Abstract
Objective
Epidemiological studies indicate that higher bone mass is associated with moderate alcohol consumption in postmenopausal women. However, the underlying cellular mechanisms responsible for the putative beneficial effects of alcohol on bone are unknown. Excessive bone turnover, combined with an imbalance whereby bone resorption exceeds bone formation, is the principal cause for postmenopausal bone loss. This study investigated the hypothesis that moderate alcohol attenuates bone turnover following menopause.
Methods
Bone mineral density (BMD) was determined by dual energy x-ray absorptiometry in 40 healthy postmenopausal women (56.3 ± 0.5 years of age, mean ± SE) who consumed 19 ± 1 g alcohol/day. Serum levels of the bone formation marker osteocalcin and resorption marker C-terminal telopeptide (CTx) were measured by immunoassay at baseline (day 0) and following alcohol withdrawal for 14 days. Participants then consumed alcohol and were assayed the following morning.
Results
BMD at the trochanter and total hip were positively correlated to level of alcohol consumption. Serum osteocalcin and CTx increased following abstinence (4.1 ± 1.6%; p = 0.01 and 5.8 ± 2.6%; p = 0.02 compared to baseline, respectively). Osteocalcin and CTx decreased following alcohol re-administration compared to the previous day (−3.4 ± 1.4%; p = 0.01 and −3.5 ± 2.1%; p = 0.05, respectively), to values that did not differ from baseline (p > 0.05).
Conclusions
Abstinence from alcohol resulted in increased markers of bone turnover whereas resumption of alcohol reduced bone turnover markers. These results suggest a cellular mechanism for the increased bone density observed in postmenopausal moderate alcohol consumers. Specifically, the inhibitory effect of alcohol on bone turnover attenuates the detrimental skeletal consequences of excessive bone turnover associated with menopause.
Keywords: osteoporosis, DXA, osteocalcin, CTx, ethanol, serum estradiol
INTRODUCTION
Postmenopausal women comprise 80% of patients with osteoporosis [1]. Postmenopausal bone loss occurs as a result of elevated and unbalanced bone turnover, in which bone resorption predominates over bone formation [2–4]. While osteoporosis is characterized by low bone mass and deterioration of bone microarchitecture [5], the elevated turnover following menopause is an independent risk factor for osteoporotic bone fractures [2, 3].
Pharmacological interventions that reduce bone turnover are commonly used for treatment of postmenopausal bone loss [6]. However, high cost and the significant potential for detrimental side effects contraindicates universal preventive drug therapy [7]. It is therefore important to identify modifiable lifestyle factors that influence the risk for osteoporosis.
Alcohol consumption is a lifestyle factor that has context-dependent effects on the skeleton. Chronic alcohol abuse is associated with reductions in bone mineral density (BMD) [8, 9], osteoblast number [10–12] and osteoblast function [8]. In contrast, moderate alcohol intake (0.5–2 drinks/day) is associated with increased BMD [13–19]. It is uncertain, however, whether the difference in BMD in the moderate alcohol consumers is due to alcohol intake or potential confounding variables.
Carefully controlled animal studies suggest that moderate alcohol decreases bone turnover while maintaining the balance between bone formation and bone resorption [20]. The purpose of the present study was to assess the effects of regular moderate alcohol consumption on bone turnover in healthy early postmenopausal women. An intervention trial was conducted over a 15 day period and evaluated the effects of alcohol withdrawal and re-administration on biochemical markers of bone formation and bone resorption. The working hypothesis was that alcohol withdrawal would result in increased bone turnover markers and subsequent return to normal alcohol consumption would decrease the turnover markers.
MATERIALS AND METHODS
Study Participants
The study protocol was approved by the Institutional Review Board at Oregon State University and written informed consent was obtained from each participant. For eligibility in the study, participants had to be: 1) postmenopausal for 10 years or less, 2) under the age of 65 years, 3) not on hormone replacement therapy (HRT) within six months of study initiation, and 4) consumers of 0.5–2 standard alcoholic drinks/day (8 – 28 g ethanol) during the year prior to study initiation. Participants were disqualified from the study if they were taking any medications known to affect bone turnover, previously suffered an osteoporosis-related fracture, or had a BMD T-score ≤ −2.5 at the femoral neck or spine [21].
Forty-four postmenopausal women consented to participate in this study and 40 completed the study; three of the participants did not meet entrance criteria and one participant withdrew prior to study initiation for reasons unrelated to the study.
Experimental Design
The intervention trial was done over a 15 day time interval and assessed the effects of alcohol withdrawal (day 0 to 14) and re-administration (day 14 to 15) on biochemical markers of bone turnover. The experimental design was based on animal studies demonstrating that alcohol has rapid but reversible effects on bone metabolism [22]. During the week prior to intervention, participants maintained their normal alcohol intake and kept a daily record of alcohol consumed. Blood samples were collected at the beginning of the intervention (day 0). During the next 14 days participants abstained from consuming alcohol and blood samples were collected on the final day of abstinence (day 14). Participants were then given a container with a pre-determined amount of alcohol as a preferred beverage, equivalent to their prior average daily intake. That evening (day 14), participants consumed the alcohol beverage as part of their normal routine, and blood was collected the following morning (day 15). To minimize diurnal variation in biochemical markers of bone turnover [23–26], blood samples from participants were collected between 0700 and 0900 hours. Participants fasted for at least 10 hours prior to blood collection. Blood samples were centrifuged within two hours of collection and serum was stored frozen at −20°C until analysis.
Methods
Bone mineral density and body composition
Dual-energy X-ray absorptiometry (DXA, Hologic QDR-4500 Elite A; Waltham, MA) was used to measure whole body composition (bone, lean mass, and fat mass) and areal BMD of the proximal femur (total hip, femoral neck, and greater trochanter) and the lumbar spine (L1-L4). Body mass index (BMI) was calculated from DXA-determined body mass and self-reported height. All scans were performed at Oregon State University and analyzed using Hologic software version 9.80 D (Hologic, Inc., Waltham, MA). Quality control was maintained using a bone phantom. The coefficient of variation for repeated DXA scans for BMD of the hip and lumbar spine in our lab is 1.0%.
Alcohol records
During the week prior to study initiation, participants kept a written record of alcoholic beverages consumed. The type and quantity of alcoholic beverage as well as the time of day the alcoholic beverage was consumed were recorded. Average daily alcohol consumption was determined according to the 2010 USDA Dietary Guidelines for typical serving sizes.
Dietary analysis
Prior to initiation of intervention, the study participants completed a Block Food Frequency Questionnaire (NutritionQuest, Berkeley, CA). Intake levels of calcium and vitamin D, two dietary factors especially important in bone metabolism, were obtained from the questionnaires. Participants submitted their Block Food Frequency Questionnaire and alcohol records at the initial blood draw (day 0).
Serum assays
Serum samples were analyzed for the resorption marker CTx and formation marker osteocalcin using ELISA (Immunodiagnostics Systems, Scottsdale, AZ). Additionally, serum estradiol levels were measured using ELISA (Calbiotech, Spring Valley, CA). To eliminate inter-assay variation, all samples for each participant were analyzed using the same assay. To reduce the impact of intra-assay variation and improve precision, all specimens were assayed in duplicate. The intra-assay variation is 1.7 – 3.0% for CTx, 1.2 – 3.3% for osteocalcin, and 5.7–8.6% for estradiol.
Statistical analysis
Simple linear regression model was used to assess the relationship between alcohol intake and BMD, osteocalcin, and CTx at baseline. F tests did not support adjustment of the primary predictor (alcohol) for age and BMI (p = 0.18 for total hip BMD; p = 0.09 for trochanter BMD; p = 0.40 for femoral neck BMD; and p = 0.74 for lumbar spine BMD). The correlation between serum osteocalcin and CTx was evaluated by linear regression using log-transformed data. During the intervention portion of the study, participants acted as their own controls and % changes compared to baseline values (day 0) and abstinence values (day 14) were calculated. Differencing was selected for our data analysis, in part, because it induced independence without requiring a model that is conditional on the strict assumptions of a parametric normal random effects distribution and equal variance over time. To determine whether osteocalcin and CTx increased after abstinence from alcohol as predicted, a one-sided paired t-test was performed. One-sided paired t-tests were also used to determine if osteocalcin and CTx decreased on day 15 as predicted after participants consumed alcohol on the evening of day 14. F tests did not support adjustment for age and BMI over the one-sample model (p = 0.22 for baseline to day 14 serum osteocalcin; p = 0.24 for day 14 to day 15 serum osteocalcin; p = 0.10 for baseline to day 14 serum CTx; and 0.56 for day 14 to day 15 serum CTx). The assumption of normality for t-tests and linear regression performed on original and log transformed data was assessed using the Anderson-Darling test and normal quantile plots. Neither indicated evidence test of data departure from normality. All statistical analyses were performed using S+ version 8.0 (TIBCO Spotfire, Somerville, MA). Differences were considered significant at p ≤ 0.05. Data are reported as mean ± SE.
RESULTS
Study population
The physical characteristics of the study population at baseline are presented in Table 1 and reflect a typical healthy population of postmenopausal women. Based on the Block Food Frequency Questionnaire, mean daily intakes of calcium were 814 ± 46 mg/d as dietary calcium and 748 ± 76 mg/d as supplemental calcium, and mean daily intakes of vitamin D were 128 ± 12 IU/d as dietary vitamin D and 383 ± 41 IU/d as supplemental vitamin D.
Table 1.
Characteristics of participants at baseline.
| N | 40 |
|---|---|
| Age (y) | 56.3 ± 0.5 |
| Number of years postmenopausal | 4.7 ± 0.5 |
| Weight (kg) | 67.8 ± 1.9 |
| Height (cm) | 164.5 ± 0.8 |
| Body mass index (kg/m2) | 25.1 ± 0.7 |
| Lean mass (kg) | 43.5 ± 0.8 |
| Fat mass (kg) | 22.1 ± 1.5 |
| Total body fat (%) | 31.5 ± 1.3 |
Values are mean ± SE
Alcohol intake
Based on one-week alcohol records prior to study initiation, the amount of alcohol consumed by participants averaged 1.4 standard (American) drinks/d or 19 ± 1 g of ethanol/d.
Bone mineral density
T-scores (comparison to young adults) for BMD ranged from −0.50 to −1.00 while Z-scores (comparison to same age adults) ranged from 0.12 to 0.29 (Table 2).
Table 2.
Bone mineral density of total hip, greater trochanter, femoral neck, and lumbar spine at baseline.
| Total Hip | |
| Bone mineral density (g/cm2) | 0.88 ± 0.02 |
| T-Score | −0.54 ± 0.13 |
| Z-Score | 0.22 ± 0.13 |
| Greater Trochanter | |
| Bone mineral density (g/cm2) | 0.65 ± 0.01 |
| T-Score | −0.50 ± 0.14 |
| Z-Score | 0.22 ± 0.13 |
| Femoral Neck | |
| Bone mineral density (g/cm2) | 0.74 ± 0.01 |
| T-Score | −1.00 ± 0.13 |
| Z-Score | 0.12 ± 0.13 |
| Total Lumbar Spine | |
| Bone mineral density (g/cm2) | 0.95 ± 0.02 |
| T-Score | −0.95 ± 0.15 |
| Z-Score | 0.29 ± 0.16 |
Values are means ± SE; N= 39 for femur and 40 for lumbar spine.
Alcohol consumption and bone mineral density
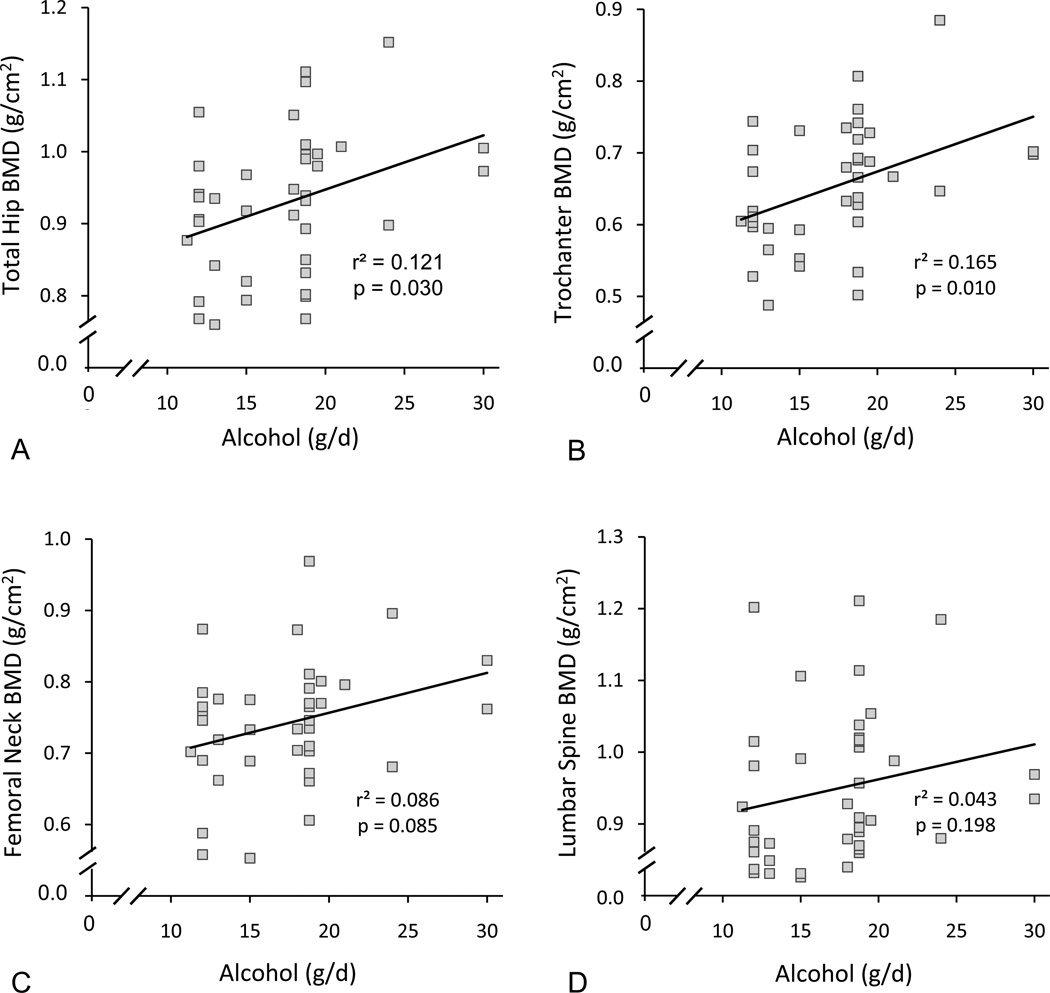
There was a positive association at baseline between alcohol consumption and total hip BMD (Figure 1a) and between alcohol consumption and trochanter BMD (Figure 1b). Significant associations were not detected between alcohol intake and BMD at the femoral neck (Figure 1c) or lumbar spine (Figure 1d). Femur measurements were not available for one participant due to a double hip replacement.
Figure 1.
Total hip (A), trochanter (B), femoral neck (C), and lumbar spine (D) BMD versus quantity of alcohol intake in early postmenopausal women. Please note the significant positive association between alcohol intake and total hip BMD (A) and trochanter BMD (B). N = 39 for femur and 40 for lumbar spine.
Alcohol consumption and biomarkers of bone turnover
A significant linear association between osteocalcin and CTx was observed at baseline (r2 = 0.36, p < 0.01). However, no relationship was noted between baseline levels of alcohol consumption and bone turnover markers.
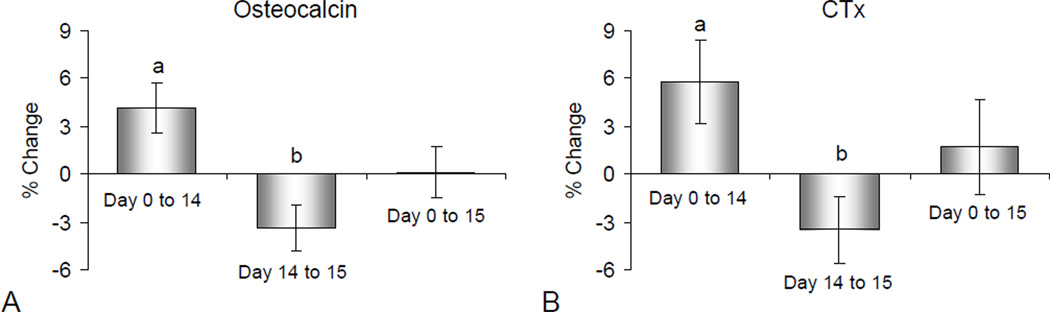
Serum osteocalcin and CTx increased following 14 days of abstinence (p = 0.01 and 0.02, respectively) (Figure 2). When alcohol was administered during the evening of day 14 and blood drawn on the morning of day 15, serum osteocalcin and CTx decreased compared to day 14 (p = 0.01 and 0.05, respectively). Day 15 serum osteocalcin and CTx levels did not differ from baseline (p = 0.94 and 0.57, respectively).
Figure 2.
Mean percent change in serum osteocalcin (A), a marker of bone formation and serum CTx (B), a marker of bone resorption, following 14 days of abstinence from alcohol and subsequent restoration of alcohol intake to pre-abstinence levels. Please note that a relatively brief interval of abstinence (14 days) resulted in significant increases in serum osteocalcin and CTx whereas resumption of alcohol consumption resulted in an overnight decrease in serum osteocalcin and CTx to levels that did not differ from baseline (day 0). Values are mean ± SE; N = 40 for each time interval. ap ≤ 0.05 compared to Day 0; bp ≤ 0.05 compared to Day 14.
Alcohol consumption and serum estradiol
Serum estradiol was evaluated in 39 of 40 participants. If all values are included in calculating the mean ± SE, estradiol levels were 7.0 ± 1.0 pg/ml on day 0, 7.2 ± 1.1 pg/ml on day 14 and 6.6 ± 1.2 pg/ml on day 15. However, 33 of 117 samples (each participant assayed at 3 time points) were below the reliable detection limit for the assay (4 pg/ml). A preliminary analysis set all values that fell below the detection limit equal to 0, and fit a general linear model with compound symmetric correlation structure using maximum likelihood methods. The null hypothesis of equal mean estradiol level at baseline, day 14, and day 15 had likelihood ratio p-value = 0.67. There also was no evidence of a difference in mean estradiol level over time when all non-measurable levels were set equal to 4 (likelihood ratio p-value = 0.65). In addition, a linear mixed model was fit using clustered normal data with undetectable values treated as interval censored between 0 and 4. There was no evidence of a difference in mean estradiol level from day 0 to day 14 (p-value = 0.52) or between days 14 and 15 (p-value = 0.40). The very low serum estradiol levels observed in this study are consistent with the expected low levels of estradiol (0–30 pg/ml) in postmenopausal women not on HRT.
DISCUSSION
This study examined the effects of alcohol withdrawal and subsequent acute restoration of alcohol intake on bone turnover markers in healthy early postmenopausal women. There was a significant increase in the bone turnover markers, osteocalcin and CTx, when alcohol was excluded for 14 days from the diets of participants who regularly consumed 8 – 28 g of ethanol/day. Within 12–14 hours of resuming alcohol consumption, osteocalcin and CTx returned to values that did not differ from baseline (pre-abstinence levels). Serum estradiol levels were low in all participants and significant differences in serum levels of this hormone were not detected with treatment.
There is no universally accepted definition for moderate alcohol consumption. Indeed, the quantities of ethanol in a standard drink vary by country and range from 8 to 19.8 g [27, 28]. In the United States, a standard drink contains 13.7 g of ethanol. A meta-analysis of all-cause mortality demonstrated a J-shaped association between alcohol consumption and total mortality [29]. The lowest mortality risk for women was between one and two drinks per day. Therefore, the two standard drinks/day used in this study is a reasonable upper limit for moderate alcohol consumption.
In the current study, alcohol consumption at baseline was positively associated with BMD at the total hip and at the trochanter; findings similar to those described in much larger cohorts of postmenopausal women in the EPIDOS and Framingham studies [15, 30]. Although our intervention study did not detect a significant effect on vertebrae, much larger observational studies investigating postmenopausal women report a beneficial effect of moderate alcohol intake on lumbar spine BMD [14, 17, 18]. Thus, an increased bone mass at one or more sites is consistently associated with moderate drinking in postmenopausal women.
We are not aware of prior intervention studies investigating the direct effects of alcohol consumption on bone turnover in postmenopausal women. In a cross-sectional study, Rapuri et al. [18] report an association between moderate alcohol intake and decreased bone turnover (lower serum osteocalcin and urinary N-telopeptide of type I collagen) in elderly postmenopausal moderate alcohol consumers. Serum bone turnover markers in postmenopausal women reflect the overall rate of bone remodeling. Bone remodeling involves a highly integrated ongoing process whereby packets of bone are resorbed and replaced. Bone remodeling is initiated by bone resorption and completed by refilling the resorption cavity with new bone. The entire process requires ~4 months [31]. Thus, abstinence for much longer than the 14 day interval used in the present study would be required to gage the full effect of alcohol consumption on bone turnover. Nevertheless, the small but significant increases in osteocalcin and CTx following short-term abstinence provide substantial evidence that moderate alcohol consumption decreases bone turnover.
A dose-response between alcohol intake and markers of bone turnover was not detected in the current study, but the study was inadequately powered to detect such changes. Also, the short duration of intervention and the relatively narrow range of alcohol consumed were limiting factors in evaluating dose-dependent effects. However, our results in postmenopausal women are consistent with a dose-response study investigating the effects of alcohol on bone turnover in an animal model. Groups of rats fed alcohol comparable to moderate drinkers (3 and 6% of total caloric intake) had balanced reductions in osteoclast and osteoblast numbers [20]. In contrast, consumption of higher levels of alcohol by rats resulted in further reductions in bone formation with a net increase in bone resorption and subsequent bone loss [20].
In the current study, we observed a reduction in bone turnover markers 12 – 14 hours following a single administration of alcohol to values that did not differ significantly from baseline. Acute effects of alcohol were also observed in a recent study by Sripanyakorn et al. that evaluated bone turnover markers in a cohort of adult (20 – 47 year old) healthy males and females [32]. Using each subject as their own control, a significant reduction in serum CTx was observed within 6 hours after ingestion of beer containing 28 g of alcohol. Thus, both studies support the conclusion that amounts of alcohol likely to be consumed in the diet result in rapid reductions in biomarkers of bone resorption. In contrast to the current study, changes in bone formation markers in response to alcohol were not observed by Sripanyakorn et al. [32]. The failure of Sripanyakorn et al. [32] to detect an inhibitory effect of alcohol on bone formation may have been due to differences in experimental design. In addition to the differences already noted, Sripanyakorn et al. [32] performed a time course (1–6 hr post alcohol administration) analysis of blood bone turnover biomarkers in a clinical setting, whereas we performed measurements 12–14 hrs following alcohol consumption in free living subjects. Alternatively, there may be important age and/or gender differences in the skeletal response to moderate alcohol.
There are several limitations in this study that should be noted. First, this was not a randomized clinical trial. Second, eligibility based on study criteria, with the exception of BMD and HRT requirements, was self-reported by participants. Third, all but one of the 40 participants were Caucasian. Therefore, the homogeneity of the sample with regard to ethnicity limits the generalizability of the study findings. Fourth, the study is likely to underestimate the effect of alcohol on bone turnover markers because the residual effects of prior long duration alcohol use on bone remodeling would be expected to persist for more than 14 days. Fifth, the study probably underestimates the acute effect of alcohol because sampling was performed at a single fixed interval following alcohol consumption. Sixth, while the great majority of the women were wine drinkers, the type of drink consumed was not controlled for. As such, the possibility that non-alcohol components of drinks influenced bone metabolism cannot be excluded. However, we and others have clearly demonstrated profound skeletal effects of ethanol. In spite of these limitations, this study demonstrates that moderate alcohol intake has rapid suppressive effects on bone turnover markers in postmenopausal women.
Moderate alcohol consumption, although associated with lower risk for cardiovascular disease, cardiovascular disease-related mortality and all-cause mortality, may increase the risk for breast cancer [33, 34]. An increase in aromatase activity has been suggested as a mechanism for the effects of alcohol on estrogen target cells [35]. However, in the present study we detected no change in serum estradiol levels. While there is substantial evidence that moderate alcohol consumption correlates with higher BMD in postmenopausal women, it is much less clear whether consuming alcohol lowers fracture rate. Therefore, even if drinking had no detrimental effects, it would be unwise to recommend drinking for the purpose of preventing fractures. On the other hand, according to WHO data, the per capita yearly consumption of alcohol for adults (15+ years) in industrialized countries ranges from 8–16 liters/year and, at least for the US, has not changed markedly (other than during Prohibition) over the last 100 years. In our study population of postmenopausal moderate drinkers, alcohol contributed an estimated 4–12 % of total energy. Thus, many women drink regularly and the great majority of these drinkers do not abuse alcohol. It is, therefore, important to understand the physiological impact of alcohol as a major highly bioactive component of the diet.
Conclusions
In summary, we show that a relatively short interval of abstinence from moderate alcohol consumption by early postmenopausal women results in increased serum levels of biochemical markers of bone formation and bone resorption, and subsequent resumption of alcohol intake in the diet rapidly reduces the levels of these markers. Thus, our findings support the hypothesis that moderate dietary alcohol consumption may slow bone loss in postmenopausal women by attenuating increased bone turnover. The observed actions provide a plausible cellular mechanism for the positive association between moderate alcohol consumption and BMD observed in postmenopausal women. Further studies are required to determine if, in addition to lowering biochemical markers of bone turnover, moderate dietary alcohol alters the negative bone remodeling balance in postmenopausal women.
SUMMARY OF ARTICLE.
Moderate alcohol consumption is associated with higher bone mass in postmenopausal women. This study suggests that moderate alcohol intake may slow bone loss in postmenopausal women by attenuating increased bone turnover.
Acknowledgments
Funding Disclosure: This work was supported by the National Institutes of Health grant AA011140 and the John C. Erkkila, M.D. Endowment for Health and Human Performance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest.
References Cited
- 1.Melton LJ, 3rd, Thamer M, Ray NF, Chan JK, Chesnut CH, 3rd, Einhorn TA, Johnston CC, Raisz LG, Silverman SL, Siris ES. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:16–23. doi: 10.1359/jbmr.1997.12.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Garnero P, Delmas PD. Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact. 2004;4:50–63. [PubMed] [Google Scholar]
- 3.Hosam K. Postmenopausal osteoporosis: etiology, current diagnosis, strategies, and nonprescription interventions. J Manag Care Pharm. 2006;12:S4–S9. doi: 10.18553/jmcp.2006.12.S6-A.S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62:781–792. doi: 10.1007/s00228-006-0174-3. [DOI] [PubMed] [Google Scholar]
- 5.General S U.S. Department of Health and Human Services. Services USDoHaH, editor. Rockville, MD: 2004. Bone Health and Osteoporosis: A Report of the Surgeon General. [Google Scholar]
- 6.Reid IR. Anti-resorptive therapies for osteoporosis. Semin Cell Dev Biol. 2008;19:473–478. doi: 10.1016/j.semcdb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Lane N. Osteoporosis: is there a rational approach to fracture prevention. Bulletin NYU Hospital for Joint Dis. 2006;64:67–71. [PubMed] [Google Scholar]
- 8.Chappard D, Plantard B, Petitjean M, Alexandre C, Riffat G. Alcoholic cirrhosis and osteoporosis in men: a light and scanning electron microscopy study. J Stud Alcohol. 1991;52:269–274. doi: 10.15288/jsa.1991.52.269. [DOI] [PubMed] [Google Scholar]
- 9.de Vernejoul MC, Bielakoff J, Herve M, Gueris J, Hott M, Modrowski D, Kuntz D, Miravet L, Ryckewaert A. Evidence for defective osteoblastic function. A role for alcohol and tobacco consumption in osteoporosis in middle-aged men. Clin Orthop Relat Res. 1983:107–115. [PubMed] [Google Scholar]
- 10.Chavassieux P, Serre CM, Vergnaud P, Delmas PD, Meunier PJ. In vitro evaluation of dose-effects of ethanol on human osteoblastic cells. Bone Miner. 1993;22:95–103. doi: 10.1016/s0169-6009(08)80221-8. [DOI] [PubMed] [Google Scholar]
- 11.Friday KE, Howard GA. Ethanol inhibits human bone cell proliferation and function in vitro. Metabolism. 1991;40:562–565. doi: 10.1016/0026-0495(91)90044-w. [DOI] [PubMed] [Google Scholar]
- 12.Klein RF, Fausti KA, Carlos AS. Ethanol inhibits human osteoblastic cell proliferation. Alcohol Clin Exp Res. 1996;20:572–578. doi: 10.1111/j.1530-0277.1996.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 13.Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Jr, Malik R, Arnsten JH. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feskanich D, Korrick SA, Greenspan SL, Rosen HN, Colditz GA. Moderate alcohol consumption and bone density among postmenopausal women. J Womens Health. 1999;8:65–73. doi: 10.1089/jwh.1999.8.65. [DOI] [PubMed] [Google Scholar]
- 15.Ganry O, Baudoin C, Fardellone P. Effect of alcohol intake on bone mineral density in elderly women: The EPIDOS Study. Epidemiologie de l'Osteoporose. Am J Epidemiol. 2000;151:773–780. doi: 10.1093/oxfordjournals.aje.a010277. [DOI] [PubMed] [Google Scholar]
- 16.Holbrook TL, Barrett-Connor E. A prospective study of alcohol consumption and bone mineral density. BMJ. 1993;306:1506–1509. doi: 10.1136/bmj.306.6891.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002;21:536–544. doi: 10.1080/07315724.2002.10719252. [DOI] [PubMed] [Google Scholar]
- 18.Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL. Alcohol intake and bone metabolism in elderly women. Am J Clin Nutr. 2000;72:1206–1213. doi: 10.1093/ajcn/72.5.1206. [DOI] [PubMed] [Google Scholar]
- 19.Wosje KS, Kalkwarf HJ. Bone density in relation to alcohol intake among men and women in the United States. Osteoporos Int. 2007;18:391–400. doi: 10.1007/s00198-006-0249-0. [DOI] [PubMed] [Google Scholar]
- 20.Turner RT, Kidder LS, Kennedy A, Evans GL, Sibonga JD. Moderate alcohol consumption suppresses bone turnover in adult female rats. J Bone Miner Res. 2001;16:589–594. doi: 10.1359/jbmr.2001.16.3.589. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 22.Turner RT, Wronski TJ, Zhang M, Kidder LS, Bloomfield SA, Sibonga JD. Effects of ethanol on gene expression in rat bone: transient dose-dependent changes in mRNA levels for matrix proteins, skeletal growth factors, and cytokines are followed by reductions in bone formation. Alcohol Clin Exp Res. 1998;22:1591–1599. doi: 10.1111/j.1530-0277.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 23.Hassager C, Risteli J, Risteli L, Jensen SB, Christiansen C. Diurnal variation in serum markers of type I collagen synthesis and degradation in healthy premenopausal women. J Bone Miner Res. 1992;7:1307–1311. doi: 10.1002/jbmr.5650071110. [DOI] [PubMed] [Google Scholar]
- 24.Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 25.Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008;19:1683–1704. doi: 10.1007/s00198-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 26.Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999;45:1858–1860. [PubMed] [Google Scholar]
- 27.International Center for Alcohol Policies Blue Book, Module 20. [Accessed June, 2011]; Available at: http://www.icap.org/PolicyTools/ICAPBlueBook/BlueBookModules/20StandardDrinks/tabid/161/Default.aspx.
- 28.Turner C. How much alcohol is in a 'standard drink'? An analysis of 125 studies. Br J Addict. 1990;85:1171–1175. doi: 10.1111/j.1360-0443.1990.tb03442.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485–492. doi: 10.1093/oxfordjournals.aje.a117664. [DOI] [PubMed] [Google Scholar]
- 31.Agerbaek MO, Eriksen EF, Kragstrup J, Mosekilde L, Melsen F. A reconstruction of the remodelling cycle in normal human cortical iliac bone. Bone Miner. 1991;12:101–112. doi: 10.1016/0169-6009(91)90039-3. [DOI] [PubMed] [Google Scholar]
- 32.Sripanyakorn S, Jugdaohsingh R, Mander A, Davidson SL, Thompson RP, Powell JJ. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J Bone Miner Res. 2009;24:1380–1388. doi: 10.1359/JBMR.090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. Jama. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. Bmj. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purohit V. Canalcoholpromotearomatizationofandrogenstoestrogens? A review. Alcohol. 2000;22:123–127. [Google Scholar]