Background: Autophagy is a conserved process of lysosome-mediated intracellular degradation.
Results: Dysregulation of autophagy is associated with retinal cell death by all-trans-retinal and by light exposure.
Conclusion: Autophagy protects the retina from light-induced retinal degeneration.
Significance: Dynamic autophagy regulation may influence retinal cell survival under stress and disease conditions.
Keywords: Alcohol Dehydrogenase, Autophagy, Retinal Degeneration, Retinal Metabolism, Rhodopsin, Atg7, Beclin1, Park2, All-trans-retinal, Light Damage
Abstract
Autophagy is a conserved feature of lysosome-mediated intracellular degradation. Dysregulated autophagy is implicated as a contributor in neurodegenerative diseases; however, the role of autophagy in retinal degeneration remains largely unknown. Here, we report that the photo-activated visual chromophore, all-trans-retinal, modulated autophagosome formation in ARPE19 retinal cells. Increased formation of autophagosomes in these cells was observed when incubated with 2.5 μm all-trans-retinal, a condition that did not cause cell death after 24 h in culture. However, autophagosome formation was decreased at concentrations, which caused cell death. Increased expression of activating transcription factor 4 (Atf4), which indicates the activation of oxidative stress, was recorded in response to light illumination in retinas of Abca4−/−Rdh8−/− mice, which showed delayed clearance of all-trans-retinal after light exposure. Expression of autophagosome marker LC3B-II and mitochondria-specific autophagy, mitophagy, regulator Park2, were significantly increased in the retinas of Abca4−/−Rdh8−/− mice after light exposure, suggesting involvement of autophagy and mitophagy in the pathogenesis of light-induced retinal degeneration. Deletion of essential genes required for autophagy, including Beclin1 systemically or Atg7 in only rod photoreceptors resulted in increased susceptibility to light-induced retinal damage. Increased photoreceptor cell death was observed when retinas lacking the rod photoreceptor-specific Atg7 gene were coincubated with 20 μm all-trans-retinal. Park2−/− mice also displayed light-induced retinal degeneration. Ultra-structural analyses showed mitochondrial and endoplasmic reticulum impairment in retinas of these model animals after light exposure. Taken together, these observations provide novel evidence implicating an important role of autophagy and mitophagy in protecting the retina from all-trans-retinal- and light-induced degeneration.
Introduction
Autophagy is an evolutionarily conserved process involving lysosome-mediated intracellular degradation (1). Autophagy occurs at a basal level mainly to maintain homeostatic function during protein and organelle turnover. Under various pathophysiological stress conditions, autophagy can be up-regulated to supply the increased demand for intracellular nutrients and energy, meet the needs of developmentally related structural remodeling, and eliminate intracellular misfolded or long-lived proteins, superfluous or damaged organelles, and invading microorganisms. Although morphological features of autophagy have been documented for decades, the functional significance of autophagy in pathophysiological conditions has only recently been appreciated due to the molecular regulators and functional analyses of autophagy-related genes (Atgs) being reported. The significance of the prosurvival function of autophagy in human disease was discovered by using mouse models that lacked genes essential for autophagosome formation, including, Beclin1, Atg5, or Atg7 (2–4). Autophagy has thus emerged as an important player in the pathophysiology in a variety of disorders such as neurodegeneration, cancer, and immune disorders (5).
The presence of autophagy occurring in retinal pigment epithelium (RPE)2 cells and photoreceptors was first described in ground squirrels under active and hibernating conditions (6). Further study confirmed that autophagy occurs ubiquitously in RPE and in photoreceptor cells under normal conditions in various species (6). Autophagosomes in retinal cells contain all types of cytoplasmic constituents and invariably display the same range of morphological features regardless of cell type or species. These observations suggest that autophagy is part of a basal cellular process common to both RPE and photoreceptor cells. Namely, autophagic events were also found to be enhanced by light stimulation after light-induced damage in RPE and photoreceptor cells, although the exact biological significance of this light-induced autophagy up-regulation was unclear (7).
Light signal is converted to electrical signal in the retina allowing visual perception, and the initial step of this conversion is photo-isomerization of the visual chromophore 11-cis-retinal to all-trans-retinal (atRAL) (8). Production of atRAL is essential for vertebrate vision; however, excess amounts of atRAL may cause retinal cell death. Delayed clearance of atRAL in Abca4−/−Rdh8−/− mice, which are deficient in both ATP binding cassette transporter 4 (ABCA4) and retinol dehydrogenase 8 (RDH8), key enzymes for atRAL clearance from photoreceptors, causes light-dependent retinal degeneration (9), indicating the important role of atRAL as an inducer of photodamage.
In the present study we showed that the protein levels of autophagosome marker LC3B-II and mitophagy regulator Park2 were significantly increased in response to light exposure at a dose able to cause retinal degeneration in Abca4−/−Rdh8−/− mice. These mice provide a model for acute light-induced retinopathy (9), thus indicating that the processes of autophagy and mitophagy are associated with light-induced retinal degeneration. Genetically modified mouse models displaying deficiencies in autophagy and mitophagy were further examined to clarify the role of autophagy in retinal degeneration. Increased susceptibility to light-induced retinal degeneration was exhibited in two different mouse lines defective in autophagy. The first line was deficient in Beclin1, whereas the second line contained a rod photoreceptor-specific deletion of Atg7. A similar retinal phenotype was observed in mice with a deficiency in the mitophagy essential gene, Park2. Our findings demonstrate inadequate autophagy and mitophagy could result in light-induced retinal degeneration, thus supporting an important role for autophagy and mitophagy in protecting the retina from photo-oxidative stress.
EXPERIMENTAL PROCEDURES
Mice
Abca4−/−Rdh8−/− mice were generated as previously described (9), and Beclin1+/− mice were originally generated by Dr. Beth Levine (UT Southwestern, Dallas, TX) and re-derived at Case Western Reserve University (10). Atg7fox/flox mice were established by Dr. Keiji Tanaka (Laboratory of Frontier Science, Tokyo Metropolitan Institute of Medical Science, Tokyo) (4). Rod photoreceptor-specific Cre mice, LMOP-Cre mice, were generated as previously described (11). Park2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were genotyped by established methods, and only those with Leu-450 in RPE65 (12) and Rd8−/− (13) were used. Mice were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were maintained either in the dark or under a 12-h light (∼10 lux)/12-h dark cycle. Manipulations in the dark were done under dim red light transmitted through a Kodak No. 1 safelight filter (transmittance >560 nm). All animal procedures and experiments were approved by the Case Western Reserve University Institutional Animal Care and Use Committees (IACUC) and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology.
Induction and Analysis of Light Damage
Six-week-old mice were dark-adapted for 48 h before exposure to light. Light damage was induced by eye exposure to diffuse white fluorescent light (150 watt spiral lamp, Commercial Electric) at the indicated intensity for indicated time periods. Before such light exposure, pupils of mice were dilated with 1% tropicamide, and after exposure animals were kept in the dark until evaluation.
Ultra-high Resolution Spectral-domain Optical Coherence Tomography (SD-OCT)
Ultra-high resolution SD-OCT (Bioptigen) was employed for in vivo imaging of mouse retinas. Mice were anesthetized by intraperitoneal injection of a mixture (20 μl/g of body weight) containing ketamine (6 mg/ml) and xylazine (0.44 mg/ml) in 10 mm sodium phosphate, pH 7.2, containing 100 mm NaCl. Pupils were dilated with 1% tropicamide. Four pictures acquired in the B-scan mode were used to construct each final averaged SD-OCT image.
LC3B-GFP Expression in ARPE19 Cells
Human-derived RPE cells, ARPE19 cells, were purchased from American Type Culture Collection (ATCC, Manassas, VA). LC3B-GFP vector is a generous gift from Dr. N. Mizushima (Tokyo Medical and Dental University, Tokyo, Japan). LC3B-GFP vector was stably transfected with FuGENE transfection reagent (Roche Applied Science). ARPE19 cells were cultured with DMEM (Thermo Scientific Hyclone, Logan, UT) with 10% fetal bovine serum. atRAL was purchased from Sigma. Bafilomycin A1, an inhibitor for lysosome degradation, was purchased from Invivogen (San Diego, CA).
Histology
Histological and immunohistochemical procedures employed were well established (14). Anti-rhodopsin 1D4 antibody was a generous gift from Dr. R. S. Molday (University of British Columbia, Vancouver CA). Peanut agglutinin and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen. Eyecups for histology were fixed in 2% glutaraldehyde, 4% paraformaldehyde and processed for embedding in Epon. Sections were cut at 1 μm and stained with toluidine blue. Electron microscopic analyses were performed as previously described (15).
Western Blotting
SDS-PAGE was performed using 12.5 or 15% polyacrylamide gels, and proteins were electrophoretically transferred onto Immobilon-P (Millipore, Bedford, MA). The membrane was blocked with 3% BSA in 10 mm phosphate, pH 7.5, containing 100 mm NaCl and incubated for 3–16 h with primary antibody. A secondary antibody conjugated with alkaline-phosphatase (Promega, Madison, WI) was used at a dilution of 1:5000. Ab binding was detected by incubation with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Promega). Anti-Beclin1, Park2, Atg5, Atg7, and β-actin antibodies were purchased from Novus Biologicals (Littleton, CO), and anti-β-tubulin antibody was obtained from Developmental Studies Hybridoma Bank (DHSB) at the University of Iowa.
RT-PCR and Quantitative RT-PCR
Total RNA was isolated using a RiboPure kit (Applied Biosystems, Austin, TX), and cDNA was synthesized using SuperScriptTMII Reserve Transcriptase (Invitrogen) following the manufacturer's instruction. Choice-TaqDNA Polymerase (Denville Scientific Inc., Metuchen, NJ) was used for PCR amplification. Primer sequences are the following: mBeclin1L (5′-atgtggaaaagaaccgcaag-3′) and mBeclin1R (5′-actccagctgctgctgcctttta-3′); mAtg5L (5′-ggagagaagaggagccaggt-3′) and mAtg5R (5′-gctgggggacaatgctaata-3′); mAtg7L (5′-accatgcagggagctagaga-3′) and mAtg7R (5′-ccactgaggttcaccatcct-3′). Real-time PCR amplification was performed using iQTM SYBR® Green Supermix (Bio-Rad). Primers were designed using web tool Primer 3 and synthesized by Eurofins MWG Operon (Hunstville, AL). Relative expression of genes was normalized by housekeeping gene Gapdh. Primer sequences are the following: mAtf4L (5′-tcctgaacagcgaagtgttg-3′) and mAtf4R (5′-acccatgaggtttcaagtgc-3′); mGapdhL (5′-gtgttcctacccccaatgtg-3′) and mGapdhR (5′-ggagacaacctggtcctcag-3′).
Ex Vivo Retinal Culture
Eyes were enucleated, washed with penicillin-streptomycin solution (Sigma), and rinsed with Hanks' balanced solution (Hyclone, Waltham, MA). Eye cups were prepared, and they were flattened by making retinal flaps. The flattened retina was transferred onto filter paper, and the retina was gently peeled away from the RPE/choroid. These procedures were performed under a surgical microscopy. Each retina on the filter paper was placed in each well of a 12-well plate filled with 0.5 ml of DMEM with 10% fetal bovine serum, and retinas were incubated for 16 h at 37 °C. Retinas were washed with 0.5 ml of fresh DMEM with 10% fetal bovine serum twice. These retinas were further incubated with/without 20 μm atRAL for 6 h at 37 °C. Lactate dehydrogenase assay was performed for calculating cell death rates (BioVision, Mountain View, CA). The percentage of cytotoxicity was calculated as ((a retina with atRAL − a retina without atRAL)/(lysis control − a retina without atRAL)) × 100.
Statistical Analyses
Data representing the means ± S.D. for the results of at least three independent experiments were compared by the one way analysis of variance test.
RESULTS
Autophagic Events in the Retina of Wild-type Mice
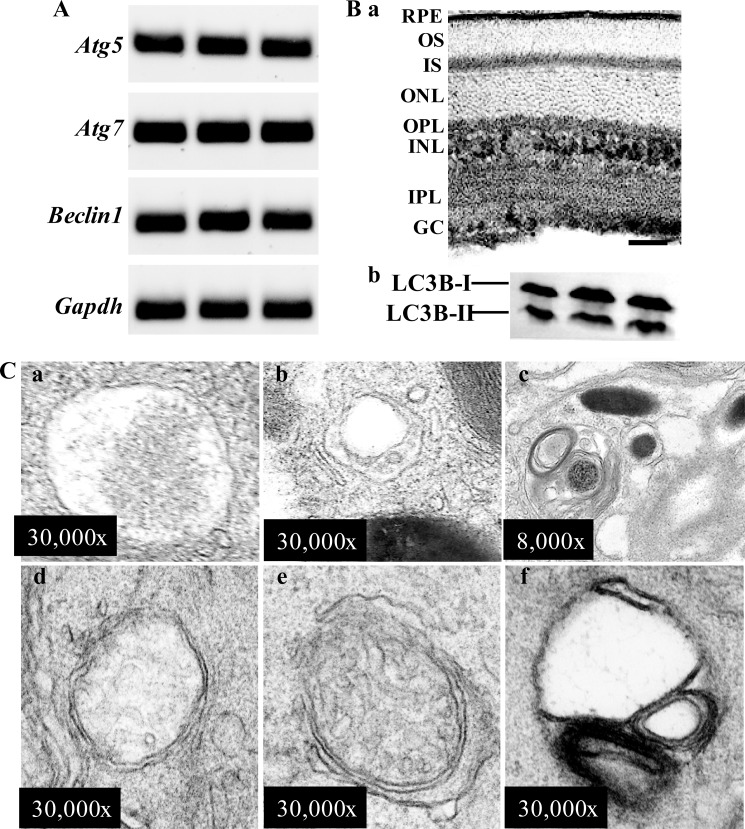
To examine the autophagy machinery in the retina, expression of genes essential for autophagy was examined in 6-week-old wild-type (WT) mice. The mRNA expression of autophagy-related genes including Atg5, Atg7, and Beclin1 was detected in the retina of WT mice by RT-PCR (Fig. 1A). Autophagy in the retina was further examined biochemically by analyzing the expression of microtubule-associated protein light chain (LC3B). Nascent LC3B (proLC3B) is undetectable under normal conditions because it is processed by ATG4 into LCB3-I right after synthesis (16). LC3B-I is localized in the cytosol, and when it is conjugated with phosphatidylethanolamine and converted to LC3-II, the latter is present on isolated membranes and autophagosomes and much less on autolysosomes (16). Therefore, the amount of LC3B-II is closely correlated with the number of autophagosomes and is widely used as a molecular marker to monitor autophagic activity (16). Immunohistochemical examination showed that LC3B was ubiquitously expressed in the retina of WT mice predominantly in RPE, photoreceptor inner segments (IS), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and ganglion cells (GC) (Fig. 1Ba). Western blotting revealed that LC3B was abundantly expressed in the retina of WT mice in the form of both LC3B-I and LC3B-II (Fig. 1Bb), providing biochemical evidence supporting the presence of autophagosomes in the retina. Electron microscopy (EM) was performed to validate the finding that autophagy is commonly present in mouse RPE and photoreceptor cells. Autophagosomes were detected in RPE cells and displayed various contents; for instance, a portion of plasma membrane included in an autophagic vacuole (Fig. 1Ca), an autophagic vacuole enclosing dilated endoplasmic reticulum and a small portion of plasma membrane (Fig. 1Cb), and an autophagosome enwrapping membranous material and a melanosome (Fig. 1Cc). Similar to the observation made in other species, autophagic events were also frequently encountered in photoreceptor inner segments in the retinas of WT mice (Fig. 1C, d and e), which showed an autophagosome enclosing a portion of plasma membrane, an autophagic vacuole containing a damaged mitochondrion, and an autophagosome wrapping up partially degraded membranous material, respectively. Taken together, our data indicate that autophagic events are present in the retina of WT mouse, particularly in RPE and photoreceptors.
FIGURE 1.
Autophagy in the retina of wild-type mice. A, the expression of genes essential for autophagy was readily detected in 6-week-old WT mouse retinas. The mRNA expression of Atg5, Atg7, Beclin1, and Gapdh was detected in isolated mouse retinas by RT-PCR done in triplicate. Ba, the expression and immunolocalization of LC3B was revealed by immunohistochemistry using the retina of albino C57BL mice, indicating the expression of LC3B in RPE, photoreceptor inner segment (IS), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and ganglion cell (GC). The bar indicates 20 μm. b, the protein expression of LC3B was detected in mouse retina. LC3B was resolved into two bands in SDS-PAGE, the cytoplasmic form LC3B-I and the autophagosome-associated form of LC3B-II. C, autophagic events were frequently encountered in the mouse retina under electromicroscopic examination. Autophagosomes containing portions of cytoplasm (a), a small amount of cytoplasm and dilated endoplasmic reticulum (ER) (b), membranous structure and a melanosome (c) were found in RPE. Autophagosomes enclosing cytoplasm (d), a damaged mitochondrion and small portion of cytoplasm (e), and partially membranous structure (f) were observed in mouse photoreceptor inner segment.
Coincubation with All-trans-retinal Modulates Authophagy and Apoptosis in ARPE19 Cells
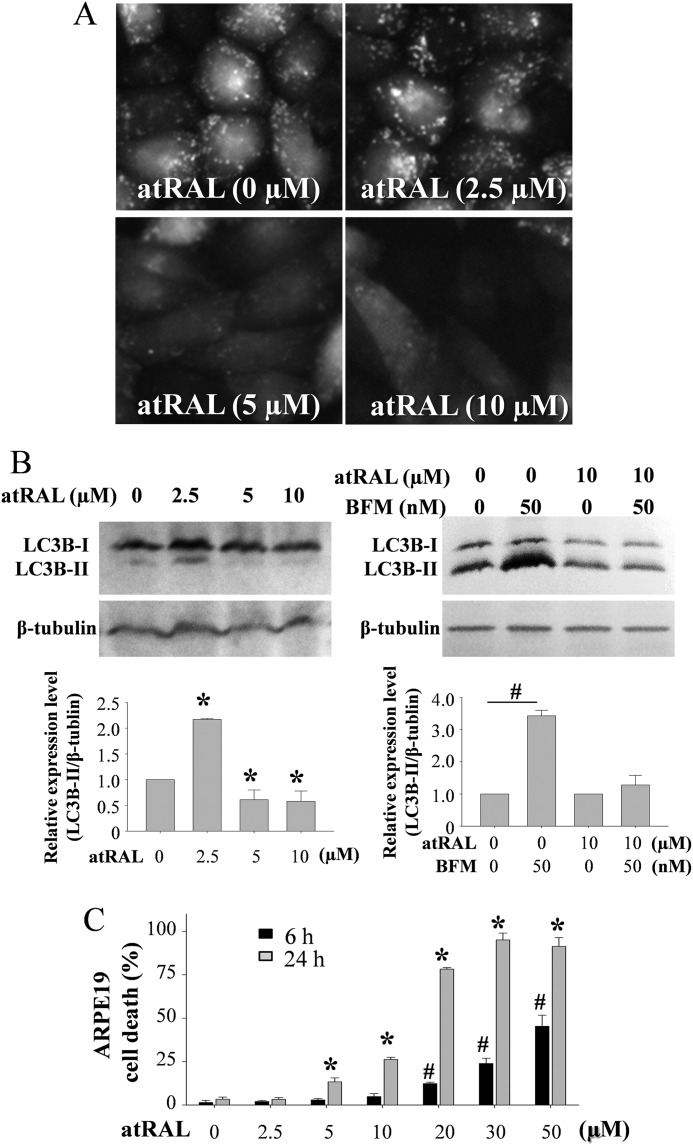
Photo-activated visual chromophore atRAL is cytotoxic and a potent inducer of light damage through mitochondrial impairment (17) and activation of NADPH oxidase (14). ARPE19 cells, human-derived RPE cells, display mitochondria-associated apoptosis induced by atRAL (17). To investigate the role of autophagy in retinal cells, expression of LC3B was examined in ARPE19 cells in the presence or absence of atRAL. ARPE19 cells with stably expressed LC3B-GFP showed autophagosomes as GFP-positive intracellular vesicles in the absence of atRAL (Fig. 2A). Increased autophagosome formation was observed when ARPE19 cells were challenged with 2.5 μm atRAL for 24 h, whereas this formation was decreased under coincubation with either 5 or 10 μm this retinoid. Western blotting also showed increased expression of LC3B-II in ARPE19 cells incubated with 2.5 μm atRAL for 24 h but decreased LC3B-II levels at 5 and 10 μm retinoid (Fig. 2B, left). Furthermore, although increased LC3B-II in ARPE19 cells was caused by 50 nm bafilomycin A1, an inhibitor for lysosome degradation, this increase was not observed when cells were treated with 10 μm atRAL (Fig. 2B, right), indicating the primary cause for decreased autophagosomes and LC3B-II expression after atRAL coincubation is the impaired autophagy and not a result of increase autophagic flux. atRAL-induced ARPE19 cell death was detected at concentrations of 5 μm and higher using a cytotoxicity assay that measures lactate dehydrogenase when cells were incubated for 24 h (Fig. 2C). These data revealed a dynamic change of autophagic events that inversely correlated with the dose-dependent cytotoxicity of atRAL, suggesting that impaired autophagy is implicated in atRAL-induced cytotoxicity. This further suggests that autophagy is mechanistically involved in retinal degeneration, especially light-induced retinopathy given that atRAL is an important mediator of retinal cell death during retinal photo-oxidative stress (9, 14, 17).
FIGURE 2.
All-trans-retinal modulates autophagosome formation. A, LC3B-GFP was stably expressed in ARPE19 cells, and cells were incubated with 0, 2.5, 5, and 10 μm atRAL for 24 h at 37 °C. GFP signals were visualized under a fluorescent microscope (Leica DMI 6000 System). B, LC3B expression in ARPE19 cells after coincubation with atRAL for 24 h was examined by Western blotting. Increased expression of LC3B-II was detected ARPE19 cells incubated with 2.5 μm atRAL, whereas decreased expression of LC3B-II was noted under coincubation with 5 and 10 μm atRAL (left panel). *, compared with cells without coincubation with atRAL, p < 0.01. Balifomycin A1 (BFM) at 50 nm, an inhibitor for lysosome/autolysosome degradation, was admixed to ARPE19 cells cultured with 0 or 10 μm atRAL (right panel). Balifomycin A1 caused increased expression of LC3B-II in ARPE19 cells, whereas this increase was not observed when cells were coincubated with 10 μm atRAL. #, compared with cells without addition of balifomycin A1, p < 0.01. C, cell death rate was calculated by measuring lactate dehydrogenase (LDH) production (LDH-Cytotoxicty Assay kit, BioVision, Milpitas, CA). ARPE19 cells were coincubated with atRAL at indicated concentrations for 6 h or 24 h. # and *, compared with vehicle-treated cells for 6 h (#) or 24 h (*), p < 0.01.
Increased LC3B-II Production Is Observed in Light-induced Retinal Damage
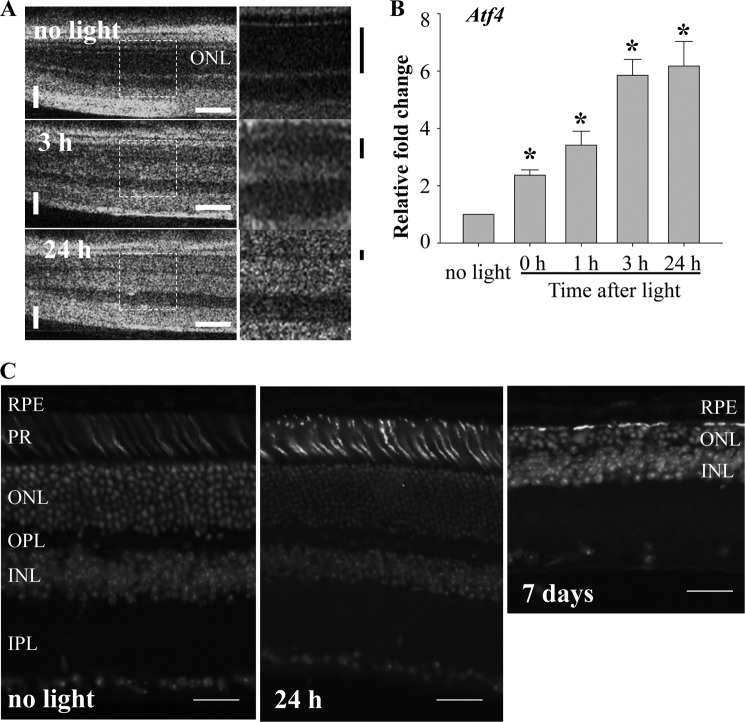
To investigate the changes of autophagy in light-induced retinopathy, protein expressions of molecules related to autophagy were examined in the Abca4−/−Rdh8−/− mouse model, which exhibits increased susceptibility to light-induced retinopathy (17). Abca4−/−Rdh8−/− mice at 6 weeks of age were exposed to light at 10,000 lux for 30 min, a condition that triggers significant retinal damage as previously described (17). The photoreceptor began to display distinct reflection in the photoreceptor outer nuclear layer (ONL) in SD-OCT images. These reflections could be associated with early changes in light-induced photoreceptor damage (Fig. 3A). Rapid and significant up-regulation of mRNA levels of activating transcription factor 4 (Atf4) in response to light exposure was observed (Fig. 3B), indicating the activation of oxidative stress by light exposure (18). Destructive retinal changes were hardly detected 24 h after light exposure, when SD-OCT displayed abnormal reflection, by conventional retinal histological analysis, and decreased ONL thickness was detected 7 days after light exposure (Fig. 3C).
FIGURE 3.
Early changes in light-induced retinal damage in Abca4−/−Rdh8−/− mice. A, Abca4−/−Rdh8−/− mice at 6 weeks of age were exposed to white light at the intensity of 10,000 lux for 30 min, and SD-OCT imaging was carried out to monitor the retinal morphology at 3 and 24 h after light exposure (left). Horizontal or vertical bars in white indicate 200 or 50 μm, respectively. Magnified images of the areas with dash lines are presented (right). Black bars indicated outside show the thickness of dark area related to ONL observed in OCT imaging. Thickness of the dark area was decreased after light exposure. B, expression of Atf4 was examined by quantitative real-time-PCR and exhibited significant and rapid up-regulation in response to light exposure of 10,000 lux for 30 min. *, compared with no light, p < 0.01. C, cryosections of Abca4−/−Rdh8−/− mice after light exposure at 10,000 lux for 30 min are presented. Cone photoreceptors and nuclei were stained with peanut agglutinin and DAPI, respectively. Although decreased ONL thickness was displayed 7 days after light, such change was not detected 24 h after light. Bars indicate 20 μm.
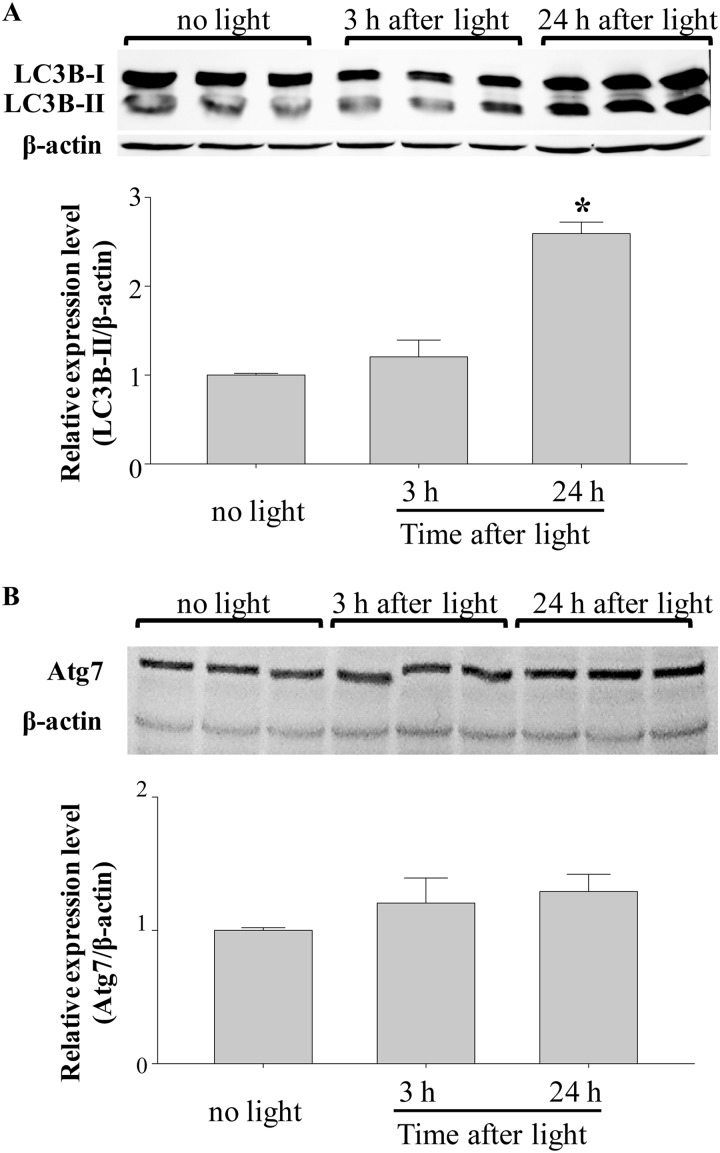
Levels of LC3B-II and protein expression of Atg7 were examined in the retinas of Abca4−/−Rdh8−/− mice. Retinas of 6-week-old Abca4−/−Rdh8−/− mice were collected either 3 or 24 h after light exposure at 10,000 lux for 30 min, which caused abnormal OCT changes and increased level of Atf4 (Fig. 3). Although the level of LC3B-II was maintained 3 h after light exposure, an increase in LC3B-II conversion was observed 24 h after light (Fig. 4A). In opposition to this finding, Atg7 protein expression was unchanged before and after light exposure (Fig. 4B), and similar observations were seen in Beclin1 protein expression (supplemental Fig. S1). Additionally, a significant increase in LC3B-II level was observed before the massive loss of the photoreceptor cells, which occurs 3–7 days after light exposure in Abca4−/−Rdh8−/− mice (19), thus suggesting that increased autophagosome formation is an early event during light-induced photoreceptor degeneration.
FIGURE 4.
Increased expression of autophagic marker LC3B-II in the retina is associated with light-induced retinopathy in Abca4−/−Rdh8−/− mice. A, expression of LC3B was examined by SDS-PAGE followed by Western blotting with triplicate samples. LC3B-II expression was significantly up-regulated by light 24 h after illumination. *, compared with no light, p < 0.01. B, expression of Atg7 was examined by SDS-PAGE followed by Western blotting with triplicate samples. Shown are retinas of 6-week-old Abca4−/−Rdh8−/− mice after exposure to white light at the intensity of 10,000 lux for 30 min.
Beclin1 Deficiency Causes Light-induced Retinopathy
As indicated above, changes in autophagic events are associated with light-induced retinal degeneration; however, it remains to be clarified whether autophagy is prosurvival or promoting cell death during light-induced retinal degeneration. To further address the functional consequence of autophagy during light-induced retinal degeneration, autophagy gene-deficient mouse model Beclin1+/− mouse was employed to examine the role of autophagy in light-induced retinal degeneration. Beclin1-deficient homozygosity results in embryonic lethality (20, 21), and Beclin1+/− mouse has been extensively studied to reveal the function of autophagy under various pathophysiological conditions. First, Beclin1 expression in the retina of WT mice was examined by immunohistochemistry. Beclin1 was expressed in RPE, ONL, inner nuclear layer (INL), and ganglion cell layer (GC) (supplemental Fig. S2A). To examine the impact of Beclin1 deficiency on retinal morphology under steady state room lighting conditions, Beclin1+/− mice together with Beclin1+/+ littermate controls were examined by SD-OCT imaging up to 12 month of age. No overt morphological changes were noted in Beclin1+/− mice as they displayed similar SD-OCT images as that from Beclin1+/+ littermate controls (supplemental Fig. S2B), although Beclin1 expression and LCB-II level was decreased by about half in Beclin1-deficient mouse retinas (supplemental Fig. S2C). These observations were supported by an immunohistochemical examination of the retinas from Belcin1+/− mice and Belcin1+/+ controls. There were no significant changes in rod photoreceptors by rhodopsin staining and in cone photoreceptors by peanut agglutinin staining (supplemental Fig. S2D). These results indicate that retinal morphology is not affected by Beclin1 deficiency under room lighting conditions.
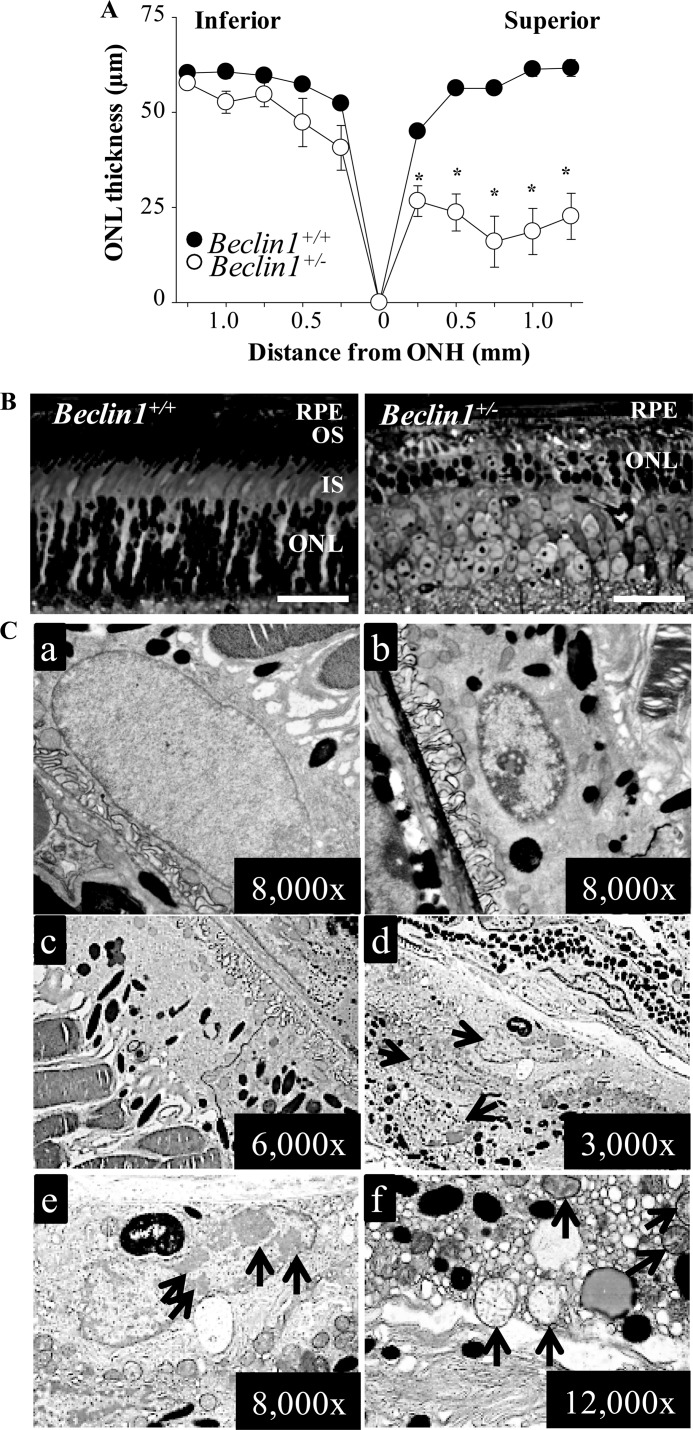
To further address the impact of defective autophagy to the retina under stress conditions, the severity of light-induced retinal degeneration was examined in 6-week-old Beclin1+/− mice and Beclin1+/+ littermate controls. When white light at 5000 lux for 2 h was applied, Beclin1+/− mice developed retinal degeneration prominent in the superior retina as revealed by SD-OCT imaging (supplemental Fig. S3A) and by ONL thickness measurements (Fig. 5A), whereas retinal morphology remained intact in Beclin1+/+ littermate controls. Compared with the intact retinal histology exhibited by light-exposed Beclin1+/+ mice, the retinas of light-exposed Beclin1+/− mice manifested severely disrupted photoreceptor architecture along with morphological changes including diminished outer segment, inner segment, and outer nuclear layer, all of which are typical of light-induced photoreceptor damage (Fig. 5B). Immunohistochemical examination revealed that contrary to the abundant and well organized expression pattern of rhodopsin and peanut agglutinin in control animals, only residual amounts of rhodopsin and peanut agglutinin expression could be detected in the retinas of Beclin1+/− mice (supplemental Fig. S3B). EM was subsequently performed to assess the impact of Beclin1 deficiency on RPE during light-induced retinal damage. Several major ultra-structural changes were noted in RPE cells of Beclin1+/− mice. First, normal euchromatin structure was displayed in an RPE nucleus from Beclin1+/+ controls 24 h after light exposure (Fig. 5Ca), but the Beclin1+/− RPE nucleus displayed heterochromatin and vacuolation (Fig. 5Cb). Moreover, in distinct contrast to the normal RPE ultra-structure displayed in the retina of Beclin1+/+ mice 10 days after light exposure (Fig. 5Cc), RPE hyperplasia marked by multiple nuclei and RPE apoptosis indicated by condensation of the chromatin in large masses at the periphery of the nucleus was noted in Beclin1+/− mice (Fig. 5C, d and e). Additionally, Beclin1+/− RPE cells also contained numerous cytoplasmic vacuoles, which occurred as a result of increased endoplasmic reticulum luminal space (Fig. 5Cf), indicating endoplasmic reticulum stress. Severely swollen mitochondria with disrupted cristae were also readily detected in Beclin1+/− RPE cells (Fig. 5C, e and f). These results indicate that in addition to an impaired response of photoreceptors to photo-oxidative stress, Beclin1 deficiency results in light-induced RPE degeneration marked by endoplasmic reticulum and mitochondrial damage, which invariably leads to elevated oxidative stress. Our results thus imply the importance of maintaining intact autophagy machinery to preserve photoreceptor and RPE integrity during photo-oxidative stress.
FIGURE 5.
Increased susceptibility to light-induced retinal damage as a result of Beclin1 deficiency. Beclin1+/− mice and Beclin1+/+ littermate controls at 6 weeks of age were exposed to white light at 5,000 lux for 2 h. A, the ONL thickness was measured by SD-OCT and plotted (*, p < 0.01). Light-induced photoreceptor degeneration was prominent in the superior retina in Beclin1+/− mice compared with intact retinal morphology displayed by Beclin1+/+ mice. ONH, optic nerve head. B, plastic sections were prepared from eye cups collected from light-exposed Beclin1+/− mice and Beclin1+/+ littermate controls, which was followed by histological examination after toluidine blue staining. Severely disrupted photoreceptor structure was marked by degenerated photoreceptor outer segment and inner segment and significantly reduced thickness of ONL in Beclin1+/− eyes compared with the intact photoreceptor structure displayed by Beclin1+/+ eyes. Bars indicate 20 μm. IS, inner segment. OS, outer segment. C, EM was carried out to examine the RPE structure in Beclin1+/− mice and Beclin1+/+ littermate control 24 h and 10 days after light exposure, respectively. a, EM images from the RPE of Beclin1+/+ mice 24 h after light exposure show normal looking nucleus. B, EM images from the RPE of Beclin1+/− mice 24 h after light exposure show changes in nucleus including chromatin condensation and vacuolation. c, shown are EM images from the RPE of Beclin1+/+ mice 10 days after light exposure. d–f, EM images from the RPE of Beclin1+/− mice 10 days after light exposure reveal chromatin condensation and accumulation of large amounts of damaged mitochondria and dilated EM causing cytoplasmic vacuolation. Arrows in d, RPE hyperplasia indicated by presence of multiple nuclei; arrow in e, nucleus showing chromatin condensation; Arrows in f, damaged mitochondria.
Rod Photoreceptor-specific Atg7 Deletion Does Not Display Abnormal Retinal Architecture under Room Lighting Conditions
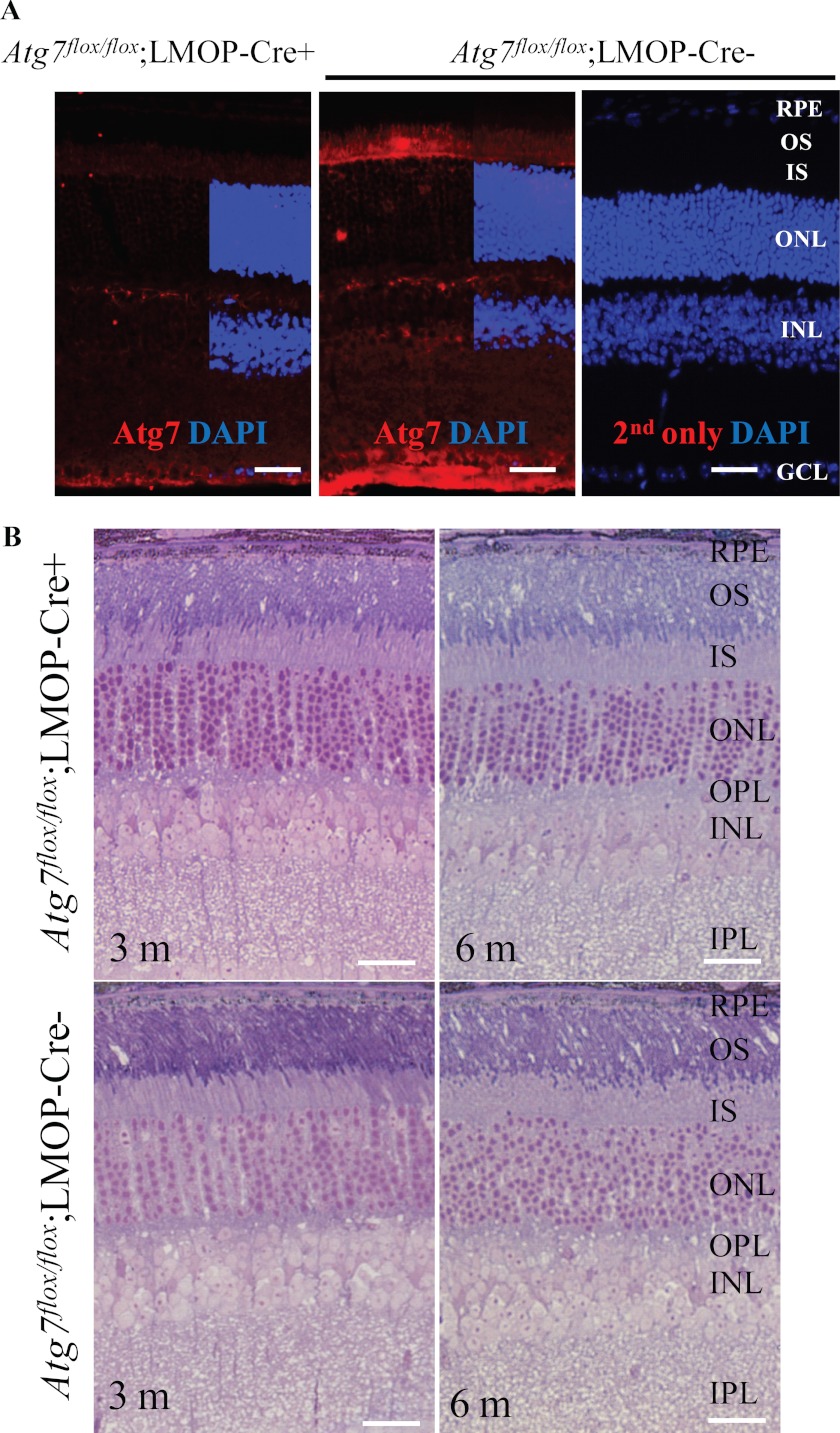
Mice lacking Atg7 are born with the expected Mendelian frequency but rapidly succumb in the first few hours of life (4). To investigate the role of Atg7, one of the essential autophagy regulators (4), in photoreceptors, conditional knock-out mice, Atg7flox/flox;LMOP-Cre+ mice, were employed. Rod photoreceptors are more susceptible to light-induced degeneration than are cone photoreceptors (22–24), and 97% of photoreceptors are rods in the mouse retina (25). LMOP-Cre can achieve rod photoreceptor-specific Cre activation (11), which results in a rod photoreceptor-specific Atg7 deletion under a combination with Atg7flox/flox manipulation. Photoreceptor inner segments in retinas of Atg7flox/flox;LMOP-Cre+ mice showed much weaker signals of Atg7 than those in retinas of Atg7flox/flox;LMOP-Cre− mice (Fig. 6A), indicating photoreceptor-specific deletion of Atg7. Retinal morphology did not show any abnormalities in retinas of Atg7flox/flox;LMOP-Cre+ mice under room lighting conditions at 3 and 6 months of age (Fig. 6B).
FIGURE 6.
Retinal morphology is maintained in mice with rod photoreceptor-specific Atg7 deletion under room lighting conditions. A, Atg7 expression was examined in Atg7flox/flox;LMOP-Cre+ and littermate Atg7flox/flox;LMOP-Cre− mice at 6 weeks of age. Immunohistochemistry was conducted with anti-Atg7 Ab (red) and DAPI (blue). Atg7 signal was much weaker in the inner segment of Atg7flox/flox;LMOP-Cre+ mice than that of Atg7flox/flox;LMOP-Cre− mice. INL, inner nuclear layer; IS, inner segment; OS, outer segment. Bars indicate 20 μm. B, the steady-state retinal morphology under room lighting conditions in Atg7flox/flox;LMOP-Cre+ mice was examined by histological analysis with epon-embedment together with their wild-type counterparts (Atg7flox/flox;LMOP-Cre−) at the ages of 3 and 6 months. No overt morphological changes were observed in Atg7flox/flox;LMOP-Cre+ mice compared with that from Atg7flox/flox;LMOP-Cre- littermate controls. Bars indicate 20 μm.
Increased Photoreceptor Death Caused by Light Exposure and All-trans-retinal in Retinas of Rod Photoreceptor-specific Atg7 Deletion
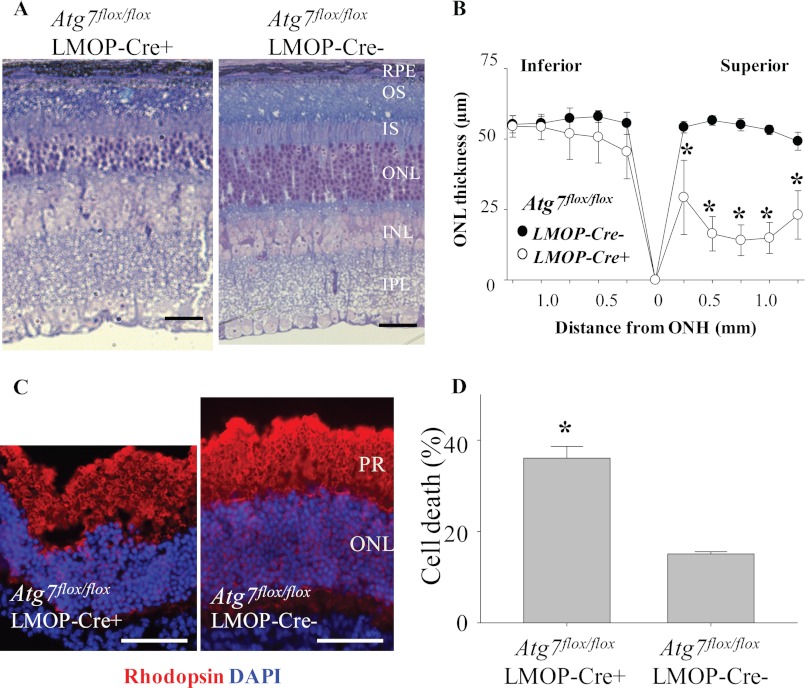
To investigate if Atg7 plays a role in light-associated stress conditions, 1) light exposure to Atg7flox/flox;LMOP-Cre+ mice and 2) coincubation of retinas of Atg7flox/flox;LMOP-Cre+ mice with atRAL were conducted. Atg7flox/flox;LMOP-Cre+ mice at 5–6 weeks of ages were exposed to 5000 lux for 2 h, which caused light-induced retinal degeneration in autophagy-deficient mice due to their Beclin1 deletion (Fig. 5). Atg7flox/flox;LMOP-Cre+ mice displayed light-induced retinal degeneration, whereas none of the littermate Atg7flox/flox;LMOP-Cre− mice showed light-induced retinal damage (Fig. 7A). The severity of light-induced retinal degeneration observed in Atg7flox/flox;LMOP-Cre+ mice is also shown (Fig. 7B).
FIGURE 7.
Increased photoreceptor death caused by light exposure and all-trans-retinal in retinas of rod photoreceptor-specific Atg7 deletion. Atg7flox/flox;LMOP-Cre+ mice and Atg7flox/flox;LMOP-Cre− littermate controls at 5–6 weeks of age were exposed to white light at 5,000 lux for 2 h. Mice were kept in the dark, and retinal morphology was examined 7 days after light exposure. A, plastic sections were prepared from eye cups collected from light-exposed Atg7flox/flox;LMOP-Cre+ mice and Atg7flox/flox;LMOP-Cre− littermate controls, which was followed by histological examination after toluidine blue staining. B, the ONL thickness was measured by SD-OCT and plotted (*, p < 0.01). Light-induced photoreceptor degeneration was prominent in the superior retina in Atg7flox/flox;LMOP-Cre+ mice compared with intact retinal morphology displayed by Atg7flox/flox;LMOP-Cre− littermate mice. C, retinas were collected from Atg7flox/flox;LMOP-Cre+ and Atg7flox/flox;LMOP-Cre− mice, and these retinas were cultured with or without 20 μm atRAL for 6 h at 37 °C. Immunohistochemistry of these retinas were conducted with anti-rhodopsin Ab (red) and DAPI (blue). Bars indicate 20 μm. PR, photoreceptor layer. D, amounts of lactate dehydrogenase (LDH) were measured by lactate dehydrogenase-cytotoxicity assay kit (BioVision) with supernatant of ex vivo retinal culture with 20 μm atRAL for 6 h at 37 °C, and cell death rate was calculated. *, compared with no light, p < 0.01. Rod photoreceptor-specific deletion of Atg7 increased the susceptibility to light-induced and atRAL-induced retinal degeneration.
Retinas of Atg7flox/flox;LMOP-Cre+ and littermate Atg7flox/flox;LMOP-Cre− mice were collected, and each of the retinas was cultured in 0.5 ml of DMEM medium with 10% fetal bovine serum with or without 20 μm atRAL for 6 h at 37 °C. Immunohistochemistry of the incubated retinas was performed with anti-rhodopsin Ab to stain for rod photoreceptors, and DAPI was used for nuclear staining. Reduced numbers of ONL with disrupted photoreceptor layers was observed in the retinas of Atg7flox/flox;LMOP-Cre+ mice, whereas retinal structure was well preserved in retinas of littermate Atg7flox/flox;LMOP-Cre− mice (Fig. 7C). The rate of cell death was calculated by measuring lactate dehydrogenase in culture supernatant of the retinas. Coincubation with 20 μm atRAL caused a higher rate of retinal cell death in retinas of Atg7flox/flox;LMOP-Cre+ mice compared with retinas of Atg7flox/flox;LMOP-Cre− mice (Fig. 7D). These data indicate that Atg7 plays a protective role in rod photoreceptors under stresses caused by light and atRAL.
Up-regulated Park2 Expression Is Associated with Light-induced Retinopathy, and Park2 Deficiency Results in Enhanced Susceptibility to Light-induced Retinopathy
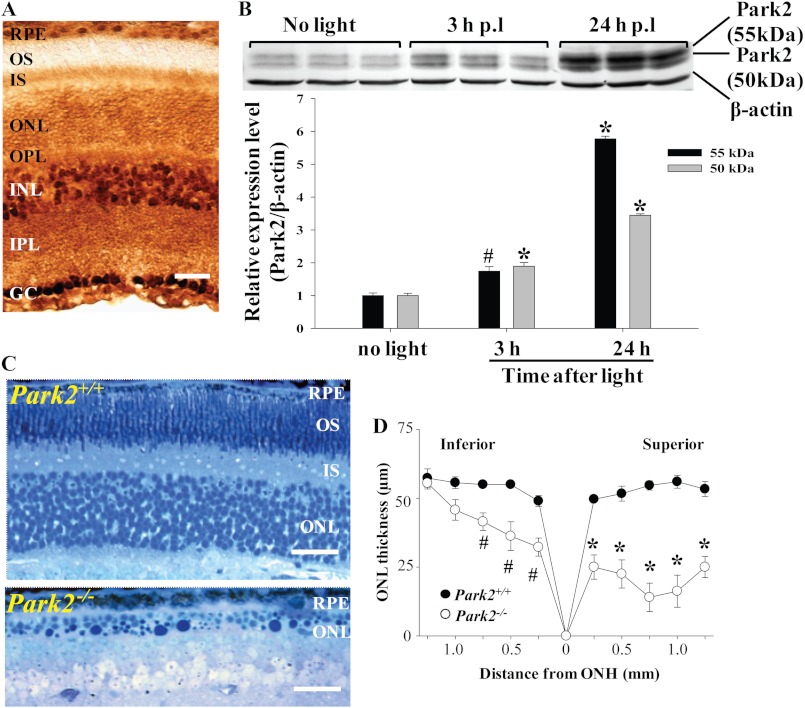
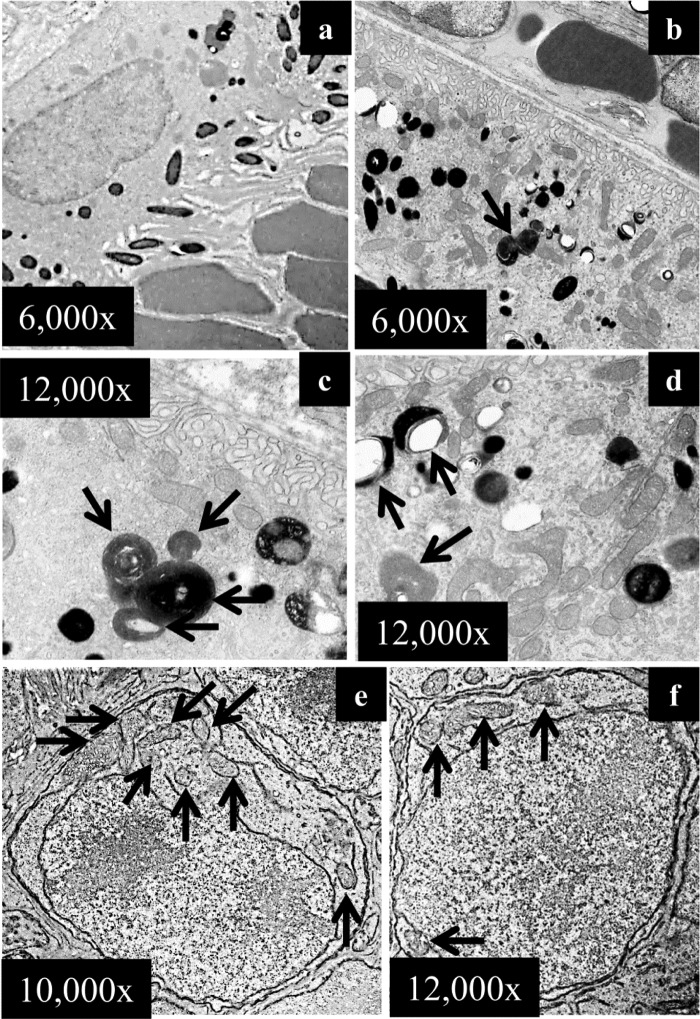
Autophagy gene deficiency results in significant photo-oxidative damage in the RPE and in photoreceptor cells including mitochondrial impairment (Figs. 5 and 7). To further delineate the contribution of mitochondria-specific autophagy, mitophagy, in this process, the expression of Park2, an essential molecular component regulating mitophagy (26), and the impact of Park2 gene deficiency upon photo-oxidative challenge were explored (27). Immunohistochemical assessment revealed that Park2 was localized in various parts of the retinas of WT mice, including RPE cells, photoreceptor inner segments, and the outer nuclear layer (Fig. 8A), which is consistent with the expression pattern reported by an independent study (28). Retinal Park2 protein expression in Abca4−/−Rdh8−/− mice was found to be significantly increased under light conditions capable of causing retinal degeneration (Fig. 8B). Park2+/+ and Park2−/− mice display similar morphologies under steady state room lighting conditions (supplemental Fig. S4A). To further clarify the role of Park2 in light-induced retinal degeneration, intermittent light at 10,000 lux was applied for 20 min with the interval of 2-h dark adaptation, and then retinal morphology was examined 7 days after light exposure. Park2−/− mice and Park2+/+ littermate controls at 6 weeks of age were used. Compared with the intact retinal morphology displayed by Park2+/+ littermate controls exposed to light, severe photoreceptor damage was observed predominantly in the superior retina in Park2−/− mice exposed to light (Fig. 8, C and D). Histological examination validated the photoreceptor damage induced by intermittent light exposure in Park2−/− mice. Compared with the well organized photoreceptor structure displayed by Park2+/+ littermate controls, histological features typical of light-induced photoreceptor damage were evident in Park2−/− mice exposed to light (Fig. 8C), including massive disorganization and reduction in the thickness of photoreceptor OS/inner segment and ONL, the presence of pyknotic photoreceptor nuclei, and nucleic acid deposition. However, when the same dose of light was given in a continuous manner, no overt retinal degeneration was detected by SD-OCT imaging (supplemental Fig. S4B). EM examinations revealed that numerous phagocytized photoreceptor outer segments at different stages of phagocytic clearance were readily detectable in Park2−/− RPE cells 24 h after light exposure (Fig. 9, b–d) when compared with Park2+/+ littermate controls (Fig. 9a). Another ultra-structural feature distinct from other light-sensitive models was the presence of juxtanuclear mitochondria in photoreceptor cells 7 days after light exposure (Fig. 9, e and f). These observations indicate that defective mitophagy is involved in the pathogenesis of light-induced retinopathy.
FIGURE 8.
Enhanced expression of Park2 is associated with light-induced retinopathy and Park2 deficiency results in light-induced retinopathy. A, Park2 expression in the retina of albino WT mice at 6 weeks of age was examined by immunohistochemistry, revealing that Park2 is ubiquitously expressed in the retina. Bars indicate 20 μm. B, Abca4−/−Rdh8−/− mice at 6 weeks of age were exposed to white light at 10,000 lux for 30 min. Park2 expression in the retina was then examined by SDS-PAGE followed by Western blotting with triplicate samples. Up-regulated Park2 expression was observed in response to light exposure. Compared with no light. *, p < 0.01; #, p < 0.05. Is, inner segment; OPL, outer plexiform layer; INL, inner nuclear layer; GC, ganglion cell; OS, outer segment. C, Park2−/− mice together with Park2+/+ littermate controls were exposed to intermittent white light for 40 min given by 20 min each with a 2-h dark adaption between each illumination. Histological examination on plastic sections after toluidine blue staining revealed the photoreceptor damage in Park2−/− mice compared with the intact photoreceptor structure in Park2+/+ mice. Bars indicate 20 μm. D, the ONL thickness was measured by SD-OCT and plotted (*, p < 0.01; #, p < 0.05).
FIGURE 9.
Impaired phagosomal digestion in RPE and ectopic mitochondria in photoreceptors in light-exposed Park2-deficient mice. Park2−/− mice together with Park2+/+ littermate controls at 6 weeks of age were exposed to intermittent light at 10,000 lux for 20 min per exposure, two exposures with a 2-h interval of dark adaptation. The ultra-structure of RPE and photoreceptors were examined 24 h after light exposure. No overt abnormality in RPE ultra-structure was displayed by Park2+/+ mice (a). In Park2−/− RPE cells, undigested or partially digested phagosomes were readily detected (arrows in b–d). A large amount of mitochondria was also present in Park2+/+ RPE cells (d). The ectopic mitochondria were also frequently detected in Park2−/− photoreceptors (e and f).
DISCUSSION
Our findings uncover a mechanistic involvement of autophagy and mitophagy in light-induced retinal degeneration. The results indicate that essential regulators of autophagy are expressed in photoreceptors and RPE cells. Up-regulated autophagic events inversely correlate with atRAL toxicity in cultured retinal cells and are associated with light-induced retinopathy in Abca4−/−Rdh8−/− mice. These findings corroborate at the biochemical level the earlier observation that autophagy events are constitutively present in photoreceptor cells and exhibit dynamic changes in response to light.
All-trans-retinal in Light-induced Retinal Degeneration
atRAL is an important intermediate of the visual cycle that is essential for vertebrate vision (8). This retinoid is highly reactive because of the aldehyde group in this molecule. One example showing the high reactivity of this molecule is the formation of condensation products of atRAL in the retina, such as di-retinoid-pyridinium-ethanolamine (A2E). Accumulation of A2E is associated with aging and retinal diseases including Stargardt disease and age-related macular degeneration (29, 30). The role of atRAL in photo-mediated damage has been suspected for decades; however, there was no experimental evidence showing a high enough concentration of atRAL to cause retinal damage after light exposure. Mice deficient in both Abca4 and Rdh8 cannot correctly clear atRAL in the retina and display light-induced retinal degeneration (9), thus uncovering the detrimental role of atRAL in the retina. Coincubation of atRAL with retinal cells causes mitochondria-associated cell apoptosis (17), and trapping of atRAL with drugs containing primary amine groups prevents retinal cell death (31). The mechanism of atRAL toxicity involves a sequential activation of Gq-G protein-coupled receptors, PLC, inositol 1,4,5-trisphosphate, and NADPH oxidase, which eventually causes oxidative stress (14). In the current study the presence of 2.5 μm atRAL increased autophagosome formation without showing cell death in human derived ARPE19 cells. Conversely, autophagosome formation was decreased in dying cells induced by coincubation with atRAL at higher concentrations including 5 and 10 μm, respectively (Fig. 2). These observations revealed dynamic changes in autophagy in response to atRAL incubation, which inversely correlates with atRAL-mediated cytotoxicity. It is possible that under relatively mild stress, autophagy could be activated to cope with cellular stress, whereas impaired autophagy is associated with increased stress by atRAL, which ultimately leads to cell death.
Autophagy in Light-induced Retinopathy
To directly address the role of autophagy in light-induced photoreceptor degeneration, the Beclin1-deficient mouse model and a rod photoreceptor-specific Atg7-deficient mouse model were employed. Autophagy is tightly regulated and mediated by an array of autophagy-related (Atg) proteins with >30 such proteins having been identified to date. Beclin1 is the mammalian ortholog of the yeast Atg6/Vps30 gene. Both Beclin1 and Atg7 are essential regulators in the initial stage of autophagosome formation (4, 32, 33). Beclin1-deficient homozygosity causes early embryonic lethality (21). Beclin1-deficient heterozygosity results in decreased autophagosome formation in various cell types and predisposes the mice to tumorigenesis (20). Decreased Beclin1 expression is associated with pathogenesis in Alzheimer disease, demonstrating the significance of autophagy in neurodegeneration (2). Involvement of autophagy in retinal degeneration was addressed by examining the retinal morphology in Beclin1+/− mice. Beclin1+/− mice displayed normal retinal morphology when compared with their wild-type counterparts under room lighting conditions. When intense light was applied, Beclin1+/− mice developed severe retinal degeneration.
Atg7 homozygous deletion causes early postnatal lethality (4). Rod photoreceptor-specific deletion of Atg7 in mice was obtained by using the Atg7flox/flox;LMOP-Cre+ combination. These mice showed decreased Atg7 protein expression in photoreceptor inner segments by immunohistochemistry (Fig. 6A) and increased susceptibility to light-induced retinal degeneration (Fig. 7, A and B). In retinal tissue cultured with atRAL, the loss of Atg7 in rod photoreceptors led to a higher rate of cell death (Fig. 7, C and D). In addition that Atg7 is one of the essential regulators of autophagy, Atg7 can also regulate proapoptotic p53. In the absence of Atg7, prolonged metabolic stress leads to augmented p53 proapoptotic activity (34). atRAL-induced cell death involves mitochondria-associated apoptosis, and inhibition of Bax activation rescues cells from atRAL-induced cell death (17). Proapoptotic p53 activation is tightly connected with activation of Bax (35, 36). The interactions of atRAL-Bax-p53-Atg7 may increase susceptibility of retinal cells dying due to light exposure and atRAL.
Taken together, our results show that Beclin1+/− and Atg7flox/flox;LMOP-Cre mice, models deficient in autophagy, are susceptible for light-induced retinal degeneration. These unique mice provide direct evidence supporting the importance of autophagy in the maintenance of retinal homeostasis during light exposure.
Park2 and Mitophagy in Light-induced Retinopathy
The role of a more specific form of autophagy, mitophagy, was also examined in retinal degeneration, considering that photoreceptor cells are heavily packed with mitochondria and atRAL causes mitochondrial impairment. Mitochondrial dysfunction is an important pathological element mediating the onset of neurodegenerative disorders, including the selective loss of dopaminergic neurons in Parkinson disease. Park2 has been determined to be strikingly and specifically recruited to dysfunctional mitochondria (26). Mitochondrial function is paraphrased as a master regulator of the life of the cell, not surprisingly due to its essential function involving energy consumption and its critical role in promoting apoptosis. Autophagy has been demonstrated to be one of the regulatory mechanisms for mitochondrial quality control, specifically involved in the removal and subsequent recycling of damaged or excess mitochondria in the cell. This autophagy-mediated elimination of mitochondria is defined as mitophagy and is specifically regulated at molecular level by several molecules such as Park2- and PTEN-induced putative kinase1 (PINK1) (37). Park2 is recruited to damaged mitochondria initiating the mitophagy process not only in cultured cell lines (38) but also in primary neurons (39). Increased expression of Park2 suppresses cell death. Conversely, Park2 deficiency is associated with mitochondrial dysfunction, which causes an increase in intracellular oxidative stress in patients with Parkinson disease (40) and in an animal model (41). Indeed, increased numbers of damaged mitochondria were observed in Park2−/− mice after light exposure. Moreover, Park2-related mitochondrial quality control has an impact on mitochondrial transport and raises the possibility that aberrant trafficking of damaged mitochondria could be one of mechanistic aspects of retinal degeneration as suggested by the observation of ectopic mitochondria being present in Park2−/− photoreceptors upon light exposure.
In summary, this study identifies an important role for autophagy and mitophagy in photoreceptor and RPE cells under photo-oxidative stress conditions and, therefore, indicates that dysregulated autophagy and mitophagy have a significant impact on the development of light-induced retinopathy.
Acknowledgments
LC3B-GFP vector was a kind gift from Dr. N. Mizushima (Tokyo Medical and Dental University). 1D4 Ab was a generous gift from Dr. R. S. Molday (University of British Columbia). Beclin1-deficient mice were originally generated by Dr. B. Levine (UT Southwestern, Dallas, TX). Atg7flox/flox mice were obtained from Dr. K. Tanaka (Laboratory of Frontier Science, Tokyo Metropolitan Institute of Medical Science, Tokyo). We thank Drs. K. Palczewski, H. Fujioka, M. Hitomi, S. Howell, and S. Roos (Case Western Reserve University) for comments and technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants K08EY019031, K08EY019880, R01EY022658, R01EY018341, R24EY021126, and P30EY011373. This work was also supported by the Research to Prevent Blindness Foundation, Foundation Fighting Blindness, Fight for Sight, Ohio Lions Eye Research Foundation, and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.

This article contains supplemental Figs. S1–S4.
- RPE
- retinal pigment epithelium
- atRAL
- all-trans-retinal
- RDH8
- retinol dehydrogenase 8
- SD-OCT
- spectral domain-optic coherence tomography
- ABCA4
- ATP binding cassette transporter 4
- ONL
- outer nuclear layer
- Ab
- antibody.
REFERENCES
- 1. Yang Z., Klionsky D. J. (2010) Eaten alive. A history of macroautophagy. Nat. Cell Biol. 12, 814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Invest. 118, 2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 [DOI] [PubMed] [Google Scholar]
- 4. Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizushima N., Komatsu M. (2011) Autophagy. Renovation of cells and tissues. Cell 147, 728–741 [DOI] [PubMed] [Google Scholar]
- 6. Remé C. E., Young R. W. (1977) The effects of hibernation on cone visual cells in the ground squirrel. Invest. Ophthalmol. Vis. Sci. 16, 815–840 [PubMed] [Google Scholar]
- 7. Remé C. E., Wolfrum U., Imsand C., Hafezi F., Williams T. P. (1999) Photoreceptor autophagy. Effects of light history on number and opsin content of degradative vacuoles. Invest. Ophthalmol. Vis. Sci. 40, 2398–2404 [PubMed] [Google Scholar]
- 8. von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maeda A., Maeda T., Golczak M., Palczewski K. (2008) Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 283, 26684–26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Portillo J. A., Okenka G., Reed E., Subauste A., Van Grol J., Gentil K., Komatsu M., Tanaka K., Landreth G., Levine B., Subauste C. S. (2010) The CD40-autophagy pathway is needed for host protection despite IFN-γ-dependent immunity and CD40 induces autophagy via control of P21 levels. PloS ONE 5, e14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Y. Z., Zheng L., Zheng W., Ash J. D., Agbaga M. P., Zhu M., Anderson R. E. (2006) Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol. Vis. 12, 389–398 [PubMed] [Google Scholar]
- 12. Wenzel A., Reme C. E., Williams T. P., Hafezi F., Grimm C. (2001) The Rpe65 L450M variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J. Neurosci. 21, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattapallil M. J., Wawrousek E. F., Chan C. C., Zhao H., Roychoudhury J., Ferguson T. A., Caspi R. R. (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells and confounds ocular-induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 53, 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y., Okano K., Maeda T., Chauhan V., Golczak M., Maeda A., Palczewski K. (2012) Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 287, 5059–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maeda A., Maeda T., Imanishi Y., Kuksa V., Alekseev A., Bronson J. D., Zhang H., Zhu L., Sun W., Saperstein D. A., Rieke F., Baehr W., Palczewski K. (2005) Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 280, 18822–18832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284, 15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lange P. S., Chavez J. C., Pinto J. T., Coppola G., Sun C. W., Townes T. M., Geschwind D. H., Ratan R. R. (2008) ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J. Exp. Med. 205, 1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda A., Golczak M., Maeda T., Palczewski K. (2009) Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest. Ophthalmol. Vis. Sci. 50, 5435–5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E. L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yue Z., Jin S., Yang C., Levine A. J., Heintz N. (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U.S.A. 100, 15077–15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okano K., Maeda A., Chen Y., Chauhan V., Tang J., Palczewska G., Sakai T., Tsuneoka H., Palczewski K., Maeda T. (2012) Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light-induced damage. J. Neurochem. 121, 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holz F. G., Gross-Jendroska M., Eckstein A., Hogg C. R., Arden G. B., Bird A. C. (1995) Colour contrast sensitivity in patients with age-related Bruch's membrane changes. Ger. J. Ophthalmol. 4, 336–341 [PubMed] [Google Scholar]
- 24. Shelley E. J., Madigan M. C., Natoli R., Penfold P. L., Provis J. M. (2009) Cone degeneration in aging and age-related macular degeneration. Arch. Ophthalmol. 127, 483–492 [DOI] [PubMed] [Google Scholar]
- 25. Jeon C. J., Strettoi E., Masland R. H. (1998) The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dagda R. K., Cherra S. J., 3rd, Kulich S. M., Tandon A., Park D., Chu C. T. (2009) Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 284, 13843–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esteve-Rudd J., Campello L., Herrero M. T., Cuenca N., Martín-Nieto J. (2010) Expression in the mammalian retina of parkin and UCH-L1, two components of the ubiquitin-proteasome system. Brain Res. 1352, 70–82 [DOI] [PubMed] [Google Scholar]
- 29. Parish C. A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. (1998) Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. U.S.A. 95, 14609–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sparrow J. R. (2010) Bisretinoids of RPE lipofuscin. Trigger for complement activation in age-related macular degeneration. Adv. Exp. Med. Biol. 703, 63–74 [DOI] [PubMed] [Google Scholar]
- 31. Maeda A., Golczak M., Chen Y., Okano K., Kohno H., Shiose S., Ishikawa K., Harte W., Palczewska G., Maeda T., Palczewski K. (2012) Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 8, 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aita V. M., Liang X. H., Murty V. V., Pincus D. L., Yu W., Cayanis E., Kalachikov S., Gilliam T. C., Levine B. (1999) Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59, 59–65 [DOI] [PubMed] [Google Scholar]
- 33. Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 [DOI] [PubMed] [Google Scholar]
- 34. Lee I. H., Kawai Y., Fergusson M. M., Rovira I. I., Bishop A. J., Motoyama N., Cao L., Finkel T. (2012) Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 336, 225–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oren M. (2003) Decision making by p53. Life, death, and cancer. Cell Death Differ. 10, 431–442 [DOI] [PubMed] [Google Scholar]
- 36. Chipuk J. E., Kuwana T., Bouchier-Hayes L., Droin N. M., Newmeyer D. D., Schuler M., Green D. R. (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010–1014 [DOI] [PubMed] [Google Scholar]
- 37. Narendra D. P., Jin S. M., Tanaka A., Suen D. F., Gautier C. A., Shen J., Cookson M. R., Youle R. J. (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y. L., Selkoe D., Rice S., Steen J., LaVoie M. J., Schwarz T. L. (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 [DOI] [PubMed] [Google Scholar]
- 41. Palacino J. J., Sagi D., Goldberg M. S., Krauss S., Motz C., Wacker M., Klose J., Shen J. (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 279, 18614–18622 [DOI] [PubMed] [Google Scholar]