Abstract
The prevalence of overweight and obesity in women has increased during the last decades. This is a serious concern since a high BMI before conception is an independent risk factor for many adverse outcomes of pregnancy. Therefore, dietary counseling, intended to stimulate weight loss in overweight and obese women prior to conception has recently been recommended. However, dieting with the purpose to lose weight may involve health risks for mother and offspring. We conducted a systematic literature review to identify papers investigating the effects of weight loss due to dietary interventions before conception. The objective of this study is to assess the effect of weight loss prior to conception in overweight or obese women on a number of health-related outcomes in mother and offspring using studies published between January 2000 and December 2011. Our first literature search produced 486 citations and, based on predefined eligibility criteria, 58 were selected and ordered in full text. Two group members read each paper. Fifteen studies were selected for quality assessment and two of them were considered appropriate for inclusion in evidence tables. A complementary search identified 168 citations with four papers being ordered in full text. The two selected studies provided data for overweight and obese women. One showed a positive effect of weight loss before pregnancy on the risk of gestational diabetes and one demonstrated a reduced risk for large-for-gestational-age infants in women with a BMI above 25 who lost weight before pregnancy. No study investigated the effect of weight loss due to a dietary intervention before conception. There is a lack of studies on overweight and obese women investigating the effect of dietary-induced weight loss prior to conception on health-related variables in mother and offspring. Such studies are probably lacking since they are difficult to conduct. Therefore, alternative strategies to control the body weight of girls and women of reproductive age are needed.
Keywords: gestational diabetes, large-for-gestational-age-infants, systematic review, weight loss before pregnancy
The optimal body weight of pregnant women has been an issue of much debate over the years. It has long been recognized that underweight women tend to deliver small infants and a low birth weight is well known to be associated with increased mortality and morbidity in children (1). Recommendations regarding weight gain during pregnancy have also been given for a long time. For example, an American textbook on obstetrics (2) stated in 1966 that ‘Excessive weight gain in pregnancy is highly undesirable for several reasons; it is essential to curtail the increment in gain to 12.5 kg at most or preferably 6.8 kg’. However, this policy of severe weight restriction during pregnancy was challenged already in the 1960s when it was realized that such a restriction is associated with an increased risk for low birth weight infants and consequently with several health problems in the offspring (3).
In 1990, the Institute of Medicine (IOM) of the National Academy of Science in the United States published a report on weight gain during pregnancy where such recommendations were based on the prepregnant BMI of the woman (4). It was recommended that lean and underweight women gain more weight than normal weight women and those were in turn recommended to gain more weight than overweight or obese women. The IOM report of 1990 (4) thus implemented the important fact that the preconceptional nutritional situation of a woman is important for her nutritional requirements during pregnancy.
The prevalence of overweight and obesity in women of reproductive age has increased considerably during the last decades. For example, in Sweden this figure increased from 25 to 36% between 1992 and 2001 in pregnant women (5). This is a serious concern since a high BMI before pregnancy confers an increased risk of maternal and perinatal complications, including preeclampsia, gestational diabetes, caesarean delivery, large-for-gestational-age-infants, stillbirth and possibly an increased risk for overweight and obesity later in life in the offspring (5–7).
In 2009, IOM revisited their recommendations for pregnancy weight gain (Table 1) (6). The following statement was an important addition to their guidelines: ‘All women should start pregnancy with a healthy body weight’. A BMI within the range of normal BMI values (18.5–24.9) is considered to be a healthy body weight. This recommendation was made since ‘evidence from the literature is remarkably clear that prepregnancy BMI is an independent predictor of many adverse outcomes of pregnancy’ (6, 7). In fact, it currently appears that, for obese women, prepregnancy BMI is more associated with an increased risk of preeclampsia, gestational diabetes mellitus, and the delivery of a large-for-gestational-age (LGA) infant than is gestational weight gain (8). The recent IOM report emphasized that the full implementation of their guidelines would mean: ‘Offering preconceptional services, such as counseling on diet and physical activity as well as access to contraception, to all overweight or obese women to help them reach a healthy weight before conceiving’ (6).
Table 1.
Weight gain during pregnancy as recommended by the Institute of Medicine 2009 (6)
| BMI (kg/m2) before conception | Recommended weight gain (kg) |
|---|---|
| <18.5 (underweight) | 12.5–18 |
| 18.5–24.9 (normal weight) | 11.5–16 |
| 25.0–29.9 (overweight) | 7–11.5 |
| >30.0 (obesity) | 5–9 |
A recently published systematic review demonstrated positive effects for mother and offspring as a result of weight reductions during pregnancy (9). Furthermore, the recent IOM report (6) presents evidence that weight loss prior to conception is associated with improved reproductive outcomes for obese women undergoing bariatric surgery (10, 11). However, no studies regarding the effect of weight loss as a result of interventions including dietary manipulations and implemented prior to conception in overweight and/or obese women were citied. This systematic literature review was conducted to identify published papers describing such studies.
Research question
The original research question was: Is there scientific evidence for positive health effects of weight loss prior to conception for overweight and obese women? Potential outcomes: weight and length of infants at birth, macrosomia, length of gestation/prematurity, malformations, stillbirth, childhood obesity/BMI, obstetric risk, preeclampsia, postpartum weight retention, gestational diabetes mellitus, hypertension, postpartum depression, lactation and lactation duration, infant growth. The strategy used to find literature relevant for this research question is shown in Table 2. Two databases (PubMed and Swe Med) were searched.
Table 2.
Search strategy for ‘Research Question’
| (‘weight loss’[All Fields] OR ‘weight management’[All Fields] OR ‘weight counseling’[All Fields] OR ‘pre-pregnancy body mass index’[All Fields] OR ‘obesity intervention’[All Fields] OR ‘following bariatric surgery’[All Fields]) AND (‘pregnancy’[All Fields] OR ‘fertilization’[All Fields] OR ‘conception’[All Fields] OR ‘infertility’[All Fields] OR ‘fertility’[All Fields]) AND (‘infant, newborn’[All Fields] OR ‘fetal macrosomia’[All Fields] OR ‘pregnancy’[All Fields] OR ‘congenital abnormalities’[All Fields] OR ‘stillbirth’[All Fields] OR ‘pre-eclampsia’[All Fields] OR ‘diabetes, gestational’[All Fields] OR ‘hypertension, pregnancy-induced’[All Fields] OR ‘depression, postpartum’[All Fields] OR ‘lactation’[All Fields] OR ‘breast feeding’[All Fields] OR ‘abortion, spontaneous’[All Fields] OR ‘bariatrics’[All Fields] OR ‘infant, low birth weight’[All Fields] OR ‘infant, very low birth weight’[All Fields] OR ‘Obstetric Risk’[All Fields] OR ‘Weight Management’[All Fields] OR ‘Obesity Intervention’[All Fields]) AND (‘2000/01/01’[PDat]: ‘2010/07/15’[PDat]) AND (‘Humans’[MH] OR Human*[TIAB]) |
Literature search
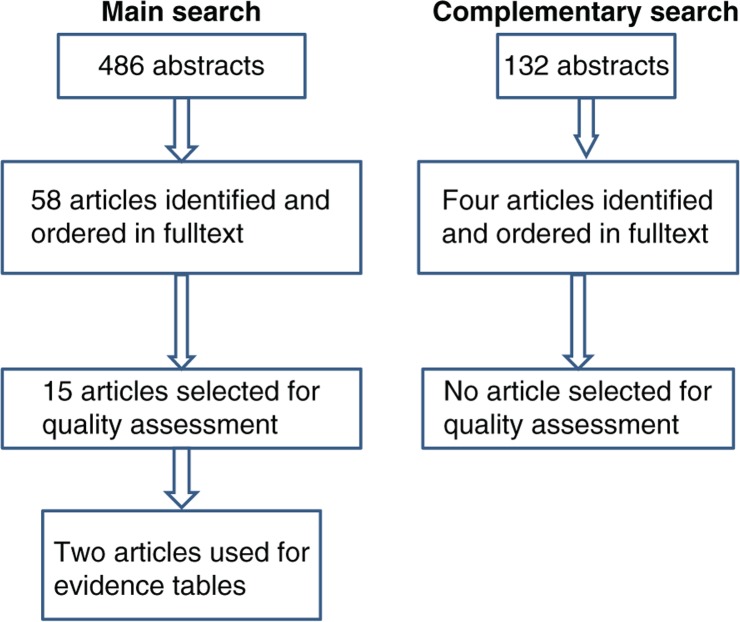
The literature search is described in Fig. 1. The main search was conducted in November 2010, covering articles published between January 1, 2000, and July 15, 2010, and identifying 486 abstracts. These articles were read by three members of the pregnancy-and-lactation-group. EF read all abstracts while IT and AS each read 50% of the abstracts. Thus, two persons read all abstracts. Abstracts were identified according to the following criteria: obesity and overweight before pregnancy (or between pregnancies) and change in body weight before pregnancy and any kind of health-related outcome including intervention trials (>1 month) but excluding weight loss by surgery. In this way, 58 articles (5, 12–68) were identified and ordered in full text. Two members of the group read each of the 58 articles and if at least one member considered an article appropriate, it was selected for quality assessment. Review articles were excluded but otherwise inclusion criteria were the same as those used to identify abstracts. Reasons for excluding 43 of the articles (12–54) are shown in the appendix. Thus, this procedure resulted in 15 articles for quality assessment (5, 55–68).
Fig. 1.
Description of literature search, including main and complementary search.
Final selection of articles and quality assessment
The 15 articles were distributed between the five group members. Each member read 5–7 articles and two members, who also carried out the quality assessment of their articles, read all articles. Among the 15 selected articles 11 (56, 58, 59, 61–68) were found not to be relevant while four (5, 55, 57, 60) were considered relevant and of sufficient quality for inclusion in evidence tables. However, one of those (57) investigated the relationship between a reduction in BMI and a preterm birth but involved mainly low-to-normal-weight women and was thus not considered relevant for our review. Another (55) was a review emphasizing the lack of relevant studies for our particular research question. Thus, two studies, Villamor and Cnattingius (5) and Glazer et al. (60) were used in evidence tables providing data for two outcomes, i.e. risk of gestational diabetes and risk of LGA infants (Table 3). To assess and rate the quality of the included studies, we applied a three-category (A–B–C) grading system based on the NNR AMSTAR quality assessment tool (QAT).
Table 3.
Table for evidence grading: risk of gestational diabetes and risk of delivering a large-for-gestational-age infant
| Reference details | Glazer N et al. (60), USA | Villamor and Cnattingius (5), Sweden | Villamor and Cnattingius (5), Sweden |
|---|---|---|---|
| Study design | Prospective cohort study | Prospective cohort study | Prospective cohort study |
| Population/subject characteristics | Obese women (heavier than 200 lbs or 90.7 kg) of mixed ethnicity with 2 singleton births who were nondiabetic at the first pregnancy | Women in Sweden giving birth to two consecutive singletons between 1992 and 2001 | Women in Sweden giving birth to two consecutive singletons between 1992 and 2001 |
| No of subjects analysed | 4,012 | 313 (from 151,025) for risk of gestational diabetes | 2,350 (from 151,025) for risk of delivering a large-for-gestational-age infant |
| Outcome measures | Risk of gestational diabetes at the second pregnancy | Risk of gestational diabetes at the second pregnancy | Risk of delivering a large-for gestational-age (LGA) infant at the second pregnancy |
| Exposure | Prepregnancy weight at an index pregnancy minus the corresponding weight at the previous pregnancy | Difference between the two pregnancies with respect to BMI recorded at the first antenatal visit | Difference between the two pregnancies with respect to BMI recorded at the first antenatal visit |
| Follow-up period, drop-out rate | Nine-months follow-up, no drop-outs | Nine-months follow-up, no drop-outs | Nine-months follow-up, no drop-outs |
| Dietary assessment method | No dietary assessment | No dietary assessment | No dietary assessment |
| Results | Women who lost at least 10 lbs (4.54 kg) between pregnancies had a decreased risk of gestational diabetes relative to women who lost less weight during this period (relative risk=0.63, 95% CI, 0.38–1.02) | Overweight and obese women who decreased their BMI more than one unit between pregnancies had no significant reduction in the risk of gestational diabetes (OR 0.96, 95% CI, 0.66–1.37) | Overweight and obese women who decreased their BMI more than one BMI-unit between pregnancies had a significant reduction in the risk of giving birth to a LGA-infant (OR 0.82, 95% CI, 0.72–0.95) |
| Confounders adjusted for | Age and weight gain during each pregnancy | Height, interpregnancy interval, age, country of origin, years of education, year of delivery and smoking | Height, interpregnancy interval, age, country of origin, years of education, year of delivery and smoking |
| Study quality and relevance | Study quality: B. The study is not quite relevant since there is no information that the women received dietary advice and we do not know why they lost weight | Study quality: A. The study is not quite relevant since there is no information that the women received dietary advice and we do not know why they lost weight | Study quality: A. The study is not quite relevant since there is no information that the women received dietary advice and we do not know why they lost weight |
Complementary search
At the end of January 2012, a complementary search (Fig. 1) was conducted covering the period between July 15, 2010, and the end of December 2011. The same search string and databases were used as in the main search. The complementary search resulted in 132 abstracts. These were read by two members of the group (EF and IT) and resulted in four articles (69–72) being ordered in full text. None of them were selected for QAT.
Results
Glazer et al. (60) provided evidence for a positive effect of weight loss (at least 10 lbs or 4.54 kg) between pregnancies on the risk of gestational diabetes during the subsequent pregnancy. Such an effect was not demonstrated by Villamor and Cnattingius (5) possibly because the women in their study weighed less and lost less weight than the women in the study by Glazer et al. (60) (Table 3). It is of interest to note, however, that the former study (5) demonstrated clearly that weight gain between pregnancies is associated with adverse health effects in mothers as well as in infants also when it occurs in normal-weight women. Furthermore, the study by Ehrlich et al. (70) confirms the findings by Glazer et al. (60) that weight loss between pregnancies, in obese and overweight women, has a positive effect on the risk for gestational diabetes in the subsequent pregnancy. Furthermore, the study by Villamor and Cnattingius (5) demonstrated a reduced risk for LGA infants in women with a BMI above 25 who lost weight equivalent to at least one BMI-unit before their next pregnancy (Table 3).
Discussion
As part of the review process, a referee alerted us about a paper by Getahun et al. (73) where changes, increases as well as decreases, in BMI during the first two pregnancies of more than 700,000 American women were analyzed in relation to LGA-births. This paper was not captured by our research question, probably since it was not presented as a paper focusing on weight loss. However, a decrease in BMI must be due to a loss of body weight. Getahun et al. (73) reported that obese women were at an increased risk for delivering LGA-infants. Furthermore, although the risk for delivering such an infant was attenuated if an obese woman lost weight between the two pregnancies, the risk was still higher than for normal weight women who maintained their body weight between their first two pregnancies.
Our research question did not include a statement requiring that weight loss should be the result of a dietary intervention. Nevertheless, it is evident from our review that studies regarding preconceptional dietary-based interventions aiming at weight reduction in overweight and obese women are currently lacking. Our literature search, including our complementary search, clearly shows that many women would benefit substantially from such a weight loss. Probable positive effects include improved reproductive outcome and improved health of mothers, for example reduced preeclampsia (71), as well as improved health of offspring. However, it is conceivable that preconceptional dietary-based interventions aiming at weight reduction in overweight and obese women also may have harmful effects, for example risks of nutritional deficiencies (i.e. iron or folate) or disorders related to eating behavior. Another concern is pointed out by Zhang et al. (74) in a recent paper where the authors discuss evidence indicating that undernutrition as well as overnutrition, imposed during the periconceptional period, may both affect the offspring negatively. Thus, it was stressed in their paper that ‘it is important to ensure that any dietary restriction interventions recommended for overweight and obese mothers are evidence-based to allow for an effective weighing up of the potential metabolic benefits and costs for the offspring’. The present paper shows that evidence-based strategies regarding how dietary interventions before conception should be carried out to be successful whilst simultaneously avoiding potentially harmful effects, are currently lacking. Although urgently needed such studies seem to be very difficult to carry out. The obvious reason for the lack of scientific evidence is a lack of data since recruiting women before conception is associated with practical problems. An alternative approach to the problem of overweight and obesity in reproductive women could be to develop public health strategies where serious efforts are made to counteract overweight and obesity in girls and young women. Additional efforts helping women to gain weight during pregnancy according to recommendations and to lose weight after delivery would be important parts of such a strategy. It should be emphasized, however, that efforts to control body weight should not occur at the prize of a nutritionally adequate dietary intake. Achieving these goals represents a difficult task but a task of considerable public health importance.
Appendix
Papers ordered in full text but not included in the systematic literature review. The reason for exclusion is also given.
| Papers excluded | Reason for exclusion |
|---|---|
| Anonymous (12) | Not relevant for research question |
| Anonymous (13) | Not relevant for research question |
| Anonymous (14) | Not relevant for research question |
| Barger et al. (15) | Not relevant for research question |
| Bellver et al. (16) | Not relevant for research question |
| Bitsko et al. (17) | Focus on safety of weight loss products, Not relevant |
| Bo et al. (18) | Study of maternal low birth weight, Not relevant |
| Caughey et al. (19) | Not a research paper, Not relevant |
| Coitinho et al. (20) | Not relevant for research question |
| Frederick et al. (21) | Deals with weight gain, not weight loss, Not relevant for research question |
| Galtier et al. (22) | Not relevant for research question |
| Gunderson et al. (23) | Not relevant for research question |
| Haugen et al. (24) | Not relevant for research question |
| Hegaard et al. (25) | Not relevant for research question |
| Jevitt (26) | Not relevant for research question |
| Johnson et al. (27) | Not relevant for research question |
| Jones et al. (28) | Not relevant for research question |
| Keller et al. (29) | Not relevant for research question |
| Kuchenbecker et al. (30) | Review, Not main topic, Not relevant |
| Kuhlmann et al. (31) | Wrong topic, Not relevant |
| Lagiou et al. (32) | Wrong topic, Not relevant for research question |
| Le Goff et al. (33) | Review |
| Lederman et al. (34) | Wrong topic, Not to the point, Not relevant for research question |
| Ly et al. (35) | Wrong topic, Not relevant |
| Maloni et al. (36) | Wrong topic, Not relevant |
| McGuire et al. (37) | Wrong topic, Not relevant |
| Metwally et al. (38) | Wrong topic, Not relevant |
| Morisset et al. (39) | Not relevant for research question |
| Nelson et al. (40) | No appropriate outcome, Review |
| Ostbye et al. (41) | Not appropriate for research question |
| Pandey et al. (42) | No appropriate outcome, Review |
| Rah et al. (43) | Not appropriate for research question |
| Rooney et al. (44) | Not appropriate for research question |
| Rooney et al. (45) | Not appropriate for research question, Not relevant |
| Saleh et al. (46) | Not appropriate for research question, Not relevant |
| Seli et al. (47) | Not appropriate for research question, Review, Not relevant |
| Tema (48) | About low birth weight, not relevant for research question |
| Turhan et al. (49) | Not relevant for research question |
| Walker et al. (50) | Not relevant for research question |
| Vallianatos et al. (51) | About weight gain, not weight loss, Not relevant |
| Weissgerber et al. (52) | Not relevant for research question, Review |
| Winkvist et al. (53) | Not relevant for research question, Review |
| Yogev et al. (54) | Not relevant for research question |
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.World Health Organization. Feto-maternal nutrition and low birth weight. 2011. http://www.who.int/nutrition/topics/feto_maternal; [cited 3 May 2012]
- 2.Eastman N, Hellman L. Williams’ obstetrics. 13th ed. New York: Appelton-Century-Crofts; 1966. p. 326. [Google Scholar]
- 3.Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. Am J Clin Nutr. 2000;71:1233S–41S. doi: 10.1093/ajcn/71.5.1233s. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine, National Research Council. Food and Nutrition Board. Washington, DC: National Academy Press; 1990. Nutrition during pregnancy, weight gain and nutrient supplements. Report of the subcommittee on nutritional status and weight gain during pregnancy. Subcommittee on dietary intake and nutrient supplements during pregnancy, Committee on nutritional status during pregnancy and lactation. [Google Scholar]
- 5.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–70. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine, National Research Council. Weight gain during pregnancy, re-examining the guidelines. In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy, Reexamining the guidelines. Washington, DC: The National Academy Press; 2009. pp. 1–13. [Google Scholar]
- 7.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care – United States. A Report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 8.Nohr EA, Vaeth M, Baker JL, Sorenson TIA, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–9. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 9.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomized evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Hum Reprod Update. 2009;15:189–201. doi: 10.1093/humupd/dmn057. [DOI] [PubMed] [Google Scholar]
- 11.Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286–96. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. Impact of physical activity during pregnancy and postpartum on chronic disease risk; Med Sci Sports Exerc; 2006. pp. 989–1006. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous. Nutrition and reproduction in women; Hum Reprod Update; 2006. pp. 193–200. [DOI] [PubMed] [Google Scholar]
- 14.Anonymous. Theme: obesity & overweight; Vårdfacket; 2005. pp. 1–24. [Google Scholar]
- 15.Barger MK, Bidgood-Wilson M. Caring for a woman at high risk for type 2 diabetes. J Midwifery Women's Health. 2006;51:222–6. doi: 10.1016/j.jmwh.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Bellver J, Busso C, Pellicer A, Remohi J, Simon C. Obesity and assisted reproductive technology outcomes. Reprod Biomed Online. 2006;12:562–8. doi: 10.1016/s1472-6483(10)61181-9. [DOI] [PubMed] [Google Scholar]
- 17.Bitsko RH, Reefhuis J, Louik C, Werler M, Feldkamp ML, Waller DK, et al. Periconceptional use of weight loss products including ephedra and the association with birth defects. Birth Defects Res A Clin Mol Teratol. 2008;82:553–62. doi: 10.1002/bdra.20472. [DOI] [PubMed] [Google Scholar]
- 18.Bo S, Marchisio B, Volpiano M, Menato G, Pagano G. Maternal low birth weight and gestational hyperglycemia. Gynecol Endocrinol. 2003;17:133–6. [PubMed] [Google Scholar]
- 19.Caughey AB. Obesity, weight loss, and pregnancy outcomes. Lancet. 2006;368:1136–8. doi: 10.1016/S0140-6736(06)69451-8. [DOI] [PubMed] [Google Scholar]
- 20.Coitinho DC, Sichieri R, D'Aquino Benicio MH. Obesity and weight change related to parity and breast-feeding among parous women in Brazil. Public Health Nutr. 2001;4:865–70. doi: 10.1079/phn2001125. [DOI] [PubMed] [Google Scholar]
- 21.Frederick IO, Rudra CB, Miller RS, Foster JC, Williams MA. Adult weight change, weight cycling, and prepregnancy obesity in relation to risk of preeclampsia. Epidemiology. 2006;17:428–34. doi: 10.1097/01.ede.0000221028.33245.0b. [DOI] [PubMed] [Google Scholar]
- 22.Galtier FI, Raingeard I, Renard E, Boulot P, Bringer J. Optimizing the outcome of pregnancy in obese women: from pregestational to long-term management. Diabetes Metab. 2008;34:19–25. doi: 10.1016/j.diabet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Gunderson EP, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes Relat Metab Disord. 2001;25:853–62. doi: 10.1038/sj.ijo.0801631. [DOI] [PubMed] [Google Scholar]
- 24.Haugen M, Alexander J. Can linoleic acids in conjugated CLA products reduce overweight problems? Tidskrift for den Norske Laegeforening. 2004;124:3051–4. [PubMed] [Google Scholar]
- 25.Hegaard HK, Petersson K, Hedegard M, Ottesen B, Dykes AK, Henriksen TB, et al. Sports and leisure-time physical activity in pregnancy and birth weight: a population-based study. Scand J Med Sci Sports. 2010;20:e96–e102. doi: 10.1111/j.1600-0838.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 26.Jevitt CM. Weight management in gynecologic care. J Midwifery Women's Health. 2005;50:427–30. doi: 10.1016/j.jmwh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DB, Gerstein DE, Evans AE, Woodward-Lopez G. Preventing obesity: a life cycle perspective. J Am Diet Assoc. 2006;106:97–102. doi: 10.1016/j.jada.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 28.Jones EJ, Roche CC, Appel SJ. A review of the health beliefs and lifestyle behaviors of women with previous gestational diabetes. J Obstet Gynecol Neonatal Nurs. 2009;38:516–26. doi: 10.1111/j.1552-6909.2009.01051.x. [DOI] [PubMed] [Google Scholar]
- 29.Keller C, Records K, Ainsworth B, Permana P, Coonrod DV. Interventions for weight management in postpartum women. J Obstet Gynecol Neonatal Nurs. 2008;37:71–9. doi: 10.1111/j.1552-6909.2007.00202.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuchenbecker WK, Ruifrok AE, Bolster JH, Heineman MJ, Hoek A. Subfertility in overweight women. Nederlands Tijdschrift voor Geneeskunde. 2006;150:2479–83. [PubMed] [Google Scholar]
- 31.Kuhlmann AK, Dietz PM, Galavotti C, England LJ. Weight-management interventions for pregnant or postpartum women. Am J Prev Med. 2008;34:523–8. doi: 10.1016/j.amepre.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Lagiou P, Hsieh CC, Trichopoulos D, Xu B, Wuu J, Mucci L, et al. Birthweight differences between USA and China and their relevance to breast cancer aetiology. Int J Epidemiol. 2003;32:193–8. doi: 10.1093/ije/dyg047. [DOI] [PubMed] [Google Scholar]
- 33.Le Goff S, Ledee N, Bader G. Obesity and reproduction: a literature review. Gynecol Obstet Fertil. 2008;36:543–50. doi: 10.1016/j.gyobfe.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Lederman SA, Alfasi G, Deckelbaum RJ. Pregnancy-associated obesity in black women in New York City. Matern Child Health J. 2002;6:37–42. doi: 10.1023/a:1014364116513. [DOI] [PubMed] [Google Scholar]
- 35.Ly CT, Diallo A, Simondon F, Simondon KB. Early short-term infant food supplementation, maternal weight loss and duration of breast-feeding: a randomised controlled trial in rural Senegal. Eur J Clin Nutr. 2006;60:265–71. doi: 10.1038/sj.ejcn.1602311. [DOI] [PubMed] [Google Scholar]
- 36.Maloni JA, Alexander GR, Schluchter MD, Shah DM, Park S. Antepartum bed rest: maternal weight change and infant birth weight. Biol Res Nurs. 2004;5:177–86. doi: 10.1177/1099800403260307. [DOI] [PubMed] [Google Scholar]
- 37.McGuire W, Dyson L, Renfrew M. Maternal obesity: consequences for children, challenges for clinicians and carers. Semin Fetal Neonatal Med. 2010;15:108–12. doi: 10.1016/j.siny.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Metwally M, Ledger WL, Li TC. Reproductive endocrinology and clinical aspects of obesity in women. Ann N Y Acad Sci. 2008;1127:140–6. doi: 10.1196/annals.1434.000. [DOI] [PubMed] [Google Scholar]
- 39.Morisset AS, St-Yves A, Veilette J, Weisnagel SJ, Tchernof A, Robitaille J. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26:17–25. doi: 10.1002/dmrr.1053. [DOI] [PubMed] [Google Scholar]
- 40.Nelson SM, Fleming RF. The preconceptual contraception paradigm: obesity and infertility. Hum Reprod. 2007;22:912–5. doi: 10.1093/humrep/del473. [DOI] [PubMed] [Google Scholar]
- 41.Ostbye T, Krause KM, Lovelady CA, Morey MC, Bastian LA, Peterson BL, et al. Active Mothers Postpartum: a randomized controlled weight-loss intervention trial. Am J Prev Med. 2009;37:173–80. doi: 10.1016/j.amepre.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey S, Bhattacharya S. Impact of obesity on gynecology. Women's Health. 2010;6:107–17. doi: 10.2217/whe.09.77. [DOI] [PubMed] [Google Scholar]
- 43.Rah JH, Shamim AA, Arju UT, Labrique AB, Klemm RD, Rashid M, et al. Difference in ponderal growth and body composition among pregnant vs. never-pregnant adolescents varies by birth outcomes. Matern Child Nutr. 2010;6:27–37. doi: 10.1111/j.1740-8709.2009.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100:245–52. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 45.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106:1349–56. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 46.Saleh AM, Khalil HS. Review of nonsurgical and surgical treatment and the role of insulin-sensitizing agents in the management of infertile women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2004;83:614–21. doi: 10.1111/j.0001-6349.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 47.Seli E, Duleba AJ. Optimizing ovulation induction in women with polycystic ovary syndrome. Curr Opin Obstet Gynecol. 2002;14:245–54. doi: 10.1097/00001703-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Tema T. Prevalence and determinants of low birth weight in Ethiopia. East Afr Med J. 2006;83:366–71. doi: 10.4314/eamj.v83i7.9448. [DOI] [PubMed] [Google Scholar]
- 49.Turhan NO, Seckin NC, Aybar F, Inegöl I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int J Gynaecol Obstet. 2003;81:163–8. doi: 10.1016/s0020-7292(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 50.Walker LO, Sterling BS, Timmerman GM. Retention of pregnancy-related weight in the early postpartum period: implications for women's health services. J Obstet Gynecol Neonatal Nurs. 2005;34:418–27. doi: 10.1177/0884217505278294. [DOI] [PubMed] [Google Scholar]
- 51.Vallianatos H, Brennand EA, Raine K, Stephen Q, Petawabano B, Dannenbaum D, et al. Beliefs and practices of First Nation women about weight gain during pregnancy and lactation: implications for women's health. Can J Nurs Res. 2006;38:102–19. [PubMed] [Google Scholar]
- 52.Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab. 2006;31:661–74. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- 53.Winkvist A, Rasmussen KM, Lissner L. Associations between reproduction and maternal body weight: examining the component parts of a full reproductive cycle. Eur J Clin Nutr. 2003;57:114–27. doi: 10.1038/sj.ejcn.1601502. [DOI] [PubMed] [Google Scholar]
- 54.Yogev Y, Langer O. Recurrence of gestational diabetes: pregnancy outcome and birth weight diversity. J Mater-Fetal Neonatal Med. 2004;15:56–60. doi: 10.1080/14767050310001650734. [DOI] [PubMed] [Google Scholar]
- 55.Birdsall KM, Vyas S, Khazaezadeh N, Oteng-Ntim E. Maternal obesity: a review of interventions. Int J Clin Pract. 2009;63:494–507. doi: 10.1111/j.1742-1241.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- 56.Callaway LK, O‘Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191:425–8. doi: 10.5694/j.1326-5377.2009.tb02876.x. [DOI] [PubMed] [Google Scholar]
- 57.Chen A, Klebaoff MA, Basso O. Prepregnancy body mass index change between pregnancies and preterm birth in the following pregnancy. Paediatr Perinat Epidemiol. 2009;23:207–15. doi: 10.1111/j.1365-3016.2009.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gesatti A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18:1928–32. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 59.Davis E, Olson C. Obesity in pregnancy. Prim Care. 2009;36:341–56. doi: 10.1016/j.pop.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiol. 2004;15:733–7. doi: 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- 61.Linné Y, Rössner S. Easy to remain overweight after pregnancy. Läkartidningen. 2003;100:4091–5. [PubMed] [Google Scholar]
- 62.Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–9. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 63.Moran LJ, Norman RJ. The obese patient with infertility: a practical approach to diagnosis and treatment. Nutr Clin Care. 2002;5:290–7. doi: 10.1046/j.1523-5408.2002.05604.x. [DOI] [PubMed] [Google Scholar]
- 64.Mutsaerts MA, Groen H, Bogt NC, Bolster JH, Land JA, Bemelmans WJ, et al. The LIFESTYLE study: costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Women's Health. 2010;10:22. doi: 10.1186/1472-6874-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olsen SF, Dragsted LO, Hansen HS, Michaelsen KF, Milman N, Nielsen MJ, et al. The scientific basis of current official dietary recommendations in relation to pregnancy. Ugeskrift for Laeger. 2005;167:2782–4. [PubMed] [Google Scholar]
- 66.Paramsothy P, Lin YS, Kernic MA, Foster-Schubert KE. Interpregnancy weight gain and caesarean delivery risk in women with a history of gestational diabetes. Obstet Gynecol. 2009;113:817–23. doi: 10.1097/AOG.0b013e31819b33ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obesity (Silver Spring) 2006;14:165–71. doi: 10.1038/oby.2006.20. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Taanila A, Ebeling H, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes. 2008;32:550–7. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- 69.Diouf I, Charles MA, Thiebaugeorges O, Forhan A, Kaminski M, Heude B, et al. Maternal weight change before pregnancy in relation to birthweight and risks of adverse pregnancy outcomes. Eur J Epidemiol. 2011;26:789–96. doi: 10.1007/s10654-011-9599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011;117:1323–30. doi: 10.1097/AOG.0b013e31821aa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mostello D, Chang JJ, Allen J, Luehr L, Shyken J, Leet T. Recurrent preeclampsia. The effect of weight change between pregnancies. Obstet Gynecol. 2010;116:667–72. doi: 10.1097/AOG.0b013e3181ed74ea. [DOI] [PubMed] [Google Scholar]
- 72.Whiteman VE, Rao K, Duan J, Alio A, Marthy PJ, Salihu HM. Changes in prepregnancy body mass index between pregnancies and risk of preterm phenotypes. Am J Perinatol. 2011;28:67–74. doi: 10.1055/s-0030-1262905. [DOI] [PubMed] [Google Scholar]
- 73.Getahun D, Ananth CV, Peltier MR, Salihu HM, Scorza WE. Changes in prepregnancy body mass index between the first and second pregnancies and risk of large-for-gestational birth. Am J Obstet Gynecol. 2007;196:530. doi: 10.1016/j.ajog.2006.12.036. e1–8. [DOI] [PubMed] [Google Scholar]
- 74.Zhang S, Rattanatray L, Morrison JL, Nicholas LM, Lie S, McMillen IC. Maternal obesity and the early origins of childhood obesity: weighing up the benefits and costs of maternal weight loss in the periconceptional period for the offspring. Exp Diabetes Res 2011. 2011:585749. doi: 10.1155/2011/585749. [DOI] [PMC free article] [PubMed] [Google Scholar]