Abstract
Context
Obesity and prostate cancer (PCa) affect substantial proportions of Western society. Mounting evidence, both epidemiologic and mechanistic, for an association between the two is of public health interest. An improved understanding of the role of this modifiable risk factor in PCa etiology is imperative to optimize screening, treatment, and prevention.
Objective
To consolidate and evaluate the evidence for an epidemiologic link between obesity and PCa, in addition to examining the proposed underlying molecular mechanisms.
Evidence acquisition
A PubMed search for relevant articles published between 1991 and July 2012 was performed by combining the following terms: obesity, BMI, body mass index and prostate cancer risk, prostate cancer incidence, prostate cancer mortality, radical prostatectomy, androgen-deprivation therapy, external-beam radiation, brachytherapy, prostate cancer and quality of life, prostate cancer and active surveillance, in addition to obesity, BMI, body mass index and prostate cancer and insulin, insulinlike growth factor, androgen, estradiol, leptin, adiponectin, and IL-6. Articles were selected based on content, date of publication, and relevancy, and their references were also searched for relevant articles.
Evidence synthesis
Increasing evidence suggests obesity is associated with elevated incidence of aggressive PCa, increased risk of biochemical failure following radical prostatectomy and external-beam radiotherapy, higher frequency of complications following androgen-deprivation therapy, and increased PCa-specific mortality, although perhaps a lower overall PCa incidence. These results may in part relate to difficulties in detecting and treating obese men. However, multiple molecular mechanisms could explain these associations as well. Weight loss slows PCa in animal models but has yet to be fully tested in human trials.
Conclusions
Obesity appears to be linked with aggressive PCa. We suggest clinical tips to better diagnose and treat obese men with PCa. Whether reversing obesity slows PCa growth is currently unknown, although it is an active area of research.
Keywords: Estrogen, Insulin, Obesity, Prostate cancer, Review, Testosterone, Weight change
1. Introduction
Prostate cancer (PCa) is the second most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality among men worldwide [1]. In the last decade, multiple epidemiologic studies have suggested that obesity is associated with increased risk and death from numerous cancer types including PCa [2–4]. Like PCa, obesity affects many men, with two-thirds of the US classified as overweight (body mass index [BMI] ≥25 kg/m2) and one-third as obese (BMI ≥30 kg/m2). These trends have stabilized in the past 10 yr [5], suggesting these levels are established as a permanent feature of US society. In Europe, the prevalence of overweight and obesity continues to rise, and current levels are comparable with those of the United States 15 yr ago [6].
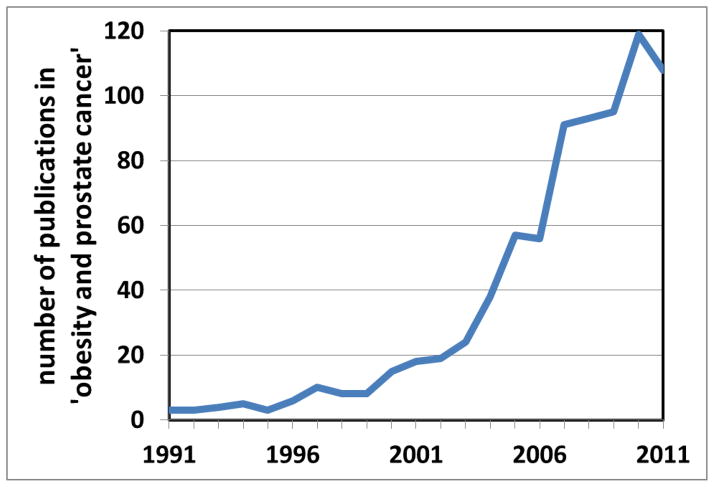
Because obesity and PCa affect substantial proportions of the male population, the association between the two is of great public health significance. Existing epidemiologic data are somewhat conflicting, and the consolidation and review of findings to date is required. Attention is increasingly turning to the elucidation of underlying molecular mechanisms, a number of which are emerging. This review focuses on the epidemiologic association between obesity and PCa incidence, treatment, and mortality, in addition to proposed underlying molecular mechanisms. It concludes with clinical recommendations for our obese patients. The contribution of specific dietary components, clearly intertwined with obesity, is the focus of our companion review. We previously reviewed the literature on this topic several years ago [7]. At that time, a PubMed search of obesity and PCa generated 237 articles. The same search in July 2012 generated 796 articles, a more than three-fold increased in the past 5 yr (Fig. 1). Thus we decided an updated review was needed.
Fig. 1.
Using the search terms obesity and prostate cancer, the number of PubMed publications has increased in the past 20 yr.
2. Evidence acquisition
Relevant literature published between January 1991 and July 2012 was identified by a search of the PubMed database using the following terms: obesity, BMI, body mass index combined with prostate cancer risk, prostate cancer incidence, prostate cancer mortality, radical prostatectomy, androgen-deprivation therapy, external-beam radiation, brachytherapy, quality of life, active surveillance, and prostate cancer combined with obesity, BMI, body mass index, and insulin, IGF, sex hormones, adipokines, leptin, adiponectin, and IL-6 (Fig. 2). Relevant English-language articles were abstracted and reviewed using the reference list to identify additional potential articles for review. Studies were selected based on clinical relevance, and so studies were excluded when the study population and/or outcome measures lacked clinical significance or were not relevant to our review (eg, cancer cachexia). Wherever possible, randomized controlled trials, prospective population-based studies, systematic reviews, and meta-analyses were selected over case-control studies and reviews. If more than one article was published in the same study population, the study with the larger sample size was selected.
Fig. 2.
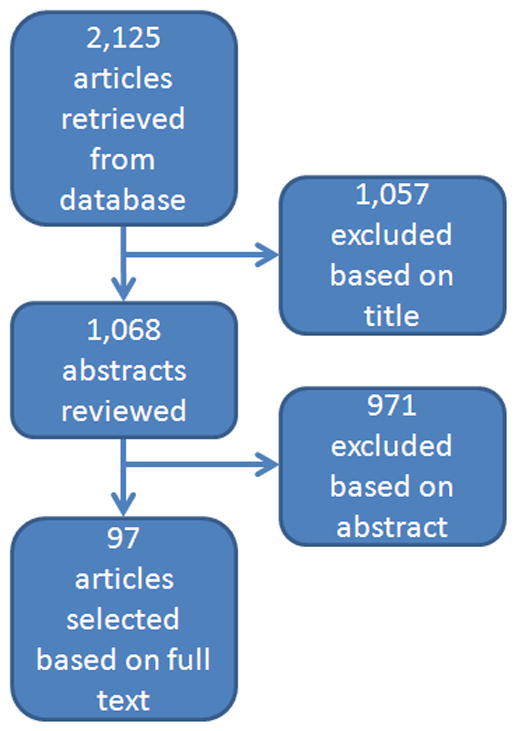
Flow diagram of search results.
3. Evidence synthesis
3.1. Measuring obesity
BMI, calculated using an individual’s height and weight (kilograms per square meter), is a straightforward and cost-effective method of measuring overall adiposity. Although BMI does not account for body mass composition or the distribution of adipose tissue, it has been repeatedly demonstrated as a reliable surrogate of obesity on a population level, with the advantage of being the most widely used measure, enabling interstudy comparisons. Thus due to ease of use and near ubiquitous use in the literature, we focused nearly exclusively on BMI, accepting the inherent limitations of this approach.
3.2. Obesity and prostate cancer incidence
All three meta-analyses to date [4,8,9] reported a positive association between obesity and PCa incidence. The relative risks (RRs) in these studies were modest yet consistent, from 1.01 (95% confidence interval [CI], 1.0–1.02) per 1 kg/m2 increase in BMI [9] to 1.05 (95% CI, 1.01–1.08) [8] and 1.03 (95% CI, 1.0–1.07) [4] per 5 kg/m2 increment in BMI. However, the findings of the individual studies contributing to these meta-analyses differ dramatically, with some reporting no association between obesity and PCa [10], some finding obesity is a risk factor [11,12], and some reporting a protective effect of obesity [13–16]. These individual studies vary greatly in size, thus contributing to the meta-analysis findings to different extents. One large Norwegian cohort study, reporting a RR of 1.09 (95% CI, 1.04–1.15) for obese versus normal weight men [11], contributed approximately 50% of PCa cases to two of the three meta-analyses [4,8]. In the third meta-analysis [9], more than half of the PCa cases came from one Swedish study that reported a RR of 1.4 (95% CI, 1.09–1.81) for obese versus normal weight men [12]. With these two Scandinavian studies contributing most of the PCa cases, the meta-analyses are weighted in favor of their positive findings.
Interestingly, when studies are examined by geographic region, separating North American studies from European, a distinct pattern emerges. Specifically, among North American studies, no effect of obesity on PCa risk was observed (RR: 1.0; 95% CI, 0.96–1.03), whereas in European and Australian studies there was a modest yet significantly positive association of obesity with PCa (RR: 1.04; 95% CI, 1.01–1.07), per 5 kg/m2 increment in BMI [4]. One possible explanation for these geographic differences relates to differential prostate-specific antigen (PSA) screening rates. In Europe, where PSA screening is not as common as in the United States, many cancers are diagnosed at more advanced stages. In the United States, where PSA screening is widespread, many cancers are diagnosed due to elevated PSA alone and are earlier stage and lower risk.
Importantly, obesity is associated with reduced PSA levels [17]. As such, in countries with widespread PSA screening programs where biopsies are largely driven by PSA, obese men have a reduced chance of undergoing biopsy compared with normal weight men, leading to detection of fewer early stage cancers in obese individuals. This detection bias can minimize or even reverse the association between obesity and PCa risk (ie, obesity becomes “protective”), as demonstrated by multiple recent large prospective cohort studies in the United States totaling data on nearly 400 000 men [14–16]. However, we can hypothesize that the association of obesity with advanced disease would still persist if obesity were biologically linked with aggressive disease. Indeed, a meta-analysis of multiple prospective studies found that although obesity has a null or slightly protective effect in localized disease, it is associated with an increased incidence of advanced PCa [18]. This dichotomous effect of obesity on PCa incidence is multifactorial: attributable in part to detection biases associated with obesity (leading to delayed diagnosis and more advanced disease at diagnosis) and also to underlying biologic mechanisms, both of which are addressed in this paper.
3.3. Detection biases
Numerous factors contribute toward the difficulty of detecting PCa in obese men. First, obese men have lower PSA values, resulting in reduced PSA-driven biopsy rates [19]. Among men with PCa, it has been reported that PSA values are 7% lower in overweight patients (BMI: 25–30 kg/m2), 14% lower in obese patients (BMI: 30–35 kg/m2), and 18% lower in severely obese patients (BMI >35 kg/m2), relative to normal weight patients (BMI <25 kg/m2) [17], with a similar reductions in PSA levels reported for overweight and obese cancer-free men [20,21]. It has been hypothesized that this is due to increased blood volume in obese individuals, leading to PSA hemodilution because PSA mass (the total amount of PSA in the blood) did not differ by BMI [17,21]. Second, obesity may make it more challenging to perform a thorough digital rectal examination (DRE), leading to yet more missed cancers [22]. Third, obese men have larger prostates, reducing the likelihood of finding cancer at biopsy [23]. Lower PSA values combined with difficulties in performing a thorough DRE can lead to lower biopsy rates among obese men; larger prostates can result in more missed cancer that collectively leads to reduced detection of early stage cancers. This allows PCa growth to continue unchecked, which could contribute to elevated occurrence of advanced disease in obese men. However, as outlined in this paper, even in the pre-PSA, premodern biopsy era, obesity was linked with PCa (particularly PCa mortality) [24], and thus detection bias cannot fully explain the link between obesity and aggressive PCa.
3.4. Obesity and prostate cancer outcome
3.4.1. Obesity and radical prostatectomy
A systematic review and meta-analysis found a 21% increase in biochemical recurrence (BCR) (RR: 1.21; 95% CI, 1.11–1.31) and a 15% increase in PCa-specific mortality (RR: 1.15; 95% CI, 1.06–1.25) following radical prostatectomy (RP), per 5 kg/m2 increase in BMI [25]. The findings of individual studies vary by geographic region, again highlighting the potentially confounding effect of obesity-related detection bias. Indeed, it has been found that the link between obesity and BCR is stronger in more recently treated men and especially those with PSA-detected cancers [26], suggesting that the more widespread the PSA screening in the underlying population, the stronger the link between obesity and BCR. Another possible explanation for the worse outcomes is that operating on obese men can be technically challenging. Data show that obesity is associated with capsular incision, reflecting a less-than-ideal operation [27]. However, obesity remains linked with poor outcome even after adjusting for pathologic features including margin status [25,28], suggesting poor technique alone cannot explain the link between obesity and aggressive PCa.
3.4.2. Obesity and nonsurgical treatment
Regarding outcomes after radiation, although a recent meta-analysis found no association between obesity and BCR for brachytherapy-treated patients (RR: 0.99; 95% CI, 0.78–1.25), obesity was associated with an increased risk of BCR for patients treated with external-beam radiation therapy (EBRT) (RR: 1.16; 95% CI, 1.08–1.26), per 5 kg/m2 increase in BMI [25]. However, studies in this area are few, and this meta-analysis is based on two brachytherapy and two EBRT studies. One possible challenge in treating obese patients with EBRT is that excess adiposity results in increased daily movement of the prostate [29]. Consequently, set-up error in obese men exceeds that of normal weight men, potentially resulting in a less-than-optimal radiation dose [30]. In contrast, excess body fat may not hinder brachytherapy to the same extent [31], although the increased use of fiducial markers in EBRT should eliminate this theoretical advantage to brachytherapy.
The impact of obesity on androgen-deprivation therapy (ADT) outcomes is understudied. One study found obesity at the time of ADT was associated with an increased risk of castrate-resistant PCa (CRPC), metastases, and PCa-specific mortality [32]. Although the exact explanation for this is unclear, one study found that testosterone levels in obese men on ADT were higher, suggesting inadequate testosterone suppression [33]. It was hypothesized that this may be because the amount of gonadotropin-releasing hormone analog given is the same regardless of body surface area, and thus obese men may be underdosed. Beyond possibly predicting a poor oncologic outcome, obesity may also increase ADT side effects: one study found a pre-ADT BMI >30 kg/m2 was associated with a 4.6-fold increased risk of new-onset type 2 diabetes [34]. To date, there are no data regarding obesity and active surveillance (AS) outcomes. Obese patients are more likely to present with comorbidities, making them poorer candidates for primary treatment, thus potentially making AS a more attractive option. However, given that obesity is linked with increased upgrading at RP [35], body size should be taken into account when considering patients for AS, highlighting the need for close surveillance in this group.
3.4.3. Obesity and health-related quality of life
Although several small observational studies reported mixed findings [36–38], a prospective multicenter study of 1201 PCa survivors treated with RP or radiotherapy reported an independent association of obesity with reduced vitality and worse health-related quality of life (HRQoL) [39]. Of note, obesity was associated with worse pretreatment vitality [38], which has a negative impact on posttreatment HRQoL. Similarly, it has been reported that erectile function following RP is determined by erectile function presurgery and is not independently associated with obesity [40]. Indeed, erectile function depends heavily on nerve-sparing status, which does not differ significantly by BMI [37]. Regarding bowel function, preliminary evidence suggests obesity is associated with delayed recovery following RP [38]. Collectively, these findings suggest that although obesity is associated with perioperative challenges and complications and poorer response to therapy, the major determinant of posttreatment HRQoL remains pretreatment HRQoL. Finally, one study found that at approximately 1 yr post-RP, obese and inactive men were 26% more likely to be incontinent versus normal weight physically active men [36]. Whether this is indicative of obesity, physical inactivity, or an interaction between the two is unclear but requires further study.
3.5. Obesity and prostate cancer–specific mortality
Multiple large cohort studies have consistently demonstrated a positive dose–response relationship between increasing BMI and fatal PCa [2,12,16]. A meta-analysis of prospective cohort studies including almost 7000 PCa-specific deaths reported 15% increased PCa-specific mortality per 5 kg/m2 increase in BMI (RR: 1.15; 95% CI, 1.06–1.25) [25]; a meta-analysis of case-control studies including almost 1000 PCa-specific deaths reported 20% increased PCa-specific mortality per 5 kg/m2 increase in BMI (RR: 1.20; 95% CI, 0.99–1.46) [25]. The similar magnitude of RR estimated using these large populations with different study designs indicates the robust association between obesity and fatal PCa. Although this effect may be attributable in part to delayed PCa diagnosis in obese versus normal weight men, obesity was associated with PCa mortality in the pre-PSA era [12,24], demonstrating that this association cannot be explained by detection bias alone and that underlying biologic mechanisms play an important role.
Although the link between obesity and PCa mortality from population-based data appears very consistent, it is noteworthy that over the past 20 yr while obesity rates have been rising, PCa mortality has been falling [41]. Much of this falling mortality rate is attributable to the introduction of PSA screening and to improved treatment options, but this may not explain the entire drop in mortality. A recent study addressed this issue. The authors, using simulation models, suggested that if obesity rates in the United States had remained stable from 1980, the PCa mortality rate in 2002 in the United States (the last year for which they had data available) would have been 23% lower [42]. In other words, the fact that mortality has declined despite the increasing prevalence of obesity suggests that an even greater improvement in mortality rates could have been seen, had obesity rates remained constant over time. Given that obesity rates have continued to climb since 2002, the impact of obesity today is likely even greater.
3.6. Mechanisms
Three mechanisms are most commonly proposed to help explain the association between obesity and aggressive PCa: the insulin/insulinlike growth factor (IGF)-1 axis, sex hormones, and adipokine signaling [43] (Fig. 3). We focus primarily on these factors, although it should be acknowledged that a myriad of effects of obesity on PCa biology is likely [44].
Fig. 3.
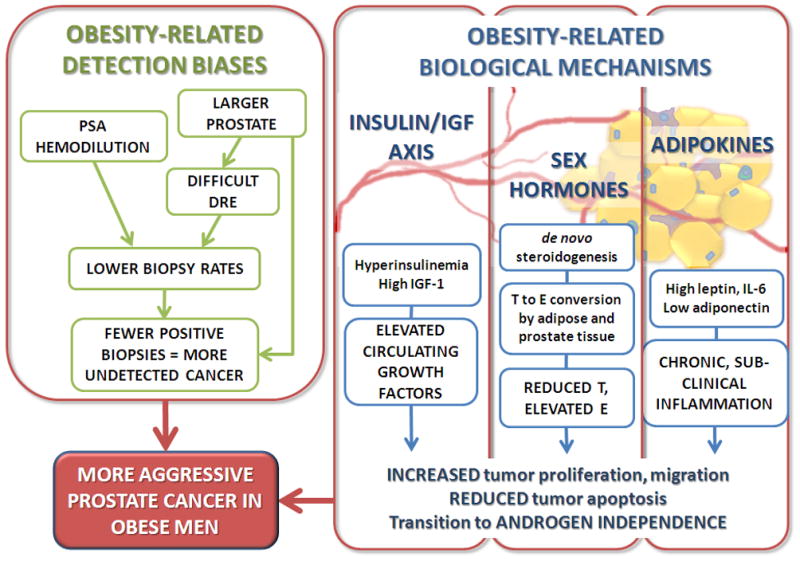
Obesity-related detection biases and biologic mechanisms contributing to the epidemiologic association between obesity and aggressive prostate cancer. DRE = digital rectal examination; E = estrogen; IGF = insulinlike growth factor; IL = interleukin; PSA = prostate-specific antigen; T = testosterone.
The insulin/IGF-1 axis has been widely implicated in obesity-fueled tumorigenesis [43], and PCa is no exception. Diet -induced hyperinsulinemia has been shown to accelerate tumor growth in different PCa xenograft models [45,46], and primary human PCa commonly expresses the insulin receptor [47], suggesting that insulin may stimulate human PCa growth. A large prospective survival analysis reported that higher serum C-peptide concentrations, a surrogate of insulin levels, were associated with increased PCa-specific mortality [48]. The insulin-lowering drug metformin has been shown to reduce PCa risk in diabetic patients[49,50]. Finally, diabetes, an end state in which insulin levels actually start to decline, has also been linked with reduced PCa risk [51]. As such, insulin is likely a key contributor to explain the link between obesity and aggressive PCa.
Obesity and hyperinsulinemia are associated with increased circulating amounts of bioactive IGF-1, a growth factor with a recognized pathogenic role in many cancers [43]. Elevated circulating IGF-1 has been linked to increased PCa incidence in meta-analyses combining PSA-detected and clinically detected PCa [52,53]; however, no association was found in the largest study to date of >5000 patients with only PSA-detected PCa [54]. One possible explanation for these contrasting findings is that IGF-1 may play a role in the progression to clinically detectable disease. Upregulation of the IGF-1 receptor accompanies the transition of androgen-dependent cell lines to androgen independence, and IGF-1 promotes PCa progression in vivo [55]. In this sense, the effects of IGF-1 on PCa risk may mirror those of obesity: associated with aggressive disease but not with more indolent PSA-detected tumors.
Beyond changes in insulin, obesity is also linked with decreased androgen levels [56]. Androgens, which play an important role in normal prostate growth and development, have long been hypothesized to influence PCa risk. Although the vast majority of the literature suggests no association between androgens and PCa risk [57], among men with PCa, those who have low testosterone (T) tend toward a more aggressive phenotype [58]. This suggests that low T may promote aggressive PCa; older men have more aggressive cancers, and older age is when T levels are at their lowest [59]. The association of low T levels with aggressive PCa is also reflected in trials of 5α-reductase inhibitors that decreased overall PCa risk but were associated with a higher Gleason score [60]. As such, the low T environment in obese men may be one plausible link between obesity and aggressive PCa, although the exact mechanisms remain unknown.
Of note, T is aromatized to estradiol (E) within adipocytes and prostate cells [61]. The greater adipose tissue mass in obese men, together with obesity-associated upregulation of this aromatization pathway, leads to elevated serum and intracellular E levels [56]. Although current epidemiologic evidence does not support an association between serum E and PCa risk [62], a substantial body of literature from preclinical studies suggests that E may play a role in promoting PCa development and progression [63]. Thus the significance of elevated E levels in obese men with respect to PCa risk and progression remains to be determined.
Obesity is a state of chronic subclinical inflammation mediated through altered levels of adipokines. Leptin is elevated in obesity and exerts a predominantly pro -tumor effect in human androgen-independent PC-3 and DU145 PCa cell lines, inducing proliferation, inhibiting apoptosis [64,65], and increasing migration [66]. Although these in vitro data suggest a link between leptin and PCa, epidemiologic studies fail to consistently demonstrate a positive association with either PCa risk or fatal PCa [10,67,68]. Thus the extent to which leptin contributes to the link between obesity and aggressive PCa is unclear.
In contrast to leptin, adiponectin has largely antitumor effects, and serum levels are reduced in obesity. A prospective study of 644 controls and 654 PCa patients found that although there was no association with overall PCa risk, reduced prediagnostic serum adiponectin levels were associated with metastatic and fatal PCa [68]. Adiponectin levels were found to be reduced significantly in metastatic PCa patients versus those with organ-confined disease [69]. Thus the limited data to date suggest that although adiponectin may not be related to PCa risk, it is associated with advanced aggressive disease, again, mirroring the overall association between obesity and PCa.
Obesity is associated with elevated serum interleukin (IL)-6 levels with adipose tissue the primary source [70]. PCa cell lines and primary human PCas produce IL-6 and express IL-6 receptor, allowing them to respond to this pleiotropic, proinflammatory adipokine in an autocrine manner [70,71]. Circulating IL-6 levels are elevated in PCa patients with metastatic relative to organ-confined disease [72,73], and they are associated with time to progression to metastatic disease [74]. However, it cannot be determined from these case-control studies whether IL-6 plays a causal or bystander role in disease progression (ie, whether IL-6 promotes tumor growth or large tumors simply produce more IL-6). Large prospective cohort studies are required to test this, but to date, only three small prospective studies have examined the association of IL-6 and PCa; two reported no association [10,75], and the third reported a modest inverse association between IL-6 and PCa risk [76]. Clinical trials of siltuximab, an IL-6 inhibitor, have proven disappointing, with no effect on PCa outcome [77]. As such, the role of IL-6 explaining the association between obesity and aggressive PCa is uncertain.
Molecular interplay between these obesity-related mechanisms has already been demonstrated. For example, IL-6 regulates androgen synthesis in PCa cell lines [78] in addition to promoting androgen receptor activation and downstream signaling [79]. This may partially counteract the treatment effect of ADT, particularly in obese men with elevated circulating IL-6. It has been suggested that obesity-associated hyperinsulinemia can directly promote de novo steroidogenesis that may contribute to the development of CRPC [80], partially explaining why obesity at the time of ADT may be linked to an increased risk of CRPC [32]. We should consider that obesity is associated with numerous changes to the serum metabolome including elevated cholesterol and free fatty acids, which are covered in detail in our companion review.
Functional single nucleotide polymorphisms (SNPs), which alter expression of genes coding for adipokines, may also influence PCa biology. A large nested case-control study found three SNPs in the leptin gene to be significantly associated with an elevated risk of PCa [81]. A study seeking to generate an adipokine genetic risk score based on multiple genetic loci for PCa found that the addition of seven SNPs in obesity -associated genes (leptin, IL-6, fibroblast growth factor 2, osteopontin, and IGF-1) significantly, albeit modestly, enhanced the predictive value of age and PSA to predict high-risk individuals [82]. Taken together, these findings suggest that gene tic variation in adipokine genes could contribute to the association between obesity and PCa.
Finally, although systemic effects appear important, paracrine effects may also play a role. The prostate is surrounded by periprostatic adipose tissue (PPAT), responsible for locally secreted growth factors and adipokines. Although it has been suggested that increased PPAT is associated with higher risk disease in radiation-treated PCa patients [83], very few mechanistic studies exploring this hypothesis have been conducted. One such study found that PPAT-derived IL-6 levels were positively associated with elevated Gleason grade in a small group of patients treated with RP, suggesting a paracrine role for PPAT in modulating PCa aggressiveness [84]. Also, treating PPAT explants with conditioned medium from the PC-3 PCa cell line induced IL-6 and tumor necrosis factor-α production, suggesting that PCa can induce a pro-tumor secretory profile in PPAT and highlighting the potential importance of paracrine cross-talk between tumor and fat [85]. In conclusion, given the multitude of obesity-related metabolic disturbances, and the tremendous interplay among them, more work is required to fully dissect the molecular links between obesity and PCa.
3.7. Interventions
3.7.1. Calorie restriction and weight loss
With an emerging epidemiologic association between obesity and aggressive PCa, we can hypothesize that weight reduction may reduce the risk of PCa-specific mortality. Calorie restriction (CR), accompanied by weight loss, has been investigated in murine PCa models. Intermittent CR slowed tumor detection and increased survival time in transgenic adenoma of the mouse prostate mice [86], and 30% CR slowed PCa progression in Hi-Myc transgenic mice [87]. However, few studies have examined the effect of weight change on PCa risk, mortality, or outcome after treatment in humans, and most of these focused on weight gain. Of these, two large prospective cohort studies, based in the United States [16] and Australia [88], reported a positive association between PCa-specific mortality and weight gain between age 18 and study entry. Two studies in the United States found that weight gain after RP was associated with an increased risk of BCR [89,90]. In contrast, a population-based cohort study in Norway examining weight gain during a decade spanning midadulthood found no association with either PCa incidence or survival [91].
Several factors may contribute to these inconsistent findings. It is possible that the amount of weight gained in the Norwegian study was insufficient to influence PCa risk compared with the larger amount of weight gained in the US and Australian studies. Alternatively, it is possible that obesity has the greatest influence earlier in life, which would explain why the studies that included data back to age 18 were —positive, whereas the study that only looked at adulthood weight was —negative. Only one study, to our knowledge, specifically examined weight loss and found that >10 lb of weight loss over a 10-yr span in midadulthood reduced the risk of nonmetastatic high-grade PCa by 45% (odds ratio: 0.55; 95% CI, 0.40–0.75) [15]. Because these results were adjusted for baseline BMI, it suggests that weight loss may be protective independent of baseline BMI. Albeit limited, the preponderance of literature suggests that weight gain is harmful for PCa, and weight loss may be beneficial. This information coupled with the clear overall health benefits of weight loss means that weight loss counseling should be standard for all overweight and obese men with newly diagnosed PCa.
3.7.2. Exercise
Three large prospective population-based studies in the United States [92] and Europe [93,94] found physical activity to be significantly protective against the risk of aggressive, but not overall, PCa. In addition, vigorous exercise postdiagnosis was shown to be associated with a reduced risk of PCa-specific mortality [95], indicating that exercise counseling in newly diagnosed PCa patients may be beneficial. Not all studies of exercise have shown benefits for PCa [96]. However, given that heart disease is the leading cause of death among men with PCa, the clear cardiovascular benefits associated with exercise should be sufficient to recommend this lifestyle intervention to our patients. Further research, in the form of mechanistic studies and randomized controlled trials, is necessary to understand the extent to which the purported benefits of exercise on PCa are real and whether they are mediated by weight reduction, improved fitness, or the combination of the two. One such mechanistic study found that although exercise did not slow tumor growth as hypothesized, it was associated with increased tumor vascularization and a shift toward reduced metastasis in an orthotopic model of murine PCa [97].
3.8. Clinical recommendations
First, for men who undergo PSA screening and for whom the absolute PSA value is used to determine whether a biopsy is indicated or not, we may consider generating BMI-adjusted PSA cut-offs for biopsy, in addition to obtaining more biopsy cores to adjust for the larger prostate volume of these patients (Fig. 4). This approach may help to diminish the detection bias associated with reduced PSA levels in obesity, thus allowing all men an equal chance of early diagnosis, no matter what their BMI. Given that obesity is associated with an increased risk of aggressive disease, as discussed in this review article, this is the population that may benefit the most from early detection. Second, it is advisable to counsel obese men regarding their elevated risk of BCR following treatment, which may be related to a combination of technical issues during treatment and underlying molecular mechanisms associated with obesity. Obesity, per se, should not factor into decisions regarding HRQoL because only baseline HRQoL appears predictive of posttreatment HRQoL, although baseline HRQoL is often lower in obese men. For many obese men with low- to intermediate-risk disease, delaying treatment by 3–6 mo to allow weight loss could be advisable because the benefits of a successful treatment may outweigh the risk of leaving the cancer untreated for that period of time. Obese men should receive counseling regarding worse outcomes following ADT, which may be partly due to inadequate T suppression. It may therefore be advisable to check T levels, especially in obese patients, at 3 mo after beginning ADT, especially for those who fail to nadir at an undetectable level. Finally, due to the growing number of disease states associated with obesity, it is advisable for all individuals to exercise and maintain a healthy weight, especially for those beginning ADT, which can lead to further weight gain and metabolic disturbances. Indeed, given that more men diagnosed with PCa die from heart disease than from PCa, it is this recommendation more than any other that is most likely to have a positive impact on our patients’ quality and quantity of life.
Fig. 4.
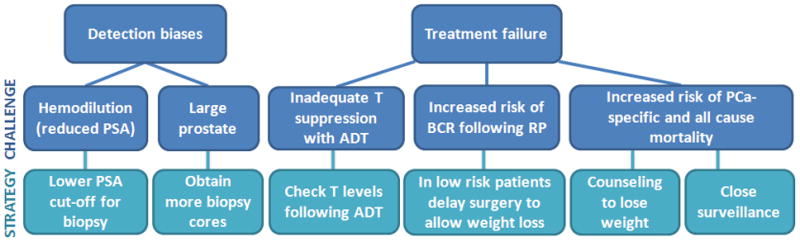
Diagram of challenges associated with obesity and possible strategies for clinical recommendations. ADT = androgen-deprivation therapy; BCR = biochemical recurrence; PCa = prostate cancer; PSA = prostate-specific antigen; RP = radical prostatectomy; T = testosterone.
4. Conclusions
The epidemiologic association of obesity and aggressive PCa is particularly relevant due to the pervasive nature of both diseases and the large numbers of men affected. Beyond age, race, and family history, there are few established risk factors for PCa. The identification of obesity as an additional risk factor for aggressive PCa is of significant public health interest due to its modifiable nature. Adipose tissue as an organ is unique in its capacity to expand and diminish throughout the lifespan of an individual, and it can be reduced through lifestyle interventions such as diet and exercise. Epidemiologic evidence linking obesity and aggressive PCa underlines the importance of taking body size into account when screening, treating, and monitoring PCa patients, as well as counseling obese patients about healthier lifestyle choices and weight loss. Unfortunately, no clinical trials specifically examining the effects of weight loss in PCa patients have been completed, nor are the molecular mechanisms linking obesity and PCa fully elucidated, although multiple pathways have been implicated. An improved understanding of the mechanisms linking PCa and obesity would likely provide valuable information regarding the etiology of aggressive PCa in general, which could in turn be used to develop better approaches for the treatment and prevention of all men with PCa.
Take-home message.
Obesity appears to be linked with aggressive prostate cancer (PCa). Small tips can be used to better diagnose and treat obese men with PCa. Whether reversing obesity slows PCa growth is currently unknown, although this area of research is active.
Acknowledgments
Funding/Support and role of the sponsor: Emma H. Allott is the recipient of NCI Grant 5R25-CA126938–03. Elizabeth M. Masko is the recipient of Department of Defense Prostate Cancer Research Program Health Disparities Training Award PC110475. Stephen J. Freedland is the recipient of NIH grant 1-R01-CA131235-01A1.
Footnotes
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Allott, Masko.
Acquisition of data: Freedland, Allott.
Analysis and interpretation of data: Freedland, Allott, Masko.
Drafting of the manuscript: Freedland, Allott.
Critical revision of the manuscript for important intellectual content: Freedland, Allott, Masko.
Statistical analysis: Freedland, Allott, Masko.
Obtaining funding: Freedland, Allott, Masko.
Administrative, technical, or material support: Freedland, Allott, Masko.
Supervision: Freedland.
Other (specify): None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079– 92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235– 41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 6.Berghofer A, Pischon T, Reinhold T, et al. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buschemeyer WC, III, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52:331– 43. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 8.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989– 1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–30. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Baillargeon J, Platz EA, Rose DP, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15:1331– 5. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 11.Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237– 42. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–9. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 13.Porter MP, Stanford JL. Obesity and the risk of prostate cancer. Prostate. 2005;62:316– 21. doi: 10.1002/pros.20121. [DOI] [PubMed] [Google Scholar]
- 14.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:63–9. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 16.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675– 84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 17.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275– 80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 18.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 19.Hekal IA, Ibrahiem EI. Obesity-PSA relationship: a new formula. Prostate Cancer Prostatic Dis. 2010;13:186–90. doi: 10.1038/pcan.2009.53. [DOI] [PubMed] [Google Scholar]
- 20.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092–5. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 21.Grubb RL, Black A, Izmirlian G, et al. Serum prostate-specific antigen hemodilution among obese men undergoing screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2009;18:748–51. doi: 10.1158/1055-9965.EPI-08-0938. [DOI] [PubMed] [Google Scholar]
- 22.Chu DI, De Nunzio C, Gerber L, et al. Predictive value of digital rectal examination for prostate cancer detection is modified by obesity. Prostate Cancer Prostatic Dis. 2011;14:346–53. doi: 10.1038/pcan.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedland SJ, Platz EA, Presti JC, Jr, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500– 4. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez C, Patel AV, Calle EE, et al. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–53. [PubMed] [Google Scholar]
- 25.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486– 501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedland SJ, Sun L, Kane CJ, et al. Obesity and oncological outcome after radical prostatectomy: impact of prostate-specific antigen-based prostate cancer screening: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102:969– 74. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 27.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 2005;174:1798– 801. doi: 10.1097/01.ju.0000177077.53037.72. [DOI] [PubMed] [Google Scholar]
- 28.Okotie OT, Aronson WJ, Wieder JA, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004;171:2260– 4. doi: 10.1097/01.ju.0000127734.01845.99. [DOI] [PubMed] [Google Scholar]
- 29.Wong JR, Gao Z, Merrick S, et al. Potential for higher treatment failure in obese patients: correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys. 2009;75:49–55. doi: 10.1016/j.ijrobp.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 30.Millender LE, Aubin M, Pouliot J, Shinohara K, Roach M., III Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:6–10. doi: 10.1016/j.ijrobp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Merrick GS, Galbreath RW, Butler WM, et al. Obesity is not predictive of overall survival following permanent prostate brachytherapy. Am J Clin Oncol. 2007;30:588– 96. doi: 10.1097/COC.0b013e318068b506. [DOI] [PubMed] [Google Scholar]
- 32.Keto CJ, Aronson WJ, Terris MK, et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2011;110:492– 498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13:241– 5. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derweesh IH, Diblasio CJ, Kincade MC, et al. Risk of new-onset diabetes mellitus and worsening glycaemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int. 2007;100:1060– 5. doi: 10.1111/j.1464-410X.2007.07184.x. [DOI] [PubMed] [Google Scholar]
- 35.Ploussard G, de la Taille A, Bayoud Y, et al. The risk of upstaged disease increases with body mass index in low-risk prostate cancer patients eligible for active surveillance. Eur Urol. 2011;61:356–62. doi: 10.1016/j.eururo.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Wolin KY, Luly J, Sutcliffe S, Andriole GL, Kibel AS. Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol. 2010;183:629– 33. doi: 10.1016/j.juro.2009.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedland SJ, Haffner MC, Landis PK, Saigal CS, Carter HB. Obesity does not adversely affect health-related quality-of-life outcomes after anatomic retropubic radical prostatectomy. Urology. 2005;65:1131– 6. doi: 10.1016/j.urology.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery JS, Gayed BA, Hollenbeck BK, et al. Obesity adversely affects health related quality of life before and after radical retropubic prostatectomy. J Urol. 2006;176:257–61. doi: 10.1016/S0022-5347(06)00504-0. [DOI] [PubMed] [Google Scholar]
- 39.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250– 61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 40.Uffort EE, Jensen JC. Impact of obesity on early erectile function recovery after robotic radical prostatectomy. JSLS. 2011;15:32– 7. doi: 10.4293/108680810X12924466009203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol. 2008;9:445–52. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fesinmeyer MD, Gulati R, Zeliadt S, et al. Effect of population trends in body mass index on prostate cancer incidence and mortality in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:808–15. doi: 10.1158/1055-9965.EPI-08-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301– 16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 44.Sharad S, Srivastava A, Ravulapalli S, et al. Prostate cancer gene expression signature of patients with high body mass index. Prostate Cancer Prostatic Dis. 2011;14:22– 9. doi: 10.1038/pcan.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 46.Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11– 9. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox ME, Gleave ME, Zakikhani M, et al. Insulin receptor expression by human prostate cancers. Prostate. 2009;69:33– 40. doi: 10.1002/pros.20852. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039– 47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617– 22. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451– 61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 51.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124:1398– 403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 53.Rowlands MA, Gunnell D, Harris R, et al. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009;124:2416–29. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowlands MA, Holly JM, Gunnell D, et al. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer Res. 2012;72:503–15. doi: 10.1158/0008-5472.CAN-11-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nickerson T, Chang F, Lorimer D, et al. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–80. [PubMed] [Google Scholar]
- 56.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol Cell Endocrinol. 2012;351:269– 78. doi: 10.1016/j.mce.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnoeller T, Jentzmik F, Rinnab L, et al. Circulating free testosterone is an independent predictor of advanced disease in patients with clinically localized prostate cancer. World J Urol. doi: 10.1007/s00345-012-0902-5. In press. [DOI] [PubMed] [Google Scholar]
- 59.Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50:935– 9. doi: 10.1016/j.eururo.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 60.Theoret MR, Ning YM, Zhang JJ, et al. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365:97– 9. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 61.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899– 911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 62.Yao S, Till C, Kristal AR, et al. Serum estrogen levels and prostate cancer risk in the prostate cancer prevention trial: a nested case-control study. Cancer Causes Control. 2011;22:1121–31. doi: 10.1007/s10552-011-9787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol. 2009;55:533– 42. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 64.Somasundar P, Frankenberry KA, Skinner H, et al. Prostate cancer cell proliferation is influenced by leptin. J Surg Res. 2004;118:71– 82. doi: 10.1016/j.jss.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Hoda MR, Popken G. Mitogenic and anti-apoptotic actions of adipocyte-derived hormone leptin in prostate cancer cells. BJU Int. 2008;102:383–8. doi: 10.1111/j.1464-410X.2008.07534.x. [DOI] [PubMed] [Google Scholar]
- 66.Huang CY, Yu HS, Lai TY, et al. Leptin increases motility and integrin up-regulation in human prostate cancer cells. J Cell Physiol. 2011;226:1274– 82. doi: 10.1002/jcp.22455. [DOI] [PubMed] [Google Scholar]
- 67.Freedland SJ, Sokoll LJ, Mangold LA, et al. Serum leptin and pathological findings at the time of radical prostatectomy. J Urol. 2005;173:773– 6. doi: 10.1097/01.ju.0000152619.96795.b2. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Stampfer MJ, Mucci L, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56:34– 43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goktas S, Yilmaz MI, Caglar K, et al. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 70.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141– 6. [PubMed] [Google Scholar]
- 71.Azevedo A, Cunha V, Teixeira AL, Medeiros R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J Clin Oncol. 2011;2:384– 96. doi: 10.5306/wjco.v2.i12.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shariat SF, Andrews B, Kattan MW, et al. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–15. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 73.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312– 6. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stark JR, Li H, Kraft P, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009;124:2683–9. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 76.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 77.Fizazi K, De Bono JS, Flechon A, et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 78.Chun JY, Nadiminty N, Dutt S, et al. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res. 2009;15:4815– 22. doi: 10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hobisch A, Eder IE, Putz T, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640– 5. [PubMed] [Google Scholar]
- 80.Lubik AA, Gunter JH, Hendy SC, et al. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011;71:5754– 64. doi: 10.1158/0008-5472.CAN-10-2470. [DOI] [PubMed] [Google Scholar]
- 81.Moore SC, Leitzmann MF, Albanes D, et al. Adipokine genes and prostate cancer risk. Int J Cancer. 2008;124:869–76. doi: 10.1002/ijc.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ribeiro R, Monteiro C, Catalan V, et al. Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue. BMC Med. 2012;10:108. doi: 10.1186/1741-7015-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Roermund JG, Hinnen KA, Tolman CJ, et al. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011;107:1775– 9. doi: 10.1111/j.1464-410X.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 84.Finley DS, Calvert VS, Inokuchi J, et al. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol. 2009;182:1621– 7. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 85.Ribeiro RJ, Monteiro CP, Cunha VF, et al. Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol Biochem. 2012;29:233–40. doi: 10.1159/000337604. [DOI] [PubMed] [Google Scholar]
- 86.Bonorden MJ, Rogozina OP, Kluczny CM, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer. 2009;61:265–75. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 87.Blando J, Moore T, Hursting S, et al. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev Res (Phila) 2011;4:2002– 14. doi: 10.1158/1940-6207.CAPR-11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bassett JK, Severi G, Baglietto L, et al. Weight change and prostate cancer incidence and mortality. Int J Cancer. 2011;131:1711– 9. doi: 10.1002/ijc.27414. [DOI] [PubMed] [Google Scholar]
- 89.Whitley BM, Moreira DM, Thomas JA, et al. Preoperative weight change and risk of adverse outcome following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. Prostate Cancer Prostatic Dis. 2011;14:361– 6. doi: 10.1038/pcan.2011.42. [DOI] [PubMed] [Google Scholar]
- 90.Joshu CE, Mondul AM, Menke A, et al. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 2011;4:544–51. doi: 10.1158/1940-6207.CAPR-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chamberlain C, Romundstad P, Vatten L, Gunnell D, Martin RM. The association of weight gain during adulthood with prostate cancer incidence and survival: a population-based cohort. Int J Cancer. 2011;129:1199– 206. doi: 10.1002/ijc.25739. [DOI] [PubMed] [Google Scholar]
- 92.Patel AV, Rodriguez C, Jacobs EJ, et al. Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2005;14:275– 9. [PubMed] [Google Scholar]
- 93.Johnsen NF, Tjonneland A, Thomsen BL, et al. Physical activity and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2009;125:902–8. doi: 10.1002/ijc.24326. [DOI] [PubMed] [Google Scholar]
- 94.Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: a prospective population-based study in Norway (the HUNT study) Int J Cancer. 2006;119:2943–7. doi: 10.1002/ijc.22184. [DOI] [PubMed] [Google Scholar]
- 95.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–32. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, Hu F, Li D, et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60:1029– 44. doi: 10.1016/j.eururo.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Jones LW, Antonelli J, Masko EM, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. 2012;113:263–72. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]