Abstract
Sarcopenia, the loss of skeletal muscle mass and function with aging, is a major contributor to frailty and morbidity in older adults. Recent evidence has emerged suggesting that endothelial dysfunction and insulin resistance of muscle protein metabolism may significantly contribute to the development of sarcopenia. In this article we review: 1) recent studies and theories on the regulation of skeletal muscle protein balance in older adults; 2) the link between insulin-resistance of muscle protein synthesis and endothelial dysfunction in aging; 3) mechanisms for impaired endothelial responsiveness in aging; and 4) potential treatments that may restore the endothelial responsiveness and muscle protein anabolic sensitivity in older adults.
Keywords: Sarcopenia, endothelial dysfunction, insulin resistance, muscle protein metabolism
Introduction
Aging is associated with the development of vascular endothelial dysfunction (1) and age-related declines in both skeletal muscle mass and function (2;3). A hallmark of endothelial dysfunction is impaired endothelial-dependent dilation (4;5). Specifically, in response to chemical (e.g. acetylcholine, insulin) or flow-mediated stimulation there is a reduced endothelial responsiveness in older adults (5;6). In addition to being a well known risk factor for the development of cardiovascular disease (7), endothelial dysfunction may play a critical role in the age-related loss of skeletal muscle mass and function, called sarcopenia (6;8;9).
The prevalence of sarcopenia is reported to range from approximately 23–27% in adults aged 65–90 years, and 30–50% in adults over 80 years of age. (10) Sarcopenia is characterized by decreased skeletal muscle mass, strength, and function (11). It is associated with increased risk of falls, fractures, and morbidity (3;12;13). As such, sarcopenia represents a significant geriatric syndrome which can negatively impact quality of life, ability to perform activities of daily living, loss of independence and ultimately result in mortality (3;14;15).
Given the increasing age of our population the prevalence and health impact of sarcopenia will surely rise over the coming decades—emphasizing the need for researchers and clinicians to better understand mechanisms underlying the development and progression of this age-related condition. Here, we will review 1) recent studies and theories relevant to the regulation of skeletal muscle protein balance in aging; 2) the link between insulin-resistance of muscle protein synthesis and endothelial dysfunction in aging; 3) mechanisms for impaired endothelial responsiveness in aging; and 4) potential treatments that may restore the endothelial responsiveness and muscle protein anabolic sensitivity in older adults.
Regulation of Muscle Protein Balance in Aging
Skeletal muscle mass largely depends upon the protein content in the muscle fibers, which is determined by the net balance between protein breakdown and synthesis. Over time, the balance between protein synthesis and breakdown can result in muscle loss (when breakdown exceeds synthesis), muscle gain (when synthesis exceeds breakdown), or muscle maintenance (when synthesis and breakdown are equivalent). Thus, it stands to reason that age-related muscle loss results from a prolonged imbalance between skeletal muscle protein synthesis and breakdown. Between meals in the postabsorptive state, net balance is negative which is indicative of an overall catabolic state. Conversely, following a meal, net balance is positive, indicating protein accretion. As dietary protein is a potent stimulator of skeletal muscle protein synthesis (16;17) via the direct effect of essential amino acids, numerous studies have been conducted to determine if there is an age-related difference in response to protein intake.
Protein Intake, Aging, and Muscle Protein Synthesis
Young and older adults have equivalent rates of net muscle protein balance in the postabsorptive state (18;19). However, the data regarding the anabolic impact of protein intake are more varied, and appear to depend on the quality and quantity of protein or essential amino acids utilized. Consistent with previous findings Katsanos et al. (20) found that young and older adults exhibit an equivalent postabsorptive net balance. However, when fed a small bolus (7g) of essential amino acids older adults displayed an attenuated anabolic response compared to young subjects. These researchers also examined the effect of leucine content on the anabolic response to essential amino acids consumption in young and older adults. Among essential amino acids, leucine is known to be the most potent stimulator of muscle protein synthesis. They found that a 7 gram bolus of essential amino acids containing 26% leucine significantly stimulated muscle protein synthesis in young but not in older adults, whereas a 7 gram bolus of essential amino acids containing 41% leucine significantly elevated muscle protein synthesis in both young and older adults (21). These findings have been supported by others showing that higher leucine levels favor elevated muscle protein synthesis in older adults (22;23). Others have conducted similar studies with intact protein sources. In a representative study, Symons et. al. (24), examined the influence of a moderate (113 g, 220 kcal, 30 g protein) and large (340 g, 660 kcal, 90 g protein) serving of 90% lean beef on muscle protein synthesis in younger (approx. 34 yrs) and older adults (approx. 68 yrs). The authors reported that muscle protein synthesis was elevated to the same extent in both young and older adults, and that the serving size did not affect the anabolic response—suggesting a ceiling effect for the influence of protein intake on synthesis. Likewise, Koopman et. al. (25) examined the impact of 35 g of intact casein on muscle protein synthesis in young and older men, and found that this level of protein consumption elicited a comparable skeletal muscle protein synthetic response in both age groups. Collectively, these data suggest that older adults may be slightly less sensitive to the anabolic effects of protein ingestion particularly at lower intakes, but that given a sufficient quantity and/or quality (i.e. higher leucine content) of protein that the anabolic response is comparable in young and older subjects.
Mixed Nutrient Intake, Aging, and Muscle Protein Synthesis
While examining the anabolic impact of protein intake alone has provided insightful data, the real-world applicability of such interventions is questionable, as both young and older adults are unlikely to consume a protein-only meal as part of their habitual diet. In an initial study from our group (26) we gave an amino acid-glucose mixture (40 g of amino acids + 40 g of glucose divided over 3 hours) to both young and older men and women. Consistent with the other findings, young and older subjects had nearly identical rates for postabsorptive muscle protein synthesis. However, during the consumption of the mixed meal, the young subjects had a significant increase in muscle protein synthesis, while muscle protein synthesis rates did not increase in the older subjects. This finding was later confirmed by others who also found defective phosphorylation of p70 S6 kinase 1 (S6K1), a key translation initiation regulator of the mTOR signaling pathway (27). Thus, it appears that aging is associated with decreased anabolic efficiency in response to a mixed meal containing carbohydrate and protein. In a follow up study, we determined if the reduced muscle protein anabolic response to mixed nutrients in older adults was due to resistance to the anabolic action of insulin—a hormone that in addition to its effects on glucose metabolism also stimulates skeletal muscle protein anabolism.
Age-related Insulin Resistance of Skeletal Muscle Protein Synthesis
To test this hypothesis, we performed hyperinsulinemic-euglycemic clamp studies in young and older adults (6). Insulin was infused directly into the femoral artery to increase the leg insulin levels to approximate post-prandial values while avoiding systemic hypoaminoacidemia. Insulin significantly stimulated muscle protein synthesis in young but not older subjects. There was no significant change in muscle protein breakdown as measured by two- and three-pool modeling. The increase in synthesis in young subjects resulted in a shift from negative to positive protein net balance across the leg—indicating overall net protein accretion during the clamp in young subjects. In the older subjects, however, the net muscle protein balance remained negative. Additionally, changes in muscle protein synthesis in all subjects were positively correlated with amino acid delivery to the leg and blood flow. Blood flow, in fact, was lower in older as compared to younger subjects at baseline and during the clamp, and tended to increase from baseline in young adults only during the clamp. This effect was likely mediated through insulin-induced vasodilation. Insulin is a potent stimulator of the endothelial-derived vasodilator, nitric oxide (28). In a subsequent study we reported that this age-related insulin resistance of muscle protein synthesis could be overcome by increasing insulin levels to approximately double the post-prandial levels via improvements in mTOR signaling (8). Consistent with our previous findings there was a very clear association between muscle protein synthesis and blood flow/amino acid delivery. Wilkes et al. (29) also reported that a euglycemic-hyperinsulinemic clamp elicited a shift from negative to positive protein net balance in young but not older subjects. However, their data indicated that this shift was driven by insulin -induced decrease in muscle protein breakdown, rather than an increase in muscle protein synthesis. These inconsistencies could be a result of the different route of administration of insulin (localized vs. systemic), and the fact that in the Wilkes study octreotide (a somatostatin mimetic) was infused along with insulin. Among its numerous pharmacological effects, octreotide is a vasoconstrictor. As insulin’s ability to stimulate muscle protein synthesis appears critically linked to its ability to stimulate blood flow and amino acid delivery, the use of octreotide may have impaired insulin’s capacity to increase muscle protein synthesis in the young subjects.
To mechanistically determine if the associations between the insulin’s effects on blood flow and amino acid delivery are essential for the hormone to exert its anabolic effect on skeletal muscle proteins, we performed a euglycemic-hyperinsulinemic clamp on healthy young subjects with or without the concomitant infusion of the nitric oxide (NO) synthase inhibitor NG-monomethyl-L-arginine (L-NMMA) (30). Blood flow was measured during the clamp and the rate of L-NMMA infusion was adjusted to clamp blood flow at basal values. Blocking the endothelial-dependent increase in blood flow during hyperinsulinemia attenuated the effects of insulin on amino acid delivery, mTOR signaling, muscle protein synthesis and net balance. Conversely, the anabolic effect of insulin in older subjects was restored when vasodilation was induced by co-infusion of insulin with the vasodilator sodium nitroprusside (SNP) (9). Overall, these data illustrate that increased blood flow and amino acid delivery are critical for insulin to exert its anabolic effects on skeletal muscle proteins. In addition to traditional large vessel blood flow, in these studies we used a novel contrast enhanced ultrasound technique to measure microvascular perfusion of leg skeletal muscle. A comparison of the young and older subjects from these two studies showed that older subjects exhibited lower postabsorptive microvascular perfusion, and a significantly blunted microvascular response to insulin compared to young subjects. Infusing SNP during the insulin clamp in older subjects restored microvascular perfusion to the level observed in young subjects. Collectively, these data show that the age-related attenuation of the anabolic action of insulin on skeletal muscle protein synthesis appears to be the result of impaired endothelial responsiveness to the vasodilatory effects of insulin.
Mechanisms for Impaired Endothelial Responsiveness in Older Adults
There are a number of theories regarding mechanisms that may contribute to impaired endothelial-dependent vasodilation in aging. Here we will briefly review the following: 1) elevated endothelin-1; 2) decrease nitric oxide availability; and 3) elevated inflammation and oxidative stress. For a more in depth review see Seals et al. (31).
Elevated Endothelin-1
Endothelin-1 (ET-1) is a powerful endothelial-derived vasoconstrictor that has been implicated in the development of hypertension, insulin resistance and heart disease. For instance, elevated ET-1 interferes with insulin receptor signaling (32), and has recently been reported to impair glucose uptake in human skeletal muscle (33). Researchers have shown that with advancing age levels of this vasonconstrictor are increased(34). As insulin is a potent vasodilator and anabolic hormone, the receptor interference by ET-1 could logically impair both insulin-induced vasodilation and skeletal muscle protein anabolism. In a rat model of diabetes, Verma at al., found that diabetic rats are hyper-reactive to ET-1 compared to control rats, and that blockade of ET-1 normalizes endothelial function.
In our studies, we found that circulating levels of ET-1 were higher in older than young subjects at baseline and during a euglycemic-hyperinsulinemic clamp (6). Not surprisingly, blood flow at baseline and during the clamp was also lower in older adults, as was the anabolic response to insulin administration. ET-1 has also been reported to be elevated in endothelial cells from healthy older compared to young men, with an inverse correlation between ET-1 expression and endothelial-dependent dilation (31).
Decreased Nitric Oxide
In contrast to ET-1, NO is an endothelial-derived vasodilator that may decrease with advancing age. Taddei et al. (35) found that aging was associated with a blunted endothelial-dependent dilatory response to acetylcholine, but not SNP, a direct NO donor and endothelium-independent vasodilator. This suggested an impairment of endothelium dependent vasodilation rather than an age-related decrease in the sensitivity to NO. They also found that aging was associated with a decrease in the ability of L-NMMA to inhibit acetylcholine-mediated endothelial-dependent dilation. In fact, in subjects greater than 60 years old, L-NMMA had almost no effect on endothelial-dependent dilation. These data indicate that decreased NO availability may be a major factor in age-related endothelial dysfunction as measured by endothelial-dependent dilation. This hypothesis if further supported by studies showing that administration of tetrahydropterin (BH4), a cofactor for NO production, selectively improves endothelial-dependent dilation in older compared to young adults (36;37).
Elevated Inflammation and Oxidative Stress
Both inflammation and oxidative stress have been reported to increase with advancing age (38;39). There is substantial evidence that both play a role in the development and progression of numerous age-related diseases and conditions including cardiovascular disease, insulin resistance, and sarcopenia (40). It has been proposed that elevated levels of the inflammatory cytokine TNF-α may contribute to impaired endothelial-dependent dilation by disrupting cell-to-cell communication through gap junctions (41). In the endothelium, this could presumably disrupt a coordinated vasodilatory response. In support of the role of inflammation in impaired endothelial-dependent dilation, researchers have shown in rodent models that inhibiting TNF-α or NF-κB, a transcription factor that facilitates increased inflammatory cytokine expression, that blood flow and endothelial-dependent dilation are restored to “young” levels in aged rodents (42;43). There is also evidence that impaired endothelial function and inflammation may create a positive feedback loop, further exacerbating these deleterious effects of inflammation. Specifically, while inflammation decreases endothelial function, it is also known that an elevated ET-1/NO ratio can lead to increased leukocyte-endothelium interaction, and thus increased inflammation (44;45). This cycle of inflammation and endothelial dysfunction underscores the link between these conditions and diseases associated with age and obesity (i.e. diabetes and cardiovascular disease), which are both associated with elevated inflammation and endothelial dysfunction.
Increased oxidative stress, can inactivate BH4 thus reducing NO availability (46). This process may be exacerbated by decreased antioxidant defense mechanisms also reported with advancing age. In both humans and animals, the use of antioxidant treatments have been shown to improve endothelial-dependent dilation, highlighting the potential contribution of oxidative stress in impaired endothelial responsiveness (35;47).
Potential Treatments to Restore Endothelial-Dependent Dilation and Skeletal Muscle Protein Anabolism in Aging
Exercise
Both acute aerobic exercise and aerobic training have a beneficial impact on endothelial-dependent dilation and the response to anabolic stimuli in older adults. For instance, a prior bout of moderate-intensity aerobic exercise restored the muscle protein anabolic effects of insulin in healthy, older adults (48). Consistent with the hypothesis that the anabolic effect of insulin is linked to its ability to stimulate blood flow and amino acid delivery, both measures were elevated during hyperinsulinemia and resulted in improved mTOR signaling only in the exercise group. A potential mechanism for this improved endothelial response to insulin is that ET-1 was significantly reduced in the exercise compared to control group. Others have reported that regular aerobic exercise improves endothelial-dependent dilation in older men possibly by increasing NO availability and decreasing oxidative stress (36)—a finding not observed in postmenopausal women (49). Similarly, as aerobic training has been reported to reduce circulating levels of inflammatory cytokines (50) and bolster anti-oxidant defense (51), the anti-inflammatory effect of exercise may underlie improvements in endothelial-dependent dilation and sensitivity of muscle proteins to anabolic stimulation in older adults. In fact, besides inhibiting endothelial-dependent dilation, elevated inflammation is reported to increase skeletal muscle protein breakdown (52).
Dietary Interventions
There is substantial evidence that healthy dietary changes (i.e. increased fruit and vegetable intake, reduced sodium and saturated fat) improve endothelial-dependent dilation in older adults (53;54). Recent studies have specifically shown a potential beneficial impact of omega-3 fatty acids on both endothelial-dependent dilation and muscle protein maintenance in older adults (55;56). For instance, Smith et al. (55;56) reported that 8 weeks of omega-3 fatty acid supplementation significantly improved the protein synthetic response to a euglycemic-hyperinsulinemic clamp. In a recent review, Egert and Stehle conclude that while promising, the reports of omega-3’s potential impact on endothelial function are varied, and may be dependent on sex, age, and health status (57). Additionally, diet-induced weight loss has been reported to improve indices of endothelial function (58) and inflammation (59). For example, Haspicova et al. have recently shown that 7% weight loss in obese, pre-menopausal women resulted in a significant improvement in endothelial responsiveness during an oral glucose load and maintenance of lean mass (60). As we have shown a strong relationship between insulin-induced blood flow and the anabolic effects of insulin, a reduction in fat mass could plausibly improve anabolic sensitivity of skeletal muscle concomitant with improved endothelial function. A recent study from Villareal et al. has in fact highlighted that a 10% weight loss improves the response of skeletal muscle protein synthesis to feeding in morbidly obese older adults (61). This warrants further investigation, with the caveat that potential changes in lean mass would have to be monitored carefully in elderly/clinical populations during a weight loss intervention.
Conclusions
Aging is associated with significant impairments in endothelial-dependent dilation and the anabolic response to insulin and mixed nutrient intake. These phenomena appear to be linked mechanistically, and may contribute to the development and progression of sarcopenia. Recent data show that vasodilators, aerobic exercise and/or dietary interventions such as omega-3 fatty acid supplementation can normalize endothelial-dependent dilation and sensitivity to anabolic stimuli in healthy, older adults. Further studies are warranted to assess the long-term efficacy of such interventions, and to more clearly define the underlying mechanisms responsible for the age-related declines in endothelial function and muscle protein maintenance. Such studies will provide invaluable basic science data, which may ultimately be translated to public health recommendations for the treatment and prevention of sarcopenia.
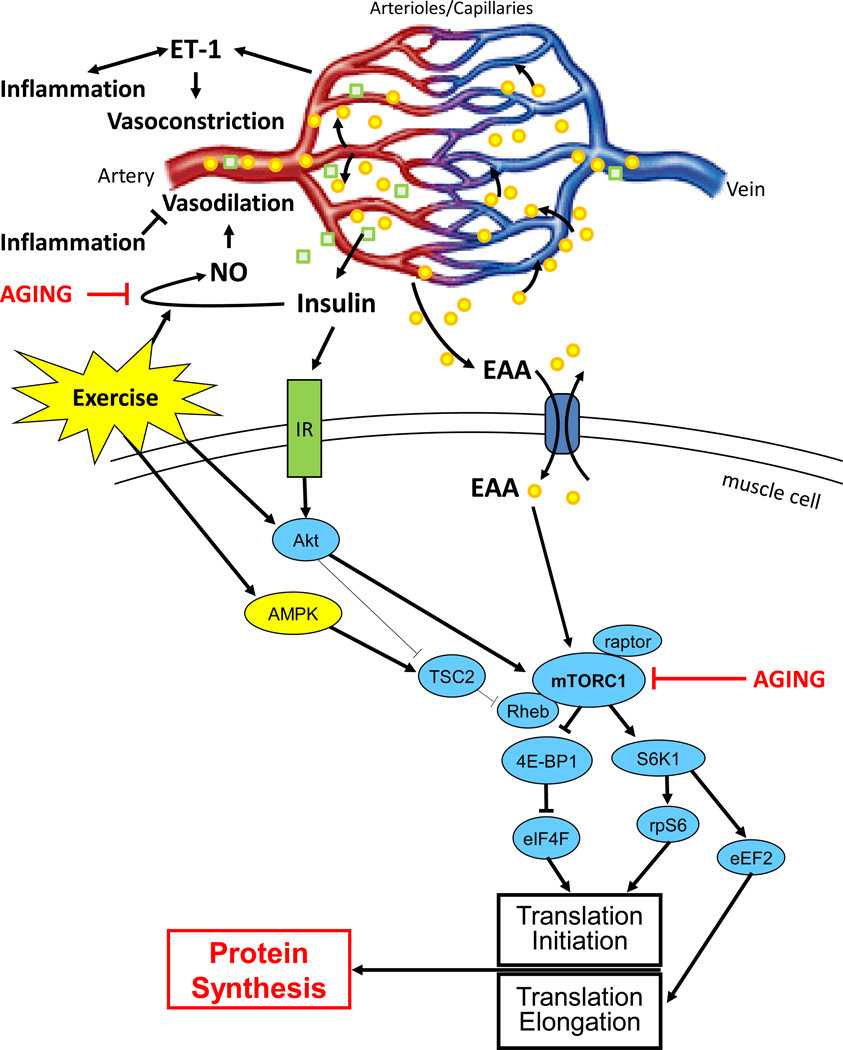
Figure 1.
Theoretical model of the impact of aging on endothelial function and muscle protein synthesis. Exercise is known to positively affect both nitric oxide (NO)_synthesis and the skeletal muscle protein anabolic sensitivity to insulin and essential amino acids (EAA).
Acknowledgements
This review article was supported by NIH grants # R01 AG018311 and # P30 AG024832.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol.Ther. 2011 doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J.Gerontol.A Biol.Sci.Med.Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J.Am.Geriatr.Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 5.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am.J.Physiol Heart Circ.Physiol. 2005;289:H308–H315. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc.Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 2009;52:1889–1898. doi: 10.1007/s00125-009-1430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmerman KL, Lee JL, Fujita S, et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59:2764–2771. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am.J.Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am.J.Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 13.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am.J.Epidemiol. 2006;164:665–671. doi: 10.1093/aje/kwj255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J.Am.Geriatr.Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 15.Rolland Y, Czerwinski S, Abellan VK, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J.Nutr.Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J.Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita S, Dreyer HC, Drummond MJ, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J.Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 20.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am.J.Clin.Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 21.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am.J.Physiol Endocrinol.Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 22.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp.Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Rieu I, Balage M, Sornet C, et al. Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition. 2007;23:323–331. doi: 10.1016/j.nut.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J.Am.Diet.Assoc. 2009;109:1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koopman R, Walrand S, Beelen M, et al. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J.Nutr. 2009;139:1707–1713. doi: 10.3945/jn.109.109173. [DOI] [PubMed] [Google Scholar]
- 26.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J.Clin.Endocrinol.Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillet C, Prod'homme M, Balage M, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 28.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr.Diab.Rep. 2003;3:279–288. doi: 10.1007/s11892-003-0018-9. [DOI] [PubMed] [Google Scholar]
- 29.Wilkes EA, Selby AL, Atherton PJ, et al. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am.J.Clin.Nutr. 2009;90:1343–1350. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman KL, Lee JL, Dreyer HC, et al. Insulin Stimulates Human Skeletal Muscle Protein Synthesis via an Indirect Mechanism Involving Endothelial-Dependent Vasodilation and Mammalian Target of Rapamycin Complex 1 Signaling. J.Clin.Endocrinol.Metab. 2010 doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin.Sci.(Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang ZY, Zhou QL, Chatterjee A, et al. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes. 1999;48:1120–1130. doi: 10.2337/diabetes.48.5.1120. [DOI] [PubMed] [Google Scholar]
- 33.Shemyakin A, Salehzadeh F, Bohm F, et al. Regulation of glucose uptake by endothelin-1 in human skeletal muscle in vivo and in vitro. J.Clin.Endocrinol.Metab. 2010;95:2359–2366. doi: 10.1210/jc.2009-1506. [DOI] [PubMed] [Google Scholar]
- 34.Donato AJ, Gano LB, Eskurza I, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am.J.Physiol Heart Circ.Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 36.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J.Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higashi Y, Sasaki S, Nakagawa K, et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp.Gerontol. 2001;37:127–136. doi: 10.1016/s0531-5565(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 39.Zanni F, Vescovini R, Biasini C, et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp.Gerontol. 2003;38:981–987. doi: 10.1016/s0531-5565(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 40.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol.Allergy Clin.North.Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 41.Payne GW. Effect of inflammation on the aging microcirculation: impact on skeletal muscle blood flow control. Microcirculation. 2006;13:343–352. doi: 10.1080/10739680600618918. [DOI] [PubMed] [Google Scholar]
- 42.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am.J.Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–1896. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- 45.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 46.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J.Clin.Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tatchum-Talom R, Martin DS. Tempol improves vascular function in the mesenteric vascular bed of senescent rats. Can.J.Physiol Pharmacol. 2004;82:200–207. doi: 10.1139/y04-010. [DOI] [PubMed] [Google Scholar]
- 48.Fujita S, Rasmussen BB, Cadenas JG, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin.Sci.(Lond) 2011;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav.Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Scheele C, Nielsen S, Pedersen BK. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol.Metab. 2009;20:95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Li YP, Chen Y, John J, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am.J.Clin.Nutr. 2009;89:485–490. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 54.McCall DO, McGartland CP, McKinley MC, et al. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation. 2009;119:2153–2160. doi: 10.1161/CIRCULATIONAHA.108.831297. [DOI] [PubMed] [Google Scholar]
- 55.Shah AP, Ichiuji AM, Han JK, et al. Cardiovascular and endothelial effects of fish oil supplementation in healthy volunteers. J.Cardiovasc.Pharmacol.Ther. 2007;12:213–219. doi: 10.1177/1074248407304749. [DOI] [PubMed] [Google Scholar]
- 56.Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am.J.Clin.Nutr. 2011;93:402–412. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr.Opin.Clin.Nutr.Metab Care. 2011;14:121–131. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 58.Kerr SM, Livingstone MB, McCrorie TA, Wallace JM. Endothelial dysfunction associated with obesity and the effect of weight loss interventions. Proc.Nutr.Soc. 2011;70:418–425. doi: 10.1017/S0029665111001674. [DOI] [PubMed] [Google Scholar]
- 59.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br.J.Nutr. 2011;106(Suppl 3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 60.Haspicova M, Milek D, Siklova-Vitkova M, et al. Post-prandial endothelial dysfunction is ameliorated following weight loss in obese premenopausal women. Med.Sci.Monit. 2011;17:CR634–CR639. doi: 10.12659/MSM.882048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villareal DT, Smith GI, Shah K, Mittendorfer B. Effect of weight loss on the rate of muscle protein synthesis during fasted and fed conditions in obese older adults. Obesity. doi: 10.1038/oby.2011.280. [Epub ahead of print, 2011 Sep 22] [DOI] [PMC free article] [PubMed] [Google Scholar]