Abstract
Two genetic instability pathways viz. chromosomal instability, driven primarily by APC mutation induced deregulated Wnt signaling, and microsatellite instability (MSI) caused by mismatch repair (MMR) inactivation, together account for greater than 90% of late-onset colorectal cancer. Our understanding of early-onset sporadic CRC is however comparatively limited. In addition, most seminal studies have been performed in the western population and analyses of tumorigenesis pathway(s) causing CRC in developing nations have been rare. We performed a comparative analysis of early and late-onset CRC from India with respect to common genetic aberrations including Wnt, KRAS and p53 (constituting the classical CRC progression sequence) in addition to MSI. Our results revealed the absence of Wnt and MSI in a significant proportion of early-onset as against late-onset CRC in India. In addition, KRAS mutation frequency was significantly lower in early-onset CRC indicating that a significant proportion of CRC in India may follow tumorigenesis pathways distinct from the classical CRC progression sequence. Our study has therefore revealed the possible existence of non-canonical tumorigenesis pathways in early-onset CRC in India.
Keywords: APC, Colorectal cancer, KRAS, MSI, p53, Wnt signaling
Introduction
Colorectal cancer (CRC) is the third most common cancer with a worldwide incidence of 1.2 million [1]. Two canonical genetic instability pathways appear to drive tumors in a majority of CRC patients namely chromosomal instability (CIN) and microsatellite instability (MSI), through a well established ‘adenoma-carcinoma’ transition sequence [2]. CIN is estimated to account for about 70–80 % of sporadic CRC and is primarily a result of protein truncating mutations in the Adenomatous polyposis coli (APC) tumor suppressor gene [3], which leads to aberrant Wnt activation, the major proliferation pathway regulating renewal of colorectal lumen epithelium. APC is the component of a multi protein complex that targets cytoplasmic β-Catenin for degradation. The complex is inhibited by Wnt activation allowing free β-Catenin to translocate to the nucleus, form a transcriptional activating complex with TCF/LEF transcription factors and activate transcription of pro-proliferative genes [4]. In addition, a truncated APC protein (devoid of the C-terminal domain that ensures proper kinetochore-microtubule interaction) has been suggested to be a possible cause for CIN [5], though this simple explanation has been challenged of late [6]. Additional common genetic aberrations, including activating KRAS and inactivating TP53 mutations, may occur sequentially after APC mutation during adenoma to carcinoma transition [7]. The microsatellite instability (MSI) pathway on the other hand drives tumors in about 15 % of sporadic CRC patients. MSI in sporadic CRC is primarily caused by inactivation of the mismatch repair (MMR) gene MLH1, induced by bi-allelic promoter CpG methylation [8]. Colorectal tumors originating due to either of the two canonical pathways differ significantly with respect to location, aggressiveness, prognosis and response to therapy [9, 10]. Taken together, either deregulated Wnt signaling or MMR inactivation appears to drive a majority of colorectal tumors. These findings are however based primarily on studies performed on age-related CRC occurring in the West.
Ethnicity-specific variations in CRC incidence and etiology are well validated. The proportion of MSI positive (MSI+) CRC in African Americans has been reported to be significantly higher than in Caucasians [11]. More importantly, analysis of the ‘Cancer Incidence in Five Continents’ (CI5) database has revealed an increase in CRC incidence in developing countries including India [12] where a high incidence of early-onset CRC is also reported [13]. Implementation of the fecal occult blood test and sigmoidoscopy/colonoscopy-based screening program [14], have led to a significant improvement in survival rates of late-onset CRC in the West. In contrast, our understanding of sporadic early-onset CRC is meager and their survival rate remains poor [15].
We have undertaken the first systematic multi pronged molecular genetic analysis of CRC from India. Our results reveal that a significant proportion of early-onset CRC patients in India, unlike their Western counterparts, harbor tumors not apparently driven by deregulated Wnt activation or MSI suggesting thereby the existence of non-canonical pathways driving oncogenesis.
Materials and Methods
Patient samples
The study was approved by independent ethics committees of each collaborating hospital as per the guidelines of the Helsinki declaration and included 298 clinically and pathologically validated sporadic colorectal adenocarcinoma samples (164 prospective samples flash frozen in liquid nitrogen immediately following surgical resection and 134 formalin fixed paraffin embedded archived samples along with corresponding matched normal for each sample) collected from four hospitals during 2003–2011 following informed consent. FFPE blocks were also generated for each fresh tumor-normal sample pair. Only colorectal adenocarcinoma (excluding mucinous/signet ring cell carcinoma, GIST, lymphoma) samples were included in the study. Samples from inadequate/poor quality tumors, from patients harboring multiple polyps or exhibiting a family history of cancer or subjected to pre-operative chemo/radiation therapy, were excluded. Majority of the tumors were well or moderately differentiated with stage I, II, III or IV (22.9%, 27.3%, 48.8% and 1%, respectively). To facilitate age-specific analysis of tumorigenesis pathways, patient samples were segregated into early-onset (age ≤50 years, median age = 44; 137 samples (84 fresh and 53 archived)) and late-onset (age ≥60 years, median age = 66; 105 samples (53 fresh and 52 archived)). Samples from patients belonging to the intermediate age group (age 51 to 59 yrs) were not included when age-specific analysis was performed. 4µm H&E sections from tumor and matched normal FFPE blocks (of both fresh and archived samples) were evaluated by the collaborating pathologist for grading and to confirm absence of discernible tumor infiltration, respectively. There was no significant variation in grade and stage distribution between samples from the two age groups.
Immuno Histochemistry (IHC)
IHC was performed as per established protocols (methods S1). A sample was scored as Wnt positive (Wnt+) if β-Catenin nuclear stain was observed in more than 35% tumor epithelial cells and Wnt negative (Wnt−) if stain was detected in less than 25% cells. Samples with intermediate stain or exhibiting a diffuse/variable staining in different sections of the same sample could not be classified and therefore were excluded from all analyses where Wnt status was compared. This ensured a clear distinction between Wnt+ and Wnt− tumors. Cytoplasmic/membrane staining was evaluated as internal control. For p53, samples with greater than 30% tumor cells exhibiting nuclear stain were classified as exhibiting p53 nuclear stabilization (NS+) and as NS− if less than 20% of tumor cells exhibited nuclear stain. Normal epithelium was confirmed for cytoplasmic membrane positivity (for β-Catenin) and negative (for p53).
MSI and mutation screening
DNA isolation was performed as per standard protocol as described in methods S1. The MSI status was determined for a subset of samples for which Wnt status was already determined (74 Wnt+, 60 Wnt− and 8 Wnt unclassified) by using the NCI panel of 5 microsatellites (BAT25, BAT26, D5S346, D17S250, D2S123), since several samples did not yield DNA of sufficient quality/quantity to accurately analyze instability status for all five microsatellites (especially for D2S123 and D17S250 since these amplicons were comparatively of higher molecular size than the other three). Samples were classified as MSI+ (MSI-high) if two or more microsatellites exhibited instability and as MSI negative (MSI−) if one (equivalent of MSI-low) or none (equivalent of Microsatellite stable (MSS)) exhibited instability. Screening for mutations in the APC mutation cluster region (MCR; codons 1286–1513) and in KRAS exon 2 were performed by PCR-DNA sequencing on the 3100 genetic analyzer (ABI Inc., USA) as described in methods S1, using DNA isolated from tumor samples. Only MSI− samples were screened for APC mutations.
Analysis of Wnt target gene expression
1µg of total RNA was reverse transcribed using the Superscript-II reverse transcription system (Invitrogen, USA) in the presence of anchored oligo dT primers (Amersham, USA) as per manufacturer’s protocol. The cDNA (diluted 10 fold) was then used for quantitative PCR (Takara Bio Inc, Japan) as described in methods S1. Only MSI− samples were chosen for Wnt target gene expression analysis.
Results
A significant proportion of early-onset CRC in India is driven neither by aberrant Wnt activation nor by MSI
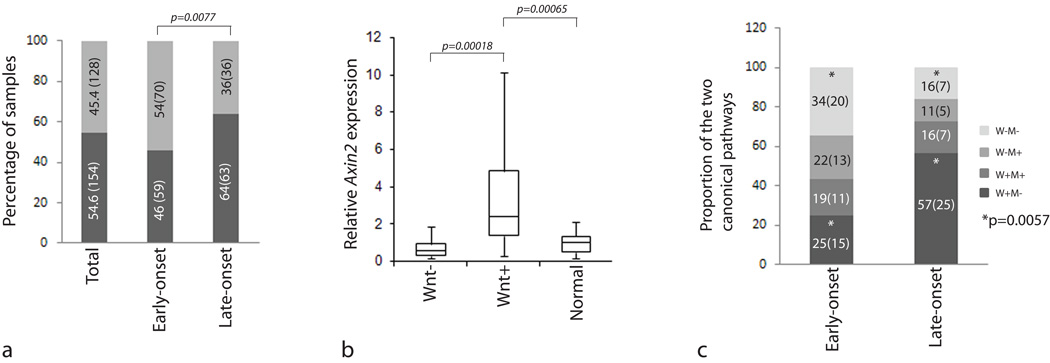
We first sought to verify whether aberrant Wnt activation was the predominant CRC initiating event among Indian patients, as is well established in patients from Western nations. Interestingly, among 282 samples (16 samples were unclassified), only 154 (54.6%) exhibited significant β-Catenin nuclear localization (fig S1a and 1a); substantially lower than the proportion reported from the West (44/52, 84.6% [16]; 100/100, 100% [17]; 38/47, 80.6% [18]). More importantly, only 46% (59/129) of early-onset as against 64% (63/99) of late-onset CRC samples exhibited β-Catenin nuclear positivity, a significant difference (p=0.0077; Fig. 1a). In order to confirm the IHC result, we performed transcript profiling of AXIN2, a previously validated Wnt target [19]. AXIN2 transcript levels were significantly higher in Wnt+ (n=19) as compared to Wnt− (n=16) and normal (n=23) samples (fig 1b). Similarly, DKK-1, a Wnt inhibitor often down-regulated in Wnt+ tumors, exhibited significantly reduced expression in Wnt+ as compared to Wnt− tumors (data not shown). In addition, 8 of 15 Wnt+ samples but only 1 of 19 Wnt− samples harbored mutation in the APC MCR region, further confirming the Wnt status. The APC mutations detected in the study are listed in Table S1 and representative sequencing results are depicted in figure S2. Our results therefore indicate a significantly reduced occurrence of Wnt+ tumors among early-onset CRC patients in India.
Figure 1. Analysis of Wnt status among Indian CRC samples.
Panel a; Result of Wnt analysis in early and late-onset CRC samples. The value inside each bar indicates the percentage as well as the actual number (in brackets) of samples. Panel b; AXIN2 transcript profiling by Q-RT-PCR validates Wnt status in CRC samples. The ‘P’ values correspond to Mann-Whitney U-Test. Panel c; Comparative analysis of early and late-onset CRC samples with respect to Wnt and MSI status. The values inside each bar indicate percentage and actual number (in brackets) of samples. The ‘P’ value (Fisher’s exact test) corresponds to comparison of Wnt+ and Wnt− samples in MSI− early and late-onset CRC samples (indicated by *). W, Wnt; M, MSI.
We investigated the MSI status in a subset of early and late-onset CRC samples for which Wnt status was already determined (representative MSI results are depicted in figure S12. The proportion of MSI+ samples was higher in early-onset (40%; 25/62) when compared to late-onset (30%; 14/47) samples (Table S2); the difference however was not significant. In order to determine the relative contribution of the two canonical pathways in early and late-onset CRC we divided samples from the two age groups into four categories with respect to Wnt and MSI status namely Wnt+ MSI−, Wnt+ MSI+, Wnt− MSI+ and Wnt− MSI−. This enabled us to compare the frequency of Wnt activation in the two age groups exclusively in MSI− samples (a more appropriate comparison, since Wnt activation in MSI+ samples is expected to have occurred as a secondary event and therefore cannot be considered as a primary CRC initiating event). The proportion of MSI− CRC samples exhibiting Wnt activation was significantly lower in early (15/35; 42.8%) as against late-onset (25/32; 78%) samples (P=0.0057; fig. 1c). More importantly, the proportion of Wnt− MSI− samples was significantly higher in early (34%; 20/59) when compared to late-onset (16%; 7/44) CRC samples (p=0.0447). Our results thereby reveal the existence of tumors not driven by either of the two (Wnt or MSI) canonical tumorigenesis pathways in a significant proportion of early-onset CRC patients.
KRAS mutation frequency is significantly lower in early as against late-onset CRC
We next evaluated p53 and KRAS status, both implicated in the CRC progression sequence [2]. p53 status was evaluated in 205 samples (of the total 298 samples) and nuclear stabilization was detected in 143 (70%) with no significant difference between samples from the two age groups (Table 1). We screened the KRAS second exon for mutations; KRAS codons 12 and 13 located in the second exon account for 90 % of all mutations detected in CRC and are known to render anti-EGFR therapy ineffective [20]. Of the 173 samples tested, KRAS mutation was detected in 51 (29.5%) (Table 1; representative sequencing results are depicted in figure S2), lower than the frequency identified from the West [21]. More importantly, the frequency in early-onset CRC samples (19/80; 24 %) was significantly lower than in late-onset samples (24/51; 47%) (p=0.0073; Table 1). The mutation spectrum was however similar to what is reported for the western population (Table S3). Interestingly, the frequency of KRAS mutation was lower (29.4%%; 19/65) than p53 nuclear stabilization (66%; 43/65) when analyzed exclusively in Wnt+ samples (Table 2) indicating perhaps that KRAS may not sequentially follow Wnt deregulation during CRC progression among Indian patients, as against the classical CRC progression sequence [2].
Table 1.
Analysis of TP53 and KRAS status among Indian CRC samples.
| MSI status | P53 | KRAS | ||
|---|---|---|---|---|
| NS+ | NS− | Mutant | Wild type | |
| Total | 143 | 62 | 51 | 122 |
| Early-onset | 63 | 31 | 19* | 61* |
| Late-onset | 51 | 16 | 24* | 27* |
p=0.0073 (fisher’s exact test)
NS, nuclear stabilization.
Table 2.
Frequency of occurrence of KRAS mutation and P53 nuclear stabilization in Wnt+ and Wnt− CRC samples.
| Wnt status1 | Total | KRAS | TP53 | KRAS mut & P53 NS+ | ||
|---|---|---|---|---|---|---|
| Mutant | Wild type | NS+ | NS− | |||
| + | 65 | 19 | 46 | 43 | 22 | 14 |
| − | 53 | 13 | 40 | 38 | 15 | 09 |
Only MSI− samples were analyzed
NS, nuclear stabilization; mut, mutant
Discussion
It is well established that deregulated Wnt signaling and MMR inactivation together account for more than 90 % of CRC. A few earlier studies revealed the existence of a minor subset of CIN−/MSI− CRC; Wnt status was however not determined. Moreover, most studies analyzed a small number of samples (<50) [22, 23], except one [24]. Our main objective in the current study was to evaluate Wnt and MSI status among Indian CRC patients. Surprisingly, our analysis has revealed a significant proportion of Wnt−/MSI− tumors in early-onset CRC from India (fig 1c). The Wnt status determined by β-Catenin IHC was confirmed by transcript profiling of AXIN2 and DKK-1 and by APC mutation screening. Though Wnt signaling results in β-Catenin induced transcriptional activation of several genes, many of these are also activated downstream of other pathways. However, AXIN2 appears to be exclusively activated by Wnt [19]. APC-MCR mutation screening identified mutations in 53% of Wnt+ samples; mutations in other regions of APC or in other components of the APC degradation complex such as AXIN1/2 or CTNNB1, may account for Wnt activation in rest of the Wnt+ samples.
Though Wnt and MSI pathways contribute independently to CRC genesis, they are not always mutually exclusive. It has been suggested that a majority of colorectal tumors (including MSI+ tumors) may exhibit active Wnt signaling either as a primary or a late event [25]. Greater than 90% of colorectal tumors have been shown to harbor genetic or epigenetic aberrations in Wnt pathway gene(s) [26]. Up-regulation of Wnt target genes in MSI+ tumors, due to mutations in APC/CTNNB1 or perturbation of a microsatellite located in the last exon of TCF4, has been documented [27]. In our analysis, 47% (17/36) of MSI+ tumors exhibited Wnt activation (Fig. 1c). More importantly, 54% of early-onset CRC samples did not exhibit Wnt activation; to the best of our knowledge, ours is perhaps the first study to reveal the possible existence of a significant proportion of colorectal tumors not exhibiting Wnt activation.
Molecular studies comparing early and late onset CRC have been rare. A recent study evaluated 45 early-onset CRC samples and showed reduced β-catenin and Cyclin E expression in MSS samples though comparison with late-onset samples was not performed [28]. An earlier study included microarray based genome-wide transcript profiling of 12 early-onset CRC samples and identified up-regulation of genes belonging to diverse tumorigenesis pathways [29]. Prognostic differences between early and late-onset CRC have been evaluated in several studies with varied results [30].
Our study has revealed a relatively high frequency of MSI among CRC tumors from India, when compared to reports from the West. Such high frequency of MSI has been reported earlier from discreet populations [11, 31]. In the current study, MSI+ tumors occurred with a comparable frequency in males and females but were associated significantly with colon as compared to rectum (p=0.04; Table S2), as reported earlier [32].
A combined effect of APC inactivation and mutation-induced KRAS activation in augmenting CRC initiation has been proposed [33]. In the current study however, majority of Wnt+ tumors (70.8%) did not harbor KRAS mutation (Table 2), suggesting perhaps that the latter may not be essential for Wnt-dependent development of CRC; similar results were obtained recently for FAP adenomas [34]. Therefore, our results appear to suggest alternative mechanism of CRC progression following Wnt activation [35].
As with other solid tumors, advanced age is an important risk factor for CRC [36]. Nevertheless, a significant increase in CRC incidence among the young in the USA (Surveillance Epidemiology and End Result (SEER) data) [37] as well as in developing nations [38] has been recently reported. Analysis of the SEER and the Office of National Statistics (UK) data has revealed an increased CRC incidence in the young and argues in favor of an age-specific approach to CRC screening [39]. The report of the Indian National Cancer registry program has revealed a steady increase in incidence of CRC in India [40]. More importantly, the ratio of early to late-onset CRC patients as per the Indian cancer registry (0.2) and as per data from several Indian oncology centres (0.52–0.64) [13], is significantly higher than international estimates [41]. Our results appear to indicate the occurrence of tumors in a significant proportion of early-onset Indian CRC patients that appear to be driven neither by canonical Wnt signaling nor by MSI, in addition to not harboring a mutant KRAS. It is imperative therefore to identify cellular pathways that drive tumorigenesis in this poorly studied CRC subtype in order to improve patient management and treatment strategies.
Supplementary Material
IHC-based detection of β-Catenin localization (cytoplasmic/nuclear in panel a and cytoplasmic/membrane in panel b) and p53 localization (nuclear stabilization in panel c and negative staining in panel d) in representative colorectal tumor samples.
Panel a, BAT25; Panel b, BAT 26; Panel c; D2S123; Panel d, D5S346; Panel e, D17S250 (for each microsatellite, result for tumor and normal samples are given in top and bottom respectively). Panel f, APC p.Q1378X (c.4132C>T); Panel g, APC c.4460delCT; Panel h, KRAS codon 15 (GGC>GAC); Panel i, KRAS codon 18 (GCC>GTC). For each mutation, mutant sequence is given on the left and normal sequence on the right; each missense mutant nucleotide is indicated by an arrow in the mutant electropherogram while the deletion mutation (panel g) is indicated by a bar in the normal electropherogram.
Acknowledgments
The authors gratefully acknowledge all patients who kindly consented to be a part of this study. RR and RA, registered PhD students of Manipal University, Karnataka, India, are thankful to the Council for Scientific and Industrial Research, Govt of India and the University Grants Commission, Govt of India, respectively, for junior and senior research fellowships. The authors thank Mr. P Ramaswamy for assistance in MSI analysis, Dr M Lavanya for help in obtaining archived tumor samples, Mr Gollapalli Chinna Thirupaul for assistance with histopathology and Ms Haleema Unnisa Begum for technical help for IHC.
Grant support: The work was supported by the NIH FIRCA grant (TW007963) to JP and MDB, the NIH grant (CA112016) to JP and a core grant from the Department of Biotechnology, Govt of India to MDB’s host institution, CDFD.
Abbreviations
- CRC
colorectal cancer
- APC
Adenomatous polyposis coli
- CIN
chromosomal instability
- MSI
microsatellite instability
- MSS
microsatellite stable
- TCF
T cell factor
- LEF
lymphoid enhancer factor
- MMR
mismatch repair
- GIST
Gastrointestinal stromal tumor
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- FFPE
formalin fixed paraffin embedded
- NCI
national cancer institute
- IHC
immunohistochemistry
- SEER
Surveillance Epidemiology and End Result
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 3.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- 5.Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3(4):429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 6.Rusan NM, Peifer M. Original CIN: reviewing roles for APC in chromosome instability. J Cell Biol. 2008;181(5):719–726. doi: 10.1083/jcb.200802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 8.de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 28(20):3380–3387. doi: 10.1200/JCO.2009.27.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355(9217):1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 10.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 11.Ashktorab H, Smoot DT, Carethers JM, et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin Cancer Res. 2003;9(3):1112–1117. [PubMed] [Google Scholar]
- 12.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Bhattacharya D, Acharya AN, Majumdar S, Ranjan P, Das S. Colorectal carcinoma in young adults: a retrospective study on Indian patients: 2000–2008. Colorectal Dis. 12(10 Online):e182–e189. doi: 10.1111/j.1463-1318.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 14.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7(7):770–775. doi: 10.1016/j.cgh.2008.12.030. quiz 711. [DOI] [PubMed] [Google Scholar]
- 15.Chan KK, Dassanayake B, Deen R, et al. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: analysis of survival and prognostic markers. World J Surg Oncol. 8:82. doi: 10.1186/1477-7819-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao X, Tomlinson I, Ilyas M, Palazzo JP, Talbot IC. Reciprocity between membranous and nuclear expression of beta-catenin in colorectal tumours. Virchows Arch. 1997;431(3):167–172. doi: 10.1007/s004280050084. [DOI] [PubMed] [Google Scholar]
- 17.Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10(4):1401–1408. doi: 10.1158/1078-0432.ccr-0157-03. [DOI] [PubMed] [Google Scholar]
- 18.Hugh TJ, Dillon SA, O'Dowd G, et al. beta-catenin expression in primary and metastatic colorectal carcinoma. Int J Cancer. 1999;82(4):504–511. doi: 10.1002/(sici)1097-0215(19990812)82:4<504::aid-ijc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Fodde R, Tomlinson I. Nuclear beta-catenin expression and Wnt signalling: in defence of the dogma. J Pathol. 221(3):239–241. doi: 10.1002/path.2718. [DOI] [PubMed] [Google Scholar]
- 20.Prenen H, Tejpar S, Van Cutsem E. New strategies for treatment of KRAS mutant metastatic colorectal cancer. Clin Cancer Res. 16(11):2921–2926. doi: 10.1158/1078-0432.CCR-09-2029. [DOI] [PubMed] [Google Scholar]
- 21.Van Schaeybroeck S, Allen WL, Turkington RC, Johnston PG. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol. 8(4):222–232. doi: 10.1038/nrclinonc.2011.15. [DOI] [PubMed] [Google Scholar]
- 22.Chan TL, Curtis LC, Leung SY, et al. Early-onset colorectal cancer with stable microsatellite DNA and near-diploid chromosomes. Oncogene. 2001;20(35):4871–4876. doi: 10.1038/sj.onc.1204653. [DOI] [PubMed] [Google Scholar]
- 23.Georgiades IB, Curtis LJ, Morris RM, Bird CC, Wyllie AH. Heterogeneity studies identify a subset of sporadic colorectal cancers without evidence for chromosomal or microsatellite instability. Oncogene. 1999;18(56):7933–7940. doi: 10.1038/sj.onc.1203368. [DOI] [PubMed] [Google Scholar]
- 24.Boardman LA, Johnson RA, Petersen GM, et al. Higher frequency of diploidy in young-onset microsatellite-stable colorectal cancer. Clin Cancer Res. 2007;13(8):2323–2328. doi: 10.1158/1078-0432.CCR-06-2739. [DOI] [PubMed] [Google Scholar]
- 25.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25(57):7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 26.Thorstensen L, Lind GE, Lovig T, et al. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia. 2005;7(2):99–108. doi: 10.1593/neo.04448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu Y, Ikeda S, Fujimori M, et al. Frequent alterations in the Wnt signaling pathway in colorectal cancer with microsatellite instability. Genes Chromosomes Cancer. 2002;33(1):73–81. doi: 10.1002/gcc.1226. [DOI] [PubMed] [Google Scholar]
- 28.Perea J, Alvaro E, Rodriguez Y, et al. Approach to early-onset colorectal cancer: clinicopathological, familial, molecular and immunohistochemical characteristics. World J Gastroenterol. 16(29):3697–3703. doi: 10.3748/wjg.v16.i29.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong Y, Ho KS, Eu KW, Cheah PY. A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: implication for tumorigenesis. Clin Cancer Res. 2007;13(4):1107–1114. doi: 10.1158/1078-0432.CCR-06-1633. [DOI] [PubMed] [Google Scholar]
- 30.Morris M, Platell C, Iacopetta B. A population-based study of age-related variation in clinicopathological features, molecular. Markers and outcome from colorectal cancer. Anticancer Res. 2007;27(4C):2833–2838. [PubMed] [Google Scholar]
- 31.Moghbeli M, Moaven O, Dadkhah E, et al. High frequency of microsatellite instability in sporadic colorectal cancer patients in Iran. Genet Mol Res. 10(4) doi: 10.4238/2011.December.14.4. [DOI] [PubMed] [Google Scholar]
- 32.Lothe RA, Peltomaki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53(24):5849–5852. [PubMed] [Google Scholar]
- 33.Janssen KP, Alberici P, Fsihi H, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131(4):1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Obrador-Hevia A, Chin SF, Gonzalez S, et al. Oncogenic KRAS is not necessary for Wnt signalling activation in APC-associated FAP adenomas. J Pathol. 221(1):57–67. doi: 10.1002/path.2685. [DOI] [PubMed] [Google Scholar]
- 35.Smith G, Carey FA, Beattie J, et al. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A. 2002;99(14):9433–9438. doi: 10.1073/pnas.122612899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soliman AS, Bondy ML, El-Badawy SA, et al. Contrasting molecular pathology of colorectal carcinoma in Egyptian and Western patients. Br J Cancer. 2001;85(7):1037–1046. doi: 10.1054/bjoc.2001.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 116(18):4354–4359. doi: 10.1002/cncr.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 39.Meza R, Jeon J, Renehan AG, Luebeck EG. Colorectal cancer incidence trends in the United States and United kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res. 70(13):5419–5429. doi: 10.1158/0008-5472.CAN-09-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramnath T, Nandakumar A. Estimating the burden of cancer. Natl Med J India. 24(2):69–71. [PubMed] [Google Scholar]
- 41.O'Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187(3):343–348. doi: 10.1016/j.amjsurg.2003.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IHC-based detection of β-Catenin localization (cytoplasmic/nuclear in panel a and cytoplasmic/membrane in panel b) and p53 localization (nuclear stabilization in panel c and negative staining in panel d) in representative colorectal tumor samples.
Panel a, BAT25; Panel b, BAT 26; Panel c; D2S123; Panel d, D5S346; Panel e, D17S250 (for each microsatellite, result for tumor and normal samples are given in top and bottom respectively). Panel f, APC p.Q1378X (c.4132C>T); Panel g, APC c.4460delCT; Panel h, KRAS codon 15 (GGC>GAC); Panel i, KRAS codon 18 (GCC>GTC). For each mutation, mutant sequence is given on the left and normal sequence on the right; each missense mutant nucleotide is indicated by an arrow in the mutant electropherogram while the deletion mutation (panel g) is indicated by a bar in the normal electropherogram.