Background: Growth factor receptors in endothelial cells are an important therapeutic target for anti-angiogenic therapy.
Results: Inhibitors of endocytosis suppress ERK1/2 activation downstream of growth factor receptors in endothelial cells.
Conclusion: Receptor internalization is required for pro-angiogenic growth factors to activate ERK1/2 in endothelial cells.
Significance: Agents that disrupt receptor internalization could be developed as a means to inhibit angiogenesis in cancer.
Keywords: Angiogenesis, Cancer, Endocytosis, Fibroblast Growth Factor (FGF), Hepatocyte Growth Factor, Signaling, Vascular Endothelial Growth Factor (VEGF)
Abstract
Vascular endothelial growth factor (VEGF) stimulates angiogenesis by binding to VEGF receptor 2 (VEGFR2) on endothelial cells (ECs). Downstream activation of the extracellular related kinases 1/2 (ERK1/2) is important for angiogenesis to proceed. Receptor internalization has been implicated in VEGFR2 signaling, but its role in the activation of ERK1/2 is unclear. To explore this question we utilized pitstop and dynasore, two small molecule inhibitors of endocytosis. First, we confirmed that both inhibitors block the internalization of VEGFR2 in ECs. We then stimulated ECs with VEGF in the presence and absence of the inhibitors and examined VEGFR2 signaling to ERK1/2. Activation of VEGFR2 and C-Raf still occurred in the presence of the inhibitors, whereas the activation of MEK1/2 and ERK1/2 was abrogated. Therefore, although internalization is not required for activation of either VEGFR2 or C-Raf in ECs stimulated with VEGF, internalization is necessary to activate the more distal kinases in the cascade. Importantly, inhibition of internalization also prevented activation of ERK1/2 when ECs were stimulated with other pro-angiogenic growth factors, namely fibroblast growth factor 2 and hepatocyte growth factor. In contrast, the same inhibitors did not block ERK1/2 activation in fibroblasts or cancer cells stimulated with growth factors. Finally, we show that these small molecule inhibitors of endocytosis block angiogenesis in vitro and in vivo. Therefore, receptor internalization may be a generic requirement for pro-angiogenic growth factors to activate ERK1/2 signaling in human ECs, and targeting receptor trafficking may present a therapeutic opportunity to block tumor angiogenesis.
Introduction
The development of new blood vessels from the existing vasculature, termed angiogenesis, is required for tumors to grow beyond a microscopic size and is an important therapeutic target in cancer (1–3). The vascular endothelial growth factor (VEGF) family of growth factors is the most studied growth factor family involved in this process. VEGF is expressed by almost all solid cancers and drives the process of angiogenesis through two cognate receptor tyrosine kinases that are expressed on vascular endothelial cells: VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2)2 (4–6). Numerous inhibitors of the VEGF signaling axis have been developed, including the VEGF-neutralizing antibody bevacizumab and the VEGF receptor tyrosine kinase inhibitor, sunitinib. These inhibitors have been shown to suppress tumor growth in preclinical models and have shown promising results in patients (1, 7–9). However, despite strong evidence that VEGF inhibition can control tumor growth, the presence of innate and acquired resistance to these drugs significantly limits their clinical efficacy (10–14).
Because VEGF is not the only growth factor that is able to stimulate tumor angiogenesis, redundancy between pro-angiogenic growth factors may be one mechanism that limits the efficacy of VEGF-targeted therapy (13–15). For example, fibroblast growth factor 2 (FGF2) is a potent pro-angiogenic growth factor that signals through two FGF receptors expressed on endothelial cells, FGFR1 and FGFR2 (16). Hepatocyte growth factor (HGF) can also stimulate angiogenesis by signaling through the MET receptor expressed on endothelial cells (17, 18). Both FGF2 and HGF can mediate resistance to VEGF receptor inhibition by providing an alternative pro-angiogenic signal for endothelial cells (18–20), and increased tumor expression of both FGF2 and HGF has been linked with resistance to VEGF pathway inhibitors in both preclinical and clinical studies (21–24). Therefore, drugs that are designed to inhibit multiple pro-angiogenic growth factors may prove to be more effective than anti-angiogenic agents that block the VEGF pathway alone (13–15). Pro-angiogenic growth factors activate multiple downstream signaling pathways that coordinate the angiogenic response in endothelial cells (6, 25). For example, VEGF, FGF2, and HGF all activate the classical Raf-MEK-ERK signaling cascade. This pathway has been reported to exert control on angiogenesis through multiple mechanisms in endothelial cells, including activation of gene transcription, stimulation of proliferation and migration, pro-survival signaling, and control of cell contractility (26–29). Moreover, suppression of the Raf-MEK-ERK signaling cascade selectively in the tumor vasculature can suppress tumor growth in animal models (28, 30, 31).
VEGFR2 is one of many receptor tyrosine kinases that undergoes endocytosis in response to ligand stimulation. After VEGF stimulation, VEGFR2 is internalized within clathrin-coated vesicles (32, 33). The fission of clathrin-coated vesicles from the plasma membrane is dependent on the GTPase dynamin II (34). These vesicles then become uncoated and deliver their contents to the early endosomes. There is a growing appreciation that the endocytosis of receptor tyrosine kinases plays a critical role in their signaling (35–37). First, ligand-induced receptor internalization can attenuate signaling both by reducing the bioavailability of the receptor at the cell surface and by mediating the delivery of receptors to degradation compartments. In the case of VEGFR2, receptor internalization has been reported to facilitate the degradation of the receptor by lysosomes and the proteasome (32, 38, 39). Second, internalization may regulate the amplitude and/or duration of downstream signaling. For example, several studies have demonstrated that inhibition of internalization can dampen VEGFR2 signaling (33, 40, 41). Third, recycling of receptors back to the plasma membrane may regulate the amplitude of the biological response. We previously showed that pharmacological stimulation of VEGFR2 recycling leads to enhanced endothelial cell migration and angiogenesis in response to VEGF (42). However, it is still not precisely clear how receptor trafficking in endothelial cells is coupled to the activation of downstream signaling pathways. Here, we utilize two small molecular inhibitors of endocytosis to address how inhibition of internalization affects activation of ERK1/2 in endothelial cells.
EXPERIMENTAL PROCEDURES
Reagents
Fetal calf serum (FCS) was from Invitrogen, and bovine brain endothelial cell mitogen was from Serotech (Kidlington, Oxfordshire, UK). The clathrin inhibitor Pitstop 2, referred to as “pitstop” throughout the manuscript, and the Pitstop 2 negative control compound, were obtained from Abcam Biochemicals (Cambridge, UK). The dynamin inhibitor dynasore monohydrate, referred to as “dynasore” throughout the manuscript, and 12-O-tetradecanolyphorbol-13-acetate (TPA), were both obtained from Sigma. Human VEGF-165A, EGF, and HGF were from R&D systems (Abingdon, Oxfordshire, UK), and human FGF2 and PDGF-BB were from Peprotech (London, UK). Antibodies were obtained from the following sources: phospho-Thr-202/Tyr-204-ERK1/2 (Sigma), dynamin II, EEA1, endomucin, HSC70, VE-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA), VEGFR2 extracellular domain (R&D Systems, Abingdon, Oxfordshire, UK), clathrin, ERK1/2, phosho-Ser-217/Ser-221-MEK1/2, VEGFR2 intracellular domain, phospho-Tyr-1175-VEGFR2 (Cell Signaling Technology, Danvers, MA), phospho-Tyr-340/Tyr-341-C-Raf (Invitrogen), C-Raf, MEK1 (BD Biosciences), and fluorescently conjugated secondary antibodies (Invitrogen). Unless otherwise stated, all other reagents were obtained from Sigma.
Tissue Culture
For standard culture, human umbilical vein endothelial cells (HUVECs) from pooled donors (TCS Cell Works, Buckingham, Buckinghamshire, UK) were seeded onto tissue culture flasks precoated with 0.1% gelatin and grown in M199 medium supplemented with 20% FCS, 0.1 mg/ml bovine brain endothelial mitogen, 1 ng/ml heparin, and antibiotics at 37 °C in 10% CO2. HUVECs were used for experiments at passages 4–8. The murine fibroblast cell line 10T1/2 and the human breast cancer cell line MDA-MB-231 were both cultured in DMEM supplemented with 10% FCS and antibiotics at 37 °C in 5% CO2.
Antibody Feeding Assay and Immunofluorescence
HUVECs were seeded onto glass coverslips precoated with 0.1% gelatin. After 48 h in culture, the cells were incubated for 2.5 h in serum-free M199 medium at 37 °C and then surface-labeled in PBS supplemented with goat anti-VEGFR2 (extracellular domain specific) antibody for 30 min at 4 °C. After labeling, coverslips were briefly washed with PBS and then either directly fixed (to confirm that the antibody labeling was restricted to the cell surface) or transferred to prewarmed serum-free M199 medium at 37 °C for 30 min to permit internalization to proceed and then fixed. For the purposes of quantifying VEGFR2 internalization, four different conditions were tested: 1) cells were antibody-labeled at 4 °C in the presence of vehicle alone (0.1% DMSO) and then allowed to internalize in the presence of vehicle alone, or 2) cells were antibody labeled at 4 °C in the presence of vehicle and then allowed to internalize in the presence of 50 ng/ml VEGF and vehicle, or 3) cells were antibody labeled at 4 °C in the presence of 10 μm pitstop and then allowed to internalize in the presence of 50 ng/ml VEGF and 10 μm pitstop, or 4) cells were antibody-labeled at 4 °C in the presence of 80 μm dynasore and then allowed to internalize in the presence of 50 ng/ml VEGF and 80 μm dynasore. Fixation was performed in 4% w/v formaldehyde for 20 min at room temperature. After washing in PBS, cells were permeabilized in PBS plus 0.1% Triton X-100 for 10 min and then incubated with primary antibody (1:500 anti-EEA1 in PBS) for 30 min. After washing 3 × 5 min in PBS, coverslips were incubated for 30 min with Alexa555-conjugated anti-goat secondary antibody to detect VEGFR2 and Alexa 647-conjugated anti-rabbit secondary antibody to detect EEA1 in PBS supplemented with DAPI. After washing 3 × 5 min in PBS, coverslips were mounted on glass slides in MOWIOL mounting medium plus anti-fade (0.1% w/v 1,4-diazabicyclo[2.2.2]octane). Images were captured using a Zeiss laser scanning confocal microscope. Quantification of VEGFR2 internalization was performed by manually counting the number of VEGFR2/EEA1 dual-positive endosomes per cell.
Western Blotting
Cells were seeded onto 10-cm-diameter tissue culture dishes and grown for 48 h to obtain 70–80% confluency. In all experiments cells were serum-deprived for 3 h in serum-free medium before stimulation. Dynasore, pitstop, pitstop negative control compound (at the indicated concentration), or vehicle alone (0.1% DMSO) was added to the medium at 15 min (pitstop or pitstop negative control) or 30 min (dynasore) before stimulation. Stimulation was achieved by adding growth factor, FCS, or TPA to the medium (final concentrations in the medium were 50 ng/ml VEGF, 25 ng/ml FGF2, 10 ng/ml HGF, 10 ng/ml platelet-derived growth factor (PDGF), 100 ng/ml epidermal growth factor (EGF), 10% FCS, or 10 μm TPA). At the appropriate time point, cells were transferred to ice, washed twice with ice-cold PBS, and lysed in 100 μl of lysis buffer (150 mm NaCl, 20 mm Tris pH 7.5, 10% glycerol, 1% IGEPAL CA-630, 1 mm Na3VO4, 10 mm NaF 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride, 0.8 mm aprotinin, 0.05 mm bestatin, 0.015 mm E-64, 0.02 mm leupeptin, and 0.01 mm pepstatin). Lysates were obtained by scraping and spun down at 15,000 × g, and the supernatant was collected and stored at −20 °C.
For Western blotting, lysates were prepared by the addition of 4× Laemmli sample buffer (final concentration 1×), then boiled at 95 °C for 5 min before loading on precast 7% or 10% SDS-PAGE gels (Invitrogen). Gels were run at 150 V for 90 min at room temperature and then transferred to nitrocellulose membranes (GE Healthcare) at 12 V overnight at 4 °C. Membranes were blocked in appropriate blocking buffer (TBS-T or PBS-T supplemented with 5% milk or 5% BSA, depending on the antibody) and incubated for either 1 h at room temperature or overnight at 4 °C with primary antibody diluted in the same blocking buffer. After 3 × 5-min washes in PBS-T or TBS-T, membranes were incubated with appropriate HRP-conjugated secondary antibody in blocking buffer. After 3 × 5-min washes in PBS-T or TBS-T, membranes were incubated with chemiluminescence substrate for 1 min before being exposed to films. Densitometry was performed using ImageJ software. For standard Western blots, the phospho immunoblot signal was normalized to the total immunoblot signal for the protein of interest. Densitometry results are presented as the fold change relative to the non-stimulated control.
Lentiviral shRNA
shRNA oligonucleotides were ligated into the pENTR/U6 Gateway system entry vector (Invitrogen) according to the manufacturer's instructions. The following shRNA oligonucleotides were utilized: clathrin (5′-AAAAGCTTCAGTACCCTGACTATGGTTCGCCATAGTCAGGGTACTGAG-3′); dynamin II, (5′-AAAAGGCTGACCATCAACAACATCATTCGTGATGTTGTTGATGGTCAGCC-3′); non-targeting (5′-CACCGGAGCCTTCAGGATTACAAGACGAATCTTGTAATCCTGAAGGCC-3′).
Oligonucleotide sequences were verified by sequencing and then transferred together with the U6 promoter into a Gateway-modified pSEW lentiviral vector backbone according to the manufacturer's instructions. Viral supernatants were generated by Lipofectamine co-transfection of the expression vector and two packaging vectors (psPAX2 and pMD2.G) into HEK293T cells. Viral supernatants were collected and stored at-80 °C until use. HUVECs were plated on 6-well plates or onto 10-cm-diameter tissue culture dishes and transduced the next day with virus. Cells were used for experiments at 72 h post-infection. Stimulation of cells and Western blotting was performed as described above.
Cell Surface Biotinylation
For assessing the effects of the inhibitors on cell surface VEGFR2 levels, HUVECs were serum-deprived for 2.5 h in serum-free medium and then treated as follows; 1) cells were treated with vehicle alone (0.1% DMSO) for 45 min and then biotinylated, or 2) cells were treated with vehicle alone for 30 min followed by 50 ng/ml VEGF and vehicle for 15 min and then biotinylated, or 3) cells were treated with 10 μm pitstop for 30 min followed by 50 ng/ml VEGF and 10 μm pitstop for 15 min and then biotinylated, or 4) cells were treated with 80 μm dynasore for 30 min followed by 50 ng/ml VEGF and 80 μm dynasore for 15 min and then biotinylated. For assessing the effects of the shRNA knockdown on cell surface VEGFR2 levels, cells were infected with lentivirus for 72 h, serum-deprived for 3 h in serum-free medium, and then treated as follows; 1) control shRNA-infected cells were directly biotinylated, or 2) control shRNA-infected cells were stimulated with 50 ng/ml VEGF for 15 min and then biotinylated, or 3) clathrin shRNA-infected cells were stimulated with 50 ng/ml VEGF for 15 min and then biotinylated, or 4) dynamin II shRNA-infected cells were stimulated with 50 ng/ml VEGF for 15 min and then biotinylated. Biotinylation was performed by washing the cells twice with ice-cold PBS then incubating for 15 min at 4 °C with 0.2 mg/ml Sulfo-NHS-Biotin in PBS (Thermo Scientific, Rockford, IL). Biotinylation was quenched with ice-cold 50 mm glycine in PBS. After further washing in ice-cold PBS, cells were lysed in 150 μl of lysis buffer (150 mm NaCl, 20 mm Tris, pH 7.5, 10% glycerol, 1% IGEPAL CA-630, 1 mm Na3VO4, 10 mm NaF, 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride, 0.8 mm aprotinin, 0.05 mm bestatin, 0.015 mm E-64, 0.02 mm leupeptin, and 0.01 mm pepstatin). Lysates were obtained by scraping and spun down at 15,000 × g, and the supernatant was collected. Protein quantification of the lysates was determined using a BCA protein quantification kit (Thermo Scientific). 20 μg of the lysate was reserved and probed for total VEGFR2 (loading control lysate). Biotinylated proteins were immunoprecipitated from 200 μg of lysate using 50 μl of NeutrAvidin beads (Thermo Scientific) at 4 °C overnight. Beads were washed 4 times with 1 ml of lysis buffer and then resuspended in Laemmli sample buffer. Immunoprecipitates were analyzed by Western blotting for VEGFR2.
Densitometry was performed using ImageJ software. Levels of immunoprecipitated VEGFR2 were normalized to total VEGFR2 levels (which were determined by blotting the loading control lysate for VEGFR2). Densitometry results are presented as the fold change relative to the non-stimulated control.
In Vitro Angiogenesis Assay
Tube formation assays were performed using a modified version of a previously published protocol (19, 43). In brief, HUVECs were cultured for 24 h in EGM2 complete medium (Lonza, Slough, Berkshire, UK), and then cells were trypsinized and resuspended in EGM2 at a concentration of 2 × 106 HUVECs per ml. Cytodex3 beads (GE Healthcare) were coated with HUVECs by incubating 2 × 106 HUVECs with 3 × 104 beads for 4 h with gentle agitation every 20 min. Coated beads were then diluted to a volume of 5 ml in EGM2 and placed in a tissue-culture incubator overnight. The next day beads were washed in EGM2 and resuspended in 50 ml of sterile 2 mg/ml fibrinogen in PBS. A volume of 500 μl of bead fibrinogen solution was then transferred to each well of a 24-well plate (∼100 beads per well) containing 6.25 μl of 50 units/ml thrombin to initiate gel polymerization. Fibrinogen gels were allowed to set for 15 min at 37 °C, and then 2 × 104 fibroblasts in EGM2 were seeded on top of each well. After 24 h, medium was exchanged for EBM2 (Lonza) plus 2.5% FCS supplemented with growth factors (10 ng/ml VEGF and 50 ng/ml FGF2) and inhibitor at the indicated concentration or vehicle (0.1% DMSO). The plates were re-fed every 2 days. After 7 days, cultures were fixed in 4% w/v formaldehyde for 20 min and then washed twice with PBS. The number of tubules sprouted per bead was counted in random fields using a light microscope. For staining of cultures, fixed preparations were permeabilized for 20 min with PBS plus 0.1% Triton X-100 and then incubated overnight at 4 °C with rhodamine-phalloidin conjugate and DAPI. After 3 × 5 min washes in PBS, cultures were transferred to PBS supplemented with anti-fade (0.1% w/v 1,4-diazabicyclo[2.2.2]octane) and then imaged on a Zeiss inverted laser scanning microscope.
In Vivo Sponge Assay of Angiogenesis
Female C57/BL6 mice were obtained from Charles River UK Ltd (Margate, Kent, UK). The assay was performed essentially as described (44, 45). Briefly, C57/BL6 mice were anesthetized with isofluorane followed by the subcutaneous implantation of 2 sterile sponge discs (measuring 10 × 5 × 5 mm) into both flanks (Caligen Foam Ltd, Accrington, Lancashire, UK). The sponges were injected 3 times per week with 100 μl of PBS containing 1) vehicle alone, 2) 160 μm dynasore, 3) 10 ng/ml VEGF, 10 ng/ml FGF-2, and vehicle or 4) 10 ng/ml VEGF, 10 ng/ml FGF-2, and 160 μm dynasore. After 21 days animals were sacrificed by cervical dislocation, and the sponges were rapidly excised, fixed in 4% w/v formaldehyde at 4 °C overnight, and then transferred to 70% ethanol the next day. For analysis of vessel infiltration, staining for endomucin was performed as described (45). In brief, paraffin-embedded, 5-μm tissue sections were deparaffinized by immersing the slides twice in 100% xylene followed by incubating twice in 100% ethanol and rehydrating with decreasing concentrations of ethanol (90 and 70%) before a final incubation in water. Sections were then incubated with 3% hydrogen peroxidase in methanol to quench endogenous peroxidase activity, and antigen retrieval was carried out using a citrate buffer. This was followed by blocking the sections using PBS supplemented with 3% BSA. Sections were incubated with endomucin antibody in PBS supplemented with 1% BSA, and after washing with PBS, sections were incubated with a biotinylated secondary antibody followed by incubation with streptavidin-biotin peroxidase solution. Antibody binding was revealed using 3,3′-diaminobenzine, and sections were lightly counterstained using Mayer's hematoxylin. Sections were dehydrated by immersing in increasing concentrations of ethanol (70, 90, 100%) followed by immersing in 100% xylene. Blood vessel infiltration was determined by counting endomucin-positive vessels across each tissue section in a blind fashion using a light microscope. Vessel density was calculated as number of vessels per section/area of section.
Ethical Approval for Animal Experimentation
Ethical approval for animal experimentation was granted by the Institute of Cancer Research Animal Ethics Committee, and all procedures were performed in accordance with UK Home Office regulations.
Statistical Analysis
Analyses of statistical significance were performed using the Student's t test (p values of less than 0.05 were considered to be statistically significant).
RESULTS
Small Molecule Inhibitors of Endocytosis Suppress the Internalization of VEGFR2 in Endothelial Cells
To address the role of receptor internalization in the activation of ERK1/2, we utilized pitstop and dynasore, two small molecule inhibitors of endocytosis (46, 47). To confirm that pitstop and dynasore can inhibit the internalization of VEGFR2 in endothelial cells, we used an “antibody feeding” assay similar to that used to monitor the fate of internalized VEGF receptors in other studies (33, 40, 41, 48). Plasma membrane VEGFR2 molecules were labeled on ice with a VEGFR2 extracellular domain-specific antibody. Examination of cells fixed directly after this labeling period demonstrated the retention of the VEGFR2 antibody at the cell surface and no colocalization with endosomes (Fig. 1A). However, when labeled cells were warmed to 37 °C for 30 min, the internalization of VEGFR2 antibody to endosomes was observed (Fig. 1B). This was expected, because VEGFR2 undergoes ligand-independent constitutive internalization in endothelial cells (36). When the labeled cells were warmed to 37 °C for 30 min in the presence of VEGF, the quantity of VEGFR2 internalized to early endosomes was significantly increased (Fig. 1C). This is consistent with a ligand-induced enhancement in receptor internalization (32, 33). However, when labeled cells were incubated with VEGF in the presence of pitstop or dynasore, the internalization of VEGFR2 to endosomes was suppressed, and the majority of VEGFR2 antibody labeling remained restricted to the plasma membrane (Fig. 1, D and E). Quantitative analysis of VEGFR2 colocalization with endosomes confirmed that both pitstop and dynasore significantly suppressed VEGFR2 internalization in endothelial cells (Fig. 1F). Importantly, when comparing treatment groups, there was no discernable difference in either the appearance of the endosomal compartment or the number of endosomes present per cell (supplemental Fig. 1).
FIGURE 1.
Pitstop and dynasore inhibit VEGFR2 internalization in endothelial cells. A–F, shown is an antibody feeding assay. Cell surface VEGFR2 was labeled in HUVECs with a VEGFR2 extracellular domain-specific antibody for 30 min at 4 °C. Cells were then either fixed directly or shifted to 37 °C for 30 min and then fixed. Fixed cells were stained with a fluorescently tagged secondary antibody to detect anti-VEGFR2 (VR2) antibody (green) and co-stained for the endosomal marker EEA1 (red). A, localization of anti-VEGFR2 antibody in cells after labeling was performed in the presence of vehicle for 30 min at 4 °C. B, localization of anti-VEGFR2 antibody after labeling in the presence of vehicle for 30 min at 4 °C followed by warming to 37 °C for 30 min in the presence of vehicle is shown. C, localization of anti-VEGFR2 antibody after labeling in the presence of vehicle for 30 min at 4 °C followed by warming to 37 °C for 30 min in the presence of VEGF and vehicle is shown. D, localization of anti-VEGFR2 antibody after labeling in the presence of 10 μm pitstop for 30 min at 4 °C followed by warming to 37 °C for 30 min in the presence of VEGF and 10 μm pitstop is shown. E, localization of anti-VEGFR2 antibody after labeling in the presence of 80 μm dynasore for 30 min at 4 °C followed by warming to 37 °C for 30 min in the presence of VEGF and 80 μm dynasore is shown. F, quantification of VEGFR2 internalization in endothelial cells treated as indicated (same conditions as shown in panels B–E) is shown. The graph shows the number of intracellular structures labeling dual positive for VEGFR2 and EEA1 per cell ± S.E. Quantification of 20 cells from 2 independent experiments was used to generate each data point. *, p < 0.05; **, p < 0.001. Scale bar = 5 μm. G, cell surface biotinylation is shown. HUVECs were treated as follows; vehicle for 45 min (lane 1), vehicle for 30 min followed by VEGF and vehicle for 15 min (lane 2), 10 μm pitstop for 30 min followed by VEGF and 10 μm pitstop for 15 min (lane 3), or 80 μm dynasore for 30 min followed by VEGF and 80 μm dynasore for 15 min (lane 4). Cells were then surface-biotinylated and lysed, and the biotinylated fraction was immunoprecipitated (IP) and probed for VEGFR2. IB, immunoblot. In panels A–E arrowheads indicate VEGFR2-positive endosomes. Arrows indicate VEGFR2 membrane staining.
We also performed surface biotinylation assays to confirm that pitstop and dynasore treatment resulted in retention of VEGFR2 at the cell surface. Cells were either incubated with vehicle alone, or they were incubated with VEGF in the presence of vehicle, pitstop, or dynasore. Cells were then subjected to cell surface biotinylation with a membrane-impermeant biotinylation reagent. The biotinylated fraction was immunoprecipitated and probed for VEGFR2. The amount of cell surface VEGFR2 was significantly reduced in cells incubated with VEGF and vehicle compared with cells incubated with vehicle alone (Fig. 1G, supplemental Fig. 2). However, in the presence of VEGF and pitstop or VEGF and dynasore, the quantity of cell surface VEGFR2 was significantly increased compared with cells treated with VEGF and vehicle (Fig. 1G, supplemental Fig. 2). These data show that both pitstop and dynasore are able to suppress VEGFR2 internalization in endothelial cells, resulting in the retention of VEGFR2 at the plasma membrane.
Internalization Is Required for Activation of MEK1/2 and ERK1/2 in VEGF-stimulated Endothelial Cells
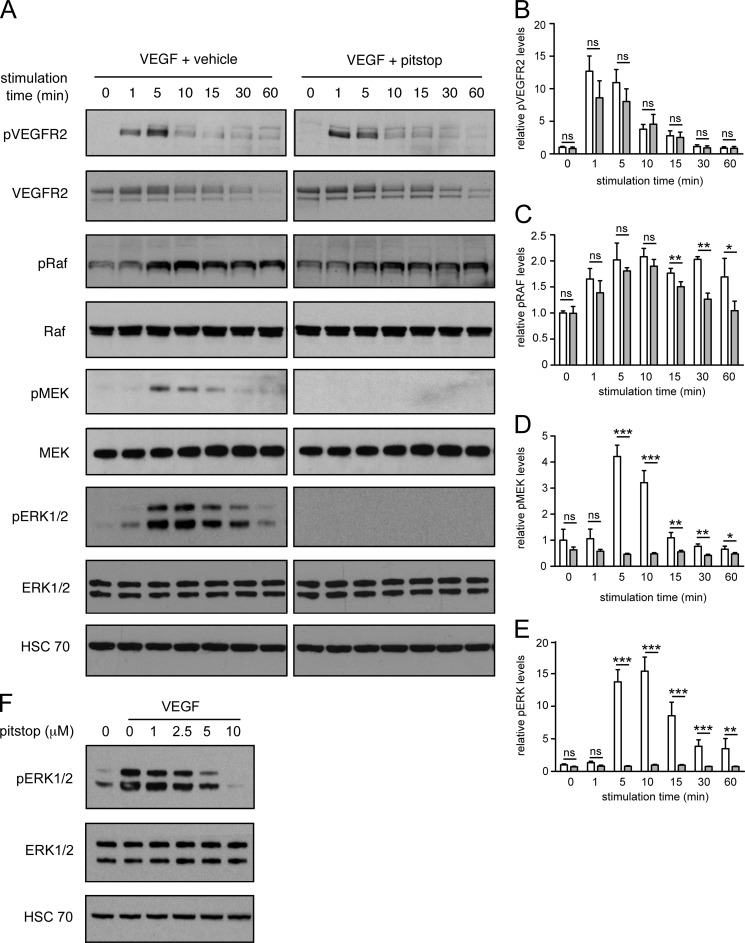
We then evaluated the activation of VEGFR2, C-Raf, MEK1/2, and ERK1/2 in VEGF-stimulated endothelial cells in the presence and absence of pitstop. To evaluate VEGFR2 activation, we blotted for Tyr(P)-1175 in VEGFR2, because phosphorylation of this residue is required for activation of ERK1/2 in endothelial cells (6, 49). To evaluate activation of C-Raf, we blotted for Tyr(P)-341 in C-Raf because phosphorylation of this site is induced by VEGF stimulation in endothelial cells, whereas phosphorylation of the Ser-338 site is not (26). Importantly, because VEGF does not significantly activate B-Raf in HUVECs, we did not examine activation of this protein (26). To evaluate MEK1/2 and ERK1/2 activation, we blotted for Ser(P)-217/Ser(P)-221 in MEK1/2 and Thr(P)-202/Tyr(P)-204 in ERK1/2, respectively, the activation loop phosphorylation sites that are required for kinase activation (50). Pitstop had no effect on the amplitude or duration of VEGFR2-Tyr(P)-1175 phosphorylation in VEGF-stimulated cells (Fig. 2, A and B). Phosphorylation of Tyr(P)-341-C-Raf was equivalent at 1, 5, 10, and 15 min of VEGF stimulation in the presence and absence of pitstop (Fig. 2, A and C). However, phosphorylation of C-Raf was suppressed in pitstop-treated cells after 30 and 60 min of VEGF stimulation (Fig. 2, A and C). Importantly, phosphorylation of the activation loop in both MEK1/2 and ERK1/2 was abrogated in cells stimulated with VEGF in the presence of pitstop (Fig. 2, A, D, and E). A pitstop negative control compound did not prevent VEGF from activating ERK1/2 in endothelial cells (supplemental Fig. 3). Moreover, the ability of pitstop to inhibit phosphorylation of ERK1/2 was pitstop dose-dependent (Fig. 2F).
FIGURE 2.
Activation of MEK1/2 and ERK1/2 in VEGF-stimulated endothelial cells is abrogated by pitstop. A, HUVECs were pretreated with vehicle or 10 μm pitstop for 15 min and then stimulated for the times indicated with 50 ng/ml VEGF. Cell lysates were analyzed by Western blotting for phosphorylated (Tyr(P)-1175) and total VEGFR2, phosphorylated (Tyr(P)-341) and total C-Raf, phosphorylated (Ser(P)-217/Ser(P)-221) and total MEK1/2, phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2. B–E, quantification of phosphorylation is shown. White bars represent vehicle, gray bars represent pitstop. n = 3 independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001, ns = no significant difference. F, HUVECs were pretreated with the indicated concentrations of pitstop for 15 min and then stimulated with 50 ng/ml VEGF for 10 min. Lysates were analyzed by Western blot for phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2.
Activation of VEGFR2, C-Raf, MEK1/2, and ERK1/2 in response to VEGF was also assessed in endothelial cells treated with dynasore. The inhibitor did not alter the amplitude or duration of VEGFR2-Tyr(P)-1175phosphorylation (Fig. 3, A and B). Moreover, although phosphorylation of Tyr(P)-341-C-Raf was significantly suppressed in dynasore-treated cells at base line and at 1 min of VEGF treatment, phosphorylation of this site at 5, 10, and 15 min of stimulation was equivalent to cells treated with VEGF alone (Fig. 3, A and C). Phosphorylation of C-Raf at the longer time points of 30 and 60 min was suppressed in dynasore-treated cells compared with VEGF alone (Fig. 3, A and C). Phosphorylation of MEK1/2 and ERK1/2 was abrogated in cells stimulated with VEGF in the presence of dynasore (Fig. 3, A, D, and E). Inhibition of ERK1/2 phosphorylation was dynasore dose-dependent (Fig. 3F).
FIGURE 3.
Activation of MEK1/2 and ERK1/2 in VEGF-stimulated endothelial cells is abrogated by dynasore. A, HUVECs were pretreated with vehicle or 10 μm dynasore for 30 min and then stimulated for the times indicated with 50 ng/ml VEGF. Lysates were analyzed by Western blotting for phosphorylated (Tyr(P)-1175) and total VEGFR2, phosphorylated (Tyr(P)-341) and total C-Raf, phosphorylated (Ser(P)-217/Ser(P)-221) and total MEK1/2, and phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2. B–E, quantification of phosphorylation is shown. White bars represent vehicle, gray bars represent dynasore. n = 3 independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns = no significant difference. F, HUVECs were pretreated with the indicated concentrations of dynasore for 30 min and then stimulated with 50 ng/ml VEGF for 10 min. Lysates were analyzed by Western blot for phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2.
Knockdown of Clathrin or Dynamin II Also Suppresses the Activation of ERK1/2 in VEGF-stimulated Endothelial Cells
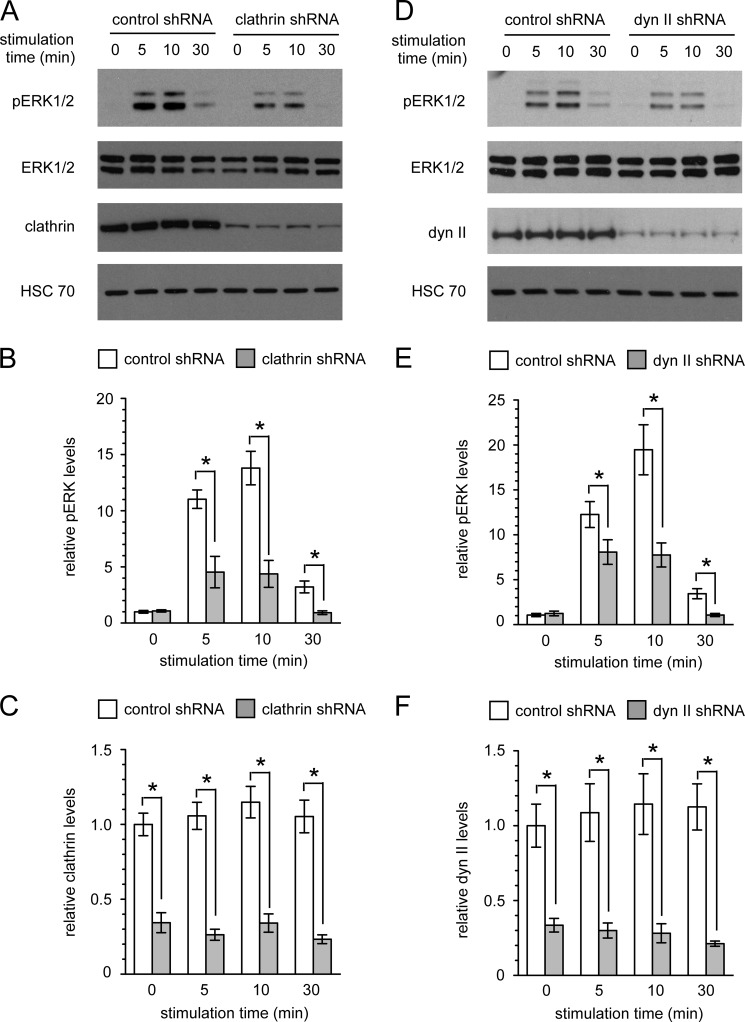
Next, we utilized an shRNA approach to independently verify whether suppressing internalization blocks signaling from VEGFR2 to ERK1/2 in endothelial cells. Importantly, shRNA knockdown of clathrin or dynamin II significantly suppressed ERK1/2 activation in endothelial cells stimulated with VEGF (Fig. 4, A–F). However, the suppression of ERK1/2 activation observed with the shRNA knockdown approach was less pronounced than that observed with the small molecule inhibitors. This is most likely because the shRNA approach only partially depletes clathrin and dynamin II protein levels, whereas the small molecule inhibitors are used at saturating concentrations that are expected to yield a stronger effect on receptor internalization. To address this, we quantified the retention of VEGFR2 at the cell surface in clathrin and dynamin II knockdown cells and compared it with that observed in the inhibitor treated cells. Significantly less VEGFR2 was retained at the plasma membrane in knockdown cells stimulated with VEGF compared with inhibitor-treated cells stimulated with VEGF (supplemental Fig. 2). These results most likely explain the difference in potency between the shRNA knockdown approach and the inhibitor treatment approach with respect to inhibition of ERK1/2 activation.
FIGURE 4.
Activation of ERK1/2 in VEGF-stimulated endothelial cells is suppressed by clathrin or dynamin II knockdown. A–F, HUVECs infected with lentivirus encoding either control non-targeting, clathrin targeting, or dynamin II (dyn II) targeting shRNA oligos were stimulated for the indicated times with 50 ng/ml VEGF. Cell lysates were analyzed by Western blotting (A and D) for phosphorylated (Thr(P)-202/Tyr(P)-204) ERK1/2, total ERK1/2, and clathrin or dynamin II. B and E, shown is quantification of ERK1/2 phosphorylation. n = 3 independent experiments. C and F, shown is quantification of clathrin or dynamin expression. n = 3 independent experiments. *, p < 0.05.
Internalization Is Required for ERK1/2 Activation in Stimulated Endothelial Cells but Not in Non-endothelial Cells
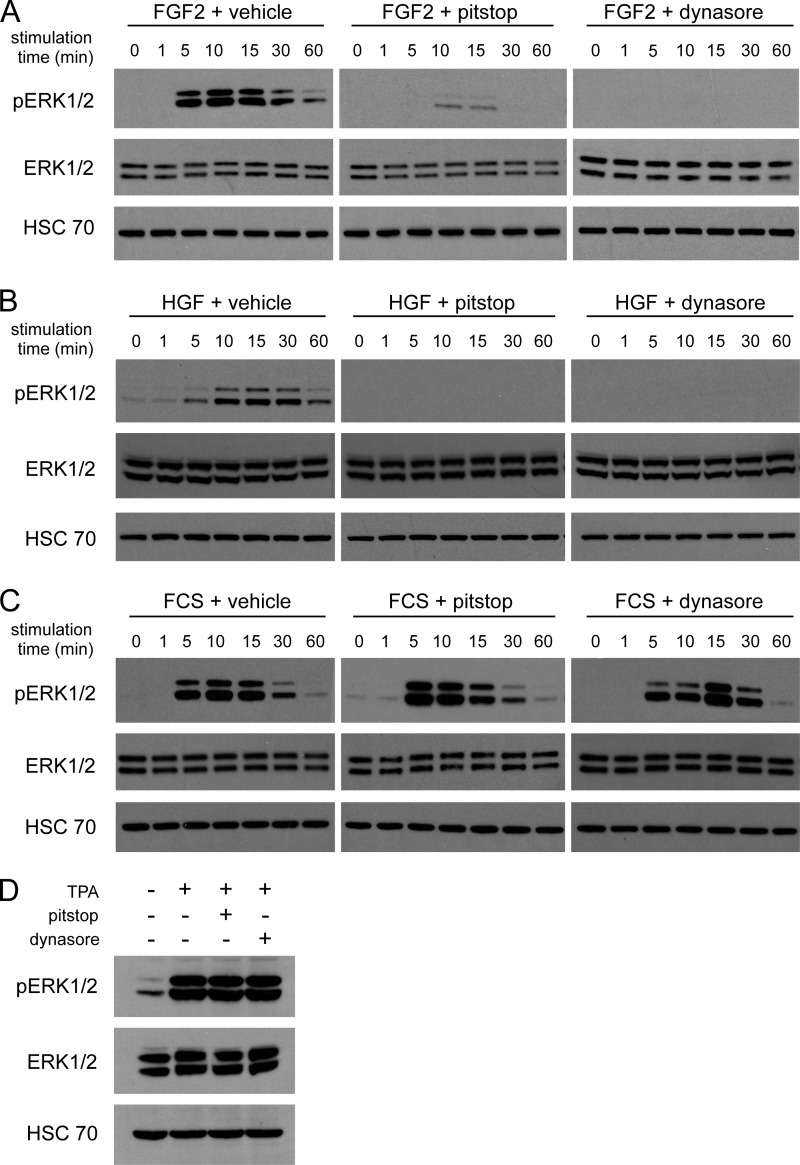
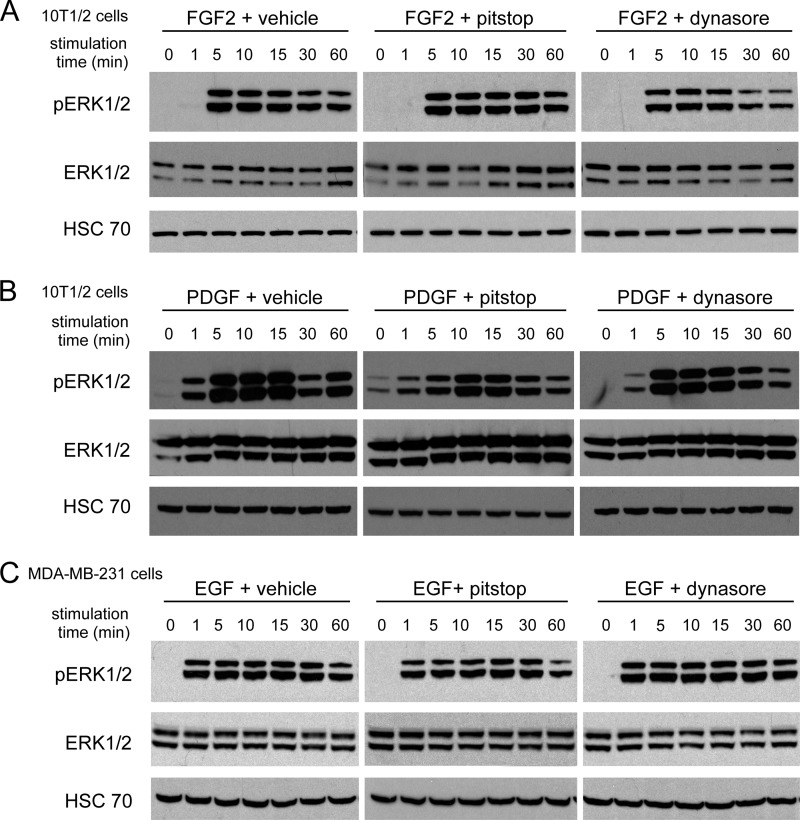
We then examined whether internalization is required for ERK1/2 activation in endothelial cells stimulated with other pro-angiogenic growth factors, namely FGF2 and HGF. Although receptors for FGF2 and HGF are well known to undergo internalization in cells (51, 52), it is not known whether internalization of these receptors is important for signal transduction in endothelial cells. Stimulation of endothelial cells with FGF2 or HGF resulted in activation of ERK1/2, but this was abrogated by pitstop and dynasore (Fig. 5, A and B). To exclude the possibility that these inhibitors block ERK1/2 activation non-specifically, endothelial cells were stimulated with FCS or with TPA. Neither pitstop nor dynasore blocked the activation of ERK1/2 that was induced by these treatments (Fig. 5, C and D). We also tested the ability of pitstop and dynasore to block signaling in non-endothelial cell types. Importantly, neither compound inhibited the activation of ERK1/2 in fibroblasts stimulated with FGF2 (Fig. 6A), fibroblasts stimulated with PDGF (Fig. 6B), or in human breast cancer cells stimulated with EGF (Fig. 6C).
FIGURE 5.
Activation of ERK1/2 in endothelial cells by FGF2 and HGF is abrogated by pitstop and dynasore, whereas activation of ERK1/2 by FCS or TPA is unimpeded. A–C, HUVECs were pretreated with vehicle, 10 μm pitstop, or 80 μm dynasore and then stimulated for the times indicated with 50 ng/ml FGF2 (A), 10 ng/ml HGF (B), or 10% FCS (C). Cell lysates were analyzed by Western blotting for phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2. D, HUVECs were pretreated with vehicle, 10 μm pitstop, or 80 μm dynasore and then stimulated for 15 min with 10 μm TPA. Cell lysates were analyzed by Western blotting for phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2.
FIGURE 6.
Activation of ERK1/2 in fibroblasts and breast cancer cells is not abrogated by pitstop or dynasore. A and B, 10T1/2 cells were pretreated with vehicle, 10 μm pitstop, or 80 μm dynasore and then stimulated for the times indicated with 50 ng/ml FGF2 (A) or 10 ng/ml PDGF-BB (B). Cell lysates were analyzed by Western blotting for phosphorylated and total ERK1/2. C, MDA-MB-231 cells were pretreated with vehicle, 10 μm pitstop, or 80 μm dynasore and then stimulated for the times indicated with 100 ng/ml EGF. Cell lysates were analyzed by Western blotting for phosphorylated (Thr(P)-202/Tyr(P)-204) and total ERK1/2.
Small Molecule Inhibitors of Endocytosis Can Suppress Angiogenesis in Vitro and in Vivo
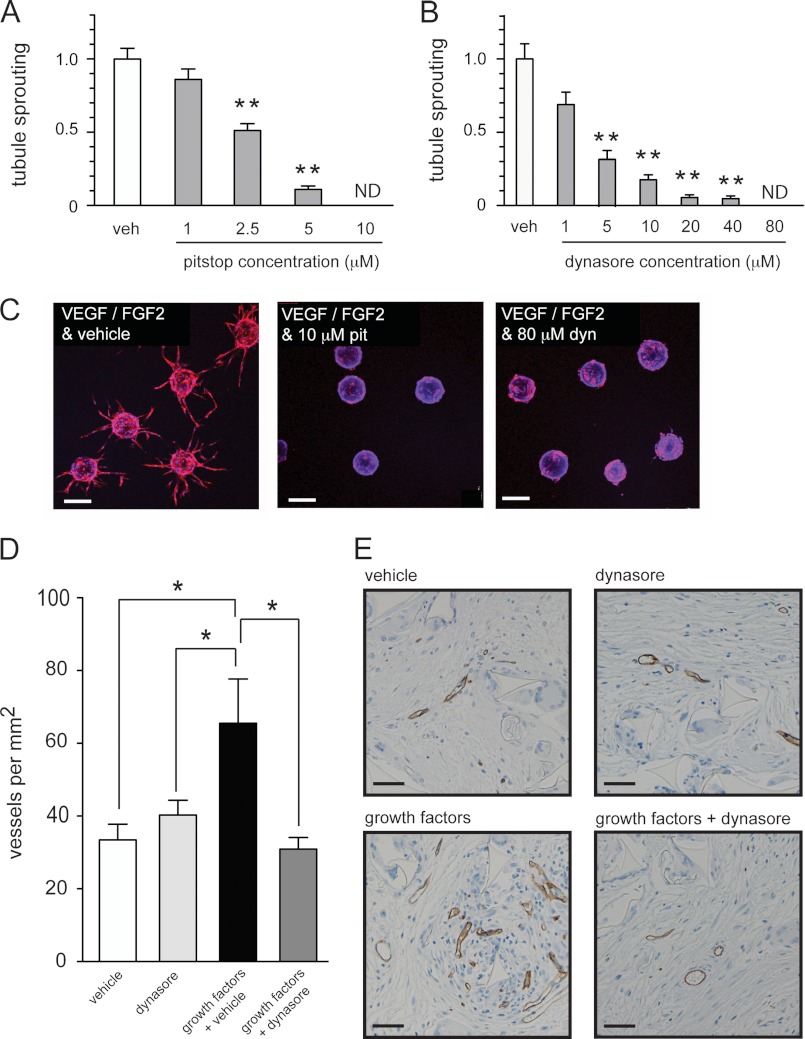
Given that endocytosis inhibitors suppressed the activation of ERK1/2 in endothelial cells stimulated with pro-angiogenic growth factors, we proceeded to examine whether these drugs could also inhibit vessel formation. First we used a well characterized three-dimensional in vitro assay of endothelial tubule formation (19, 43). Latex beads coated with endothelial cells were embedded in a three-dimensional fibrinogen matrix and then incubated with VEGF and FGF2 in the presence of vehicle, pitstop, or dynasore. Tubule formation was inhibited by dynasore and pitstop in a dose-dependent fashion (Fig. 7, A–C). We then examined the ability of dynasore to inhibit angiogenesis in vivo using the subcutaneous sponge assay (44). Inert sponges implanted subcutaneously under the back skin of mice were injected three times a week with control solution (vehicle in PBS), dynasore (dynasore in PBS), growth factors (VEGF, FGF2, and vehicle in PBS), or growth factors plus dynasore (VEGF, FGF2, and dynasore in PBS). Microvessel density in the group receiving growth factor treatment was significantly enhanced compared with the group that received control solution (Fig. 7, D and E). However, microvessel density in the group receiving growth factors plus dynasore was significantly impaired when compared with the group that received growth factors plus vehicle (Fig. 7, D and E). These data confirm that small molecule inhibitors of endocytosis can suppress endothelial tubule formation in vitro and angiogenesis in vivo.
FIGURE 7.
Small molecule inhibitors of internalization suppress angiogenesis. A–C, beads coated with adherent HUVECs were embedded in a fibrinogen gel and then cultured in the presence of growth factors (VEGF and FGF2) and vehicle or growth factors and the indicated concentrations of drug for 7 days. Graphs in A and B show the number of tubules formed per bead quantified after 7 days ± S.E. Quantification of 20 beads from 2 independent experiments was used to generate each data point. Examples of beads treated as indicated after 7 days are shown in C. Beads are stained with rhodamine-phalloidin (red) and DAPI (blue). D and E, sponges were subcutaneously implanted into mice and were injected three times per week with vehicle alone, dynasore alone, growth factors and vehicle (VEGF, FGF2, and vehicle), or growth factors and dynasore (VEGF, FGF2, and dynasore). After 21 days sponges were removed for analysis of blood vessel infiltration by staining for endomucin. The graph in D shows the number of blood vessels per mm2 quantified in sponges from mice treated as indicated (n = 6 mice per treatment group). Representative images of the endomucin staining are shown in E. *, p < 0.05; **, p < 0.001. ND = none detected. Scale bar = 200 μm.
DISCUSSION
The intracellular trafficking of growth factor receptors can control the amplitude and duration of downstream signaling (35, 53). Although receptor internalization has been implicated in the control of VEGFR2 signaling, the precise details of how this occurs are unclear (36). Here we examine how receptor internalization regulates activation of ERK1/2 in endothelial cells stimulated with VEGF. Our data indicate that although internalization is not required for the activation of VEGFR2 and C-Raf, internalization is required for the activation of MEK1/2 and ERK1/2. This suggests that the efficient activation of MEK1/2 by C-Raf requires receptor endocytosis. We also show that internalization is required for FGF2 and HGF to activate ERK1/2 in endothelial cells. However, internalization was not required for growth factors to activate ERK1/2 in fibroblasts or breast cancer cells. Inhibition of internalization blocked the angiogenic response to growth factors both in vitro and in vivo. These data suggest that internalization may be a generic requirement for pro-angiogenic growth factor receptors to activate angiogenesis in endothelial cells.
Previously published studies have examined the role of internalization in VEGFR2 signaling. Lampugnani et al. (33) showed that siRNA silencing of clathrin attenuated phosphorylation of both VEGFR2 and ERK1/2 in VE-cadherin−/−, but not in wild type, mouse endothelial cells. In a more recent study dynasore treatment abrogated the phosphorylation of both VEGFR2 and Akt in VEGF-stimulated mouse endothelial cells (40). Moreover, Lanahan et al. (41) used mouse aortic endothelial cells deficient in synectin or myosin to show that delayed internalization of VEGFR2 suppressed the phosphorylation of VEGFR2, Akt and ERK1/2. These studies suggest that VEGFR2 internalization is required for optimal phosphorylation of VEGFR2 and subsequent optimal activation of downstream signaling. However, this is in contrast to other work demonstrating that internalization is not required for optimal phosphorylation of VEGFR2 (54, 55). Therefore, it is not precisely clear how receptor internalization couples VEGFR2 to the activation of downstream signaling pathways.
In the current study we addressed this issue by carefully examining how inhibition of internalization affects signal transduction from VEGFR2 to ERK1/2. Importantly, we show that phosphorylation of VEGFR2 at Tyr-1175, which is required for ERK1/2 activation in endothelial cells (49), is not suppressed when internalization is blocked. Shc and Grb2 bind to phosphorylated Tyr-1175, which in turn bind SOS, leading to activation of Ras (6, 50). The subsequent activation of C-Raf involves the recruitment of C-Raf to the plasma membrane by activated Ras followed by phosphorylation of C-Raf at Ser-338 and Tyr-341 (50). Previous studies have shown that although VEGF stimulation of endothelial cells does not induce phosphorylation of the Ser-338 site in C-Raf, VEGF stimulation does induce phosphorylation of the Tyr-341 site (26). Importantly, we found that phosphorylation of C-Raf at Tyr-341 still occurs in response to VEGF stimulation when internalization is blocked. However, downstream phosphorylation of MEK1/2 and ERK1/2 in response to VEGF was completely abrogated when internalization was blocked.
These data suggest that although internalization is not required for activation of either VEGFR2 or C-Raf in human endothelial cells stimulated with VEGF, internalization is necessary for activation of the more distal kinases in the cascade, i.e. MEK1/2 and ERK1/2. Interestingly, similar results were observed when the endocytosis of G-protein-coupled receptors was inhibited in HEK-293 cells, which led the authors to conclude that internalization is required for Raf to activate MEK (56). We speculate that a signaling complex containing both VEGFR2 and activated C-Raf must become internalized for C-Raf to efficiently phosphorylate MEK1/2 in VEGF-stimulated endothelial cells. However, the mechanism may not be as simple as this. For example, although the activation of MEK1/2 and ERK1/2 can occur at the plasma membrane in HeLa cells stimulated with EGF, the sustained activation of these kinases was shown to require their relocalization to endosomes (57). Therefore, another interpretation of our data is that although activated C-Raf can transiently phosphorylate MEK1/2 at the plasma membrane in endothelial cells stimulated with VEGF, sustained phosphorylation of MEK1/2 and ERK1/2 may require endocytosis of VEGFR2-Raf-MEK-ERK signaling complexes in endothelial cells.
In contrast to these findings, recently published work indicates that receptor internalization is not required for growth factors to activate ERK1/2 in cells (58–60). Across these studies, several methods were employed to block receptor internalization, including treatment of cells with dynasore, deletion of the dynamin II gene, dynamin II shRNA, or the use of internalization-defective EGF receptors. Regardless of the method utilized, activation of ERK1/2 in response to EGF was unimpeded when internalization was blocked. However, only immortalized cells were used in these studies, and none examined growth factor signaling in primary endothelial cells (58–60). Interestingly, although we observe here that inhibition of endocytosis prevented growth factors from activating ERK1/2 in endothelial cells, these same inhibitors did not prevent ERK1/2 activation when immortalized fibroblasts or cancer cells were stimulated with growth factors. Therefore, although our data indicate that receptor internalization is required for growth factors to activate ERK1/2 in primary endothelial cells, receptor internalization may not be required to activate this pathway in other cell types.
There must be a strong biological rationale as to why endothelial cells need to internalize growth factor receptors to complete signal transduction to ERK1/2. One possible explanation is that this mechanism may help to limit the chance that angiogenesis is activated inappropriately in endothelial cells in vivo. Of note, the internalization and recycling of VEGF receptors in endothelial cells is controlled by interactions with numerous other transmembrane proteins involved in angiogenesis, including αvβ3-integrin, EphB2, neuropilin-1, and VE-cadherin (33, 40, 42, 48, 61). Because internalization is required for the activation of ERK1/2, these receptor interactions may be able to control the amplitude and duration of ERK1/2 signaling in endothelial cells through control of receptor trafficking. This extra level of control may be required not only to control the onset of angiogenesis but also to coordinate one or more aspects of the complex process that is blood vessel formation, such as cell migration, cell proliferation, cell survival, tube formation, vessel branching, vessel anastomosis, or vessel maturation (3, 37).
Agents that target VEGF receptor signaling have taken center stage in the clinical translation of angiogenesis inhibitors. However, multiple pro-angiogenic growth factors exist in the tumor microenvironment. Therefore, agents that inhibit signaling from a broader range of pro-angiogenic growth factor receptors may inevitably prove to be more effective than drugs that target VEGF signaling alone (10, 13–15). This can be achieved by designing kinase inhibitors that target multiple receptors. For example, kinase inhibitors that target signaling from both VEGF and FGF receptors or from both VEGF and MET receptors have been developed and are currently being tested in the clinic (62–64). Another potential strategy may be to target an alternative process besides kinase activity that is also a generic requirement for signaling from pro-angiogenic growth factor receptors. The data presented here suggest that disrupting the trafficking of growth factor receptors in endothelial cells may be an alternative strategy for abrogating tumor angiogenesis.
Acknowledgments
We are exceedingly grateful to Clare Isacke for critical comments on the manuscript. We also thank Claus Jørgensen, Stephanie Kermorgant, Harry Mellor, Jim Norman, and Christiana Ruhrberg for useful discussions. We thank Ann-Marie Baker for assistance with immunohistochemistry.
This work was supported by Breakthrough Breast Cancer and the Royal Marsden NIHR Biomedical Research Centre.

This article contains supplemental Figs. 1–3.
- VEGFR2
- VEGF receptor 2
- HGF
- hepatocyte growth factor
- TPA
- 12-O-tetradecanolyphorbol-13-acetate
- HUVECs
- human umbilical vein endothelial cells.
REFERENCES
- 1. Ferrara N., Kerbel R. S. (2005) Angiogenesis as a therapeutic target. Nature 438, 967–974 [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D., Folkman J. (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P., De Smet F., Loges S., Mazzone M. (2009) Branching morphogenesis and antiangiogenesis candidates. Tip cells lead the way. Nat. Rev. Clin. Oncol. 6, 315–326 [DOI] [PubMed] [Google Scholar]
- 4. Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) VEGF receptor signaling. In control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 5. Ferrara N., Gerber H. P., LeCouter J. (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 6. Koch S., Tugues S., Li X., Gualandi L., Claesson-Welsh L. (2011) Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 [DOI] [PubMed] [Google Scholar]
- 7. Kerbel R. S. (2008) Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrara N., Hillan K. J., Gerber H. P., Novotny W. (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 3, 391–400 [DOI] [PubMed] [Google Scholar]
- 9. Ellis L. M., Hicklin D. J. (2008) VEGF-targeted therapy. Mechanisms of anti-tumour activity. Nat. Rev. Cancer 8, 579–591 [DOI] [PubMed] [Google Scholar]
- 10. Bergers G., Hanahan D. (2008) Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 8, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azam F., Mehta S., Harris A. L. (2010) Mechanisms of resistance to antiangiogenesis therapy. Eur. J. Cancer 46, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 12. Rini B. I., Atkins M. B. (2009) Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 10, 992–1000 [DOI] [PubMed] [Google Scholar]
- 13. Bottsford-Miller J. N., Coleman R. L., Sood A. K. (2012) Resistance and escape from antiangiogenesis therapy. Clinical implications and future strategies. J. Clin. Oncol. 30, 4026–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sennino B., McDonald D. M. (2012) Controlling escape from angiogenesis inhibitors. Nat. Rev. Cancer 12, 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kerbel R. S. (2005) Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell 8, 269–271 [DOI] [PubMed] [Google Scholar]
- 16. Presta M., Dell'Era P., Mitola S., Moroni E., Ronca R., Rusnati M. (2005) Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16, 159–178 [DOI] [PubMed] [Google Scholar]
- 17. Bussolino F., Di Renzo M. F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P. M. (1992) Hepatocyte growth factor is a potent angiogenic factor that stimulates endothelial cell motility and growth. J. Cell Biol. 119, 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silvagno F., Follenzi A., Arese M., Prat M., Giraudo E., Gaudino G., Camussi G., Comoglio P. M., Bussolino F. (1995) In vivo activation of met tyrosine kinase by heterodimeric hepatocyte growth factor molecule promotes angiogenesis. Arterioscler. Thromb. Vasc. Biol. 15, 1857–1865 [DOI] [PubMed] [Google Scholar]
- 19. Welti J. C., Gourlaouen M., Powles T., Kudahetti S. C., Wilson P., Berney D. M., Reynolds A. R. (2011) Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib. Oncogene 30, 1183–1193 [DOI] [PubMed] [Google Scholar]
- 20. Shojaei F., Lee J. H., Simmons B. H., Wong A., Esparza C. O., Plumlee P. A., Feng J., Stewart A. E., Hu-Lowe D. D., Christensen J. G. (2010) HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 70, 10090–10100 [DOI] [PubMed] [Google Scholar]
- 21. Casanovas O., Hicklin D. J., Bergers G., Hanahan D. (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8, 299–309 [DOI] [PubMed] [Google Scholar]
- 22. Batchelor T. T., Sorensen A. G., di Tomaso E., Zhang W. T., Duda D. G., Cohen K. S., Kozak K. R., Cahill D. P., Chen P. J., Zhu M., Ancukiewicz M., Mrugala M. M., Plotkin S., Drappatz J., Louis D. N., Ivy P., Scadden D. T., Benner T., Loeffler J. S., Wen P. Y., Jain R. K. (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porta C., Paglino C., Imarisio I., Ganini C., Sacchi L., Quaglini S., Giunta V., De Amici M. (2013) Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology 84, 115–122 [DOI] [PubMed] [Google Scholar]
- 24. Kopetz S., Hoff P. M., Morris J. S., Wolff R. A., Eng C., Glover K. Y., Adinin R., Overman M. J., Valero V., Wen S., Lieu C., Yan S., Tran H. T., Ellis L. M., Abbruzzese J. L., Heymach J. V. (2010) Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer. Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J. Clin. Oncol. 28, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cross M. J., Claesson-Welsh L. (2001) FGF and VEGF function in angiogenesis. Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 22, 201–207 [DOI] [PubMed] [Google Scholar]
- 26. Alavi A., Hood J. D., Frausto R., Stupack D. G., Cheresh D. A. (2003) Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301, 94–96 [DOI] [PubMed] [Google Scholar]
- 27. Eliceiri B. P., Klemke R., Strömblad S., Cheresh D. A. (1998) Integrin αvβ3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J. Cell Biol. 140, 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mavria G., Vercoulen Y., Yeo M., Paterson H., Karasarides M., Marais R., Bird D., Marshall C. J. (2006) ERK-MAPK signaling opposes Rho kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 9, 33–44 [DOI] [PubMed] [Google Scholar]
- 29. Wei G., Srinivasan R., Cantemir-Stone C. Z., Sharma S. M., Santhanam R., Weinstein M., Muthusamy N., Man A. K., Oshima R. G., Leone G., Ostrowski M. C. (2009) Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood 114, 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hood J. D., Bednarski M., Frausto R., Guccione S., Reisfeld R. A., Xiang R., Cheresh D. A. (2002) Tumor regression by targeted gene delivery to the neovasculature. Science 296, 2404–2407 [DOI] [PubMed] [Google Scholar]
- 31. Reynolds A. R., Moein Moghimi S., Hodivala-Dilke K. (2003) Nanoparticle-mediated gene delivery to tumour neovasculature. Trends Mol. Med. 9, 2–4 [DOI] [PubMed] [Google Scholar]
- 32. Ewan L. C., Jopling H. M., Jia H., Mittar S., Bagherzadeh A., Howell G. J., Walker J. H., Zachary I. C., Ponnambalam S. (2006) Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting, and degradation in endothelial cells. Traffic 7, 1270–1282 [DOI] [PubMed] [Google Scholar]
- 33. Lampugnani M. G., Orsenigo F., Gagliani M. C., Tacchetti C., Dejana E. (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174, 593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Damke H., Baba T., Warnock D. E., Schmid S. L. (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorkin A., von Zastrow M. (2009) Endocytosis and signaling. Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott A., Mellor H. (2009) VEGF receptor trafficking in angiogenesis. Biochem. Soc. Trans 37, 1184–1188 [DOI] [PubMed] [Google Scholar]
- 37. Eichmann A., Simons M. (2012) VEGF signaling inside vascular endothelial cells and beyond. Curr. Opin. Cell Biol. 24, 188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duval M., Bédard-Goulet S., Delisle C., Gratton J. P. (2003) Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. Consequences on nitric oxide production from endothelial cells. J. Biol. Chem. 278, 20091–20097 [DOI] [PubMed] [Google Scholar]
- 39. Murdaca J., Treins C., Monthouël-Kartmann M. N., Pontier-Bres R., Kumar S., Van Obberghen E., Giorgetti-Peraldi S. (2004) Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J. Biol. Chem. 279, 26754–26761 [DOI] [PubMed] [Google Scholar]
- 40. Sawamiphak S., Seidel S., Essmann C. L., Wilkinson G. A., Pitulescu M. E., Acker T., Acker-Palmer A. (2010) Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465, 487–491 [DOI] [PubMed] [Google Scholar]
- 41. Lanahan A. A., Hermans K., Claes F., Kerley-Hamilton J. S., Zhuang Z. W., Giordano F. J., Carmeliet P., Simons M. (2010) VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell 18, 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reynolds A. R., Hart I. R., Watson A. R., Welti J. C., Silva R. G., Robinson S. D., Da Violante G., Gourlaouen M., Salih M., Jones M. C., Jones D. T., Saunders G., Kostourou V., Perron-Sierra F., Norman J. C., Tucker G. C., Hodivala-Dilke K. M. (2009) Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 15, 392–400 [DOI] [PubMed] [Google Scholar]
- 43. Nakatsu M. N., Hughes C. C. (2008) An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol. 443, 65–82 [DOI] [PubMed] [Google Scholar]
- 44. Mahadevan V., Hart I. R., Lewis G. P. (1989) Factors influencing blood supply in wound granuloma quantitated by a new in vivo technique. Cancer Res. 49, 415–419 [PubMed] [Google Scholar]
- 45. da Silva R. G., Tavora B., Robinson S. D., Reynolds L. E., Szekeres C., Lamar J., Batista S., Kostourou V., Germain M. A., Reynolds A. R., Jones D. T., Watson A. R., Jones J. L., Harris A., Hart I. R., Iruela-Arispe M. L., Dipersio C. M., Kreidberg J. A., Hodivala-Dilke K. M. (2010) Endothelial α3β1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am. J. Pathol. 177, 1534–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. von Kleist L., Stahlschmidt W., Bulut H., Gromova K., Puchkov D., Robertson M. J., MacGregor K. A., Tomilin N., Tomlin N., Pechstein A., Chau N., Chircop M., Sakoff J., von Kries J. P., Saenger W., Kräusslich H. G., Shupliakov O., Robinson P. J., McCluskey A., Haucke V. (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 [DOI] [PubMed] [Google Scholar]
- 47. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 48. Wang Y., Nakayama M., Pitulescu M. E., Schmidt T. S., Bochenek M. L., Sakakibara A., Adams S., Davy A., Deutsch U., Lüthi U., Barberis A., Benjamin L. E., Mäkinen T., Nobes C. D., Adams R. H. (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 [DOI] [PubMed] [Google Scholar]
- 49. Takahashi T., Yamaguchi S., Chida K., Shibuya M. (2001) A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J. 20, 2768–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kolch W. (2000) Meaningful relationships. The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351, 289–305 [PMC free article] [PubMed] [Google Scholar]
- 51. Kermorgant S., Parker P. J. (2005) c-Met signaling. Spatiotemporal decisions. Cell Cycle 4, 352–355 [DOI] [PubMed] [Google Scholar]
- 52. Wesche J., Haglund K., Haugsten E. M. (2011) Fibroblast growth factors and their receptors in cancer. Biochem. J. 437, 199–213 [DOI] [PubMed] [Google Scholar]
- 53. Miaczynska M., Pelkmans L., Zerial M. (2004) Not just a sink. Endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16, 400–406 [DOI] [PubMed] [Google Scholar]
- 54. Jakobsson L., Kreuger J., Holmborn K., Lundin L., Eriksson I., Kjellén L., Claesson-Welsh L. (2006) Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell 10, 625–634 [DOI] [PubMed] [Google Scholar]
- 55. Anderson S. M., Shergill B., Barry Z. T., Manousiouthakis E., Chen T. T., Botvinick E., Platt M. O., Iruela-Arispe M. L., Segura T. (2011) VEGF internalization is not required for VEGFR-2 phosphorylation in bioengineered surfaces with covalently linked VEGF. Integr Biol. (Camb) 3, 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Daaka Y., Luttrell L. M., Ahn S., Della Rocca G. J., Ferguson S. S., Caron M. G., Lefkowitz R. J. (1998) Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 273, 685–688 [DOI] [PubMed] [Google Scholar]
- 57. Teis D., Wunderlich W., Huber L. A. (2002) Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3, 803–814 [DOI] [PubMed] [Google Scholar]
- 58. Sousa L. P., Lax I., Shen H., Ferguson S. M., De Camilli P., Schlessinger J. (2012) Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 109, 4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luwor R. B., Chin X., McGeachie A. B., Robinson P. J., Zhu H. J. (2012) Dynamin II function is required for EGF-mediated Stat3 activation but not Erk1/2 phosphorylation. Growth Factors 30, 220–229 [DOI] [PubMed] [Google Scholar]
- 60. Goh L. K., Huang F., Kim W., Gygi S., Sorkin A. (2010) Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J. Cell Biol. 189, 871–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ballmer-Hofer K., Andersson A. E., Ratcliffe L. E., Berger P. (2011) Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood 118, 816–826 [DOI] [PubMed] [Google Scholar]
- 62. Sarker D., Molife R., Evans T. R., Hardie M., Marriott C., Butzberger-Zimmerli P., Morrison R., Fox J. A., Heise C., Louie S., Aziz N., Garzon F., Michelson G., Judson I. R., Jadayel D., Braendle E., de Bono J. S. (2008) A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin. Cancer Res. 14, 2075–2081 [DOI] [PubMed] [Google Scholar]
- 63. Jonker D. J., Rosen L. S., Sawyer M. B., de Braud F., Wilding G., Sweeney C. J., Jayson G. C., McArthur G. A., Rustin G., Goss G., Kantor J., Velasquez L., Syed S., Mokliatchouk O., Feltquate D. M., Kollia G., Nuyten D. S., Galbraith S. (2011) A phase I study to determine the safety, pharmacokinetics and pharmacodynamics of a dual VEGFR and FGFR inhibitor, brivanib, in patients with advanced or metastatic solid tumors. Ann. Oncol. 22, 1413–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kurzrock R., Sherman S. I., Ball D. W., Forastiere A. A., Cohen R. B., Mehra R., Pfister D. G., Cohen E. E., Janisch L., Nauling F., Hong D. S., Ng C. S., Ye L., Gagel R. F., Frye J., Müller T., Ratain M. J., Salgia R. (2011) Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 29, 2660–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]