosis of CAV (all grades). When the subset of patients with Grade 1 CAV was compared with controls, the results were similar (Table 3). In this instance, the combination of VEGF-A and VEGF-C provided nearly perfect discrimination between mild CAV (Grade 1) and controls (AUC = 0.979; 95% CI 0.931 to 1.000; p < 0.001).
Discussion
In this study we have demonstrated that soluble proteins involved in vascular remodeling are associated with established CAV in patients with angiographically apparent disease. Specifically, we found that a combination of serum levels of VEGF-C, VEGF-A and PF-4 can identify patients with established CAV in a sensitive and specific manner. In addition, levels of these three proteins allowed for a sensitive and specific diagnosis of even mild CAV. Our findings support the hypothesis that serum levels of VEGF-C, VEGF-A and PF-4 may serve as the basis for the development of a clinical diagnostic test for CAV. A larger prospective study will allow for identification of optimal cut-off values so that patients can be risk-stratified in the future.
To date, no non-invasive blood test exists for use in CAV screening.29,30 Chronic rejection with the development of CAV continues to be the most prominent cause of late allograft loss in heart transplant recipients,2 and its prevention and treatment is a priority for the development of novel therapeutics in the field. Unfortunately, a major impediment to progress relates to a lack of tools to predict disease initiation. State-of-the-art approaches continue to rely heavily on invasive testing, such as coronary angiographic and IVUS.5,6,31 Although other less invasive imaging studies are available, including computed tomographic (CT) angiography, dobutamine stress echocardiography and nuclear imaging, they have limitations due to their lack of sensitivity and/or their limited ability to detect early and small-vessel disease.5 Thus, it is generally appreciated that sensitive and clinically useful biomarkers are needed to advance our ability to detect and treat this condition.
Our data suggest that multiple proteins involved in angiogenesis are associated with CAV in human heart transplant recipients. We believe that these findings lay the groundwork for the development of a quantitative blood-based assay for the diagnosis of CAV. Consistent with other reports,27,28 we found high serum levels of VEGF-A in patients with angiographically apparent CAV. However, a new observation in our data set is that the combination of VEGF-A with VEGF-C and PF-4 has better diagnostic test performance characteristics than VEGF-A alone. In addition, related molecules, including angiopoietin-1, artemin, urokinase-type plasminogen activator and vasohibin, showed strong statistical associations with CAV and should be measured quantitatively in a larger prospective study, along with VEGF-A, VEGF-C and PF-4, in order to validate the findings of this pilot cross-sectional analysis. Although our findings support an association between these proteins and established CAV, the cross-sectional study design does not allow inference to be made regarding when serum levels of these biomarkers increase relative to the development of CAV. Nevertheless, the association between these biomarkers and mild CAV (Grade 1) supports the hypothesis that changes in these biomarkers will precede the development of clinically apparent disease. A prospective cohort study examining these biomarkers at multiple time-points relative to the appearance of CAV, assessed by both IVUS and angiography, is needed to test this hypothesis.
VEGF-A is an important pro-angiogenic molecule that is well established to promote the survival and proliferation of vascular endothelial cells. It also has potent proinflammatory effects, which include its ability to act as a chemoattractant for monocytes and lymphocytes,32–35 and its ability to elicit vascular permeability.36 Consistent with our findings, VEGF-A has emerged as an important molecule in the rejection process, and its expression has been reported by our group as well as several others in association with both acute and chronic allograft rejection.33,34,37–39 Torry et al found that enhanced VEGF-A expression was confined to areas of the allograft myocardium in association with monocyte/macrophage infiltrates and also that expression of VEGF-A was associated with fibrin deposition.38 In previous studies, we observed increases in VEGF-A expression within human cardiac allografts, and we found that the expression of VEGF-A was spatially associated with infiltrates. Furthermore, we noted that high levels of VEGF-A expression correlated with the development of both acute and chronic allograft rejection, and that persistent overexpression of intragraft VEGF-A identified risk for the development of CAV.40 Other investigators reported that genotypes associated with high VEGF production confer increased risk for development of chronic rejection.41–43 Finally, in experimental animal models, VEGF-A has been shown to be involved in the development of CAV. In these models, forced overexpression of VEGF-A within the myocardium of cardiac allografts results in monocyte recruitment, vascular disease and CAV development.37 The current findings, taken together with previously published data, provide compelling evidence that VEGF-A is overexpressed in association with rejection and that VEGF-A may serve as a biomarker of alloimmune-mediated graft injury as well as CAV disease activity.
An intriguing finding in this study is that VEGF-C was also associated with CAV. VEGF-C binds VEGF receptor (R)-2 and VEGFR-3 to mediate its biologic effects, and it is well-established to be a dominant factor stimulating lymphangiogenesis. Consistent with our findings, increased production of VEGF-C has been reported in experimental models of chronic allograft rejection44 and recent studies have linked increased lymphangiogenesis to chronic allograft rejection after both kidney and lung transplantation in humans.45–48 In addition, Nykänen et al demonstrated that inhibition of VEGFR-3 leads to a decrease in lymphatic vessel activation, intragraft inflammation and graft vasculopathy in a rat and mouse model of chronic cardiac rejection.44 Our study has provided the first clinical evidence that measurement of serum levels of VEGF-C may provide useful information after heart transplantation. Based on these data in both animals and humans, we speculate that VEGF-C/VEGFR-3 interactions represent an important area for mechanistic investigation.
PF-4 was one of the first chemokines shown to be an angiogenesis inhibitor.49 At sites of vascular injury, circulating platelets adhere to the naked basement membrane, resulting in aggregation and release of their alpha granule contents, which include PF-4. In this manner, high levels of PF-4 have been reported to be released within minutes of endothelial injury.49 Once bound to its receptor CXCL4, PF-4 functions in diverse biologic processes through its effects on multiple cell types, including immune cells and endothelial cells. Its effects on endothelial cells inhibit angiogenesis and vascular repair, whereas its chemoattractant effects on leukocytes promote inflammation, notably monocyte-dependent inflammation. Although PF-4 has not yet been reported to function in CAV development, it is well established to promote atherosclerosis.50,51 The presence of pro-thrombotic factors, including tissue plasminogen activator, fibrin and anti-thrombin, in early post-transplant endomyocardial biopsy specimens has been associated with later development of CAV.52,53 Thus, it is possible that the ongoing pro-thrombotic response within cardiac allografts in patients with CAV leads to elevated PF-4 concentrations. Collectively, these findings provide further rationale to support PF-4 as a biomarker of vascular injury and CAV development.49
The current study has several limitations that require consideration. The arrays used did not allow for generation of a standard curve and therefore do not permit well-defined cut-offs expressed as a concentration of each molecule in serum. This methodologic limitation is partially obviated by the fact that all assays were run by the same laboratory using a standard protocol. However, it is likely that a multiplex bead Luminex or ELISA-based assay would have allowed for more quantitative results and standardization of our findings. Although bead-based assays for the molecules reported in this study are being developed, we performed a multiplex Luminex assay using a commercially available kit to assess levels of VEGF-A in a subset of our cohort. We also performed a VEGF-A ELISA on these serum samples. Using both techniques, we found a significant increase in VEGF-A concentrations in patients with CAV compared with controls. It should also be noted that VEGF-A and other sequestered proteins are released from platelets during the process of clotting, and therefore measurements using serum samples may not be as reliable as plasma measurements.54 Thus, it is possible that biomarker testing in plasma may reveal more significant changes than those reported in this study.
Although our study involved a small number of patients with CAV, the findings suggest that testing for VEGF-A, VEGF-C and PF-4 in combination may be a highly sensitive approach to screening for established CAV. Our sample size is consistent with earlier pilot studies in transplantation that have laid the groundwork for larger observational cohort studies.55,56 Thus, we believe this pilot study provides valuable data supporting the hypothesis that monitoring blood concentrations of VEGF-A, VEGF-C and PF-4 can be used to non-invasively diagnose established CAV. An alternative hypothesis, which must be considered, is that elevated levels of VEGF-A, VEGF-C and PF-4 in the CAV cohort may be the result of atherosclerotic vascular disease affecting the peripheral vasculature or coronary arteries themselves. There is a clear overlap between risk factors for coronary artery disease and CAV.
In conclusion, in this pilot discovery biomarker study, we have identified distinct patterns of soluble proteins associated with EC injury, repair and proliferation in stable adult heart transplant recipients with angiographically apparent CAV. The data provide a basis for future studies in which these candidate biomarkers can be validated using high-throughput quantitative assays with a rapid turnaround time.
Supplementary Material
Acknowledgments
We thank Dr Peter Ganz for helping to establish this collaboration and for advice and thoughts pertaining to study design. We also thank Dr Elizabeth Blume for mentoring to K.P.D. and for her support and thoughtful comments, and Dave Finkel (R&D Systems, Minneapolis, MN) for help with the protein array experiments. Finally, we acknowledge the support provided by Drs. Waleed Alhabeeb and Ravi Thadhani.
This work was funded in part by grants from the National Institutes of Health (NIH; UL1 RR 025758 [Harvard Catalyst], 5U01AI063623, 5U01AI063594 and 3R01AI046756-10S1). K.P.D. was supported by NIH grants (T32 HL07572 and K12HD052896-06) and the Boston Children’s Hospital Cardiac Transplant and Education Fund. M.S. was supported by NIH grant T32DK007726.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
Supplementary data
Supplementary data associated with this article can be found in the online version, at www.jhltonline.org.
References
- 1.Kirk R, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: fourteenth pediatric heart transplantation report—2011. J Heart Lung Transplant. 2011;30:1095–1103. doi: 10.1016/j.healun.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report—2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Arora S, Ueland T, Wennerblom B, et al. Effect of everolimus introduction on cardiac allograft vasculopathy—results of a randomized, multicenter trial. Transplantation. 2011;92:235–243. doi: 10.1097/TP.0b013e31822057f1. [DOI] [PubMed] [Google Scholar]
- 4.Raichlin E, Bae JH, Khalpey Z, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 5.Schmauss D, Weis M. Cardiac allograft vasculopathy—recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer RJ, Lee MS. Transplant coronary artery disease. JACC Cardiovasc Interv. 2010;3:367–377. doi: 10.1016/j.jcin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 8.Briscoe DM, Yeung AC, Schoen FJ, et al. Predictive value of inducible endothelial cell adhesion molecule expression for acute rejection of human cardiac allografts. Transplantation. 1995;59:204–211. [PubMed] [Google Scholar]
- 9.Briscoe DM, Alexander SI, Lichtman AH. Interactions between T lymphocytes and endothelial cells in allograft rejection. Curr Opin Immunol. 1998;10:525–531. doi: 10.1016/s0952-7915(98)80218-5. [DOI] [PubMed] [Google Scholar]
- 10.Pober JS. Immunobiology of human vascular endothelium. Immunol Res. 1999;19:225–232. doi: 10.1007/BF02786490. [DOI] [PubMed] [Google Scholar]
- 11.Denton MD, Davis SF, Baum MA, et al. The role of the graft endothelium in transplant rejection: evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr Transplant. 2000;4:252–260. doi: 10.1034/j.1399-3046.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 12.Pober JS. Endothelial activation: intracellular signaling pathways. Arthritis Res. 2002;4(suppl 3):S109–S116. doi: 10.1186/ar576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos IH, Briscoe DM. Endothelial injury: cause and effect of alloimmune inflammation. Transpl Infect Dis. 2002;4:152–159. doi: 10.1034/j.1399-3062.2002.t01-1-02002.x. [DOI] [PubMed] [Google Scholar]
- 14.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459–2465. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruneau S, Woda C, Daly K, et al. Key features of the intragraft microenvironment that determine long-term survival following transplantation. Front Immunol. 2012:3. doi: 10.3389/fimmu.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P, Zhao DX. Allograft arteriosclerosis and immune-driven angiogenesis. Circulation. 2003;107:1237–1239. doi: 10.1161/01.cir.0000059744.64373.08. [DOI] [PubMed] [Google Scholar]
- 19.Pober JS, Orosz CG, Rose ML, et al. Can graft endothelial cells initiate a host anti-graft immune response? Transplantation. 1996;61:343–349. doi: 10.1097/00007890-199602150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Valujskikh A, Heeger PS. Emerging roles of endothelial cells in transplant rejection. Curr Opin Immunol. 2003;15:493–498. doi: 10.1016/s0952-7915(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 21.Kreisel D, Krupnick AS, Gelman AE, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 22.Sis B, Jhangri GS, Bunnag S, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 23.Billingham ME. Dilemma of variety of histopathologic grading systems for acute cardiac allograft rejection by endomyocardial biopsy. J Heart Transplant. 1990;9:272–276. [PubMed] [Google Scholar]
- 24.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Nagji AS, Hranjec T, Swenson BR, et al. Donor age is associated with chronic allograft vasculopathy after adult heart transplantation: implications for donor allocation. Ann Thorac Surg. 2010;90:168–175. doi: 10.1016/j.athoracsur.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramson LP, Pahl E, Huang L, et al. Serum vascular endothelial growth factor as a surveillance marker for cellular rejection in pediatric cardiac transplantation. Transplantation. 2002;73:153–156. doi: 10.1097/00007890-200201150-00030. [DOI] [PubMed] [Google Scholar]
- 28.Bayliss J, Bailey M, Leet A, et al. Late onset antibody-mediated rejection and endothelial localization of vascular endothelial growth factor are associated with development of cardiac allograft vasculopathy. Transplantation. 2008;86:991–997. doi: 10.1097/TP.0b013e318186d734. [DOI] [PubMed] [Google Scholar]
- 29.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 30.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 31.Cai QJ, Rangasetty UC, Barbagelata A, et al. Cardiac allograft vasculopathy advances in diagnosis. Cardiol Rev. 2011;19:30–35. doi: 10.1097/CRD.0b013e3181fbde2f. [DOI] [PubMed] [Google Scholar]
- 32.Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 33.Reinders ME, Sho M, Izawa A, et al. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003;112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 35.Edelbauer M, Datta D, Vos IH, et al. Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo. Blood. 2010;116:1980–1989. doi: 10.1182/blood-2009-11-252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158–163. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- 37.Lemstrom KB, Krebs R, Nykanen AI, et al. Vascular endothelial growth factor enhances cardiac allograft arteriosclerosis. Circulation. 2002;105:2524–2530. doi: 10.1161/01.cir.0000016821.76177.d2. [DOI] [PubMed] [Google Scholar]
- 38.Torry RJ, Labarrere CA, Torry DS, et al. Vascular endothelial growth factor expression in transplanted human hearts. Transplantation. 1995;60:1451–1457. doi: 10.1097/00007890-199560120-00014. [DOI] [PubMed] [Google Scholar]
- 39.Pilmore HL, Eris JM, Painter DM, et al. Vascular endothelial growth factor expression in human chronic renal allograft rejection. Transplantation. 1999;67:929–933. doi: 10.1097/00007890-199903270-00024. [DOI] [PubMed] [Google Scholar]
- 40.Reinders ME, Fang JC, Wong W, et al. Expression patterns of vascular endothelial growth factor in human cardiac allografts: association with rejection. Transplantation. 2003;76:224–230. doi: 10.1097/01.TP.0000071363.55007.D0. [DOI] [PubMed] [Google Scholar]
- 41.Girnita DM, Brooks MM, Webber SA, et al. Genetic polymorphisms impact the risk of acute rejection in pediatric heart transplantation: a multi-institutional study. Transplantation. 2008;85:1632–1639. doi: 10.1097/TP.0b013e3181722edc. [DOI] [PubMed] [Google Scholar]
- 42.Girnita DM, Ohmann EL, Brooks MM, et al. Gene polymorphisms impact the risk of rejection with hemodynamic compromise: a multicenter study. Transplantation. 2011;91:1326–1332. doi: 10.1097/TP.0b013e31821c1e10. [DOI] [PubMed] [Google Scholar]
- 43.Shahbazi M, Fryer AA, Pravica V, et al. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol. 2002;13:260–264. doi: 10.1681/ASN.V131260. [DOI] [PubMed] [Google Scholar]
- 44.Nykanen AI, Sandelin H, Krebs R, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth actor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- 45.Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 46.Dashkevich A, Heilmann C, Kayser G, et al. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 2010;90:406–412. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Thaunat O, Patey N, Caligiuri G, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185:717–728. doi: 10.4049/jimmunol.0903589. [DOI] [PubMed] [Google Scholar]
- 48.Yin N, Zhang N, Lal G, et al. Lymphangiogenesis is required for pancreatic islet inflammation and diabetes. PLoS One. 2011;6:e28023. doi: 10.1371/journal.pone.0028023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandercappellen J, van Damme J, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2011;22:1–18. doi: 10.1016/j.cytogfr.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Koenen RR, von Hundelshausen P, Nesmelova IV, et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15:97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 51.Pitsilos S, Hunt J, Mohler ER, et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost. 2003;90:1112–1120. doi: 10.1160/TH03-02-0069. [DOI] [PubMed] [Google Scholar]
- 52.Labarrere CA, Woods JR, Hardin JW, et al. Early prediction of cardiac allograft vasculopathy and heart transplant failure. Am J Transplant. 2011;11:528–535. doi: 10.1111/j.1600-6143.2010.03401.x. [DOI] [PubMed] [Google Scholar]
- 53.Labarrere CA, Woods JR, Hardin JW, et al. Value of the first post-transplant biopsy for predicting long-term cardiac allograft vasculopathy (CAV) and graft failure in heart transplant patients. PLoS One. 2012;7:e36100. doi: 10.1371/journal.pone.0036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klement GL, Yip TT, Cassiola F, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson JA, Kim EJ, Begley B, et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant. 2011;11:2228–2234. doi: 10.1111/j.1600-6143.2011.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strehlau J, Pavlakis M, Lipman M, et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci USA. 1997;94:695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.