Abstract
The discoidin domain receptors (DDRs) are receptor tyrosine kinases that recognize collagens as their ligands. DDRs display unique structural features and distinctive activation kinetics, which set them apart from other members of the kinase superfamily. DDRs regulate cell-collagen interactions in normal and pathological conditions and thus are emerging as major sensors of collagen matrices and potential novel therapeutic targets. New structural and biological information has shed light on the molecular mechanisms that regulate DDR signaling, turnover, and function. This minireview provides an overview of these areas of DDR research with the goal of fostering further investigation of these intriguing and unique receptors.
Keywords: Collagen, Extracellular Matrix, Phosphotyrosine Signaling, Protease, Signal Transduction, Tyrosine Protein Kinase (Tyrosine Kinase)
Introduction
The discoidin domain receptor (DDR)2 family comprises two distinct members, DDR1 and DDR2, which were initially discovered in the early 1990s and characterized as receptor tyrosine kinases (RTKs) based on the presence of a catalytic kinase domain (KD) (1–7). Subsequently, collagens were identified as ligands for DDRs (8), thus establishing the unique characteristic of these receptors among other members of the RTK superfamily. Upon collagen binding, DDRs undergo tyrosine autophosphorylation with distinctive activation kinetics, which elicits genetic and cellular programs that regulate a variety of cell-collagen interactions. Despite their unique characteristics, the biochemical and cellular mechanisms by which DDRs mediate their multiple biological effects remain poorly defined. This minireview provides an overview of current information on DDR structure, regulation, and signaling. For information on specific DDR biological functions in processes such as cell adhesion, migration, and invasion over collagen matrices and their role in normal and pathological processes, the reader is directed to the following recent reviews (9–11)
DDR Structure
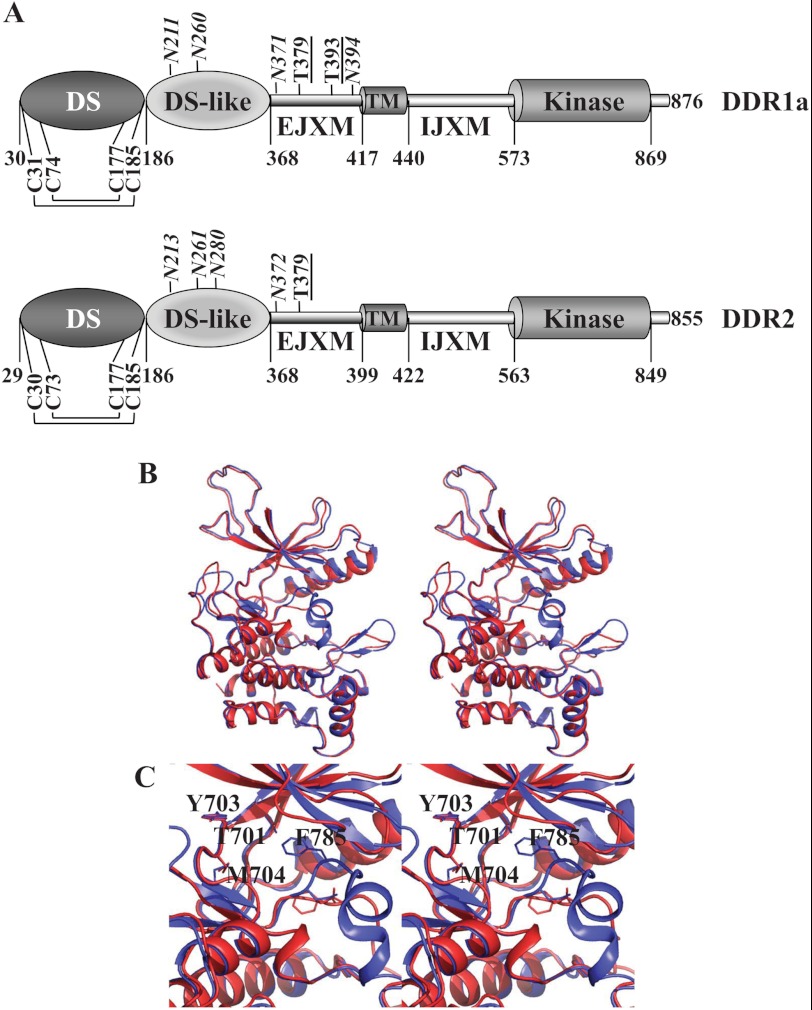
The DDR1 subfamily is composed of five membrane-anchored isoforms, and the DDR2 subfamily is represented by a single protein. The five DDR1 isoforms are generated by alternative splicing. DDR1a, DDR1b, and DDR1c are full-length functional receptors, and DDR1d and DDR1e are truncated or kinase-inactive receptors (10, 12). Two additional secreted splice variants of DDR1 have also been identified (13). DDR1b and DDR1c contain an additional 37 residues within the intracellular juxtamembrane (IJXM) region. With the exception of the two secreted DDR1 isoforms, all DDRs are single-pass type I transmembrane glycoproteins that are characterized by the presence of six distinct protein domains: a discoidin (DS) domain, a DS-like domain, an extracellular juxtamembrane (EJXM) region, a transmembrane (TM) segment, a long IJXM region, and an intracellular KD (Fig. 1A). The presence of the N-terminal DS and DS-like domains is the defining feature of the DDR RTK subfamily. The DS domain exhibits high homology to a protein module originally identified in proteins from Dictyostelium discoideum (14). In this organism, the DS domain functions as a galactose-binding lectin, which is important for the maintenance of cell morphology and cytoskeletal organization (14). In eukaryotes, many unrelated proteins contain DS domains, which, depending on the protein, recognize distinct ligands and thereby fulfill distinct biological functions. In DDRs, the DS domain contains the collagen-binding region and is responsible for mediating DDR specificity for fibrillar and non-fibrillar collagens (15–18). NMR (19) and x-ray (20, 21) structural studies of the DS domain of DDRs revealed that it is structurally similar to all DS domains, consisting of two antiparallel sheets for a total of eight β-strands in a jellyroll configuration, with six loops at the top, forming a collagen-binding motif. The structure is further stabilized by four cysteine residues forming two intramolecular disulfide bonds: Cys-74 (Cys-73 in DDR2) and Cys-177 link loops 2 and 6, whereas Cys-31 (Cys-30 in DDR2) and Cys-185 are located near the N- and C-terminal regions. Sequence differences in these loops define the specificity for each ligand among different DS domain-containing proteins. Conserved residues within loops 1, 2, and 4 of the DS domain were shown to be involved in fibrillar collagen binding (19, 20), and thus mediate interactions of DDRs with their common ligands. In contrast, unique residues located within loops 4 and 6 of the DS domain of DDR1 were shown to be critical for collagen IV-mediated activation (18). Thus, distinct regions within the DS domain can discriminate between fibrillar and non-fibrillar collagens. It has been suggested that a patch of conserved surface residues in the DS domain that is away from the collagen-binding site is also required for collagen-mediated activation, possibly by affecting receptor dimerization or alternatively acting as a second, weak affinity collagen-binding site (21).
FIGURE 1.
A, domain structure of DDRs. Only cysteine residues involved in intramolecular disulfide bond formation are shown. Predicted N-glycosylation (italic) and O-glycosylation (underlined) sites are indicated. B, ribbon representation of the modeled DFG-in (red) and DFG-out (blue) conformations of the DDR1a KD shown in stereo. C, close-up stereoview of the catalytic pocket with several key residues in stick representation.
Following the DS domain, there is a 182-residue-long DS-like domain unique to DDRs. This domain shows two antiparallel β-sheets comprising a total of eight β-strands with jellyroll topography. In addition, the DS-like domain of DDR1 contains five additional strands protruding between the β1- and β2-strands, which contain two N-glycosylation sites, Asn-211 and Asn-260, and a calcium-binding site (21). The function of the DS-like domain of DDRs is not fully understood, but recent data suggest that it contributes to collagen-induced receptor activation (21). The EJXM region of human DDRs (49 residues in DDR1 and 31 residues in DDR2), which connects the DS domain to the TM segment, is of unknown structure. The EJXM region contains several putative N- and O-glycosylation sites (Fig. 1A), which may regulate receptor trafficking, turnover, and/or ligand-induced activation (15). Recent evidence indicates that the EJXM region of DDR1 is cleaved by membrane-type matrix metalloproteinases (MT-MMPs) MMP-14, MMP-15, and MMP-16, which release the entire ectodomain, consequently regulating collagen-induced receptor activation,3 consistent with previous studies reporting shedding of DDR1 by a metalloproteinase-dependent mechanism (7, 22, 23). Interestingly, in contrast to DDR1, DDR2 is not cleaved by MT-MMPs at the EJXM region, possibly due to the significant lack of sequence homology in this area. Thus, DDRs evolved to be differentially regulated by ectodomain shedding, a process that may alter signaling. A short TM helical segment (∼20 residues) links the ectodomain and the intracellular domains of DDRs. The TM segment plays a role in receptor dimerization (24), and in DDR1, it contains a series of leucine residues, thought to constitute a leucine zipper motif, critical for receptor signaling (24). An unusually large (130–140 residues) IJXM region connects the TM segment with the KD. The IJXM region contains several tyrosine residues that serve as docking sites for cytoplasmic effectors and regulators that are essential for signal transduction. In DDR1b and DDR1c, the IJXM region contains an insertion of 37 residues that includes an extra tyrosine residue within an NPXY motif, which serves as a docking site for the ShcA adaptor molecule (8) and possibly for other proteins containing phosphotyrosine-binding (PTB) domains (25). Thus, these isoforms may recruit different adaptor proteins and consequently activate distinct signaling and/or endocytic pathways.
A classical KD (∼300 residues) follows the IJXM region in the DDR1a, DDR1b, and DDR1c isoforms and in DDR2. Although the structure of the KD has not been solved, adequate sequence similarities to kinases with structural information made homology models feasible, which we generated. Our KD homology models illustrate structural features that are common to kinases, such as N- and C-terminal lobes, an αC-helix, a P-loop, an activation loop, an F-helix, and the DFG motif (26). Two models were generated based on the two well known conformations of the DFG motif, DFG-in and DFG-out, which bind type I and II inhibitor classes, respectively (Fig. 1, B and C) (27). The DFG-in model was based on the crystal structure of the insulin-like growth factor 1 receptor (36% overall sequence identity; Protein Data Bank code 1K3A) (28), and the DFG-out model was based on TrkA (45% overall sequence identity; code 4F0I) (29). We observed two loop areas with low conformational reliability due to multiple amino acid insertions in the KD of DDR1a compared with the templates. However, both areas are remote from the catalytic pocket. The secondary structure of one of these regions, Gly-821–Arg-825, was predicted to add an extra α-helical turn to the existing helix of the template. The insertion at the other region, Ser-594–Asn-605, was too large to make reliable predictions. Although it is difficult to reliably predict the function of the Ser-594–Asn-605 region based solely on our models given its well exposed position, the proximity to the IJXM region, and the flexibility observed during modeling, it could be playing a key role in DDR kinase activity. The catalytic site of the DDR1a KD shows several conserved residues in the kinase family, such as Glu-672, Lys-655, and Asp-784. The hinge region is composed of amino acid residues 701–705, with Thr-701 serving as the gatekeeper residue as in Abelson (Abl) kinase and the stem cell factor receptor KD. Additionally, Day et al. (30) used a homology model based on multiple templates to illuminate the structural basis for the inhibition of DDR1 by imatinib, nilotinib, and dasatinib. One of these templates, the Abl kinase, shares a 61% sequence identity at the binding pocket with DDR1. Furthermore, the authors indicate that DDRs could potentially form a hydrophobic cage-like pocket as in the Abl kinase to interact with type II inhibitors. Compared with the crystal structure of the Abl kinase bound to dasatinib, 11 of the 14 residues that have protein-ligand contacts are conserved in DDR1 (31), including the threonine gatekeeper residue, which forms important hydrogen bonds with type II inhibitors. Interestingly, other phylogenetically related kinases such as muscle-specific kinase, the insulin receptor, and the insulin-like growth factor 1 receptor have a methionine at this position and thus are not inhibited by these drugs. Consistent with this observation, DDR2 harboring the T654M gatekeeper mutation is resistant to dasatinib inhibition (32). The elucidation of the crystal structure of the DDR KD will aid in the design of specific kinase inhibitors, which may be beneficial in various pathological conditions in which DDRs have been identified as potent potential therapeutic targets.
DDR Regulation
Collagen Specificity
Both DDR1 and DDR2 bind to and are activated by fibrillar collagens I–III and V. Basement membrane collagen IV activates DDR1 but not DDR2 (8, 16), whereas non-fibrillar collagen X primarily activates DDR2 (33). Four DDR2-binding sites (34) and one DDR1-binding site (35) have been identified in collagens II and III so far. The primary binding site for both DDR1 and DDR2 in collagens II and III contains the GVMGFO motif (where O is hydroxyproline), which is also conserved in the α1(I) (35) and α1(V) (36) chains. However, none of the six α(IV) chains contain the GVMGFO motif (37), suggesting the possibility that other sites in non-fibrillar collagens are involved in DDR1 binding. DDRs bind to the native triple helix of collagens and not to individual collagen α-chains or denatured collagens (8, 16). Likewise, deglycosylated or degraded collagen cannot support DDR activation (8, 38, 39). It is also likely that DDR-collagen interactions may be influenced by factors post-fibrillogenesis, such as matrix mineralization, glycation, and cross-linking. Although DDRs preserve the capacity to bind to both monomeric (40, 41) and polymerized (42) collagen I, cells cultured on monomeric versus polymerized collagen exhibit reduced DDR2 activation (38, 43). Thus, structural alterations during conditions that promote collagen remodeling may alter DDR signaling.
The importance of DDRs as collagen receptors is demonstrated by the phenotype of DDR knock-out mice, which exhibit a variety of skeletal abnormalities (44, 45). Interestingly, DDR-deficient mice also display various reproductive abnormalities (44, 46, 47), which highlight an unexpected impact of DDRs in normal reproductive processes. Also, studies with DDR-deficient mice showed a key role for these receptors in inflammatory and fibrotic responses in various conditions (for a detailed description of DDR roles in knock-out mice and other biological functions, see Ref. 10).
Activation
Upon collagen binding, DDRs undergo tyrosine autophosphorylation. The two distinguishing features of DDR phosphorylation dynamics are a delayed and a sustained response. Rapid quenching experiments have shown that classical RTKs such as the EGF receptor (EGFR) and FGF receptor undergo tyrosine autophosphorylation within seconds of ligand binding (48, 49). In contrast, depending on the cell type, the DDRs require a remarkably long period to achieve a similar activated state (minutes to hours) (8). After receptor activation, many classical RTKs undergo negative regulation via mechanisms such as receptor/ligand internalization and subsequent degradation or dephosphorylation by phosphatases (50, 51). However, in the case of the DDRs, phosphorylation levels persist for days, with no apparent means for signal attenuation (8). The molecular basis and the biological effects of these two intriguing characteristics of DDR phosphorylation are poorly understood, but lessons from classical RTKs may provide some clues to these outstanding questions. The kinetic profile of DDR phosphorylation is reminiscent of delayed negative feedback mechanisms commonly elicited to down-regulate immediate-early RTK activation (52). It has previously been reported that DDR1 serves to counteract the signaling effects of the α2β1 integrin by reducing the activation of STAT1/3 (signal transducers and activators of transcription 1/3) and Cdc42 (53, 54). Because α2β1 integrin activation and formation of focal adhesion signaling complexes occur within minutes of collagen binding, it is plausible that DDRs have evolved as a late-wave negative feedback mechanism for the down-regulation of integrin signaling. The network motif of slow accumulation of late effectors for negative regulation has been postulated to facilitate the transition to a new cellular state (52). This idea raises questions as to the functional relevance of the sustained DDR phosphorylation profile and the apparent lack of negative regulation of the receptor itself. Previous RTK studies have shown that sustained signaling often results in contrasting biological outcomes compared with transient signals. In classical experiments with EGF versus NGF signaling in PC12 cells, transient ERK activation via EGFR led to cell proliferation, whereas sustained ERK phosphorylation through the activation of TrkA (the NGF receptor) promoted neuronal differentiation (55, 56). Sustained activation of DDR1 in this cellular system did not promote neuronal differentiation, likely due to a failure of DDR1 to maintain sustained ERK activation (57). This negative finding is likely to be due to the choice of biological system rather than the lack of a functional role in DDR-mediated signaling. However, this study suggests that, in addition to their role as active signaling kinases, DDRs may act as molecular scaffolds similar to other kinases such as integrin-linked kinase and KSR1 (kinase suppressor of Ras1) (58, 59). Chimeras comprising the ligand-binding domain of PDGF receptor β fused to the IJXM region of DDR1 and the KD of TrkA were capable of promoting PC12 cell differentiation through the recruitment of adaptors FRS2 and Shc to the DDR1 IJXM region (57). There is precedence for such DDR1 scaffold function in interactions with the PDZ domains of Par3 and Par6 at cell-cell junctions (60). Perhaps one role of DDR-sustained phosphorylation is to sequester a high local concentration of adaptor and signaling proteins such as Shc and PI3K in close proximity to classical RTKs. Upon growth factor activation, the increased affinity of the SH2 (Src homology 2) and PTB domains of these adaptor/signaling proteins for classical RTK tyrosine phosphorylation sites would result in the competitive recruitment of proteins from the DDRs to these RTKs, thereby facilitating rapid propagation of signaling networks (61). Once the phosphorylation levels of these classical RTK have diminished as a result of negative feedback regulations, the adaptor proteins could then revert to the still activated DDRs, maintaining steady-state protein levels.
The delayed/maintained receptor phosphorylation profile would likely contribute to both the extracellular ligand binding and intracellular signaling dynamics. In contrast to growth factors, which are normally secreted in an autocrine or paracrine manner in response to stimuli, collagen is a relatively stable and abundant ligand. Thus, the slow activation rate of the DDRs could represent an evolutionary mechanism to avoid rapid and excessive receptor activation in the presence of ubiquitous collagen levels until a threshold of ligand concentration or exposure time is achieved. Intracellular effects are also critical, as DDR1 is hyperphosphorylated in the presence of pervanadate, suggesting that the slow DDR phosphorylation dynamics is due at least in part to regulation by tyrosine phosphatases (62). Studies with EGFR demonstrate that six different EGF ligand family members display distinct receptor tyrosine phosphorylation patterns, dynamics, and intensities (63). These differences are independent of ligand affinity and are thought to be due to subtle differences in receptor-ligand binding conformations, which result in distinct endocytic trafficking, recycling, and degradation characteristics (63, 64). These data may shed light on the mechanisms of DDR-sustained activation, as persistence in EGFR tyrosine phosphorylation largely correlates with the ability of the individual ligand to promote receptor ubiquitination and degradation (63).
Receptor Endocytosis and Shedding
Receptor internalization upon ligand-induced activation is a common cellular response aimed at regulating RTK signaling (65). Several RTKs are known to be internalized following ligand binding by clathrin-dependent and clathrin-independent mechanisms, leading to degradation of the receptor-ligand complex in lysosomes or recycling of the receptor to the cell surface. Studies with DDR1b fused to fluorescent proteins have shown that exposure of cells to collagen I induces receptor aggregation of preformed dimers, followed by receptor internalization into Rab5a-positive endosomes and recycling to the cell surface (66). Interestingly, the onset of DDR1 endocytosis and recycling precedes receptor phosphorylation, suggesting that full receptor activation and signaling occur partly within the endocytic vesicles (67). Thus, it is possible that DDR activation and signaling proceed away from the plasma membrane. It will be interesting to determine whether activated DDR1 continues to transmit signals within the endocytic pathway, as reported with EGFR (50). In the case of integrins, the presence of an NPXY motif on the integrin tail has been shown to regulate clathrin-dependent endocytosis and to play a role in integrin recycling during cell adhesion, spreading, migration, and epithelial morphogenesis (68, 69). Interestingly, DDR1b and DDR1c, but not DDR1a, contain an NPXY motif within the IJXM region, suggesting that the internalization and intracellular fate of these isoforms may be unique among members of the DDR1 subfamily. It will therefore be important to determine whether DDRs display isoform type-specific endocytic regulation and, if so, how this process regulates receptor function in collagen- and cell context-dependent manners.
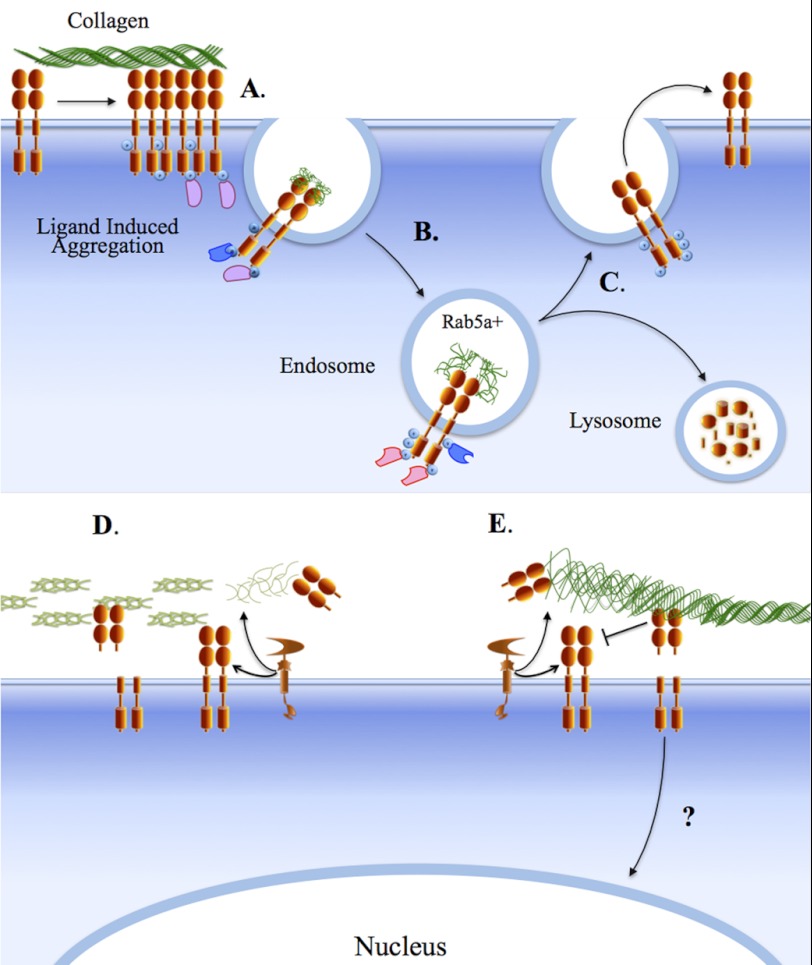
Evidence indicates that the pool of DDR1 on the cell surface is also regulated by ectodomain shedding, a process generally mediated by the action of metalloproteases, which causes the release of the extracellular domain of membrane proteins. Ectodomain shedding can modulate RTK signaling by reducing the amount of functional receptors on the cell surface and/or controlling the availability of membrane-anchored ligands (70). DDR1 is shed both in a collagen-dependent (23, 71) and collagen-independent (7) manner by a metalloprotease, which cleaves the ectodomain of DDR1 and generates a membrane-anchored C-terminal fragment. Recent evidence shows that the membrane-anchored collagenases (MMP-14, MMP-15, and MMP-16) are all capable of DDR1 shedding by cleaving at the EJXM region, whereas the secreted collagenases (MMP-1 and MMP-13) are not.3 However, additional evidence suggests that ADAM proteases also shed DDR1, indicating that cleavage of the DDR1 ectodomain is mediated by multiple metalloproteases. Functionally, DDR1 shedding may terminate receptor signaling by releasing activated receptors and/or reducing receptor availability. The released ectodomain may also act as a “decoy receptor,” which could negatively impact collagen binding to surface receptors. The released ectodomain could also modulate the organization of the neighboring collagen matrix. Indeed, it has been shown that both membrane-anchored DDR1 and soluble DDR1 inhibit fibrillogenesis by reducing the rate and quantity of collagen I deposition and altering fiber morphology and matrix mineralization (41, 72, 73). Thus, DDR1 shedding may represent a major process of receptor regulation. At present, it is unclear whether DDR2 is also regulated by proteolytic processing. However, our evidence suggests that DDR2 is not cleaved by MT-MMPs at the EJXM region,3 consistent with the lack of homology in this area between DDRs, which possibly evolved to confer a differential sensitivity to the action of sheddases. The relationship between MT-MMPs and DDRs may go beyond differential sensitivity to receptor shedding. MT-MMPs are major pericellular collagenases capable of hydrolyzing both basement membrane and interstitial collagens (74). As such, MT-MMPs may regulate DDRs by altering the integrity of the collagen matrix, exposing or degrading collagen-binding sites, which consequently may impact receptor activation. Fig. 2 summarizes the current understanding of DDR1 endocytosis and ectodomain shedding.
FIGURE 2.
DDR1 endocytosis and shedding. Activation of DDR1 dimers by collagen induces receptor aggregation (A) and triggers endocytosis of the receptor-ligand complex into Rab5-positive endosomes (B), which can lead to lysosomal degradation or recycling of the receptor to the cell surface (C). The presence of the NPXY motif within the IJXM region of the DDR1b and DDR1c isoforms may confer on these receptors a unique endocytic trafficking route by mediating association with specific adaptor/scaffold proteins (pink and blue shapes). Within the endosomal compartment, DDR1 may display continued signaling. DDR1 is cleaved by metalloproteinases, including MT-MMPs and ADAMs, at the cell surface, releasing the ectodomain and generating a C-terminal fragment (D and E). Together with the full-length receptor, the released DDR1 ectodomain can play a role in regulation of collagen fibrillogenesis (D) and or block ligand binding (E). The action of MT-MMPs on collagen may also regulate DDR1 signaling by altering the integrity of the collagen matrix (D and E). The C-terminal fragment of DDR1 may translocate to the nucleus (E). At present, there is no information on DDR2 internalization and shedding.
DDR Signaling
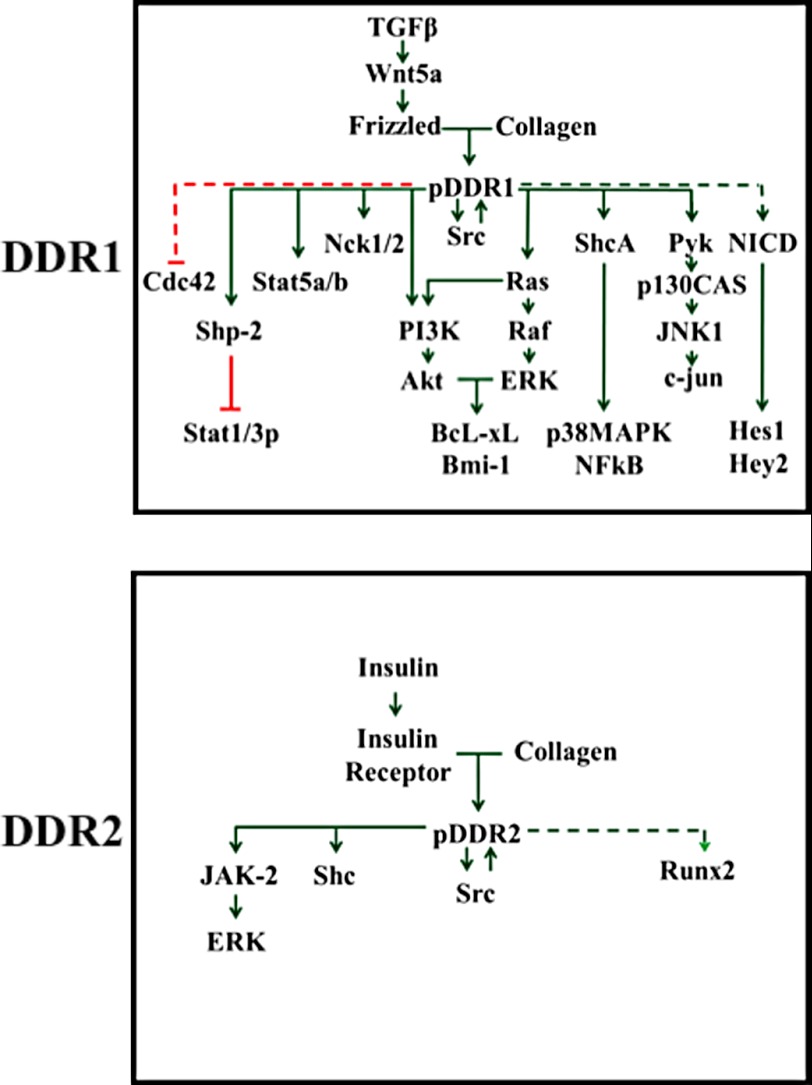
Phosphorylation of tyrosine residues within the intracellular domains of activated DDRs generates docking sites for SH2, SH3, and PTB domain-containing proteins (8, 53, 62, 75–78). These protein complexes, as in all RTKs, result in the activation of distinct DDR-initiated signaling pathways, some of which are summarized in Fig. 3. Evidence so far suggests that stimulation of DDR1 with collagen is coupled to the activation of the PI3K/Akt and Ras/ERK MAPK cascades (79–81). In the case of DDR2, the evidence points to a role for Src as a downstream effector and regulator of DDR2 signaling (82–84). Although more needs to be done to understand DDR signaling, it is becoming evident that defining the signal transduction pathways coupled to DDR activation by collagens is a challenging task. First, DDRs bind to multiple collagen types, which exhibit both unique and common structural and biological properties, and consequently different collagen types may produce different receptor responses (81). Second, collagens display multiple cell interaction sites that recognize different binding proteins (e.g. integrins) (85). Indeed, collagen-induced DDR1 activation has been shown to synergize or antagonize phenotypic responses upon integrin activation (53, 80, 86–88). Third, DDR signaling is cell/tissue type-specific and context-dependent (53, 54, 79–81). Fourth, DDRs may act in concert with other signaling receptors, including the Wnt5a/Frizzled (76, 89, 90) and Notch1 (91) receptors and the insulin receptor (92, 93), in the case of DDR1 and DDR2, respectively. Finally, the signaling pathways activated by DDR1 and DDR2 are likely to be influenced by the acquisition of point mutations and gene amplifications, as recently reported in several human cancers (32, 94). However, understanding whether and how these DDR alterations may potentially contribute to cancer development and progression would require identifying signaling pathways that are specifically activated by DDRs in cancer cells and their functional consequences.
FIGURE 3.
DDR signaling. The wiring diagram depicts signaling effectors of DDR activated in response to collagen. Solid and dashed green arrows indicate direct and indirect positive signaling effectors upstream and downstream of phosphorylated DDRs (pDDR), respectively. Red lines indicate direct (solid) and indirect (dashed) negative effects of phosphorylated DDRs on signaling effectors.
Concluding Remarks
Considerable structural and functional knowledge exists on many members of the RTK family, yet we are only beginning to comprehend the unique properties and functions of DDRs. These receptors are intriguing because of their distinctive activation kinetics in response to a unique class of ligands, the collagens, setting them apart from the other RTK family members. The accumulating evidence indicates that DDRs are major cellular sensors of environmental cues and thus are critical for normal development as revealed by the phenotype of DDR-deficient mice (44, 45). Moreover, the reported alterations in DDR genes in human cancer (32, 94) suggest that these receptors could impact disease progression, and therefore, DDRs may represent new therapeutic targets worth exploring. Despite these exciting new developments, the data so far provide only a glimpse into the mechanisms of DDR action at the cell-collagen interface. Eventually, only a multidisciplinary approach comprised of genomic, structural, and biological methods will help to shed light on how DDRs signal and function in physiological and pathological conditions.
This work was supported, in whole or in part, by National Institutes of Health Grant CA61986 from NCI (to R. F.).
H.-L. Fu, A. Sohail, R. R. Valiathan, B. D. Wasinski, M. Kumarasiri, K. V. Mahasenan, M. M. Bernardo, D. Tokmina-Roszyk, G. B. Fields, S. Mobashery, and R. Fridman, manuscript submitted for publication.
- DDR
- discoidin domain receptor
- RTK
- receptor tyrosine kinase
- KD
- kinase domain
- IJXM
- intracellular juxtamembrane
- DS
- discoidin
- EJXM
- extracellular juxtamembrane
- TM
- transmembrane
- MT-MMP
- membrane-type matrix metalloproteinase
- PTB
- phosphotyrosine-binding
- EGFR
- EGF receptor.
REFERENCES
- 1. Johnson J. D., Edman J. C., Rutter W. J. (1993) A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc. Natl. Acad. Sci. U.S.A. 90, 5677–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Marco E., Cutuli N., Guerra L., Cancedda R., De Luca M. (1993) Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. J. Biol. Chem. 268, 24290–24295 [PubMed] [Google Scholar]
- 3. Zerlin M., Julius M. A., Goldfarb M. (1993) NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene 8, 2731–2739 [PubMed] [Google Scholar]
- 4. Perez J. L., Jing S. Q., Wong T. W. (1996) Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines. Oncogene 12, 1469–1477 [PubMed] [Google Scholar]
- 5. Laval S., Butler R., Shelling A. N., Hanby A. M., Poulsom R., Ganesan T. S. (1994) Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line. Cell Growth Differ. 5, 1173–1183 [PubMed] [Google Scholar]
- 6. Sánchez M. P., Tapley P., Saini S. S., He B., Pulido D., Barbacid M. (1994) Multiple tyrosine protein kinases in rat hippocampal neurons: isolation of Ptk-3, a receptor expressed in proliferative zones of the developing brain. Proc. Natl. Acad. Sci. U.S.A. 91, 1819–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alves F., Vogel W., Mossie K., Millauer B., Höfler H., Ullrich A. (1995) Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene 10, 609–618 [PubMed] [Google Scholar]
- 8. Vogel W., Gish G. D., Alves F., Pawson T. (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1, 13–23 [DOI] [PubMed] [Google Scholar]
- 9. Leitinger B. (2011) Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 27, 265–290 [DOI] [PubMed] [Google Scholar]
- 10. Valiathan R. R., Marco M., Leitinger B., Kleer C. G., Fridman R. (2012) Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 31, 295–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh Y. C., Lin H. H., Tang M. J. (2012) A tale of two collagen receptors, integrin β1 and discoidin domain receptor 1, in epithelial cell differentiation. Am. J. Physiol. Cell. Physiol. 303, C1207–C1217 [DOI] [PubMed] [Google Scholar]
- 12. Alves F., Saupe S., Ledwon M., Schaub F., Hiddemann W., Vogel W. F. (2001) Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. FASEB J. 15, 1321–1323 [DOI] [PubMed] [Google Scholar]
- 13. Jin P., Zhang J., Sumariwalla P. F., Ni I., Jorgensen B., Crawford D., Phillips S., Feldmann M., Shepard H. M., Paleolog E. M. (2008) Novel splice variants derived from the receptor tyrosine kinase superfamily are potential therapeutics for rheumatoid arthritis. Arthritis Res. Ther. 10, R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kiedzierska A., Smietana K., Czepczynska H., Otlewski J. (2007) Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim. Biophys. Acta 1774, 1069–1078 [DOI] [PubMed] [Google Scholar]
- 15. Curat C. A., Eck M., Dervillez X., Vogel W. F. (2001) Mapping of epitopes in discoidin domain receptor 1 critical for collagen binding. J. Biol. Chem. 276, 45952–45958 [DOI] [PubMed] [Google Scholar]
- 16. Leitinger B. (2003) Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J. Biol. Chem. 278, 16761–16769 [DOI] [PubMed] [Google Scholar]
- 17. Abdulhussein R., McFadden C., Fuentes-Prior P., Vogel W. F. (2004) Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J. Biol. Chem. 279, 31462–31470 [DOI] [PubMed] [Google Scholar]
- 18. Xu H., Raynal N., Stathopoulos S., Myllyharju J., Farndale R. W., Leitinger B. (2011) Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 30, 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ichikawa O., Osawa M., Nishida N., Goshima N., Nomura N., Shimada I. (2007) Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J. 26, 4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carafoli F., Bihan D., Stathopoulos S., Konitsiotis A. D., Kvansakul M., Farndale R. W., Leitinger B., Hohenester E. (2009) Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure 17, 1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carafoli F., Mayer M. C., Shiraishi K., Pecheva M. A., Chan L. Y., Nan R., Leitinger B., Hohenester E. (2012) Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure 20, 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogel W. F. (2002) Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 514, 175–180 [DOI] [PubMed] [Google Scholar]
- 23. Slack B. E., Siniaia M. S., Blusztajn J. K. (2006) Collagen type I selectively activates ectodomain shedding of the discoidin domain receptor 1: involvement of Src tyrosine kinase. J. Cell. Biochem. 98, 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noordeen N. A., Carafoli F., Hohenester E., Horton M. A., Leitinger B. (2006) A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J. Biol. Chem. 281, 22744–22751 [DOI] [PubMed] [Google Scholar]
- 25. Uhlik M. T., Temple B., Bencharit S., Kimple A. J., Siderovski D. P., Johnson G. L. (2005) Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J. Mol. Biol. 345, 1–20 [DOI] [PubMed] [Google Scholar]
- 26. Taylor S. S., Kornev A. P. (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J., Yang P. L., Gray N. S. (2009) Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 9, 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Favelyukis S., Till J. H., Hubbard S. R., Miller W. T. (2001) Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Struct. Biol. 8, 1058–1063 [DOI] [PubMed] [Google Scholar]
- 29. Bertrand T., Kothe M., Liu J., Dupuy A., Rak A., Berne P. F., Davis S., Gladysheva T., Valtre C., Crenne J. Y., Mathieu M. (2012) The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J. Mol. Biol. 423, 439–453 [DOI] [PubMed] [Google Scholar]
- 30. Day E., Waters B., Spiegel K., Alnadaf T., Manley P. W., Buchdunger E., Walker C., Jarai G. (2008) Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur. J. Pharmacol. 599, 44–53 [DOI] [PubMed] [Google Scholar]
- 31. Rix U., Hantschel O., Dürnberger G., Remsing Rix L. L., Planyavsky M., Fernbach N. V., Kaupe I., Bennett K. L., Valent P., Colinge J., Köcher T., Superti-Furga G. (2007) Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 110, 4055–4063 [DOI] [PubMed] [Google Scholar]
- 32. Hammerman P. S., Sos M. L., Ramos A. H., Xu C., Dutt A., Zhou W., Brace L. E., Woods B. A., Lin W., Zhang J., Deng X., Lim S. M., Heynck S., Peifer M., Simard J. R., Lawrence M. S., Onofrio R. C., Salvesen H. B., Seidel D., Zander T., Heuckmann J. M., Soltermann A., Moch H., Koker M., Leenders F., Gabler F., Querings S., Ansén S., Brambilla E., Brambilla C., Lorimier P., Brustugun O. T., Helland A., Petersen I., Clement J. H., Groen H., Timens W., Sietsma H., Stoelben E., Wolf J., Beer D. G., Tsao M. S., Hanna M., Hatton C., Eck M. J., Janne P. A., Johnson B. E., Winckler W., Greulich H., Bass A. J., Cho J. (2011) Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 1, 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leitinger B., Kwan A. P. (2006) The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 25, 355–364 [DOI] [PubMed] [Google Scholar]
- 34. Farndale R. W., Lisman T., Bihan D., Hamaia S., Smerling C. S., Pugh N., Konitsiotis A., Leitinger B., de Groot P. G., Jarvis G. E., Raynal N. (2008) Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochem. Soc. Trans. 36, 241–250 [DOI] [PubMed] [Google Scholar]
- 35. Gu T. L., Deng X., Huang F., Tucker M., Crosby K., Rimkunas V., Wang Y., Deng G., Zhu L., Tan Z., Hu Y., Wu C., Nardone J., MacNeill J., Ren J., Reeves C., Innocenti G., Norris B., Yuan J., Yu J., Haack H., Shen B., Peng C., Li H., Zhou X., Liu X., Rush J., Comb M. J. (2011) Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS ONE 6, e15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giudici C., Raynal N., Wiedemann H., Cabral W. A., Marini J. C., Timpl R., Bächinger H. P., Farndale R. W., Sasaki T., Tenni R. (2008) Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J. Biol. Chem. 283, 19551–19560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parkin J. D., San Antonio J. D., Pedchenko V., Hudson B., Jensen S. T., Savige J. (2011) Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum. Mutat. 32, 127–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhadriraju K., Chung K. H., Spurlin T. A., Haynes R. J., Elliott J. T., Plant A. L. (2009) The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness in the down-regulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials 30, 6687–6694 [DOI] [PubMed] [Google Scholar]
- 39. Ith B., Wei J., Yet S. F., Perrella M. A., Layne M. D. (2005) Aortic carboxypeptidase-like protein is expressed in collagen-rich tissues during mouse embryonic development. Gene Expr. Patterns 5, 533–537 [DOI] [PubMed] [Google Scholar]
- 40. Agarwal G., Kovac L., Radziejewski C., Samuelsson S. J. (2002) Binding of discoidin domain receptor 2 to collagen I: an atomic force microscopy investigation. Biochemistry 41, 11091–11098 [DOI] [PubMed] [Google Scholar]
- 41. Agarwal G., Mihai C., Iscru D. F. (2007) Interaction of discoidin domain receptor 1 with collagen type 1. J. Mol. Biol. 367, 443–455 [DOI] [PubMed] [Google Scholar]
- 42. Ferri N., Carragher N. O., Raines E. W. (2004) Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am. J. Pathol. 164, 1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wall S. J., Werner E., Werb Z., DeClerck Y. A. (2005) Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J. Biol. Chem. 280, 40187–40194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vogel W. F., Aszódi A., Alves F., Pawson T. (2001) Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell. Biol. 21, 2906–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Labrador J. P., Azcoitia V., Tuckermann J., Lin C., Olaso E., Mañes S., Brückner K., Goergen J. L., Lemke G., Yancopoulos G., Angel P., Martínez C., Klein R. (2001) The collagen receptor DDR2 regulates proliferation, and its elimination leads to dwarfism. EMBO Rep. 2, 446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kano K., Kitamura A., Matsuwaki T., Morimatsu M., Naito K. (2010) Discoidin domain receptor 2 (DDR2) is required for maintenance of spermatogenesis in male mice. Mol. Reprod. Dev. 77, 29–37 [DOI] [PubMed] [Google Scholar]
- 47. Kano K., Marín de Evsikova C., Young J., Wnek C., Maddatu T. P., Nishina P. M., Naggert J. K. (2008) A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol. Endocrinol. 22, 1866–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dengjel J., Akimov V., Olsen J. V., Bunkenborg J., Mann M., Blagoev B., Andersen J. S. (2007) Quantitative proteomic assessment of very early cellular signaling events. Nat. Biotechnol. 25, 566–568 [DOI] [PubMed] [Google Scholar]
- 49. Furdui C. M., Lew E. D., Schlessinger J., Anderson K. S. (2006) Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol. Cell 21, 711–717 [DOI] [PubMed] [Google Scholar]
- 50. Avraham R., Yarden Y. (2011) Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 [DOI] [PubMed] [Google Scholar]
- 51. Peschard P., Park M. (2007) From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 26, 1276–1285 [DOI] [PubMed] [Google Scholar]
- 52. Amit I., Citri A., Shay T., Lu Y., Katz M., Zhang F., Tarcic G., Siwak D., Lahad J., Jacob-Hirsch J., Amariglio N., Vaisman N., Segal E., Rechavi G., Alon U., Mills G. B., Domany E., Yarden Y. (2007) A module of negative feedback regulators defines growth factor signaling. Nat. Genet. 39, 503–512 [DOI] [PubMed] [Google Scholar]
- 53. Wang C. Z., Su H. W., Hsu Y. C., Shen M. R., Tang M. J. (2006) A discoidin domain receptor 1/SHP-2 signaling complex inhibits α2β1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol. Biol. Cell 17, 2839–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang C. Z., Yeh Y. C., Tang M. J. (2009) DDR1/E-cadherin complex regulates the activation of DDR1 and cell spreading. Am. J. Physiol. Cell Physiol. 297, C419–C429 [DOI] [PubMed] [Google Scholar]
- 55. Marshall C. J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 [DOI] [PubMed] [Google Scholar]
- 56. Santos S. D., Verveer P. J., Bastiaens P. I. (2007) Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 9, 324–330 [DOI] [PubMed] [Google Scholar]
- 57. Foehr E. D., Tatavos A., Tanabe E., Raffioni S., Goetz S., Dimarco E., De Luca M., Bradshaw R. A. (2000) Discoidin domain receptor 1 (DDR1) signaling in PC12 cells: activation of juxtamembrane domains in PDGFR/DDR/TrkA chimeric receptors. FASEB J. 14, 973–981 [DOI] [PubMed] [Google Scholar]
- 58. Hannigan G. E., McDonald P. C., Walsh M. P., Dedhar S. (2011) Integrin-linked kinase: not so 'pseudo' after all. Oncogene 30, 4375–4385 [DOI] [PubMed] [Google Scholar]
- 59. Zhang H., Photiou A., Grothey A., Stebbing J., Giamas G. (2012) The role of pseudokinases in cancer. Cell. Signal. 24, 1173–1184 [DOI] [PubMed] [Google Scholar]
- 60. Hidalgo-Carcedo C., Hooper S., Chaudhry S. I., Williamson P., Harrington K., Leitinger B., Sahai E. (2011) Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat. Cell Biol. 13, 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gordus A., Krall J. A., Beyer E. M., Kaushansky A., Wolf-Yadlin A., Sevecka M., Chang B. H., Rush J., MacBeath G. (2009) Linear combinations of docking affinities explain quantitative differences in RTK signaling. Mol. Syst. Biol. 5, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lemeer S., Bluwstein A., Wu Z., Leberfinger J., Müller K., Kramer K., Kuster B. (2012) Phosphotyrosine-mediated protein interactions of the discoidin domain receptor 1. J. Proteomics 75, 3465–3477 [DOI] [PubMed] [Google Scholar]
- 63. Roepstorff K., Grandal M. V., Henriksen L., Knudsen S. L., Lerdrup M., Grøvdal L., Willumsen B. M., van Deurs B. (2009) Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 10, 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilson K. J., Gilmore J. L., Foley J., Lemmon M. A., Riese D. J., 2nd (2009) Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol. Ther. 122, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abella J. V., Park M. (2009) Breakdown of endocytosis in the oncogenic activation of receptor tyrosine kinases. Am. J. Physiol. Endocrinol Metab. 296, E973–E984 [DOI] [PubMed] [Google Scholar]
- 66. Mihai C., Chotani M., Elton T. S., Agarwal G. (2009) Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J. Mol. Biol. 385, 432–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murphy J. E., Padilla B. E., Hasdemir B., Cottrell G. S., Bunnett N. W. (2009) Endosomes: a legitimate platform for the signaling train. Proc. Natl. Acad. Sci. U.S.A. 106, 17615–17622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Margadant C., Monsuur H. N., Norman J. C., Sonnenberg A. (2011) Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 23, 607–614 [DOI] [PubMed] [Google Scholar]
- 69. Bogdanović O., Delfino-Machín M., Nicolás-Pérez M., Gavilán M. P., Gago-Rodrigues I., Fernández-Miñán A., Lillo C., Ríos R. M., Wittbrodt J., Martínez-Morales J. R. (2012) Numb/Numbl-opo antagonism controls retinal epithelium morphogenesis by regulating integrin endocytosis. Dev. Cell 23, 782–795 [DOI] [PubMed] [Google Scholar]
- 70. Hayashida K., Bartlett A. H., Chen Y., Park P. W. (2010) Molecular and cellular mechanisms of ectodomain shedding. Anat. Rec. 293, 925–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Curat C. A., Vogel W. F. (2002) Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J. Am. Soc. Nephrol. 13, 2648–2656 [DOI] [PubMed] [Google Scholar]
- 72. Flynn L. A., Blissett A. R., Calomeni E. P., Agarwal G. (2010) Inhibition of collagen fibrillogenesis by cells expressing soluble extracellular domains of DDR1 and DDR2. J. Mol. Biol. 395, 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sivakumar L., Agarwal G. (2010) The influence of discoidin domain receptor 2 on the persistence length of collagen type I fibers. Biomaterials 31, 4802–4808 [DOI] [PubMed] [Google Scholar]
- 74. Rowe R. G., Weiss S. J. (2009) Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu. Rev. Cell Dev. Biol. 25, 567–595 [DOI] [PubMed] [Google Scholar]
- 75. L'hôte C. G., Thomas P. H., Ganesan T. S. (2002) Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. FASEB J. 16, 234–236 [DOI] [PubMed] [Google Scholar]
- 76. Dejmek J., Dib K., Jönsson M., Andersson T. (2003) Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int. J. Cancer 103, 344–351 [DOI] [PubMed] [Google Scholar]
- 77. Koo D. H., McFadden C., Huang Y., Abdulhussein R., Friese-Hamim M., Vogel W. F. (2006) Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 580, 15–22 [DOI] [PubMed] [Google Scholar]
- 78. Yang G., Li Q., Ren S., Lu X., Fang L., Zhou W., Zhang F., Xu F., Zhang Z., Zeng R., Lottspeich F., Chen Z. (2009) Proteomic, functional and motif-based analysis of C-terminal Src kinase-interacting proteins. Proteomics 9, 4944–4961 [DOI] [PubMed] [Google Scholar]
- 79. Lu K. K., Trcka D., Bendeck M. P. (2011) Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovasc. Pathol. 20, 71–76 [DOI] [PubMed] [Google Scholar]
- 80. Suh H. N., Han H. J. (2011) Collagen I regulates the self-renewal of mouse embryonic stem cells through α2β1 integrin- and DDR1-dependent Bmi-1. J. Cell. Phyisiol. 226, 3422–3432 [DOI] [PubMed] [Google Scholar]
- 81. Ongusaha P. P., Kim J. I., Fang L., Wong T. W., Yancopoulos G. D., Aaronson S. A., Lee S. W. (2003) p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 22, 1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ikeda K., Wang L. H., Torres R., Zhao H., Olaso E., Eng F. J., Labrador P., Klein R., Lovett D., Yancopoulos G. D., Friedman S. L., Lin H. C. (2002) Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J. Biol. Chem. 277, 19206–19212 [DOI] [PubMed] [Google Scholar]
- 83. Olaso E., Lin H. C., Wang L. H., Friedman S. L. (2011) Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang K., Kim J. H., Kim H. J., Park I. S., Kim I. Y., Yang B. S. (2005) Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J. Biol. Chem. 280, 39058–39066 [DOI] [PubMed] [Google Scholar]
- 85. Sweeney S. M., Orgel J. P., Fertala A., McAuliffe J. D., Turner K. R., Di Lullo G. A., Chen S., Antipova O., Perumal S., Ala-Kokko L., Forlino A., Cabral W. A., Barnes A. M., Marini J. C., San Antonio J. D. (2008) Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 283, 21187–21197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shintani Y., Fukumoto Y., Chaika N., Svoboda R., Wheelock M. J., Johnson K. R. (2008) Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 180, 1277–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yeh Y. C., Wang C. Z., Tang M. J. (2009) Discoidin domain receptor 1 activation suppresses α2β1 integrin-dependent cell spreading through inhibition of Cdc42 activity. J. Cell. Physiol. 218, 146–156 [DOI] [PubMed] [Google Scholar]
- 88. Xu H., Bihan D., Chang F., Huang P. H., Farndale R. W., Leitinger B. (2012) Discoidin domain receptors promote α1β1- and α2β1-integrin-mediated cell adhesion to collagen by enhancing integrin activation. PLoS ONE 7, e52209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jönsson M., Andersson T. (2001) Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J. Cell Sci. 114, 2043–2053 [DOI] [PubMed] [Google Scholar]
- 90. Roarty K., Serra R. (2007) Wnt5a is required for proper mammary gland development and TGF-β-mediated inhibition of ductal growth. Development 134, 3929–3939 [DOI] [PubMed] [Google Scholar]
- 91. Kim H. G., Hwang S. Y., Aaronson S. A., Mandinova A., Lee S. W. (2011) DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J. Biol. Chem. 286, 17672–17681 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92. Iwai L. K., Chang F., Huang P. H. (2013) Phosphoproteomic analysis identifies insulin enhancement of discoidin domain receptor 2 phosphorylation. Cell Adh. Migr. 7, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Huang P. H. (2012) Phosphoproteomic studies of receptor tyrosine kinases: future perspectives. Mol. Biosyst. 8, 1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]