Background: Recent evidence suggests that adjuvant-mediated cell death contributes to adjuvant activities.
Results: We found that the prototypical adjuvant, alum, triggers a novel form of cathepsin-mediated necrosis. We found that agents that trigger this cell death pathway trigger, like alum, a Th2-biased immune response. We also found that inhibiting this cell death pathway prevented adjuvant-mediated immunity.
Conclusion: Selective induction of necrosis is a powerful inducer of adaptive immunity.
Significance: Understanding how adjuvant-mediated necrotic cell death controls immunity should improve the design of more powerful but safe adjuvants.
Keywords: Cell Signaling, Cellular Immune Response, Inflammation, Innate Immunity, Necrosis (Necrotic Death), Caspase-1, Cathepsins, Nod-like Receptors
Abstract
Immunologic adjuvants are critical components of vaccines, but it remains unclear how prototypical adjuvants enhance the adaptive immune response. Recent studies have shown that necrotic cells could trigger an immune response. Although most adjuvants have been shown to be cytotoxic, this activity has traditionally been considered a side effect. We set out to test the role of adjuvant-mediated cell death in immunity and found that alum, the most commonly used adjuvant worldwide, triggers a novel form of cell death in myeloid leukocytes characterized by cathepsin-dependent lysosome-disruption. We demonstrated that direct lysosome-permeabilization with a soluble peptide, Leu-Leu-OMe, mimics the alum-like form of necrotic cell death in terms of cathepsin dependence and cell-type specificity. Using a combination of a haploid genetic screen and cathepsin-deficient cells, we identified specific cathepsins that control lysosome-mediated necrosis. We identified cathepsin C as critical for Leu-Leu-OMe-induced cell death, whereas cathepsins B and S were required for alum-mediated necrosis. Consistent with a role of necrotic cell death in adjuvant effects, Leu-Leu-OMe replicated an alum-like immune response in vivo, characterized by dendritic cell activation, granulocyte recruitment, and production of Th2-associated antibodies. Strikingly, cathepsin C deficiency not only blocked Leu-Leu-OMe-mediated necrosis but also impaired Leu-Leu-OMe-enhanced immunity. Together our findings suggest that necrotic cell death is a powerful mediator of a Th2-associated immune response.
Introduction
Immunologic adjuvants are essential components of modern vaccines that generally determine the strength and type of immune response directed at specific antigens (1). Despite their importance, very few immunologic adjuvants have been developed that are effective and safe enough for human use. Aluminum salts (collectively known as alum) were the first applied adjuvants and remain the most widely used adjuvants worldwide (2). Alum is a component of all adjuvant-containing vaccines approved in the United States either by itself or mixed with lipid A (AS-04). Alum skews the immune response toward a T helper type 2 (Th2) response associated with strong production of IL-4 and the IgG1 antibody subtype (3, 4). Despite near-ubiquitous use of adjuvants, it remains unclear how they activate and direct the adaptive immune response. Nlrp3 signaling pathways have been implicated in alum adjuvant activities (5, 6). However, the contribution of the Nlrp3 signaling pathway in alum-mediated immune responses has been challenged by recent studies (7–10).
More recently, alum-mediated immunity has been linked to the release of non-cytokine biomolecules, including uric acid and double-stranded DNA, suggesting an alternate model for alum-mediated immunity (10, 11). Current studies suggest that upon necrotic release these biomolecules act as endogenous “danger” signals and stimulate the adaptive immune response (12–15). Necrotic cell death has been implicated in immune responses against multiple microbial pathogens and in tumor immunity (16–18). As nearly all immunologic adjuvants have cytotoxic activities, it is conceivable that cytotoxic adjuvants, such as alum, enhance the adaptive immune response through induction of necrosis (9, 19, 20). Specifically, alum has been associated with necrosis, myofascitis, and granuloma formation at the site of injection. Supporting this model, recent studies have shown that the local release of uric acid and double-stranded DNA from the site of alum injection drives T cell responses (9–11). However, the mechanism of adjuvant-mediated cell death and its contribution to adaptive immunity remains poorly understood.
Here we describe a mechanism for alum-mediated necrosis and examined its contribution to the adaptive immune response. We found that alum specifically kills myeloid leukocytes by inducing cathepsin-mediated lysosome rupture. We found that the lysosome-permeabilizing dipeptide methyl-ester Leu-Leu-OMe (LLOMe)3 mimics this phenotype. Using a combination of a haploid screen and cathepsin-deficient macrophages, we identified specific cathepsins that control cell death mediated by these lysosome-disrupting agents. We found that in addition to mimicking alum-mediated necrosis, LLOMe also recapitulated alum-mediated immune responses with a Th2 bias. Finally, cathepsin C deficiency impaired both LLOMe-mediated necrosis and immune potentiation. Together, these data suggest that adjuvant-mediated necrotic cell death is a powerful inducer of a Th2-associated adaptive immune response.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Imject alum and silica crystals (MIN-U-SIL-15) were purchased from Thermo Scientific and US Silica, respectively. CA-074-Me and E64d were from Peptides International. The caspase-1 inhibitor aspartic acid-OBzl-chloromethylketone (Boc-D-CMK) and the lysosome-destabilizing agent LLOMe were purchased from Bachem. The activity-based cathepsin probe GB123 was a generous gift of Dr. L. Santambrogio (Albert Einstein College of Medicine). Propidium iodide was from Sigma, LysoTracker was from Invitrogen, DMEM and RPMI were from Cellgro, and fetal bovine serum was from Atlanta Biologicals. Antibodies for analysis of cell type specificity were: anti-mouse CD45/B220-allophycocyanin-Cy7 (clone RA3–6B2), CD3e-PE (clone 145–2C11), CD8a-PerCP (clone 53–6.7), CD11c-PE-Cy7 (clone HL3), CD11b-allophycocyanin (clone M1/70), Ly6G-V450 (clone 1A8:B&D), F4/80-FITC (clone BM8). All antibodies were from eBioscience, except for CD4-Pacific Orange (clone RM4–5), which was from Invitrogen. Secondary antibodies and IgG2c standards were from Southern Biotech, and IgG, IgG1, and IgG2a standards were from Santa Cruz.
Mice
Wild type BALB/c and C57BL/6 mice were purchased from The Jackson Laboratory in Ben Harbor. Nlrp3-, Ipaf-, ASC-, and caspase-1-deficient mice have been previously described (21). Cathepsin-deficient mice were generously provided by Drs. Johanna Joyce and Thomas Reinheckel (22, 23). All animal experiments were performed in compliance with the institutional guidelines of the Institute for Animal Studies and approved by the Animal Institute Committee of the Albert Einstein College of Medicine (protocol 20090601).
Generation of Primary Cell Lines
For the generation of primary macrophages, bone marrow was isolated from femurs and tibias of mice as described previously (24). For the preparation of primary dendritic cells, cells were conditioned in RPMI with 20 ng/ml granulocyte-macrophage colony-stimulating factor as described previously (25). Primary B and T cells were purified by positive selection from splenic homogenates using magnetically labeled antibodies against CD45/B220 and CD90.2 (Miltenyi Biotec), respectively.
Generation of Splenic Cell Suspensions
Splenic cell suspensions generated from spleens of age-matched C57BL/6 and cathepsin-deficient mice were incubated in RPMI medium containing 2 mg/ml collagenase and 30 μg/ml DNase. After LLOMe exposure, cells were stained with the Blue LIVE/DEAD viability dye (BluVID; Invitrogen) and incubated with PBS containing 2% FCS, 0.05% NaN3 (w/v) and monoclonal antibodies against cell type-specific markers. B cells were identified as CD45/B220pos CD11bneg CD3eneg, and CD4 and CD8 T cells were identified as CD45/B220neg CD11bneg CD3epos CD4pos or CD8pos, respectively. Macrophages, monocytes, neutrophils, and dendritic cells (DCs) were CD45/B220neg CD3eneg, macrophages were CD11cneg F4/80pos and divided further into CD11bpos and CD11bneg subsets. Neutrophils were CD11bhigh and Ly6Gpos, dendritic cells were identified as CD11cpos and F4/80neg, and monocytes were identified as CD11bhigh/intermediate CD11cneg Ly6Gneg and F4/80neg. Cells were fixed in 1% paraformaldehyde and analyzed by using a BD LSRII flow cytometer.
Haploid-genetic Screen for LLOMe Resistance
A positive enrichment screen for cells resistant to (3 mm) LLOMe-induced cell death was performed using quasi-haploid KBM7 cells (26, 27). KBM7 cells were mutagenized using a gene trap retrovirus and exposed to LLOMe. Surviving cells were selected and expanded to 30 million cells for genomic DNA isolation. Inverse PCR was used to amplify genomic regions flanking the insertion sites. Insertion sites were sequenced on an Illumina HiSeq2000 and 50-bp single-reads were aligned to the human genome (hg18). Genes significantly enriched for mutations were identified by comparing the number of retrieved sense insertions to an unselected control dataset using a one-sided Fisher exact test as described previously (26, 27).
Cell Death Assays
Lymphocytes and splenic cells suspensions were exposed to lysosome-disrupting agents, and cell death was determined by measuring PI uptake or lactate dehydrogenase (LDH) activity as described previously (28). For ex vivo cytotoxicity measurements, splenic cell suspensions were treated with CA-074-Me for 2 h followed by LLOMe challenge. Cells were then stained and fixed for flow cytometry.
Histopathology
8-Week-old female C57BL/6 mice were anesthetized with isoflurane. Mice were then injected subcutaneously with 2 mg of alum or 1 mg of LLOMe diluted in PBS. The skin was excised over a 1.5-cm area around the injection site and fixed in 10% formalin for 72 h and embedded into paraffin. Five-μm sections were stained by hematoxylin and eosin and evaluated by a board-certified veterinary pathologist.
In Vivo Spleen Depletion
For in vivo spleen depletion assays 8-week-old female C57BL/6 mice were injected with alum (750 μg) and LLOMe (1 mg) by tail vein injection. After 24 h, splenic cells were then stained and fixed for flow cytometry as described above.
Flow Cytometry and ELISA
Macrophages were labeled with CD11b-PE, lysosome membrane potential was measured with LysoTracker Green, and membrane impairment was determined by using PI. IL-1β levels were measured using the Ready-Set-Go ELISA kit from eBioscience. Anti-OVA titers were determined by using Costar ELISA plates coated with 10 μg/ml ovalbumin.
Immunization and Antibody Production
Eight-week-old C57BL/6 and cathepsin-deficient mice were immunized twice (after 2 weeks) with PBS, adjuvant alone, or 50 μg of ovalbumin with varying doses of alum and LLOMe. 21 days after injection, serum was retrieved by retro-orbital bleeding to determine serum antibody titers using isotype-specific sandwich ELISA.
Statistical Analysis
Leukocyte depletion data were analyzed by a two-way analysis of variance with a Bonferroni post-test to assess statistically relevant differences. Antibody titers were analyzed with a one-way analysis of variance with an individual column post-test comparison to PBS-immunized mice. Normality was confirmed for all analyses by using a D'Agastino and Pearson omnibus normality test before analysis. Nonparametric data were put through a log 10 transformation, and normality was confirmed. Immunization titers were determined by a two-tailed t test. IgG titers were determined by taking the average of wild type titers divided by individual titers.
RESULTS
Lysosome-disrupting Agents Trigger a Novel Form of Programmed Necrosis
The molecular mechanism by which adjuvants and, specifically, alum stimulate the immune system remains poorly understood. Recent studies have linked the necrotic release of intracellular components to stimulation of the adaptive immune response and adjuvant activities (13–15). Accordingly, nearly all adjuvants, including alum, trigger cytotoxic effects (19). Based on these findings, we hypothesized that adjuvant-mediated cell death could drive the adaptive immune response. To test our hypothesis, we set out to identify genes that control adjuvant-induced cell death and the adaptive immune response.
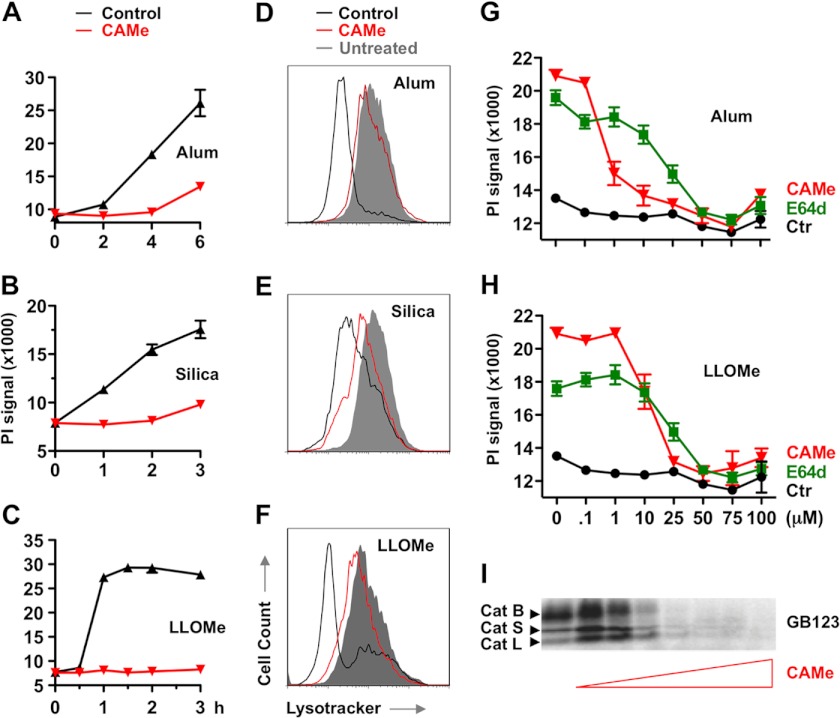
Consistent with previous studies, we found that alum crystals induced necrosis of murine macrophages. Alum has previously been reported to activate IL-1β release through the activation of caspase-1, a protein strongly associated with necrotic cell death. As cathepsin inhibitors had been reported to block alum-induced IL-1β release and cell death (29–32), we tested whether these inhibitors also blocked alum-mediated necrosis. We found that the cathepsin inhibitors CA-074-Me and E64d prevented alum-induced necrosis (Fig. 1, A, D, and G). Previous studies have suggested that crystal compounds, such as alum, trigger lysosome rupture (30, 33). Consistent with these studies, we found that alum-challenged macrophages showed lysosome impairment that correlated with cell death induction (Fig. 1, A and D). Intriguingly, CA-074-Me impaired both alum-induced lysosome impairment and cell death (Fig. 1, A and D). We then examined whether this was a general phenomena associated with lysosome-disrupting crystals. We found that CA-074-Me also blocked lysosome rupture and necrosis mediated by silica crystals in murine macrophages, which has been reported to destabilize lysosomes similarly to alum (Fig. 1, B and E).
FIGURE 1.
Cathepsin inhibitors block lysosome disruption and macrophage death mediated by alum, silica, and LLOMe. A–F, LPS-primed C57BL/6-derived macrophages were exposed to 150 μg/ml alum (A and D), 200 μg/ml silica (B and E), or 2.5 mm LLOMe (C and F) in the presence of 100 μm CA-074-Me (CAMe). Cell death (A–C) was determined by PI exclusion assays. D–F, the lysosome membrane potential was measured by using the fluorescent dye LysoTracker using flow cytometry at 5, 3, and 1 h for alum, silica, and LLOMe, respectively. G and H, macrophages were treated with 150 μg/ml alum (G) or 1.5 mm LLOMe (H) for 6 and 2 h, respectively, in the presence of increasing concentrations of CA-074-Me or E64d. Cell death was measured by PI exclusion. I, macrophages were pretreated with increasing concentrations of CA-074-Me for 2 h and labeled with the activity-based probe GB123 (1 μm). cat, cathepsin.
Lysosomes are lytic organelles that contain constituently active hydrolytic enzymes whose activity is primarily constrained by containment within lysosomes (34). Previous studies have shown that many hydrolases remain biologically active upon lysosomal rupture and release into the cytosol (30). Given the broad lytic activity of lysosomal hydrolases, we hypothesized that release of these constituently active enzymes into the cytosol may be sufficient to induce necrotic cell death. To test this hypothesis, we treated cells with the lysosome-destabilizing dipeptide, LLOMe (35). LLOMe has been shown to trigger an apoptotic pathway at low and necrotic cell death at high concentrations (36, 37). We found that 2 mm LLOMe induced rapid lysosome rupture and necrotic cell death, which was also blocked by CA-074-Me (Fig. 1, C, F, and H). Taken together our findings suggested that alum, silica, and LLOMe trigger cathepsin-dependent lysosome rupture and necrotic cell death.
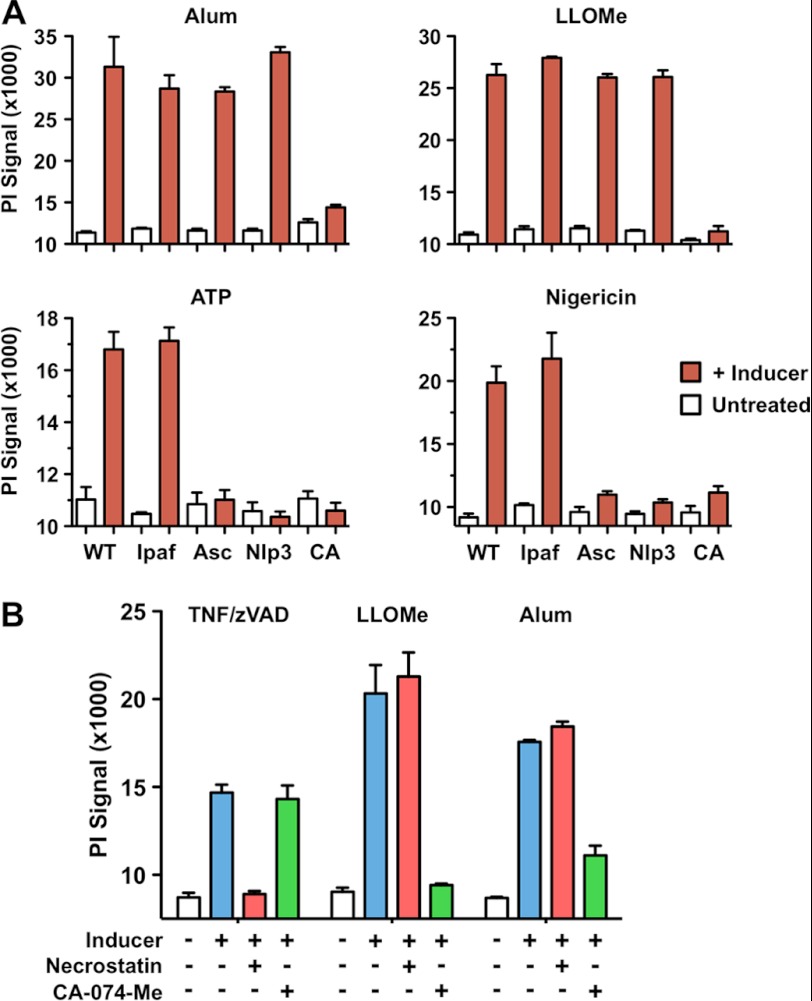
The cathepsin dependence we observed suggested that the alum-, silica-, and LLOMe-mediated necrotic cell death follows a program with specific checkpoints. Lysosomal function has been implicated in two mechanisms of programmed necrotic cell death: pyroptosis (caspase-1-mediated necrosis) and necroptosis (RIP-1-mediated necrosis) (32, 38–40). Recently, lysosome-destabilizing agents have been reported to activate caspase-1 (the critical effector for pyroptotic cell death) through stimulation of the Nod-like receptor Nlrp3 (5, 30). To test whether caspase-1 activation was involved in alum and LLOMe-mediated necrosis, we challenged LPS-primed macrophages with alum and LLOMe. Consistent with inflammasome activation (5, 30), we found that the caspase-1 inhibitor t-butoxycarbonyl-d-chloromethyl ketone and the cathepsin inhibitor CA-074-Me blocked IL-1β release (as a readout of caspase-1 activation) by alum, LLOMe, ATP, and nigericin (supplemental Fig. 1A). Although all inducers activated caspase-1, we found that t-butoxycarbonyl-d-chloromethyl ketone and Nlrp3 or Asc deficiency did not block cell death mediated by alum, LLOMe, and silica crystals (Fig. 2A and supplemental Fig. 1, B–D). As expected, t-butoxycarbonyl-d-chloromethyl ketone and Nlrp3 and Asc deficiency blocked necrotic cell death induced by ATP and nigericin (Fig. 2A and supplemental Fig. 1B), which were used as positive controls for pyroptotic cell death (21, 41). Deficiency of the irrelevant NLR (nucleotide binding domain and leucine-rich repeat-containing) Ipaf, however, did not prevent ATP and nigericin-induced necrosis, demonstrating the Nlrp3 specificity of this process (Fig. 2A). Together, our findings suggested that the lysosome-destabilizing agents, alum, LLOMe, and silica, trigger caspase-1- and Nlrp3-independent necrosis.
FIGURE 2.
Alum and LLOMe induce inflammasome- and RIP-1-independent cell death. A, wild type, and Ipaf-, Asc-, and Nlrp3-deficient macrophages were primed with 250 ng/ml LPS in the presence or absence of 100 μm CA-074-Me (CA, wild type cells only) for 2 h. Macrophages were then exposed to 150 μg/ml alum, 1.5 mm LLOMe, 5 mm ATP, or 10 μm nigericin for 2 h (6 h for alum), or left untreated. Cell death was determined by PI exclusion. B, alum- and LLOMe-mediated cell death is independent of necroptosis. C57BL/6 macrophages were primed with LPS for 2 h and treated with 2.5 mm LLOMe for 2 h or 150 μg/ml of alum for 6 h. Cells were challenged in the presence or absence of the necroptosis inhibitor necrostatin-1 (Necrost, 100 μm) or the cathepsin inhibitor CA-074-Me (100 μm). CAMe, CA-074-Me; zVAD, benzyloxycarbonyl-VAD-fluoromethyl ketone.
As alum and LLOMe failed to trigger pyroptosis, we investigated whether they induced necroptosis, the other recognized form of programmed necrosis (32). We found that the RIP-1 inhibitor necrostatin-1 failed to block alum or LLOMe-induced cell death (Fig. 2B), whereas it efficiently blocked necrosis mediated by the necroptosis inducers TNFα and benzyloxycarbonyl-VAD-fluoromethyl ketone (32). Likewise, CA074-Me blocked alum- and LLOME-mediated necrosis, whereas it did not prevent TNFα and benzyloxycarbonyl-VAD-fluoromethyl ketone-mediated cell death (Fig. 2B). Together these data suggested that alum and LLOMe initiate a mechanism of programmed necrosis that is distinct from pyroptosis and necroptosis.
Specific Cathepsins Control Cell Death by Alum and LLOMe
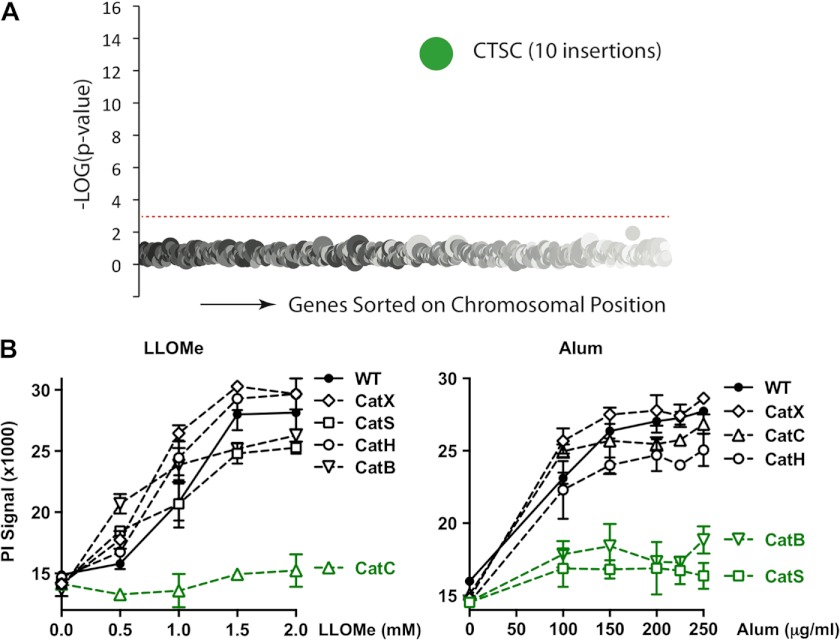
Our studies indicated that alum and LLOMe trigger a novel form of programmed cell death. To identify genes that control this form of programmed necrosis and possibly also the immune response, we performed a genetic screen. Toward this we employed a mutagenesis-based genetic approach with human KBM7 cells that have a single copy of all human chromosomes except for chromosome 8. The near-haploid phenotype of KBM7 cells allows generation of null alleles for nearly all nonessential human genes by a single mutation event. To identify critical genes required for induction of necrotic cell death, we performed random insertional mutagenesis using a gene-trap vector (26, 27). This procedure is based on the idea that cells lacking critical genes for cell death induction survive exposure to the lysosome-disrupting agents. As alum generated a substantial background of surviving KBM7 cells, we relied on LLOMe for the haploid screen, as it triggers an alum-like necrotic cell death but kills macrophages with much higher efficiency. We subjected mutagenized KBM7 cells to LLOMe and expanded surviving cells. After sequencing of gene-trap insertion sites, we found that only one gene, CTSC (cathepsin C), was significantly (p < 10−13) enriched for mutations as compared with the control dataset (Fig. 3A). This result suggested that cathepsin C is required for LLOMe-induced cell death consistent with previous pharmacological studies (35). To confirm that cathepsin C is critical for LLOMe-mediated cell death, we used a panel of murine macrophages deficient in predominant macrophage-associated cathepsins (B, C, H, S, and X). Consistent with the haploid screen, we found that cathepsin C deficiency completely prevented LLOMe-mediated necrosis, whereas cathepsins B, H, S, and X deficiency had no impact on this process (Fig. 3B, left panel). Together, this genetic evidence indicated that a single gene, cathepsin C, controls LLOMe-mediated cell death. We next tested the specificity of this process by challenging cathepsin-deficient macrophages with alum. Intriguingly, cathepsin C was not required for alum-mediated necrosis, but the process was instead jointly dependent on cathepsins B and S (Fig. 3B, right panel). These results were consistent with the cathepsin B dependence of alum-induced IL-1β release and the fact that alum-mediated cell death was blocked by CA-074-Me at concentrations that also blocked cathepsin B activity (Fig. 1, G and I) (30).
FIGURE 3.
Specific cathepsins control macrophage death by alum and LLOMe. A, a haploid genetic screen identified cathepsin C as critical for LLOMe-mediated cell death. Genes statistically enriched for gene-trap insertions in the LLOMe-treated KBM7 cells are compared with unselected cells. Circles represent genes, and the size of these circles corresponds to the number of independent gene-trap mutations detected in the gene of interest in the LLOMe-resistant cell population. Genes are sorted on the x axis based on the chromosomal position. B, macrophages lacking cathepsin B, C, H, S, or X were challenged with increasing concentrations of alum (μg/ml) or LLOMe (mm), and membrane impairment was determined 6 h (alum) or 3 h (LLOMe) post challenge by PI exclusion assays. Cat, cathepsin.
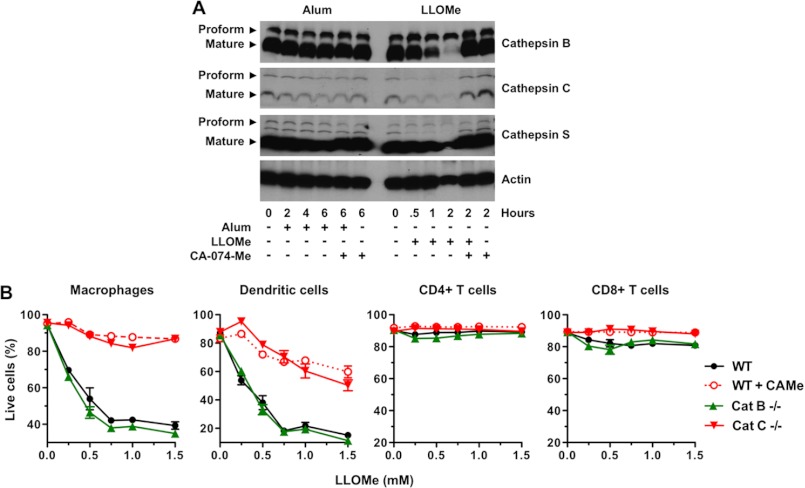
Consistent with previous studies, we found that these predominant cysteine cathepsins are constituently active in murine macrophages (Fig. 1I). Here we tested whether lysosome-disrupting agents further stimulate cathepsin activation as assessed by cathepsin cleavage. We found that LLOMe did not further activate cathepsin C (Fig. 4A). In fact, levels of activated cathepsins C, B, and S decreased in LLOMe-treated macrophages concurrent with necrotic cell death, presumably due a release of these cathepsins after lysosome rupture (Fig. 4A). Accordingly, cathepsin levels recovered after CA-074-Me exposure at concentrations that block LLOMe-induced cell death (Figs. 1I and 4A). Together we demonstrated that specific cathepsins control necrosis mediated by the lysosome-destabilizing agents, LLOMe and alum.
FIGURE 4.
Alum and LLOMe kill specific immune cells in a cathepsin-dependent fashion. A, alum and LLOMe do not activate cathepsins B, C, and S. Cells were exposed to 100 μm CA-074-Me for 2 h and subsequently treated with 150 ng/ml alum or 2.5 mm LLOMe. Lysates were probed for cathepsins B, C, and S levels by immunoblotting at different time points post alum/LLOMe exposure. B, cytopathic effects were analyzed in wild type and cathepsin (Cat) B- and C-deficient splenocytes by live/dead stain and flow cytometry 2 h post-LLOMe exposure. Wild type macrophages treated with 100 μm CA-074-Me served as a control. Immune cell specificity was determined using cell type-specific antibodies and flow cytometry.
Alum and LLOMe Specifically Kill Myeloid Leukocytes in a Cathepsin-dependent Fashion
Our hypothesis that adjuvant-mediated cytotoxicity could trigger an adaptive immune response seems counterintuitive, as it entails killing of cells that are required for induction of an immune response. To identify cells that are actually killed by cytotoxic agents, we challenged an array of primary immune cells isolated from C57BL/6 mice with alum and LLOMe. In addition to being toxic in primary macrophages (Fig. 1), we found that alum and LLOMe also killed primary dendritic cells in a cathepsin-dependent manner while sparing primary B and T lymphocytes (supplemental Fig. 2, B and C).
To determine toxicity under more physiological conditions in a multicellular milieu, we challenged splenic cell suspensions with LLOMe. Consistent with our findings with primary cells, we found that LLOMe specifically killed macrophages and DCs while sparing CD4 and CD8 T cells (Fig. 4B). We also found that alum and LLOMe killed neutrophils (data not shown). In agreement with our inhibitor and knock-out studies, CA074-Me and cathepsin C deficiency prevented LLOMe killing of antigen-presenting cells, whereas cathepsin B deficiency had no impact on this process (Fig. 4B). Consistent with our ex vivo data, we found that intravenous injection of alum and LLOMe significantly depleted macrophages and DCs, whereas the lymphocyte populations remained largely unchanged (supplemental Fig. 2A). In keeping with LLOMe-induced cytopathic effects in vivo, we found a significant increase in lactate dehydrogenase levels after intraperitoneal injection of LLOMe (supplemental Fig. 3A). Although CA-074-Me is highly efficient in blocking LLOMe-mediated cytopathic effects in vitro, CA-074-Me only partially blocked the lactate dehydrogenase signal in vivo (supplemental Fig. 3A). As many agents such as CA-074-Me are rapidly removed from the blood stream and accumulate in the liver (42), we assessed LLOMe toxicity within the liver. Although many chemical agents are rapidly removed from the blood and accumulated in the liver, we found only minimal cytopathic effects and CA-074-Me recovery in liver macrophages (Kupffer cells) (supplemental Fig. 3, B and C). Together, our findings indicate that LLOMe and alum trigger a similar form of necrotic cell death with similar host cell specificity.
LLOMe Generates Alum-like Inflammatory Responses in Vivo
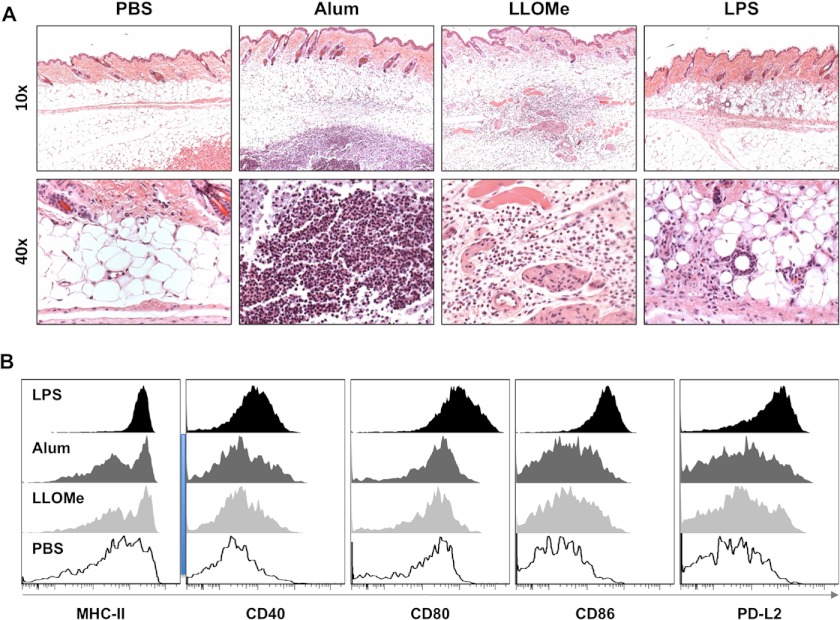
We found that LLOMe mimics alum-induced necrosis on multiple levels, as both agents trigger necrotic cell death by inducing lysosome rupture, specifically kill myeloid leukocytes, and induce cathepsin-dependent cell death. Based on these findings we postulated that if LLOMe replicates the alum-induced necrotic cell death, then the chemically unrelated LLOMe might also trigger an alum-like immune response. Within 1 day of injection, alum is known to recruit of inflammatory leukocytes to the site of injection and to induce DC maturation (3, 10). To measure the acute immune response to alum or LLOMe, we subcutaneously injected C57BL/6 mice with these agents and assessed the localized inflammatory response by H&E staining 24 h post challenge. As a control, we used the adjuvant LPS, which has been shown to trigger Th1 responses (43).
Each adjuvant generated a distinct pattern of inflammation and swelling at the injection site (Fig. 5A). We found that alum injection generated a nodular infiltrate (typical of insoluble agents) consisting of macrophages surrounding degenerating neutrophils and eosinophils (Fig. 5A), which have been specifically associated with Th2 responses (44). Consistent with being a soluble agent, LLOMe generated a diffuse infiltrate scattered throughout the subcutis comprised primarily of macrophages, neutrophils, and eosinophils (Fig. 5A). In contrast to alum and LLOMe, LPS recruited primarily monocytes, without any recruitment of eosinophils and only few granulocytes (Fig. 5A), characteristic of a Th1 immune response (45). In addition, exposure of alum, LLOMe, and LPS triggered the up-regulation of specific T-cell interacting molecules (Fig. 5B). DCs isolated from lymph nodes of alum-, LLOME-, and LPS-treated mice showed enhanced surface staining for MHC class II as well as the co-stimulatory molecules CD40, CD80, CD86, and PD-L2 (Fig. 5B). The potent Toll-like receptor activator, LPS, produced the strongest up-regulation of surface markers, whereas alum- and LL-treated DCs showed similar but more moderate phenotypes (Fig. 5B). Despite being cytopathic to dendritic cells (Fig. 4B), no signs of DC depletion were observed in distal lymph nodes and spleen after alum and LLOMe exposure. Taken together, these data suggested that alum and LLOMe induce similar acute inflammatory responses.
FIGURE 5.
Alum and LLOMe trigger similar immune responses in vivo. A, histopathologic skin sections were taken from the site of subcutaneous injection and stained with H&E from mice injected with a 200-μl solution containing PBS, 1.75 mg/ml alum, 2 mm LLOMe, or 100 μg of LPS. Representative images are shown at 10× and 40×. Images are representative of 2 separate experiments (n = 3 each experiment, n = 6 total). Close-up images focus on adipose sections of tissue. B, dendritic cells isolated from draining (axillary) lymph nodes from alum, LLOME (LL), and LPS-treated mice were assessed for maturation markers by flow cytometry. The data are shown by representative histograms of activation markers.
Cathepsin-mediated Necrosis Enhances the Adaptive Immune Response
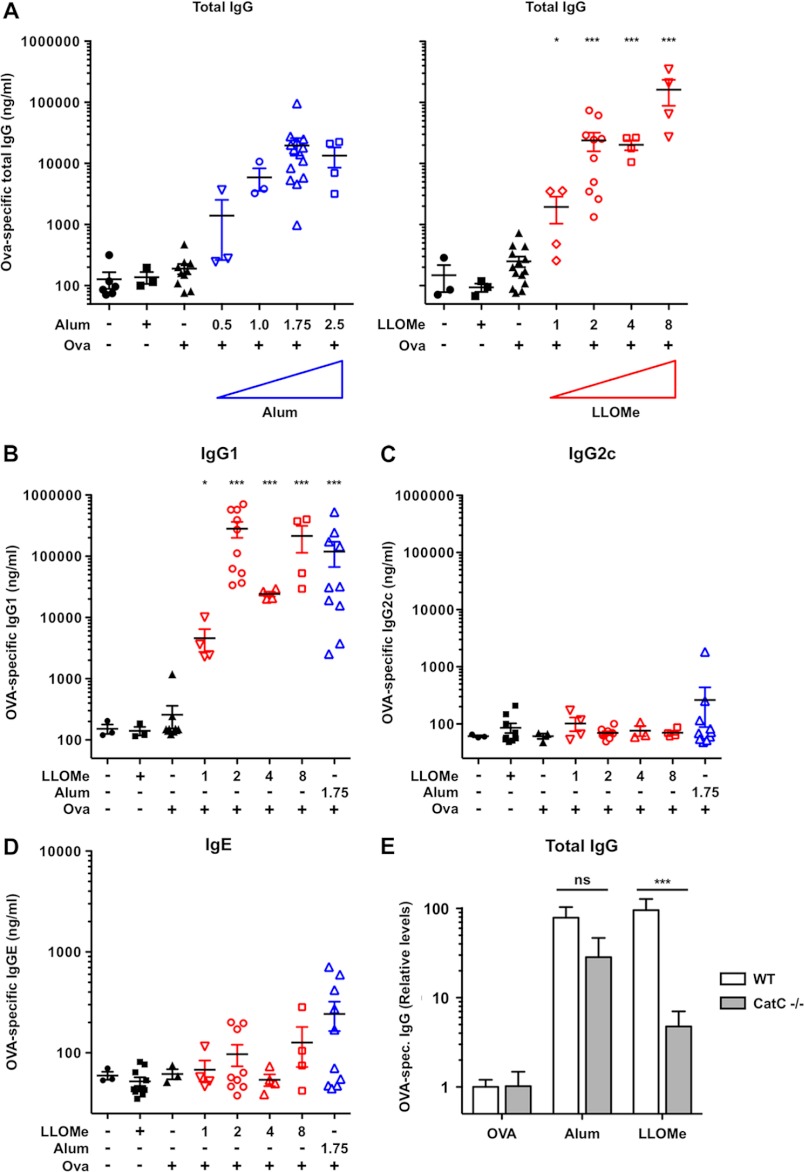
Our initial hypothesis was that alum-mediated necrosis triggers an adaptive immune response, and agents that mimic alum, such as LLOMe, should have adjuvant activities. To determine if LLOMe triggers, like alum, an adaptive immune response, we subcutaneously immunized C57BL/6 mice with 50 μg of ovalbumin in the presence of increasing amounts of alum and LLOMe. Mice immunized with PBS only or ovalbumin in PBS served as controls. As expected, alum induced a robust increase in total OVA-specific IgG in a dose-dependent fashion, showing a maximum response at 1.75 mg of alum per injection (Fig. 6A). Alum triggered a significant increase in OVA-specific IgG1 antibodies with only minimal induction of IgG2c consistent with a polarized Th2 immune response (Fig. 6, B and C). Like alum, LLOMe enhanced antibody production in a dose-dependent manner (Fig. 6, A and B). LLOMe also mimicked the alum-associated polarized production of Th2-associated IgG1 antibodies while failing to trigger Th1-associated IgG2c antibodies (Fig. 6, B and C). At the highest concentration tested, LLOMe induced ∼10-fold higher OVA-specific IgG levels than saturating concentrations of alum, suggesting that LLOMe is a more powerful adjuvant than alum (Fig. 6, A and B). Although LLOMe is a soluble peptide with a very limited biological half-life, it also generated, like alum, a long-lasting Th2-associated immune response that retained its strength 7 months from the initial immunization (supplemental Fig. 4, A and B). Although LLOMe generated stronger IgG titers than alum, it generated notably less IgE (Fig. 6, A and D), an antibody isotype strongly linked to allergic hypersensitivity reactions and potential adverse reactions to immunizations (46). Together these data indicate that LLOMe induced a strong long-lasting production of Th2-associated antibodies.
FIGURE 6.
LLOMe triggers Th2-associated antibody production in a cathepsin C-dependent fashion. A, C57BL/6 mice were injected with a 200-μl mixture containing 50 μg of OVA and increasing doses of alum (mg/ml) or LLOMe (mm). Mice were boosted with identical doses on day 15, and total IgG production was assessed on day 21. Data were normalized by log transformation and assessed for variance analyzed by one-way analysis of variance showing significance with subsequent post-test assessing for significant difference against PBS-only mice. Statistical significance is represented as follows: ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. B–D, C57BL/6 mice were injected twice with 50 μg of OVA and increasing doses of alum (mg/ml) or LLOMe (mm). IgG1 (B), IgG2c (C), and IgE (D) production was assessed on day 21 post immunization. E, wild type mice (n = 6 each) and cathepsin C−/− mice were immunized against OVA and adjuvanted with PBS PBS (n = 4), alum (n = 3), or LLOMe (n = 3) according to the same schedule. Induction of total Ova-specific IgG antibodies relative to PBS-OVA is shown in WT and cathepsin C−/− mice. Significance was determined by two-way analysis of variance.
Our findings suggested a correlation between cell death and adaptive immunity in alum- and LLOMe-treated mice. To conclusively link lysosome-mediated necrosis with adjuvant function, we tested whether cathepsin C deficiency blocked not only LLOMe-mediated necrosis but also an LLOMe-induced adaptive immune response. Consistent with our hypothesis, cathepsin C deficiency strongly reduced the production of total OVA-specific IgG in LLOMe-treated mice (Fig. 6E). Compared with mice receiving OVA alone, LLOMe triggered a 95-fold increase of total OVA-specific antibodies in wild type mice but only a 4.5-fold increase in cathepsin C−/− mice (Fig. 6E). As expected, cathepsin C deficiency had no significant impact on OVA-specific antibody production in alum-treated mice (Fig. 6E). Intriguingly, cathepsin C−/− mice generated higher OVA-specific antibody titers than wild type mice in response to OVA alone (supplemental Fig. 4C). These results indicate that cathepsin C−/− mice are not immuno-compromised but are in fact hyper-responsive to immunological stimuli (supplemental Fig. 4C).
Taken together, our data demonstrate that LLOMe is a powerful mimetic of alum-mediated necrosis in vitro and that LLOMe-induced cell death triggers a significant adjuvant effect that are impaired by cathepsin C deficiency. Our findings suggest that necrotic cell death is both necessary and sufficient for induction of the adaptive immune response by LLOMe and that adjuvant-induced necrosis is a powerful inducer of a Th2-associated immune response.
DISCUSSION
Specific Cathepsins Are Critical for Necrosis Mediated by the Lysosome-destabilizing Agents, Alum and LLOMe
Alum, like most adjuvants tested to date, is a cytotoxic compound (20, 47). Because necrosis has generally been considered an unwanted epiphenomenon in adjuvant effects (20, 47), adjuvant-mediated necrosis has been poorly studied. In light of recent evidence demonstrating the immunogenicity of necrotic cells (13–15), we examined adjuvant-mediated cytotoxicity in detail. We found that alum triggers a novel form of necrotic cell death that is cell type-specific and dependent on specific cathepsins and lysosome rupture. We further demonstrated that lysosome-destabilizing agents, such as the dipeptide LLOMe, mimic alum-induced cell death. Using a combination of a haploid genetic screen and cathepsin-deficient mice, we found that cathepsin activity is critical for adjuvant-induced necrosis. Here we provide genetic evidence that cathepsin activity is not only critical for adjuvant induced-induced cell death but also for the immune response mediated by these agents.
We found cathepsin specificity of necrotic cell death was highly inducer-specific, as cathepsin C controlled LLOMe-induced cell death, whereas cathepsins B and S were required for cytotoxicity associated with alum. It has been demonstrated that lysosomal accumulation of particulate compounds, such as alum, leads to lysosome rupture and cytopathic effects (30). We found that alum and LLOMe do not promote activation of their corresponding cathepsins but that the constituent activity of these cathepsins is crucial for lysosome rupture mediated by these agents. Pharmacological studies have suggested that the carboxypeptidase activity of cathepsin C converts the LLOMe dipeptide-methyl ester into a membranolytic polymer responsible for its lysosome-disrupting effect (35). Here we provide evidence that cathepsin C is, in fact, critical for LLOMe-induced cell death. It remains to be shown how cathepsins B and S promote alum-induced lysosome rupture and cytotoxic effects. It is conceivable that a measured cathepsin release from lysosomes triggers a forward loop promoting lysosome rupture and cell death in alum-treated cells. However, the precise role of cathepsins in particulate-mediated lysosome rupture and cell death awaits further investigations.
Alum and LLOMe Induce a Novel Form of Programmed Necrosis
Here we demonstrate that alum and LLOMe induce a form of cell death that is distinct from pyroptosis and necroptosis yet also cathepsin-dependent (32, 38, 48, 49). Our findings suggest that specific cathepsins control alum and LLOMe-induced lysosomal rupture and necrotic cell death. Lysosomes contain hydrolytic highly promiscuous enzymes that include proteases, lipases, amylases, and nucleases, whose enzymatic activity is primarily controlled by subcellular compartmentalization. It is reasonable to assume that release of these constitutively active hydrolases from lysosomes drives plasma membrane impairment and cell death. The lysosome-mediated necrosis (LMN) caused by alum and LLOMe may be relevant to a broad array of cytotoxic compounds. It has been shown that many insoluble particles, including silica crystals, monosodium urate crystals, asbestos, cholesterol plaques, and ultra-high molecular weight polyethylene (a component of many orthopedic implants), have been shown to be phagocytosed by macrophages and induce lysosomal impairment (30, 33). As many of these compounds have been reported to trigger tissue necrosis and granuloma formation (50, 51), it remains to be shown whether necrotic cell death contributes to the inflammatory diseases associated with these agents (47).
LLOMe Is a Potent Immunologic Adjuvant
We postulated that alum-mediated necrosis is responsible for its immunostimulatory properties and agents inducing necrotic cell death exhibit adjuvant activities. Consistent with this hypothesis, we found that, like alum, the lysosome-disrupting agent LLOMe triggered a novel form of necrotic cell death and granulocytosis and eosinophilia at the injection site and potentiation of adaptive immune responses when used as an adjuvant. Just as LLOMe induced more robust necrosis than alum in vitro, LLOMe also triggered a stronger antibody response than alum in vivo. The high efficiency of the LLOMe-induced immune response was surprising, as LLOMe is soluble and has an extremely short biological half-life in vivo. Our findings suggest that the transient cell death signal induced by LLOMe is sufficient to potentiate a strong and long-lasting immune response as would be necessary for a pharmacologically relevant immunologic adjuvant. In contrast to LLOMe, alum forms a depot and might trigger cell death over an extended period of time. However, within a month, a granuloma has been shown to form around alum depots, which presumably prevents further necrosis and immunogenic signaling (52, 53). Furthermore, removal of the alum depot does not reduce alum immunogenicity (52, 53), indicating that transient cell death might also be sufficient in the case of alum.
The immunostimulatory effect of necrotic myeloid cell death by adjuvants might initially seem counterintuitive, as it involves killing the very cells that are crucial for induction of a protective immune response. Although alum and LLOMe efficiently kill myeloid cells in vitro, our studies suggest that killing of these cells appears to be limited to a local burst at the injection site when used as adjuvants in vivo. We demonstrated that subcutaneous challenge did not interfere with general immune functions, such as DC activation in distal lymph nodes and spleen. These results suggest that adjuvant-induced killing of immune cells is local and does not interfere with immune functions at distal sites.
It is conceivable that our immune system has evolved to sense damage and to rapidly respond to microorganisms and agents that trigger damages in the host. Cell death is the most fundamental form of such damage, and multiple studies have shown that the immune system is able to respond to specific compounds released from necrotic cells. Based on our findings and results from other laboratories, we hypothesize that adjuvant- and microorganism-mediated programmed necrosis activates the adaptive immune system. Our studies indicate that the adjuvants, alum and LLOMe, activate the adaptive immune response by inducing necrosis in myeloid cells.
Cathepsin-mediated Necrosis Is Necessary and Sufficient to Generate Adjuvant Effects
The main hypothesis of this paper is that adjuvant-mediated necrosis is sufficient to generate adaptive immune responses. To this end we identified an agent (LLOMe) that mimics alum-mediated necrosis and generates an alum-like adaptive immune response. To directly assess the contribution of necrotic cell death to immunity, we took advantage of the fact that cathepsin C deficiency completely blocks LLOMe-mediated cell death. As predicted, we found impaired antibody production in cathepsin C-deficient mice in response to LLOMe, strongly supporting our premise that necrotic cell death is a powerful inducer of the adaptive immune response. As described above, cathepsins B and S jointly control alum-mediated cell death, as deficiency of either cathepsin does not completely prevent alum-mediated necrosis. Cathepsin B and S double knock-out mice are likely to be highly efficient in blocking cell death and presumably in preventing an immune response mediated by alum. However, these double knock-out mice cannot be used for adjuvant studies, as cathepsin S is critical for antigen presentation by MHC class II (54).
Cytotoxic Adjuvants Balance Cell Death and with Immunopotentiation
Alum, the gold standard of safe and effective adjuvants, appears to limit toxicity through two distinct mechanisms. First, it appears to restrict cell death to a region proximal to the injection site by forming a deposit at the injection site. A second mechanism to limit collateral damage might be the cell type specificity, as alum kills only specific immune cells while sparing CD4- and CD8-positive T cells. It remains to be shown whether the immunostimulatory effect of cytotoxic adjuvants requires killing of specific (immune) cells. It is conceivable that each cell type releases a unique, characteristic set of factors with distinct immunostimulatory activities. For example, necrotic B16 melanoma cells have been shown to trigger a cytotoxic T-cell response (12), whereas alum and LLOMe generate a humoral response, possibly by killing myeloid cells. Uric acid, DNA, and HMGB1 are cellular factors released from necrotic cells that have been associated with an adaptive immune response (9–11). It remains to be shown whether any of these compounds contribute to an LLOMe-mediated immune response, as suggested for alum (9–11).
Because necrosis has generally been considered an unwanted epiphenomenon of adjuvants (20, 47), adjuvant toxicity has been considered a fault that should be minimized. Based on our findings and studies by others, we suggest that the criteria for adjuvant selection and approval for clinical usage should be reevaluated. Our studies indicate that the ability of adjuvant compounds to induce cell death should not by itself be considered a detractor. Although necrosis of myeloid cells appears to be important for alum and LLOMe-mediated immunity, complete depletion of these immune cells apparently would be detrimental for induction of an immune response (20). Therefore, further research is required to understand how these fundamental processes are controlled and contained, how killing of bystander cells is avoided, and how adjuvant-induced killing is limited to critical cell types. Therefore, generating a safe-but-effective adjuvant would require balancing cytotoxicity with immune cell functions. Taken together, our findings indicate that it is possible to design highly efficient adjuvants by inducing limited, localized necrosis. Understanding the mechanisms that control adjuvant-mediated necrotic cell death and the accompanying inflammatory processes should improve our ability to design highly efficient but safe adjuvants.
Acknowledgments
We thank O. Olson (Sloan-Kettering Institute) for technical support and S. Porcelli (Albert Einstein College of Medicine) and Wolfgang Leitner for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants 1R56AI092497-01A1 (to J. B.), 5R01CA125162-05 (to J. A. J.), 5R01AI087630-02 (to F. S. S.), and 5R01AI088027-02 (to K. C.). This work was also supported by the Martin Turkish Foundation, Inc. and the M-O'Connor-Foundation.

This article contains supplemental Figs. 1–4
- LLOMe
- l-leucyl-l-leucine methyl ester
- OVA
- ovalbumin
- PI
- propidium iodide
- DC
- dendritic cell.
REFERENCES
- 1. Gupta R. K., Siber G. R. (1995) Adjuvants for human vaccines. Current status, problems, and future prospects. Vaccine 13, 1263–1276 [DOI] [PubMed] [Google Scholar]
- 2. Singh M., O'Hagan D. (1999) Advances in vaccine adjuvants. Nat. Biotechnol. 17, 1075–1081 [DOI] [PubMed] [Google Scholar]
- 3. McKee A. S., Munks M. W., MacLeod M. K., Fleenor C. J., Van Rooijen N., Kappler J. W., Marrack P. (2009) Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183, 4403–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ulanova M., Tarkowski A., Hahn-Zoric M., Hanson L. A. (2001) The common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect. Immun. 69, 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenbarth S. C., Colegio O. R., O'Connor W., Sutterwala F. S., Flavell R. A. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H., Willingham S. B., Ting J. P., Re F. (2008) Cutting edge. Inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 181, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchi L., Núñez G. (2008) The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spreafico R., Ricciardi-Castagnoli P., Mortellaro A. (2010) The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur. J. Immunol. 40, 638–642 [DOI] [PubMed] [Google Scholar]
- 9. Lambrecht B. N., Kool M., Willart M. A., Hammad H. (2009) Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 21, 23–29 [DOI] [PubMed] [Google Scholar]
- 10. Kool M., Soullié T., van Nimwegen M., Willart M. A., Muskens F., Jung S., Hoogsteden H. C., Hammad H., Lambrecht B. N. (2008) Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205, 869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K., Lekeux P., Coban C., Akira S., Ishii K. J., Bureau F., Desmet C. J. (2011) DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 17, 996–1002 [DOI] [PubMed] [Google Scholar]
- 12. Shi Y., Evans J. E., Rock K. L. (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 [DOI] [PubMed] [Google Scholar]
- 13. Kono H., Rock K. L. (2008) How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iyer S. S., Pulskens W. P., Sadler J. J., Butter L. M., Teske G. J., Ulland T. K., Eisenbarth S. C., Florquin S., Flavell R. A., Leemans J. C., Sutterwala F. S. (2009) Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. U.S.A. 106, 20388–20393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rovere-Querini P., Capobianco A., Scaffidi P., Valentinis B., Catalanotti F., Giazzon M., Dumitriu I. E., Müller S., Iannacone M., Traversari C., Bianchi M. E., Manfredi A. A. (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 5, 825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hara H., Kawamura I., Nomura T., Tominaga T., Tsuchiya K., Mitsuyama M. (2007) Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection. Distinct role of listeriolysin O determined by cytolysin gene replacement. Infect. Immun. 75, 3791–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sauter B., Albert M. L., Francisco L., Larsson M., Somersan S., Bhardwaj N. (2000) Consequences of cell death. Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willingham S. B., Bergstralh D. T., O'Connor W., Morrison A. C., Taxman D. J., Duncan J. A., Barnoy S., Venkatesan M. M., Flavell R. A., Deshmukh M., Hoffman H. M., Ting J. P. (2007) Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrovsky N. (2008) Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev. Vaccines 7, 7–10 [DOI] [PubMed] [Google Scholar]
- 20. Gupta R. K., Relyveld E. H., Lindblad E. B., Bizzini B., Ben-Efraim S., Gupta C. K. (1993) Adjuvants. A balance between toxicity and adjuvanticity. Vaccine 11, 293–306 [DOI] [PubMed] [Google Scholar]
- 21. Sutterwala F. S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G. S., Grant E. P., Bertin J., Coyle A. J., Galán J. E., Askenase P. W., Flavell R. A. (2006) Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 [DOI] [PubMed] [Google Scholar]
- 22. Halangk W., Lerch M. M., Brandt-Nedelev B., Roth W., Ruthenbuerger M., Reinheckel T., Domschke W., Lippert H., Peters C., Deussing J. (2000) Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Invest. 106, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roth W., Deussing J., Botchkarev V. A., Pauly-Evers M., Saftig P., Hafner A., Schmidt P., Schmahl W., Scherer J., Anton-Lamprecht I., Von Figura K., Paus R., Peters C. (2000) Cathepsin L deficiency as molecular defect of furless. Hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 14, 2075–2086 [DOI] [PubMed] [Google Scholar]
- 24. Muehlbauer S. M., Evering T. H., Bonuccelli G., Squires R. C., Ashton A. W., Porcelli S. A., Lisanti M. P., Brojatsch J. (2007) Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6, 758–766 [DOI] [PubMed] [Google Scholar]
- 25. Alileche A., Serfass E. R., Muehlbauer S. M., Porcelli S. A., Brojatsch J. (2005) Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLOS Pathog. 1, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carette J. E., Guimaraes C. P., Varadarajan M., Park A. S., Wuethrich I., Godarova A., Kotecki M., Cochran B. H., Spooner E., Ploegh H. L., Brummelkamp T. R. (2009) Haploid genetic screens in human cells identify host factors used by pathogens. Science 326, 1231–1235 [DOI] [PubMed] [Google Scholar]
- 27. Carette J. E., Raaben M., Wong A. C., Herbert A. S., Obernosterer G., Mulherkar N., Kuehne A. I., Kranzusch P. J., Griffin A. M., Ruthel G., Dal Cin P., Dye J. M., Whelan S. P., Chandran K., Brummelkamp T. R. (2011) Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Squires R. C., Muehlbauer S. M., Brojatsch J. (2007) Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J. Biol. Chem. 282, 34260–34267 [DOI] [PubMed] [Google Scholar]
- 29. Kuroda E., Ishii K. J., Uematsu S., Ohata K., Coban C., Akira S., Aritake K., Urade Y., Morimoto Y. (2011) Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity 34, 514–526 [DOI] [PubMed] [Google Scholar]
- 30. Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujisawa A., Kambe N., Saito M., Nishikomori R., Tanizaki H., Kanazawa N., Adachi S., Heike T., Sagara J., Suda T., Nakahata T., Miyachi Y. (2007) Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood 109, 2903–2911 [DOI] [PubMed] [Google Scholar]
- 32. Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. (2010) Molecular mechanisms of necroptosis. An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 [DOI] [PubMed] [Google Scholar]
- 33. Sharp F. A., Ruane D., Claass B., Creagh E., Harris J., Malyala P., Singh M., O'Hagan D. T., Pétrilli V., Tschopp J., O'Neill L. A., Lavelle E. C. (2009) Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. U.S.A. 106, 870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Duve C., Wattiaux R. (1966) Functions of lysosomes. Annu. Rev. Physiol. 28, 435–492 [DOI] [PubMed] [Google Scholar]
- 35. Thiele D. L., Lipsky P. E. (1990) The action of leucyl-leucine methyl ester on cytotoxic lymphocytes requires uptake by a novel dipeptide-specific facilitated transport system and dipeptidyl peptidase I-mediated conversion to membranolytic products. J. Exp. Med. 172, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thiele D. L., Lipsky P. E. (1992) Apoptosis is induced in cells with cytolytic potential by l-leucyl-l-leucine methyl ester. J. Immunol. 148, 3950–3957 [PubMed] [Google Scholar]
- 37. Stoka V., Turk B., Schendel S. L., Kim T. H., Cirman T., Snipas S. J., Ellerby L. M., Bredesen D., Freeze H., Abrahamson M., Bromme D., Krajewski S., Reed J. C., Yin X. M., Turk V., Salvesen G. S. (2001) Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276, 3149–3157 [DOI] [PubMed] [Google Scholar]
- 38. Bergsbaken T., Fink S. L., Cookson B. T. (2009) Pyroptosis. Host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., Tschopp J. (2004) NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 40. Günther C., Martini E., Wittkopf N., Amann K., Weigmann B., Neumann H., Waldner M. J., Hedrick S. M., Tenzer S., Neurath M. F., Becker C. (2011) Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hentze H., Lin X. Y., Choi M. S., Porter A. G. (2003) Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 10, 956–968 [DOI] [PubMed] [Google Scholar]
- 42. Guicciardi M. E., Deussing J., Miyoshi H., Bronk S. F., Svingen P. A., Peters C., Kaufmann S. H., Gores G. J. (2000) Cathepsin B contributes to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Przetak M., Chow J., Cheng H., Rose J., Hawkins L. D., Ishizaka S. T. (2003) Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine 21, 961–970 [DOI] [PubMed] [Google Scholar]
- 44. Seubert A., Monaci E., Pizza M., O'Hagan D. T. (2008) The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J. Immunol. 180, 5402–5412 [DOI] [PubMed] [Google Scholar]
- 45. Moore A., McCarthy L., Mills K. H. (1999) The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 17, 2517–2527 [DOI] [PubMed] [Google Scholar]
- 46. Kelso J. M., Jones R. T., Yunginger J. W. (1993) Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J. Allergy Clin. Immunol. 91, 867–872 [DOI] [PubMed] [Google Scholar]
- 47. Petrovsky N., Aguilar J. C. (2004) Vaccine adjuvants. Current state and future trends. Immunol. Cell Biol. 82, 488–496 [DOI] [PubMed] [Google Scholar]
- 48. Newman Z. L., Leppla S. H., Moayeri M. (2009) CA-074Me protection against anthrax lethal toxin. Infect. Immun. 77, 4327–4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang H., Zhong C., Shi L., Guo Y., Fan Z. (2009) Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to necroptosis. J. Immunol. 182, 6993–7000 [DOI] [PubMed] [Google Scholar]
- 50. Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. (1990) Pulmonary response to silica or titanium dioxide. Inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am. J. Respir. Cell Mol. Biol. 2, 381–390 [DOI] [PubMed] [Google Scholar]
- 51. Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., Wright S. D., Hornung V., Latz E. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Noe S. M., Green M. A., HogenEsch H., Hem S. L. (2010) Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine 28, 3588–3594 [DOI] [PubMed] [Google Scholar]
- 53. McKee A. S., Munks M. W., Marrack P. (2007) How do adjuvants work? Important considerations for new generation adjuvants. Immunity 27, 687–690 [DOI] [PubMed] [Google Scholar]
- 54. Riese R. J., Mitchell R. N., Villadangos J. A., Shi G. P., Palmer J. T., Karp E. R., De Sanctis G. T., Ploegh H. L., Chapman H. A. (1998) Cathepsin S activity regulates antigen presentation and immunity. J. Clin. Invest. 101, 2351–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]