Abstract
Several lines of evidence have converged to indicate that memory formation involves plasticity of dendritic spines in the medial prefrontal cortex (PFC) and the hippocampus. Memory varies with estrogen levels throughout the lifespan of the female. Generally, increased levels of estrogen are related to greater dendritic spine density on pyramidal cells in the PFC and hippocampus and to improved memory function. Brain derived neurotrophic factor (BDNF) is a growth factor which increases dendritic spines and enhances memory function. Estrogens increase BDNF levels in the PFC and hippocampus. In the present review we provide evidence that estradiol and BDNF may work in concert to enhance cognition. In adult females, fluctuations in recognition memory following ovariectomy and estradiol replacement, during the estrous cycle, in pregnancy and with aging are accompanied by similar changes in circulating estradiol, BDNF levels and spine density alterations in the PFC and hippocampus. In addition, both estradiol and BDNF induce spine plasticity via rapid membrane effects and slower transcriptional regulation via the CREB pathway. Moreover, estradiol increases BDNF levels through action on nuclear receptors. While the exact mechanism(s) for the influence of estrogens and BDNF on memory remain unclear, this combination may provide the basis for new and more effective strategies for treating age-related and neurodegenerative memory loss.
Keywords: Aging, BDNF, Dendritic spines, Estradiol, Memory
1. Introduction
Cognition, i.e. learning and memory, undergoes significant change over the lifespan in both males and females whether they are humans, non-human primates or rodents. The ability to learn and remember begins early during the developmental period, is molded by hormonal influences during puberty, reaches its apex during adulthood and finally declines with advanced age. In contrast to males, females of all species exhibit significant fluctuations in cognition related to their reproductive status and to the processes of reproduction. Thus, females provide a rich physiologic model system in which to study the influence of hormones or other factors which may contribute to the maintenance of cognitive function.
Our laboratory and others have documented cognitive changes in female rodent models in relation to gonadal status and estrous cyclicity, pregnancy and multiparity, estrapause and aging (Dohanich, 2002; Frick, 2009; Luine, 2006; Luine, 2008; Luine and Frankfurt, 2012). Many of these cognitive changes are associated with alterations in the density of synaptic spines in the dendritic trees of pyramidal neurons located in the prefrontal cortex (PFC) and hippocampus, areas known to contribute to learning and memory (Churchwell et al., 2010; Luine et al., 2011; Luine and Frankfurt, 2012, Luine and Frankfurt, in press). The mechanism by which hormones, predominantly estrogens, cause these changes in brain morphology and cognitive function are not well understood. Brain-derived neurotrophic factor (BDNF), which is known to play an important role in development, maintenance and plasticity of the brain, especially in these regions may ultimately contribute to cognitive function (Driscoll et al., 2012). Moreover, BDNF also contributes to regulation of neuronal maturation and survival, axonal and dendritic arborization and the maintenance of dendritic spine density (Poo, 2001). In this paper, we review evidence for the role of BDNF in regulating dendritic spine density as one way in which estrogens promote cognition in female rats and provide a working model for this interaction.
2. Estrogenic regulation of neural functions
a) Estrogens increase BDNF levels
The distribution of BDNF within neurons of the basal forebrain-diagonal band region, hippocampus and frontal cortex suggested that BDNF might serve a prominent role in cognitive function. An extensive literature now supports this view with many studies showing effects on learning and memory when BDNF levels are altered pharmacologically or through gene knockouts (Kiss et al., 2012; Li et al., 2012; Vigers et al., 2012). In addition, many studies indicate that estradiol regulates BDNF levels in the brain (For review see Scharfman and MacLusky, 2006). Gibbs (1999), using RT-PCR for BDNF mRNA and ELISA for BDNF protein levels, reported that two days of estradiol treatment increased mRNA levels in pyriform cortex and hippocampus of ovariectomized (Ovx) rats, but no changes were detected in either BDNF protein or mRNA in the frontal cortex or nucleus basalis; however, increased septal and decreased hippocampal BDNF protein was found. Singh et al (l995) also found regional differences in estradiol’s induction of BDNF mRNA; increases by estradiol in Ovx rats were found in hippocampal but not cortical regions. When Ovx rats received a longer treatment, 8 weeks, with estradiol or soybean phytoestrogens, BDNF mRNA was increased in the frontal cortex (Pan et al., 1999). Thus, a longer treatment interval may be required for estrogens to alter BDNF function in the PFC as compared to the hippocampus. However, discrepancies in published data are present concerning estradiol’s regulation of BDNF, and it is important to consider whether experiments were performed in adult or developing subjects or in vitro as such differences could be critical for regulatory processes. Moreover, as indicated by other reviews in this volume, BDNF levels are subject to multiple steroid hormone regulators which could complicate expression profiles. Nonetheless, results of current studies appear consistent with the notion that BDNF is synthesized in forebrain targets such as the frontal cortex, olfactory bulb and hippocampus, and retrogradely transported from these areas to the basal forebrain (Jezierski and Sohrabji, 2003). Presumably, BDNF is then released to act post synaptically but pre-synaptic actions cannot be ruled out (see section 3 for further discussion). A more in depth discussion of BDNF regulation by estradiol can be found in Carbone and Handa (2012, this volume). In the hippocampus, progesterone treatment following estradiol treatment reversed the estrogen dependent increase in BDNF (Gibbs, 1999). Bimonte-Nelson et al. (2008) and Engler-Chiurazzi et al. (2011) demonstrated that estradiol or Premarin (a commonly used hormone replacement drug which contains a mixture of estrogens) treatments for 16 days increased BDNF (also NGF and NT3) levels in the entorhinal and perirhinal but not the PFC and that progesterone reversed this change in Ovx 13 month old rats (Bimonte-Nelson et al., 2004
Over the estrous cycle (4–5 days), gonadal hormones wax and wane in adult, female rats. Estradiol increases in late diestrus and peaks in proestrus at which time progesterone secretion synergizes with estradiol to stimulate ovulation and sexual behavior. Working with Scharfman, we developed a low dose estradiol paradigm (three doses which were given at 8:30 AM and PM and the following morning at 8:30 AM to Ovx rats) which simulates this pre-ovulatory estrogen surge (Scharfman et al., 2007). Fig. 1 shows hippocampal BDNF levels in Ovx females and in Ovx females 4 h after receiving the last estradiol injection. As shown in either the Ovx subject (Fig 1A) or the Ovx subject receiving estradiol (Fig 1B), BDNF expression is highest in the mossy fiber pathway. Overall, pyramidal neurons in CA1 region show less BDNF than dentate neurons, but a significant increase in expression is seen in both areas following estradiol treatment. This increase is presumably a reflection of increased synthesis of BDNF in CA3 pyramidal cells which is anterogradely transported to Schaffer collateral nerve terminals. This pattern of estrogen-dependent increases in BDNF expression in response to estradiol treatment is similar to that found in the intact rat on proestrous morning (Scharfman and MacLusky, 2006). Thus, BDNF levels show a remarkable upregulation with estradiol treatment or on proestrous morning when estradiol levels are also high. This study (Scharfman et al., 2007) extended previous studies in which it had been demonstrated that Ovx decreased BDNF mRNA in the rat hippocampus (Liu et al., 2001; Pan et al., 1999; Solum and Handa, 2002) and estrogen treatment restored it (Berchtold et al., 2001; Singh et al., 1995). Increases in BDNF protein or mRNA by estradiol were consistent with an earlier demonstration that the gene encoding BDNF contained an estrogen response element (ERE) (Sohrabji et al., 1995) which provides a mechanism for estrogen’s influence on BDNF levels. This mechanism is shown in Fig. 2A where circulating estradiol enters the cell nucleus and binds to an ERE on the BDNF gene which leads to an increase in BDNF levels.
Figure 1. Physiological levels of estradiol increase BDNF expression in Ovx rats.
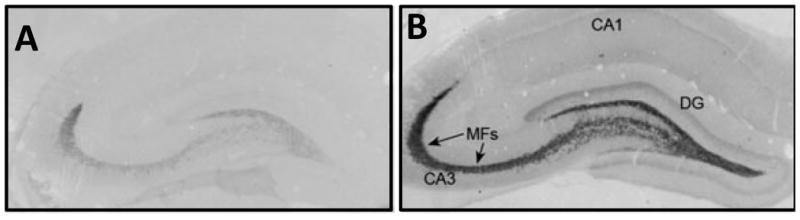
(A) BDNF immunoreactivity in a coronal section through the dorsal hippocampus in an Ovx rat treated with vehicle. (B) BDNF immunoreactivity in a section from an Ovx rat treated with estradiol doses that mimic proestrous levels. BDNF expression throughout the hippocampus is increased in estrogen-treated rats and BDNF expression is highest in mossy fibers (MFs; arrows) and the inner molecular layer (IML, single arrow in B). DG, dentate gyrus. Figure from Scharfman et al, (2007); reprinted by permission from Blackwell Publishing, Ltd.
Figure 2. Schematic mechanisms for estrogen and BDNF dependent increases in spines and memory.
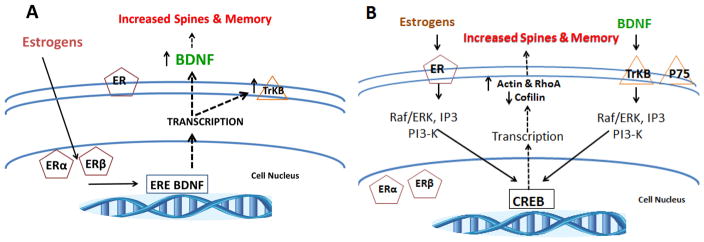
(A) Circulating estrogens enter the cell nucleus where they can bind to two types of receptors, ERα and ERβ. The complexes act as nuclear transcription factors by binding to an estrogen response element (ERE) on the BDNF gene on DNA and stimulating transcription which leads to increases in BDNF. Increased levels of BDNF are associated with increased spines and memory.
(B) Shown on the left side, circulating estrogens can bind to membrane receptors, ER, and through membrane-mediated signaling activate cellular kinase cascades including the extracellular signal-regulated kinase/mitogen-activated protein kinase (Raf/ERK), phosphatidylinositol 3-kinase/Akt (P13-K) and inositol triphosphate (IP3) pathways which then activate transcription factors through CREB (cAMP response element-binding) and thereby increase or decrease the transcription of downstream genes, including the BDNF gene. Increased transcription of actin (a filament protein in spines) and Rho (Ras homolog gene family, member A), a small GTPase protein known to regulate the actin cytoskeleton, and decreased transcription of cofilin (a family of actin-binding proteins which disassembles actin filaments) leads to increased spines and memory. Shown on the right side, BDNF can initiate a similar cascade resulting in increased dendritic spines and enhanced memory by signaling through its membrane receptor, TrKB.
Estrogens also influence expression of BDNF’s receptors, tyrosine kinase receptor A and B (TrKA and TrKB) which are present in the membrane of some neurons (See Fig. 2B). Ovx rats given estradiol show increased TrKB (Spencer et al., 2008), and estradiol or phytoestrogens, given for 12 weeks, increase TrKB mRNA levels in the hippocampus (Pan et al., 2010). Recently Spencer-Segal, et al. (2012) demonstrated that estradiol’s effects on TrKB were abolished in estrogen receptor (ER) knockout mice. Interactions with GABAergic neurons may contribute to estrogenic regulation of BDNF in the PFC (Blurton-Jones and Tuszynski, 2006). While consensus in the literature is lacking and the exact mechanisms remain unclear, a preponderance of studies show that estradiol alters mRNA for and protein expression of BDNF and its receptor TrKB throughout the basal forebrain, and therefore, increased BDNF could exert powerful effects on function, including learning and memory, within the basal forebrain and its targets.
b) Estrogens increase dendritic spines
Since the seminal observation by Cajal that most neurons have dendrites that are covered extensively by spines, it has become increasing evident that dendritic spines are critically important mediators of neural plasticity (reviewed by Urbanska et al., 2012). Indeed, the number and type of dendritic spines in many brain areas fluctuate in response to a myriad of stimuli (reviewed by Bourne, and Harris, 2008; Leuner et al., 2003; Frankfurt et al., 2009; Luine and Frankfurt 2012). In the hippocampus and medial PFC there is increasing evidence that the mechanism(s) underlying learning and memory involve dendritic plasticity. In the hippocampus, which is critical to short-term memory, the acquisition of new memories in a conditioning paradigm is associated with increases in dendritic spine density in the CA1 region in adult male rats (Jedlicka et al., 2008; Leuner et al., 2003) and female rats (Beltran-Campos et al., 2011). In addition, evidence shows that existing spines undergo structural alterations that result in LTP (Jedlicka, et al., 2008; Leuner et al., 2003).
In over twenty years of study, it has been clearly demonstrated that estrogens increase dendritic spine density in many brain regions (See McEwen and Alves, 1999). In adult cycling female rats, fluctuations of approximately 30% occur in dendritic spine density on neurons in the ventromedial hypothalamic nucleus (VMN) (Frankfurt, et al.,1990) and pyramidal cells in the CA1 region of the hippocampus (CA1) (Woolley and McEwen, 1992; Smith et al., 2010; Velazquez-Zamora et al., 2012b). In both regions, dendritic spine density is greatest when estrogen levels are highest on proestrus. When adult female rats are Ovx, there is a decrease in dendritic spine density in the VMN (Frankfurt, et al., 1990), CA1 (Gould et al., 1990, Wallace et al., 2006) and the medial PFC (Wallace et al., 2006). Administration of estradiol to Ovx rats restores the levels of dendritic spines in the VMN (Calizo and Flanagon-Cato, 2003, Frankfurt, et al., 1990), CA1 (Gould et al., 1990; Smith et al., 2010) and the amygdala (de Castilhos et al., 2008; Rasio-Filha, et al., 2012). In mice, five days of estradiol treatment to Ovx subjects increased mushroom shaped spines in CA1 (Li et al., 2004). Similar results have been obtained in non-human primates for CA1 (Hajszan and Leranth, 2010) and the PFC, where estradiol has been shown to increase both dendritic spines and spine synapse density in monkeys (Hajszan et al., 2007a, 2007b; MacLusky et al., 2005).
Recent studies have shown that estrogens can rapidly, within minutes, increase spine density in the brain. Ovx rats received 20μg/kg 17β-estradiol and were sacrificed 30 min later for spine density measurements (Inagaki et al., 2012). In pyramidal cells in CA1 and the medial PFC, spine density increased 30 minutes after estradiol administration (Fig. 3A). In CA1, 17β-estradiol increased basal spine density by 29% compared to control but did not alter apical spine density. In the PFC, 17β-estradiol administration resulted in a 16% increase in apical and a 27% increase in basal spine density as compared to control after 30 minutes. In the rat arcuate nucleus, an area involved in reproductive function, administration of 50 μg of estradiol benzoate resulted in a large increase in dendritic spines after 4h that persisted through 48h (Christensen et al., 2011).
Figure 3. Acute estrogen treatments increase spine density and enhance place memory.
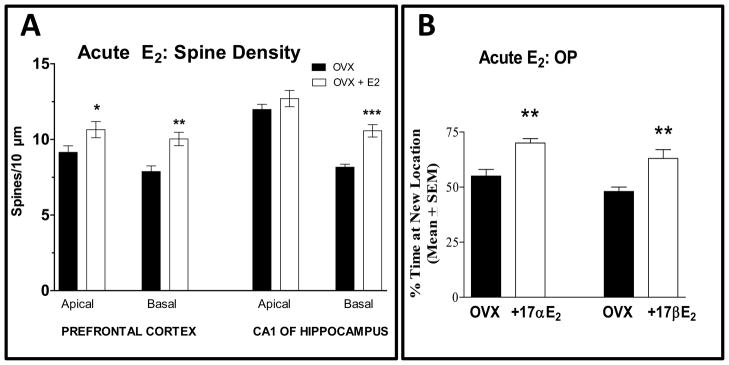
(A) Spine density is shown in areas of brain from Ovx rats at 30 min after treatment with corn oil (OVX) or 20 μg/kg of estradiol. Entries are mean ± SEM. ** p < 0.01, * p < 0.05. Data from Inagaki et al. (2012).
(B) Object Placement (OP) is shown after treatment with estrogens. The % time with the object at new location is plotted. Scores of 50% indicate chance exploration of old and new objects while a higher % indicates significant discrimination between objects in the old and new locations. OVX rats received a 3 min. sample trial followed by injection of corn oil (OVX), 5 μg/kg, 17α-E2 or 20 β-E2, and memory (recognition/retention trial) was tested 4 h later. ** p < 0.01. Data from Inagaki et al. (2012).
Consistent with these in vivo results, Srivastava (2012) demonstrated that administration of WAY-0700, an ERβ agonist, induced an increase in spinogenesis, as well as an increase in spine size within 30 minutes in cortical cell cultures. A concomitant increase in PSD-95 was also observed in these cells after 30 minute exposure to WAY-0700. Studies using hippocampal slice cultures confirmed that estradiol increases the expression of PSD-95 in the stratum lucidum of CA3 neurons and increases spine density at proximal sites of CA3 apical dendrites where PSD-95 was clustered on these spine heads (Sato et al., 2007). When cultures of Ammon’s horn neurons and DG neurons were used, estradiol increased the number of presynaptic sites in the cultures that contained DG neurons. The drug K252a, an inhibitor of the TrKB receptor, suppressed the estradiol dependent increase in BDNF release but the estrogen receptor antagonist did not. Overall, these data suggest that estradiol induces synaptogenesis between the mossy fibers and CA3 neurons by enhancing BDNF release from DG granule cells in an ER-independent manner. However, this result does not preclude action of estradiol on membrane receptors to alter BNDF release (see below). Thus, a broad range of in vivo and in vitro studies have shown that acute and chronic exposure, from min to months, to circulating estrogens are accompanied by alterations in dendritic spine density.
3. BDNF regulates spines
As part of a family of nerve growth factors which bind to TrKB and other receptors, both BDNF and TrKB have been shown to be important modulators of synaptic plasticity and are critical to the development of the CNS (Cohen-Cory et al., 2011; Poo, 2001). Experiments have also demonstrated that BDNF is required for the maintenance of dendritic spines in the adult CNS (Vigers, et al., 2012). In addition LTP, which requires alterations in spine strength, also requires BDNF (Kiprianova et al., 1999; reviewed by Santos et al., 2010). Plasticity of dendritic spines includes both the generation of “new” spines and the process of maturation from thin filipodial projections to more complex structures that can make synapses (reviewed by Ziv and Smith, 1996). This gradual process requires the mobilization of many proteins, especially the reorganization of actin (reviewed by Penzes and Rafalovich, 2012), which is highly concentrated in dendritic spines (Matus et al., 1982) and actin associated proteins which extend into the post synaptic density (reviewed by Fortin, et al., 2011).
Many experiments have shown that BDNF–TrKB signaling can influence dendritic spine and synapse density in cortical and hippocampal neurons (Cohen-Cory, 2010; Gottmann et al., 2009; Yoshii and Constantine-Paton, 2010). BDNF-TrKB signaling increases dendritic spine density on the apical dendrites of pyramidal cells in CA1 (Tyler and Pozzo-Miller 2001). Tyler and Pozzo-Miller (2003) also demonstrated that BDNF shifts the proportion of spine types towards the thin and mushroom-shaped spines in hippocampal slice cultures. However, in a recent study, Chapleau and Pozzo-Miller (2012) demonstrated that the TrkB inhibitor K-252a actually increased dendritic spine density in CA1 but that the spines were of the thin, immature highly unstable variety. The authors conclude that BDNF plays an important role in overall spine turnover or pruning of immature spines and that requires the activation of the TrkB, but not the p75NTR receptor.
How does BDNF increase dendritic spines? It appears that BDNF mediated effects on dendritic spines occur through both rapid membrane effects and slower transcriptional regulation via the CREB pathway (See Figure 2). Binding of BDNF to the TrKB receptor results in the activation of several pathways including the Ras/ERK (extracellular signal-regulated protein kinase), the PI3-K (phosphatidylinositol 3-kinase) and phospholipase C-g pathways (Kelly and Lynch, 2000; Horwood et al., 2006; see Santos et al., 2010 for review). Alonso et al. (2004) have shown that the increase in dendritic spine density by BDNF requires ERK activation after binding to the TrKB receptor.
Estrogen’s trophic effects on dendritic spine density and memory appear to be due to both membrane mediated and genomic effects (Figure 2). Although there are differences between the amygdala and other brain areas in terms of estrogen effects on dendritic spine density, a comprehensive review by Rasio-Filho et al. (2012), supports the idea that the neuroprotective effects of estrogen are mediated by the generation of CREB related gene products as well as estrogen induced alterations in signal transduction that result in manipulation of the cytoskeletal proteins involved in spine plasticity. Estrogen has been shown to stimulate the assembly of actin as well as associated proteins important to spine plasticity (Sandstrom and Williams, 2001; Srivastava, 2012). Using hippocampal slices, Kramar et al., (2009) found that 20 minute administration of estrogen promoted the assembly of actin by decreasing cofilin, a protein responsible for separating actin polymers. Further, estrogen effects on cofilin appear to be due to the activation of the RhoA pathway, and Ovx depressed RhoA activity and spine cytoskeletal plasticity. Administration of 10ug of estradiol benzoate increases CaMK IV in the amygdala (Zhou et al., 2001), which can phosphorylate CREB.
Thus, a great deal of data supports the idea that estrogen and BDNF can alter dendritic spine density through both rapid membrane mediated second messenger systems and via genomic mechanisms. It is likely that in some brain regions estrogen and BDNF converge to alter dendritic spines in the same cell and that they may amplify one another. However it is equally likely that there are differential effects of estrogen and BDNF on dendritic spines, especially given that BDNF is transported through nerve cells. Therefore another important question that remains is whether the effects of estrogen and BDNF are pre – or post synaptic.
4. Cognitive function – a role for estrogen acting through BDNF and spines?
a) Cognitive Function and estrogens
A substantial literature demonstrates that gonadal hormones, mainly estradiol, influence cognitive function during development, at adulthood and during aging in rodents (Dohanich, 2002; Frick, 2009; Luine, 2008; Luine et al., 2011, Luine and Frankfurt, 2012; Luine and Frankfurt, in press) and in humans (Sherwin, 2007). In rats, spatial memory tasks have been widely used in many cognitive studies, including those utilizing estradiol. These tasks test both learning and memory. Spatial memory tasks rely on rats making relationships between a reinforcement site and cues in the environment, hence their designation as spatial learning. The reinforcement could be food hidden in a cup (radial maze) or a submerged platform in a pool of water on which to escape (water maze), and cues are usually objects, posters and furniture in the testing room. Spatial memory depends on an intact hippocampus, but also relies on input from cortical sites including the prefrontal, entorhinal and perirhinal cortices (Broadbent et al., 2004; Ennaceur and Aggleton, 1994; Ennaceur et al., 1997). Other tasks such as object recognition and conditioned avoidance or tasks using visual or olfactory cues are more dependent on cortical areas and less dependent on the hippocampus and are commonly referred to as non-spatial memory tasks (Ennaceur et al., 1997). The results of research using radial arm mazes, water mazes, recognition memory tasks and avoidance tests all show that estradiol enhances cognition. In this review, we discuss estrogen’s effects on BDNF and spines in relation to both spatial and non-spatial memory.
Estrogen’s influence on learning and memory, similar to its reproductive effects, occurs through binding to classical nuclear receptors. There are two nuclear estrogen receptors, estrogen receptor alpha (ERβ) and estrogen receptor beta (ERβ), present in the brain with somewhat different distributions (Kuiper et al., 1997). Both receptors are ligand dependent transcription factors which act as nuclear transcription factors by binding to estrogen response elements (ERE) on DNA and stimulating transcription. Transcription culminates in expression of unique proteins and factors in many tissues including the brain (Fig. 2A). A wealth of data shows that ERβ mediates effects on memory but a role for ERβ cannot be discounted (Luine and Frankfurt, in press). Genomic actions in the nucleus result in long lasting and sustained effects on neural function. In adult females, genomic effects most likely underlie changes in neural functions that occur during the menstrual and estrous cycles, pregnancy, menopause and aging.
Receptor(s) for estrogens are also present outside of the cell nucleus in the membranes of neural cells as well as in peripheral estrogen target tissues (Blaustein, 1992; Milner et al, 2001; Towart et al, 2003 and Fig. 2B). Membrane receptors mediate rapid hormonal effects (sec to min) through signal transduction pathways (See Levin, 2002] for review of early work on the topic). Estrogens can also enhance memory through actions at membrane receptors (Inagaki et al., 2010; Inagaki et al, 2012; Luine and Frankfurt, 2012). The significance of rapid alterations in memory function by estradiol, which is the subject of a recent review (Luine and Frankfurt, in press), remains to be determined.
Thus, estrogen effects can be rapid and short-lived as well as delayed in onset and long lasting. Later in this review, we will consider both genomic and non-genomic mechanisms that may underlie estrogen’s effects on BDNF and spines and their involvement in cognitive function.
(b) Relation of BDNF and spines to estrogen dependent changes in cognition in adult females
As discussed in section 2, estradiol regulates BDNF levels and spine density in the hippocampus and medial PFC, areas critical for memory formation, and also influences memory. Thus, these neural functions vary with changes in estradiol levels. However, a major unanswered question remaining is whether these variations are causal or simply represent parts of the constellation of estradiol-induced changes in the brain with no relationship to one another. The next sections review the relationship(s) between these variables in adult female rats in an attempt to shed light on the question.
(1). Changes in memory, BDNF and spines over the estrous cycle and following estradiol treatments
The low dose estrogen treatment paradigm (Scharfman et al., 2007), which simulates the rise of estradiol on proestrus and also increases hippocampal BDNF, was used to assess estrogen effects on recognition memory. In Ovx subjects, estrogen treatment enhanced object recognition (Fig. 4A) and spatial memory in the object placement task (Fig. 4B). In both tasks, estrogen treatments did not increase initial exploration of objects by subjects, but treatments did increase the amount of time that rats spent exploring new objects or objects in new locations 2–4 h after the initial exploration trial. This result shows better memory in estrogen treated as compared to Ovx rats. Longer treatments, two days, with higher doses of estradiol, 50 ug/kg, also enhance object recognition and placement memory (Jacome et al., 2010), and Velazquez-Zamora et al., (2012a) showed that estradiol or selective estrogen agonists enhanced allocentric working memory in the Y-maze when given to Ovx rats 24 h earlier.
Figure 4. Physiological replacement of estrogen improves performance on object place and object recognition. Exploration of objects (sec) is shown during T1 sample trials and during T2 recognition/retention trials in Ovx rats given three vehicle injections (V-V-V) or three injections of estrogens (3–4–3, to simulate the proestrous rise of estradiol).
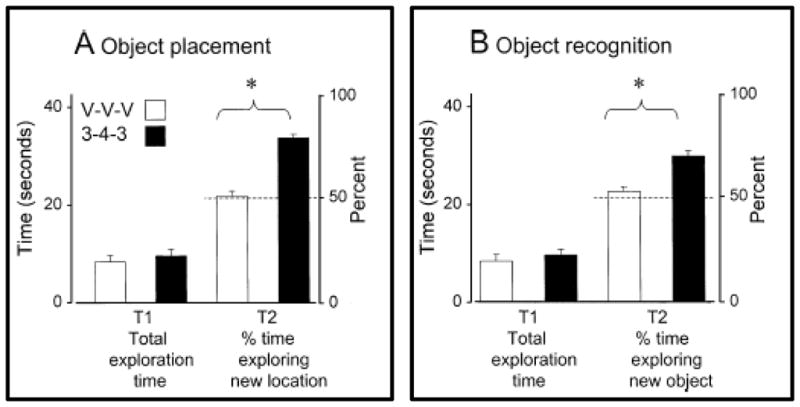
A. Object Placement - Estrogen treated rats did not spend more time exploring in T1 than vehicle treated rats but spent significantly more time exploring objects in new locations in T2 while vehicle treated rats did not, p < 0.05.
B. Object Recognition - Estrogen treated rats did not spend more time exploring in T1 than vehicle treated rats but spent significantly more time exploring objects in new locations in T2 while vehicle treated rats did not, p < 0.05. Figure from Scharfman et al, (2007); reprinted by permission from Blackwell Publishing, Ltd.
Estrogens appear to enhance memory following acute as well as chronic treatments. In a series of experiments, we gave various estrogens - estradiol, diethylstilbestrol and estrogen receptor specific agonists - immediately after a sample trial, and recognition memory (both object and place recognition) was tested 2–4 h later (Inagaki et al., 2010; Inagaki et al., 2012; Jacome et al.; 2010, Luine et al., 2003). As shown in Fig. 3B, both 17α-E2 and 17β-E2 enhanced object placement memory. If injections are delayed until 45 min after the sample trial, then subjects cannot significantly discriminate in recognition memory tasks (Inagaki et al., 2010). Thus, estradiol must be present during the memory consolidation phase in order to enhance memory. This time course for memory activation is similar to the time course for the increase in spines in the PFC and CA1 (Fig. 3A). The ability of both isomers of estradiol to enhance memory suggests estrogen action at membrane receptors because the dose of 17α-E2, 5 μg/kg, is too low to activate nuclear receptors.
The ability of female rats to perform memory tasks such as the water maze task (Frick and Berger-Sweeney, 2001), object placement (Frye et al., 2007) and object recognition (Walf et al., 2006) changes over the estrous cycle. In general, performance is best on late diestrus or proestrus when estrogen levels are high. We recently showed that object recognition fluctuates over the estrous cycle with subjects significantly discriminating between novel and old objects, i.e. remembering the old object, only on proestrus (Inagaki et al., 2012).
Thus, both estradiol treatments and high levels of estradiol on proestrus are associated with increases in hippocampal BDNF and enhancements in memory in rats. As indicated earlier, dendritic spines in a number of brain areas are increased on proestrus and following estradiol treatments. In addition, Li et al. (2004) reported that 1 ug/day of estradiol for 5 days, the same dose of estradiol that increases spine density in CA1, also enhances place memory, and Velazquez-Zamora et al. (2012a) showed that estradiol increased spine density in layer III of the PFC at the same dose which enhanced allocentric working memory in the Y-maze. However, in previous experiments spine density in the memory-tested subjects after chronic estrogen treatments was not assessed. Therefore, we determined whether two days of estradiol treatment to OVX rats results in enhanced spatial memory and increased spine density in brain regions as compared to Ovx rats. Given that learning or memory might alter spine density, we included a non-behaviorally tested group as well. Ovx rats received vehicle or estradiol for two days and behavioral tests for object placement or only vehicle and estradiol treatments. Fifty-two hours after the last estradiol injection and immediately following object placement testing, subjects were sacrificed (Non-behavior subjects were sacrificed at the same time after estradiol or vehicle injection). Fig 5A shows spine density and Fig 5B shows object placement tests in the subjects. For spine density, statistical analyses by ANOVA indicated significant main effects of area and treatment but not behavior. Therefore, spine density for the behavior and non-behavior groups was combined in Fig 5. Consistent with previous studies in non-behaviorally tested subjects (Frankfurt et al., 1990; Gould et al., 1990) estrogen increased spine density in basal, but not apical, dendrites of CA1 and in the VMN of the hypothalamus of estrogen treated Ovx rats. No changes in either dendritic tree of PFC pyramidal neurons were seen following estradiol treatment. The estrogen treated Ovx subjects showed better object placement memory than vehicle treated subjects (Fig 5B). The observation that spine density was not altered in PFC after estradiol treatment suggests that estrogen induced enhanced spatial memory may not rely on changes in spine density in this area. Another possibility is that the total number of spines may not change but that the spine type may be altered. However, we have found that object memory is impaired and PFC spine density is decreased 7 weeks after Ovx (Wallace et al., 2006 and see next paragraph). Taking together results from the current study with the previous Ovx study suggests that spine density in the PFC maybe related to long term changes in memory such as after OVX rather than after a few days of altered estradiol levels. In contrast, spines in CA1 may be important for both short and long term changes in memory induced by estradiol. The increased spine density observed in the VMN after estradiol treatment is related to estradiol’s well-documented maintenance of reproductive function (Frankfurt et al., 1990).
Figure 5. Chronic estrogen treatment increases spine density and enhance place memory.
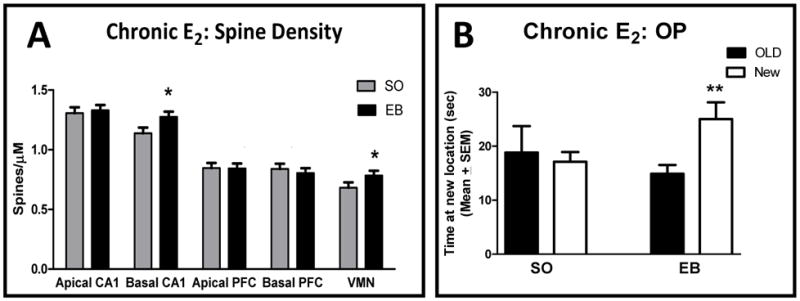
(A) Spine density is shown in areas of brain from Ovx rats that received vehicle or estradiol (50 ug/kg) for two days and behavioral tests for object placement or only vehicle and estradiol treatments. Fifty-two hours after the last estradiol injection and immediately following object placement testing, subjects were sacrificed. Non-behavior subjects were sacrificed at the same time after estradiol or vehicle injection. As ANOVA showed no effect of behavior (F1,23= 0.10, p= 0.75), the treated only and treated + behavior groups were combined in the graph. * p < 0.05. Data from Monde, Frankfurt and Luine, unpublished.
(B) Object placement data for subjects in (A) is shown. The time spent with objects at the old (solid bars) and new (open bars) locations is shown. ** p < 0.01. Data from Monde, Frankfurt and Luine, unpublished.
Consistent with the neural and behavioral observations described in cycling or hormone treated females, Ovx (which lowers circulating levels of estrogens) impairs memory and decreases spine density and BDNF levels as compared to gonadally intact rats. For example, we assessed the effects of Ovx on object recognition, object placement and spine density in the PFC and hippocampal sub-regions (Wallace et al., 2006). Prior to Ovx, rats could significantly discriminate between old and new objects and between objects in new and old locations when tested 4 hours after first viewing the objects. One week after surgery, the Ovx group showed impaired object recognition memory, i.e. were unable to discriminate between old and new objects while gonadally intact females continued to discriminate (Wallace et al., 2006). In contrast, place memory was not affected, but by four weeks post-Ovx, rats could not significantly discriminate between old and new locations. These results suggest recognition memory may be more sensitive to ovarian steroid effects than spatial memory since performance of object recognition was lost faster after Ovx. The subjects were sacrificed 7 weeks post Ovx and brain morphology was analyzed following Golgi impregnation. In the PFC and CA1 (but not CA3), Ovx females had lower spine density in both apical and basal dendrites than intact rats, ranging from 17% decreases in apical CA1 to 53% decreases in apical PFC. Thus, impaired memory in the Ovx subjects, as compared to the gonadally intact subjects, was associated with lower spine density in the hippocampus and PFC. A time course documenting loss in spine densities after Ovx would provide further information as to the relationship between estrogens, BDNF and spine density. Similar effects on spine density have been reported by Beltran-Campos et al (2011) who found that CA1 apical dendrite spines were 55% lower in Ovx as compared to intact rats. Moreover, the Ovx rats were impaired in learning the location of the platform in the Morris Water Maze spatial memory task. Also consistent with these observations, phytoestrogens, present in soy meal and used in laboratory rat chow, influence spine density in the PFC and hippocampus and memory. Ovx rats fed a high phytoestrogen diet for two months had greater spine density in CA1 and the medial PFC, 48% and 20% higher respectively, than rats on the low phytoestrogen diet (Luine et al., 2006). Rats receiving the high phytoestrogen diet also performed better on object placement but not object recognition. As indicated in section 2(a), Ovx decreases BDNF, and estradiol or phytoestrogens increase BDNF.
In conclusion, there is a strong association between circulating estradiol levels, BDNF levels and spine density in the hippocampus and memory: when estrogen levels are high (proestrous day of estrous cycle), when estrogen is given to Ovx rats, or when gonadally intact rats are compared to Ovx rats, BDNF levels and spine density are higher, and spatial and non-spatial memory is enhanced. The relationship between changes in spines and memory is less clear in the PFC. Long, but not short, term changes in circulating estradiol lead to similar changes in BDNF, spines and memory. Yet, estradiol induced changes in PFC BDNF levels show the same temporal relationship as the spine changes. Two days of estradiol did not alter BDNF mRNA or protein in the PFC (Gibbs, 1999) but an eight week treatment of estradiol or phytoestrogens increased PFC BDNF levels (Pan et al., 2010). Likewise, two days of EB treatment did not alter spines in the PFC (Figure 5), but long-term phytoestrogen treatment increased spine density (Luine et al, 2006). Estrogens also acutely enhance PFC spines and recognition memory (Figure 3; Inagaki et al, 2010; 2012), but PFC BDNF has not been measured under these conditions. Nonetheless, because short-term EB treatments enhance recognition memory without changes in spines or BDNF, it is difficult to ascribe a direct role for PFC BDNF or spines in enhancing memory. Because the PFC has received much less investigation than the hippocampus in relation to these variables, future experiments may provide a better understanding of whether BDNF and spines in this area contribute to all or only some aspects of estrogen’s enhancement in recognition memory.
(2) Changes in estradiol, memory, BDNF and spines over the female life cycle
At the end of pregnancy, circulating estrogens rise and remain at the highest levels exhibited over the lifespan of females; estrogens remain elevated for many days following parturition (Watson et al., 2010). Dams have higher levels of dendritic spines in both apical and basal branches of pyramidal cells in CA1 and the medial PFC, 20 days after parturition, when compared to virgin females (Kinsley, 2006). Toward the end of pregnancy, gestation days 16–18, dams also show enhanced object placement memory as compared to non-pregnant females (Macbeth, et al., 2008a). Levels of BDNF have not been measured in pregnant rats, but both BDNF and recognition memory have been compared in multiparous females (underwent 5–6 pregnancies and rearing bouts) and nulliparous females (virgins) (Macbeth et al., 2008b). Multiparous females had better object and place memory than nulliparous females. In addition, BDNF levels were approximately two times higher in CA1 and septum of the multiparous as compared to the nulliparous females. No changes were found in CA3 or DG, and PFC was not measured. Thus, pregnant and repeatedly pregnant rats show a convergence of elevated estradiol, dendritic spines, and BDNF along with enhanced recognition memory.
With advanced age, females (and males) show a decline in most aspects of cognitive function (See Frick, 2009 for review) and a convergence of changes in properties previously discussed including reduced circulating estradiol, lowered levels of BDNF and decreased dendritic spine density. Specifically, estrous cyclicity is lost beginning around 12 months of age, and estradiol gradually declines to approximately 20% of the level in proestrus rats at 21 months of age (Luine et al, 2011). Compared to young adult rats (2 – 4 months old), object (Wallace et al, 2007) and place memory (Luine et al, 2011) is impaired, and spine densities are reduced by 16% in apical, but not basal, dendrites in both CA1 and PFC; density was not altered in CA3 (Wallace et al, 2007; Luine et al, 2011). In addition, BDNF is decreased in the hippocampus and frontal cortex in 24 month old as compared to 4 month old rats (Bimonte-Nelson et al, 2008).
Middle aged rats (12–14 months of age) are often used as a model of aging in order to explore the effectiveness and the mechanisms of various hormone replacement regimens for menopause, and these results are pertinent for possible BDNF changes. Kiss et al., (2012) compared the effects of estradiol treatment for 18 days to 3, 7 and 12 month old Ovx rats. Spatial memory on the Morris water maze and hippocampal BDNF was increased in all age groups by the estradiol treatment. Premarin was given to 13 month old rats for 16 days, and enhanced memory on the water radial arm maze was reported (Engler-Chiurazzi et al., 2011). While Premarin increased BDNF in the perirhinal cortex, no changes were found in the prefrontal cortex or hippocampus. Kramar et al. (2012) reported that BDNF, applied acutely to the hippocampus, or an ampakine, given for 4 days to elevate hippocampal BDNF levels, restored LTP and actin polymerization in middle aged Ovx rats. Thus, beginning at middle age and extending through old age, comprised hippocampal memory functions appear related to declines in both spines and BDNF in the hippocampus and PFC.
5. Are E and BDNF additive, synergistic, or not convergent in enhancing memory?
As shown schematically in Fig. 2B, both BDNF and estrogen alter proteins involved in spine assembly and maturation. BDNF effects are mediated via TrKB receptors while estrogen effects are mediated by membrane and intracellular ERα and ERβ receptors. Both estrogen (Scharfman and MacLusky, 2006; Zhou et al, 2005) and BDNF activate CREB (Poo, 1999; Raisa-Filho, 2012; Yoshii and Constantine-Paton, 2010). Thus, estrogens and BDNF could be additive or synergistic in increasing spines and memory function through a common action on CREB. In addition, there is increasing data that estrogen’s trophic effects on dendritic spines may be mediated by an increase in BDNF (Fig. 2A) which then causes increases in spines and memory. Administration of 10μg of 17β-estradiol benzoate or vehicle to Ovx rats for 2 weeks increased the levels of BDNF, CaMK IV and pCREB in the CA1 and CA3 regions of the hippocampus (Zhou, et al, 2005). Comparison of the effects of a single 5μg dose of estradiol benzoate for 6h and 2 doses given for 48h reveals that there are differential effects on proteins implicated in synaptic plasticity (Spencer-Segal et al., 2012). Six hours after estradiol administration there was an increase in pAKT in the hippocampus, but it took 48h for TrKB to be increased. Since spines are increased within 1–4h of estrogen administration (Christianson et al., 2011; Inagaki et al., 2012;), the change in TrKB levels maybe too late to mediate spine changes. On the other hand, increases in BDNF could alone intensify estrogen’s effects. Both of these effects were eliminated in estrogen receptor alpha and beta knockout mice indicating that both receptors are at least active in the modulation of estrogen induced synaptic (Spencer-Segal et al., 2012). Estradiol increases the expression of BDNF in the developing cerebellum and promotes spinogenesis in purkinje cells (Haraguchi et al., 2012). This effect is blocked by tamoxifen and is decreased in cytochrome P450 aromatase knock-out mice which have decreased estradiol levels. The increased expression of TrKB receptor by estradiol in cultured hypothalamic neurons is blocked in cultures in which TrKB induction is blocked with an TrKB antisense nucleotide (Carrer, et al., 2003). In addition, immune-blotting of E2-treated cultures showed increased levels of phosphorylated ERK1 and ERK2, suggesting MAPKs involvement in the estrogen induced increase in TrKB. It is important to consider that estrogen dependent increases in memory (Inagaki et al., 2010) and spine synapses (MacLusky et al., 2005) are extremely sensitive to estrogen levels and exhibit a sharp inverted U shaped dose-response curve. Thus, dose and timing appear to influence the mechanism of estrogen action and therefore may be responsible for some of the apparent differences found in the expression of spines and BDNF in response to estrogen.
Overall, the exact mechanism(s) for enhancements of dendritic spines and memory by estrogens and BDNF are unclear. Further research to delineate the mechanisms is important because cognitive function declines with normal aging and is precipitously comprised in neurodegenerative diseases. Current treatments for enhancing cognition are largely ineffective; thus, new strategies based on estrogen, BDNF or, more likely, their combination might provide better therapeutic outcomes.
Highlights.
Estrogens increase BDNF levels, dendritic spine densities and enhance memory
BDNF increases spine densities and enhances memory
Reproductive and age related memory changes are associated with altered estradiol, BDNF and spines
Estrogens and BDNF may work in concert to enhance memory
Acknowledgments
Supported by NIH Grants MD007599 from NIMHD, UL1-RR024996 from CTSC and a CUNY Collaborative grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–8. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Campos V, Pado-Alcala RA, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte GL, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Research. 2011;1369:119–130. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Granholm AC, Nelson ME, Moore AB. Patterns of neurotrophin protein levels in male and female Fischer 344 rats from adulthood to senescence: how young is “young” and how old is “old”? Exp Aging Res. 2008;34:13–26. doi: 10.1080/03610730701761908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen- induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–63. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–12. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Balancing structure and function at hippocampal dendriticspines. Ann Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Flanagon-Cato LM. Hormonal-neural integration in the female rat ventromedial hypothalamus: triple labeling for estrogen receptor-alpha, retrograde tract tracing from the periaqueductal gray, and mating-induced Fos expression. Endocrinology. 2003;144:5430–40. doi: 10.1210/en.2003-0331. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neurosci. doi: 10.1016/j.neuroscience.2012.10.073. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer HF, Cambiasso MJ, Brito V, Gorosito S. Neurotrophic factors and estradiol interact to control axogenic growth in hypothalamic neurons. Ann N Y Acad Sci. 2003;1007:306–16. doi: 10.1196/annals.1286.029. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Pozzo-Miller L. Divergent roles of p75NTR and Trk receptors in BDNF’s effects on dendritic spine density and morphology. Neural Plast. 2012;2012:578057. doi: 10.1155/2012/578057. Epub 2012 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-Initiated Estradiol Signaling Induces Spinogenesis Required for Female Sexual Receptivity. J Neurosci. 2011;30:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93:415–21. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–88. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castilhos J, Forti CD, Achaval M, Rasia-Filho AA. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: a Golgi study. Brain Res. 2008;1240:73–81. doi: 10.1016/j.brainres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RI, editors. Hormones, brain and behavior. San Diego: Academic Press; 2002. pp. 265–327. [Google Scholar]
- Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLos One. 2012;7(4):e35217. doi: 10.1371/journal.pone.0035217. Epub 2012 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32:680–97. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Srivastava T, Soderling TR. Structural modulation of dendritic spines during synaptic plasticity. Neuroscientist. 2011;50:496–501. doi: 10.1177/1073858411407206. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51:530–5. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Wang HY, Marmolejo N, Bakshi K, Friedman E. Prenatal cocaine increases dendritic spine density in cortical and subcortical brain regions of the rat. Dev Neurosci. 2009;31:71–75. doi: 10.1159/000207495. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–37. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CA, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. Treatment with estrogen and progesterone affects relative levels of brain derived neurotrophic factor mRNA and protein in different regions of the adult brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–34. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Sasahara K, Shikimi H, Honda S, Harada N, Tsutsui K. Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. Cerebellum. 2012;11(2):416–7. doi: 10.1007/s12311-011-0342-6. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol. 2010;31:519–30. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology. 2007a;148:1963–7. doi: 10.1210/en.2006-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 2007b;53:638–46. doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–84. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol a in adult female rats: role of dendritic spines. Endocrinology. 2012;153:335767. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute Estrogen Treatment Facilitates Recognition Memory Consolidation and Alters Monoamine levels in Memory-related Brain Areas. Hormones & Behavior. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiology of learning and memory. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behav Brain Res. 2008;192:12–9. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Estrogen enhances retrograde transport of brain-derived neurotrophic factor in the rodent forebrain. Endocrinology. 2003;144:5022–9. doi: 10.1210/en.2003-0724. [DOI] [PubMed] [Google Scholar]
- Kelly A, Lynch MA. Long-term potentiation in dentate gyrus of the rat is inhibited by the phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology. 2000;39:643–51. doi: 10.1016/s0028-3908(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kiprianova I, Sandkühler J, Schwab S, Hoyer S, Spranger M. Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol. 1999;159:511–9. doi: 10.1006/exnr.1999.7109. [DOI] [PubMed] [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–8. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging. 2012;33:708–19. doi: 10.1016/j.neurobiolaging.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Rex CS, Gall CM, Lynch G. Estrogen’s Place in the Family of Synaptic Modulators. Mol Cell Pharmacol. 2009;5:258–262. [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduta J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory tasks in female mice. PNAS. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Dai FR, Du XP, Yang QD, Zhang X, Chen Y. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behavioural Brain Research. 2012;231:146–153. doi: 10.1016/j.bbr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Luine V. Neuroendocrinology of memory and cognition. In: Lajtha Abel, Blaustein Jeff D., editors. Handbook of Neurochemistry and Molecular Neurobiology. 3. Berlin: Springer; 2006. pp. 775–800. Behavioral Neurochemistry and Neuroendocrinology. [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinology. 2008;20:866–72. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex ofovariectomized rats. Brain Research. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. An Integrative Review of Estradiol Effects on Dendritic Spines and Memory over the Lifespan. In: Kahn Scott M., editor. Sex Steroid. Intech Open Access Publishing; 2012. http://www.intechopen.com/articles/show/title/an-integrative-review-ofestrogeneffectson-memory-and-dendritic-spines-over-the-lifespan. [Google Scholar]
- Luine V, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and spine density changes. Front Neuroendocrinology. doi: 10.1016/j.yfrne.2012.07.004. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Wallace ME, Frankfurt M. Age-related deficits in spatial memory and hippocampal spines in virgin, female Fischer 344 rats. Curr Gerontol Geriatr Res. 2011;2011:Article ID 316386. doi: 10.1155/2011/316386. 7 pages, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Gautreaux C, Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. 2008a;1241:136–47. doi: 10.1016/j.brainres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, MacLusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Hormones & Behavior. 2008b;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environmental health perspectives. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A, Ackerman M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. PNAS. 1982;79:7590–4. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Pan Y, Anthony M, Clarkson TB. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neurosci Letts. 1999;261:17–20. doi: 10.1016/s0304-3940(98)00994-x. [DOI] [PubMed] [Google Scholar]
- Pan M, Li Z, Yeung V, Xu RJ. Dietary supplementation of soy germ phytoestrogens or estradiol improves spatial memory performance and increases gene expression of BDNF, TrkB receptor and synaptic factors in ovariectomized rats. Nutr Metab (Lond) 2010;7:75–83. doi: 10.1186/1743-7075-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Rafalovich I. Regulation of the Actin Cytoskeleton in Dendritic Spines. Advances in Experimental Medicine and Biolog. 2012;970:81–95. doi: 10.1007/978-3-7091-0932-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Dalpian F, Menezes IC, Brusco J, Moreira JE, Cohen RS. Dendritic spines of the medial amygdala: plasticity, density, shape, and subcellular modulation by sex steroids. Histol Histopathol. 2012;8:985–1011. doi: 10.14670/HH-27.985. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Santos AR, Comprido D, Duarte CB. Regulation of local translation at the synapse by BDNF. Prog Neurobiol. 2010;92:505–16. doi: 10.1016/j.pneurobio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–20. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J Steroid Biochem Mol Biol. 2007;106:151–6. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–4. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen’s ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA. 2010;107:19543–8. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–4. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–9. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–19. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–46. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP. Two-Step Wiring Plasticity - A mechanism for estrogen-induced rewiring of cortical circuits. J Steroid Biochem Mol Biol. 2012;131:17–23. doi: 10.1016/j.jsbmb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–58. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553(Pt 2):497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska M, Swiech L, Jaworski J. Developmental plasticity of the dendritic compartment: focus on the cytoskeleton. Adv Exp Med Biol. 2012;970:265–84. doi: 10.1007/978-3-7091-0932-8_12. [DOI] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, Garcia-Segura LM, González-Burgos I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm Behav. 2012a;61:512–7. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, González-Tapia D, González-Ramírez MM, Flores-Soto ME, Vázquez-Valls E, Cervantes M, González-Burgos I. Plastic changes in dendritic spines of hippocampal CA1 pyramidal neurons from ovariectomized rats after estradiol treatment. Brain Res. 2012b;1470:1–10. doi: 10.1016/j.brainres.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellano A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in hippocampus and prefrontal cortex. Brain Research. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010;2:153–66. doi: 10.2147/ijwh.s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–22. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cohen RS, Pandey CS. Estrogen affects the expression of Ca2+/calmodulin- dependent protein kinase IV in amygdala. Neuroreport. 2001;12:2987–90. doi: 10.1097/00001756-200109170-00046. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role in dendritic filopodia in synaptogensis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]


